Abstract
Purpose
To describe the clinical course of advanced juxtapapillary retinal capillary hemangioblastomas (RCH) associated with von Hippel-Lindau (VHL) disease treated with systemic sunitinib malate, an agent that inhibits both anti-vascular endothelial growth factor and anti-platelet-derived growth factor signaling.
Design
Observational case review.
Participants
Three patients with advanced VHL-related juxtapapillary RCH treated with systemic sunitinib malate.
Methods
Patient 1 was followed routinely every 4 months while on systemic sunitinib prescribed by her oncologist for metastatic pancreatic neuroendocrine and kidney tumors. Patients 2 and 3 were part of a prospective clinical trial evaluating the use of systemic sunitinib for ocular VHL lesions during a period of 9 months. Visual acuity, size of RCH, and degree of exudation were recorded at each visit. Optical coherence tomography (OCT) and fluorescein angiography were also obtained at some visits.
Main Outcome Measures
Visual acuity, size of RCH, and degree of exudation.
Results
Three patients with advanced VHL-associated juxtapapillary RCH were treated with systemic sunitinib malate. While none of the patients lost vision during therapy, treatment with sunitinib malate did not improve visual acuity or reduce the size of RCH. Improvements in RCH-associated retinal edema were observed in two patients. All patients experienced multiple adverse effects, including thyroid toxicity, thrombocytopenia, nausea, fatigue, jaundice, and muscle aches. Two of the three patients had to discontinue treatment prematurely and the third required dose reduction.
Conclusions
Systemic sunitinib malate may be useful in slowing progression of ocular disease from VHL-associated RCH. However, significant systemic adverse effects limited its use in this small series, and systemic sunitinib malate may not be safe for treatment of RCH when used at the doses described in this report. Further studies are required to determine if this medication used at lower doses with different treatment strategies, other medications in the same class or drugs directed at multiple targets in the tumor, may be safer and more effective for the treatment of advanced VHL-associated RCH.
Keywords: von Hippel-Lindau disease, retinal capillary hemangioblastoma, vascular endothelial growth factor, platelet-derived growth factor, sunitinib
Introduction
Von Hippel-Lindau (VHL) disease is a heritable multisystem cancer syndrome characterized by hemangioblastomas of the retina and central nervous system (CNS), renal cell carcinomas, pheochromocytomas, as well as tumors of the pancreas, epididymis, and broad ligment.1 Mutations in the VHL tumor suppressor gene are transmitted in an autosomal dominant manner, and VHL disease is highly penetrant. Retinal capillary hemangioblastomas (RCH) are the most common ocular lesion found in patients with VHL disease and may be located anywhere in the retina from the juxtapapillary region to the periphery.2 RCH cause vision loss through structural damage to the retina or optic nerve from the tumor itself, from subretinal and intraretinal exudation, or from fibroglial membrane contraction with formation of tractional retinal detachment. In a large series of 406 VHL patients (205 of which had ocular VHL lesions), approximately 8% of eyes with VHL lesions had visual acuity <20/200, and approximately 8% of the entire population had unilateral enucleation.3 Standard therapy for RCH remains ablation with either laser photocoagulation or cryotherapy.4 Other treatment modalities, including photodynamic therapy (PDT), radiotherapy, and anti-vascular endothelial growth factor (VEGF) intravitreal injections, have also been attempted with varying degrees of success.2, 5-7
VHL disease is caused by mutations in the VHL tumor suppressor gene product (pVHL). Normally, pVHL functions as an E3 ligase that ubiquinates hypoxia-inducible factor alpha (HIFα) leading to HIFα degradation.8, 9 Mutations in pVHL result in reduced degradation and increased protein levels of HIFα. As a crucial transcriptional regulator of cellular responses to hypoxia, HIFα modulates the expression of hundreds of proteins, including pro-angiogenic factors, such as VEGF and platelet-derived growth factor (PDGF). Increased production of pro-antigenic and cell proliferative factors is thought to play a role in formation of hemangioblastomas of the retina and CNS, and high expression of VEGF has been found in pathologic RCH specimens.10, 11
Blockade of VEGF signaling with pegaptanib,6 a small molecule inhibitor of the VEFG165 isoform, and ranibizumab,7 an anti-VEGF monoclonal antibody, in patients with VHL-associated RCH has been investigated with variable results, which suggested modest beneficial effects in terms of reduced exudation from RCH in some patients but showed no change in the overall size or lesion burden of RCH. VEGF and PDGF function through binding to their respective receptors, which belong to a family of proteins known as receptor tyrosine kinase (RTK). Sunitinib malate is a tyrosine kinase inhibitor (TKI) that blocks multiple RTK, including those for VEGF and PDGF.12 We hypothesized that blocking both VEGF and PDGF signaling might exert greater inhibitory effects on RCH than antagonism of VEGF alone. Here we report the clinical course of three patients with advanced juxtapapillary RCH associated with VHL disease who underwent treatment with systemic sunitinib malate.
Methods
The clinical characteristics, including age, systemic VHL disease involvement, and details of sunitinib malate therapy, as well as ocular testing, including visual acuity, optical coherence tomography, color fundus photography, and fluorescein angiography were collected for three consecutive VHL patients treated with sunitinib malate. Patient 1 was started on sunitinib malate by her oncologist for VHL-associated kidney and metastatic pancreatic neuroendocrine tumors. She was initially started on sunitinib 50mg/day orally in cycles of 4 weeks followed by a two-week rest period according to the standard chemotherapy regimen. However, she developed nausea, fatigue, jaundice, and muscle aches associated with treatment that led to a dose reduction to 25mg/day. Patient 1 was seen approximately every four months at the National Eye Institute under an IRB-approved protocol for ocular examination and testing while on sunitinib therapy.
Patients