Abstract
Background:
We sought to determine if a structured educational program for neurology residents can lower door-to-needle (DTN) times at an academic institution.
Methods:
A neurology resident educational stroke boot camp was developed and implemented in April 2013. Using a prospective database of 170 consecutive acute ischemic stroke (AIS) patients treated with IV tissue plasminogen activator (tPA) in our emergency department (ED), we evaluated the effect of the intervention on DTN times. We compared DTN times and other process measures preintervention and postintervention. p Values < 0.05 were considered significant.
Results:
The proportion of AIS patients treated with tPA within 60 minutes of arrival to our ED tripled from 18.1% preintervention to 61.2% postintervention (p < 0.001) with concomitant reduction in DTN time (median 79 minutes vs 58 minutes, p < 0.001). The resident-delegated task (stroke code to tPA) was reduced (75 minutes vs 44 minutes, p < 0.001), while there was no difference in ED-delegated tasks (door to stroke code [7 minutes vs 6 minutes, p = 0.631], door to CT [18 minutes in both groups, p = 0.547]). There was an increase in stroke mimics treated (6.9% vs 18.4%, p = 0.031), which did not lead to an increase in adverse outcomes.
Conclusions:
DTN times were reduced after the implementation of a stroke boot camp and were driven primarily by efficient resident stroke code management. Educational programs should be developed for health care providers involved in acute stroke patient care to improve rapid access to IV tPA at academic institutions.
National guidelines specify that eligible acute ischemic stroke (AIS) patients should be treated with IV tissue plasminogen activator (tPA) within 60 minutes of arrival to an emergency department (ED),1 as the odds for good outcomes are reduced with delays in time to treatment.2,3
In 2011, Get With The Guidelines introduced the Target Stroke program, 11 best practice strategies to reduce door-to-needle (DTN) times.4–6 This program successfully reduced DTN times to less than 60 minutes at hospitals across the country with improvement in patient outcomes.7,8 International studies of process improvement have shown even more impressive reductions in DTN times, but these results required the presence of stroke attending physicians to interpret neuroimaging, obtain consent, and administer IV tPA.9,10 Having stroke attending physician presence in the ED at all times is unrealistic for most hospitals in the United States.
Even with participation in the Target Stroke program, academic medical centers (AMCs) face additional challenges. Neurology residents are usually the first responders to stroke codes and evaluate and manage AIS patients in the ED. A prior single-center study showed that resident-run stroke codes and thrombolytic decision-making by a senior resident can effectively lower DTN times without an increase in symptomatic intracranial hemorrhage (sICH).11 At our institution, junior neurology residents often staff the ED consultation service, making it difficult to implement a senior resident–led protocol. We hypothesized that a stroke boot camp for all residents could improve resident efficiency during acute stroke code management, resulting in lower DTN times.
METHODS
Intervention design and implementation
A neurology resident educational curriculum was developed and implemented in April 2013. All residents were required to attend, and were free of all clinical responsibilities during the 3 hours of the educational program. Subsequently, the incoming junior residents attended the boot camp during their orientation in July. All residents were required to complete formal NIH Stroke Scale (NIHSS) training prior to the program. We created a Socratic case-based stroke boot camp consisting of 4 patient scenarios: (1) a patient with contraindications for IV tPA, (2) a patient who received IV tPA (appendix e-1 at Neurology.org/cp), (3) a patient eligible for endovascular therapy, and (4) an inpatient stroke code. While endovascular therapy was discussed in the boot camp, the purpose of this study was to evaluate the effect on DTN times for IV tPA administration. We presented each case and paused to ask residents how they would proceed with obtaining focused neurologic history and examination, medication history, and decision-making for acute thrombolytic and endovascular therapy. Key teaching points were emphasized, including the distinction between symptom discovery and last known well times, evaluation of early infarct signs and hemorrhage on noncontrast head CT, IV tPA exclusions, criteria for premixing IV tPA, and evaluation for large vessel syndromes. We also discussed the challenges involved in obtaining this information when the patient was aphasic, had neglect, or family members were not present. We highlighted the need to confirm historical data through collateral sources. Appendix e-1 shows an example of our case presentation, which includes some protocols specific to our institution, but this case could be modified to apply to other hospitals. We used various methods including patient cases, mnemonics, pocket cards with IV tPA risks/benefits (appendix e-2), and talking points for discussion with patients and families (table 1). The boot camp was facilitated by senior residents, stroke fellows, and stroke attending physicians. Pretests and posttests were conducted to assess resident knowledge and familiarity with stroke code management and decision-making.
Table 1.
Components of stroke code boot camp
Demographic and clinical variables
Our study was a cross-sectional study evaluating consecutive patients presenting to the ED with acute stroke symptoms who were treated with IV tPA from January 2010 through August 2015. Patient data including demographic information, NIHSS scores, relevant times in the DTN process, complications of tPA, and outcomes were collected in a prospective database during this time period. We compared demographic data, NIHSS scores, relevant times, complications, and outcomes preintervention (January 2010–March 2013) and postintervention (April 2013–August 2015). We also evaluated the month of July separately, as this is the first month of residency training, to ensure that this was not a source of bias. As we did not collect discharge modified Rankin Scale scores, our favorable outcome was based on discharge location, with favorable outcome being defined as discharge to home or acute rehabilitation, and unfavorable outcome being defined as discharge to skilled nursing facility or in-hospital death. We used the National Institute of Neurological Disorders and Stroke IV tPA trial to define sICH as a hemorrhage not seen on a previous CT scan with either a suspicion of hemorrhage or any decline of neurologic status.12
All patients without contraindications had MRI of the brain during their hospital admission. For those patients without evidence of restricted diffusion on MRI, 2 independent stroke neurologists (M.B.M. and F.Z.C.) who were unfamiliar with the patients evaluated case summaries and independently rated the cases as stroke mimic or aborted stroke. Stroke mimic was defined as a patient with neurologic symptoms that did not appear to be secondary to vascular etiology and were likely secondary to an alternative cause. An aborted stroke was defined as a patient who presented with neurologic symptoms and examination consistent with ischemia to a vascular territory (clinical transient ischemic attack) without another explanation after diagnostic workup and imaging. All discrepancies were adjudicated by a third stroke neurologist (F.A.S.).
Standard protocol approvals, registrations, and patient consents
The data collection was approved by the Northwestern University Institutional Review Board. Because this was a quality improvement initiative, waiver of informed consent was granted.
Statistical analysis
Means and medians were reported for continuous variables. Fisher exact tests and Pearson χ2 were used for dichotomized variables. Mann-Whitney U and Kruskal-Wallis tests were used for all continuous variables aside from age, which was compared via the Student t test. We assessed differences in relevant times, proportion meeting DTN time <60 minutes, adverse outcomes, and discharge outcomes in preintervention and postintervention groups. We also compared the same measures in subgroups by postgraduate year (PGY) level. We considered p < 0.05 to be statistically significant. Statistical analysis was performed using IBM SPSS Statistics version 23.0 (IBM, Armonk, NY).
RESULTS
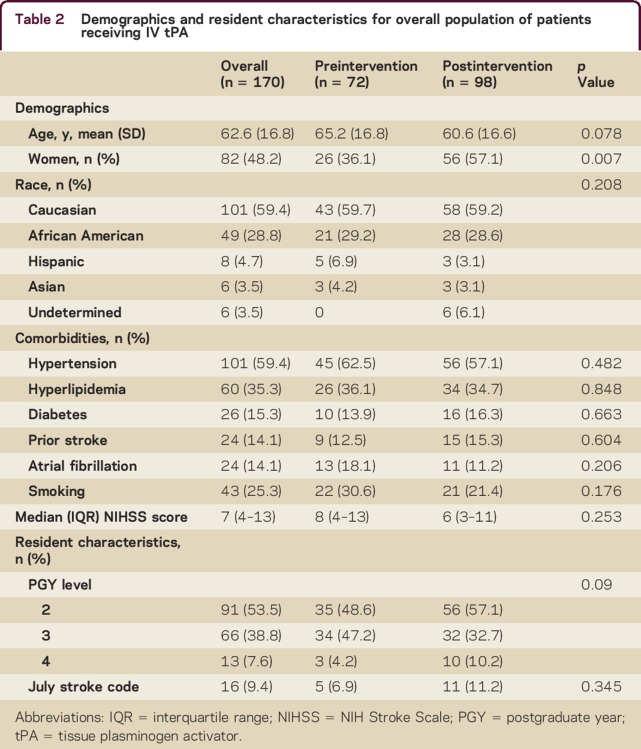
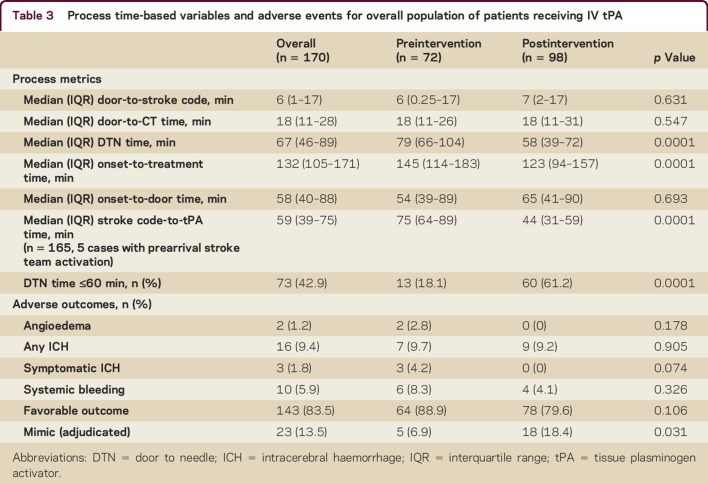
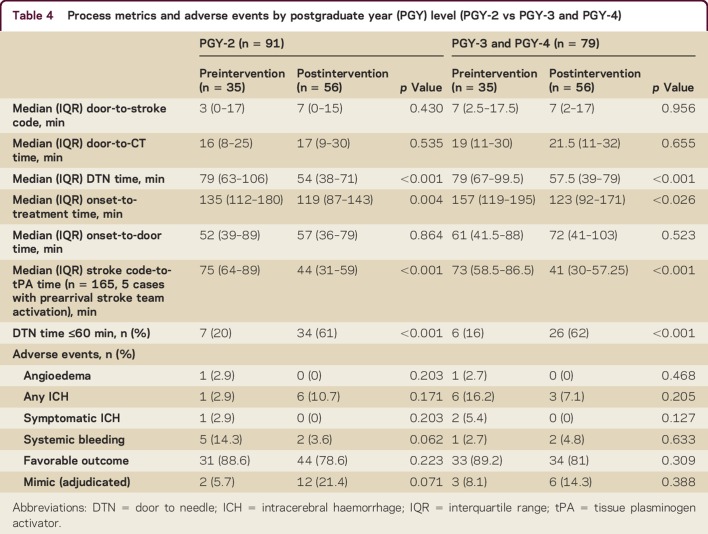
We analyzed 170 consecutive AIS patients treated with IV tPA in our ED during the study period. The mean age was 63 years, 48% were women, and 59% were Caucasian. The preintervention and postintervention groups did not differ by any demographic variables except sex; there were more women in the postintervention group (36.1% vs 57.1%, p = 0.007, table 2). There were no differences in vascular comorbidities, baseline NIHSS scores, PGY level, or July effect. The proportion of patients treated within 60 minutes increased from 18.1% preintervention to 61.2% postintervention (p < 0.001) with a major reduction in median DTN time from 79 minutes to 58 minutes (p < 0.001). Door to stroke code (median 7 minutes vs 6 minutes, p = 0.631) and door to CT (median 18 minutes in both groups, p = 0.547) were not different in the preintervention and postintervention periods, but stroke code to tPA time was reduced (75 vs 44 minutes; p < 0.001, table 3). The time reductions occurred in both junior residents (PGY-2) and senior residents (PGY-3 and PGY-4) without increase in adverse outcomes (table 4). Residents performed a knowledge test before and after the educational module. While this was a small sample size of 15 residents with incomplete data, there was an improvement in aggregate test scores with a mean pretest score of 71% (n = 14) and mean posttest score of 78% (n = 11). We were not able to compare individual scores of residents, as the data were de-identified.
Table 2.
Demographics and resident characteristics for overall population of patients receiving IV tPA
Table 3.
Process time-based variables and adverse events for overall population of patients receiving IV tPA
Table 4.
Process metrics and adverse events by postgraduate year (PGY) level (PGY-2 vs PGY-3 and PGY-4)
There was a reduction in sICH (4.2% vs 0%, p = 0.074, table 3) following the intervention. There was no difference in other adverse outcomes or favorable discharge outcomes comparing the preintervention and postintervention periods (table 3). In the final adjudication, there were 23 stroke mimic cases (13.5%). There was an increase in stroke mimics (6.9% vs 18.4%, p = 0.031, table 3) in the postintervention period. There were more mimics treated with tPA among junior residents (21.4% vs 5.7%, p = 0.071, table 4) in the postintervention period. There was no difference noted among senior residents.
DISCUSSION
Our study demonstrated that a neurology resident education program, stroke boot camp, reduced DTN times at an AMC. Process intervals influenced by the ED such as door-to-stroke code and door-to-imaging times did not change after our intervention, while stroke code to IV tPA time, a process reflecting neurology resident management, was reduced by over 30 minutes. This held true for both senior and junior neurology residents, suggesting equal benefit in both experienced and new residents. The importance to patient care cannot be overstated, as faster onset-to-treatment times have been shown to reduce disability, mortality, and sICH, and improve ambulation and discharge to home for acute stroke patients receiving IV tPA.2–4,8
Our study confirms the result of a prior study conducted at another AMC, Washington University, which demonstrated that a resident-based acute stroke protocol can reduce DTN times without increasing adverse events including sICH.11 In that study, senior residents (PGY-4) were given autonomy to make stroke thrombolysis decisions. At our institution, both junior and senior residents are required to discuss all patients with a board-certified stroke attending physician prior to thrombolytic administration.
AMCs face a unique challenge in acute stroke management in that our first responders are often junior residents. While requiring attending physician presence prior to administration of IV tPA improves diagnostic accuracy and treatment formulation, it may delay DTN time, especially during nights and weekends. At our institution, junior residents are the first stroke team responders in over 50% of cases. Our residency program leadership has placed emphasis on earlier experience with neurologic emergencies to ensure that our senior residents can appropriately supervise junior residents on inpatient services. In addition, like many other neurology residency programs across the country, our program has modified the curriculum to ensure that residents obtain a balanced education in both inpatient and outpatient neurology subspecialties and research. In order to ensure this balance and comply with duty hour restrictions, there is increased exposure to inpatient rotations as junior residents (PGY-2) and more outpatient rotations and research as senior residents (PGY-3 and PGY-4). Hence, our junior residents are often on the front line of acute stroke management.
Leading stroke codes requires rapid neurologic evaluation, review of eligibility criteria, and discussion of IV tPA risks and benefits with a patient and family during a quick encounter. Without formal training, this experience can be daunting for neurology residents, especially earlier in their training. Based on our results, it is clear that a structured, case-based simulation course for neurology residents can provide the knowledge base, problem-solving skills, and confidence necessary to make acute stroke management decisions more rapidly. It is reassuring that in our study, there were considerable reductions in DTN times for both junior and senior residents managing acute stroke patients in the ED without an increase in adverse events. Our findings potentially extend outside the academic realm, to hospitals with advanced practice providers and ED physicians who are an essential part of the acute stroke team. Ensuring that all providers who participate in acute stroke management are prepared for their tasks is important, and standardized training can help improve system efficiency and quality.
Ongoing training will be required as national and international organizations and guidelines set increasingly loftier targets for DTN performance.
AMCs also have a mission to educate neurology residents. Residents are required to be exposed to acute stroke decision-making during their training. Neurology residents are evaluated every 6 months along a continuum of milestones created by the Accreditation Council for Graduate Medical Education. The cerebrovascular disorders patient care milestone outlines that residents should learn the indications/contraindications for IV tPA therapy, understand how to appropriately use thrombolytic therapy, and appropriately refer patients for interventional therapy as part of their training.13 Implementation of a resident-run stroke protocol serves both the patient care and educational missions of AMCs. It facilitates delivery of rapid stroke care to patients while providing residents the first-hand training experiences and learning opportunities necessary for matriculation from residency.
We observed an unintended consequence of our intervention: an increase in stroke mimic treatment. The increased mimic treatment rate did not result in more adverse outcomes, but it raises the concern of increased diagnostic error that may occur with less experienced physicians. Alternatively, increased mimic treatment may have been a consequence of lower diagnostic accuracy in the setting of reduced time to treatment. We have previously reported on this experience at our institution, noting the relationship between increased mimic treatment and decreased DTN at our center.14 Our rate of mimic treatment is similar to that of other institutions, which ranges from 1% to 25%.15–18 One study using a CT-based protocol found an increase of mimics in the postintervention period after a DTN intervention.19 A recent study using an MRI-based protocol prior to IV tPA administration demonstrated that DTN times could be lowered to <60 minutes with a mimic rate of 0%.19 While stroke mimic treatment is reduced or even eliminated by MRI, this may not be a practical solution, as most hospitals do not have the required resources to obtain MRI prior to thrombolysis. While the administration of IV tPA to a stroke mimic has potential risks of systemic and intracranial hemorrhage and angioedema, as well as increased costs of stroke unit care and wasted drug resources, a recent multicenter retrospective observational study and review of the literature confirmed that adverse outcomes for stroke mimics treated with IV tPA were minimal.16 Nevertheless, given this unintended consequence, stroke mimic treatment rates should be monitored before and after quality initiatives to reduce DTN times.
Sixty-one percent of the adjudicated stroke mimic cases were in women. It is possible that the increase in women treated in the postintervention period may be explained by the uptick in treated stroke mimics. Prior studies have shown that women are more likely to present with atypical stroke symptoms.20,21 In addition, our boot camp may have also taught our residents to recognize more atypical stroke symptoms, resulting in an increase in stroke mimic treatment.
Our study was limited as it was a single-center study without a control group. We did not control for the fact that DTN times have been reduced across the country due to accumulated experience. The frequency of IV tPA administration also increased in the postintervention period, which could reflect similar developments across the country and contribute to improved DTN times. In addition, there was a concurrent DTN project in our ED that began 6 months after our education intervention. However, given that our door-to-stroke code and door-to-CT times did not change, it is likely that the stroke boot camp had the strongest effect on DTN times by lowering stroke code-to-tPA times. Finally, it should be noted that we reported DTN times from consecutive patients presenting to the ED with acute focal neurologic deficits, without excluding cases for acceptable delay (e.g., blood pressure lowering with IV agents).
DTN times improved after implementation of a stroke boot camp program and were driven primarily by efficient resident stroke code management. At AMCs where residents are first-line providers, specialized residency training should be developed and implemented to reduce DTN times while also increasing resident exposure and competence in acute stroke management. In addition, education should be developed for all health care providers who participate in acute stroke management, as these findings would likely apply to settings outside of the academic realm.
Supplementary Material
Footnotes
Supplemental data at Neurology.org/cp
AUTHOR CONTRIBUTIONS
I.M. Ruff: drafting/revising manuscript for content including medical writing for content, study concept/design, analysis and interpretation of data. A.L. Liberman: drafting/revising manuscript for content including medical writing for content, study concept/design, analysis and interpretation of data. F.Z. Caprio: drafting/revising manuscript for content including medical writing for content. M.B. Maas: drafting/revising manuscript for content including medical writing for content. S.J. Mendelson: drafting/revising manuscript for content including medical writing for content. F.A. Sorond: drafting/revising manuscript for content including medical writing for content. D. Bergman: drafting/revising manuscript for content including medical writing for content. R.A. Bernstein: drafting/revising manuscript for content including medical writing for content. Y. Curran: drafting/revising manuscript for content including medical writing for content. S. Prabhakaran: drafting/revising manuscript for content including medical writing for content, study concept/design, analysis and interpretation of data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
I.M. Ruff, A.L. Liberman, and F.Z. Caprio report no disclosures. M.B. Maas serves on a scientific advisory board for Hyperfine Research and receives research support from NIH. S.J. Mendelson receives research support from the University of Chicago. F.A. Sorond serves on scientific advisory boards for North American Thrombosis Forum and Boston Biomedical Associates and receives research support from NIH (NIA, NINDS). D. Bergman reports no disclosures. R.A. Bernstein serves on scientific advisory boards and speakers' bureaus for and has received funding for travel and speaker honoraria from Boehringer Ingelheim and Medtronic. Y. Curran reports no disclosures. S. Prabhakaran has served as a Section Editor for Current Atherosclerosis Reports; receives publishing royalties from UpToDate, Inc.; and receives research support from NIH/NINDS and PCORI. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Jauch EC, Saver JL, Adams HP, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 2.Meretoja A, Keshtkaran M, Saver JL, et al. Stroke thrombolysis: save a minute, save a day. Stroke 2014;45:1053–1058. [DOI] [PubMed] [Google Scholar]
- 3.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–1703. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013;309:2480–2488. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation 2011;123:750–758. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association's Target: stroke initiative. Stroke 2011;42:2983–2989. [DOI] [PubMed] [Google Scholar]
- 7.Ruff IM, Ali SF, Goldstein JN, et al. Improving door-to-needle times: a single center validation of the target stroke hypothesis. Stroke 2014;45:504–508. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632–1640. [DOI] [PubMed] [Google Scholar]
- 9.Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012;79:306–313. [DOI] [PubMed] [Google Scholar]
- 10.Meretoja A, Weir L, Ugalde M, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology 2013;81:1071–1076. [DOI] [PubMed] [Google Scholar]
- 11.Ford AL, Connor LT, Tan DK, et al. Resident-based acute stroke protocol is expeditious and safe. Stroke 2009;40:1512–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 13.The Neurology Milestone Project. Available at: acgme.org/portals/0/pdfs/milestones/neurologymilestones.pdf. Accessed January 25, 2017. [Google Scholar]
- 14.Liberman AL, Liotta EM, Caprio FZ, et al. Do efforts to decrease door-to-needle time risk increasing stroke mimic treatment rates? Neurol Clin Pract 2015;5:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmen TM, Meyer BC, McClean TL, Lyden PD. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, Stroke Center. J Stroke Cerebrovasc Dis 2008;17:23–25. [DOI] [PubMed] [Google Scholar]
- 16.Zinkstok SM, Engelter ST, Gensicke H, et al. Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke 2013;44:1080–1084. [DOI] [PubMed] [Google Scholar]
- 17.Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med 2003;42:611–618. [DOI] [PubMed] [Google Scholar]
- 18.Chernyshev OY, Martin-Schild S, Albright KC, et al. Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology 2010;74:1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota's lean manufacturing principles and value stream analysis. Stroke 2012;43:3395–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah S, Luby M, Poole K, et al. Screening with MRI for Accurate and Rapid Stroke Treatment: SMART. Neurology 2015;84:2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisabeth L, Brown DL, Hughes R, Majersik JJ, Morgenstern LB. Acute stroke symptoms comparing women and men. Stroke 2009;40:2031–2036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.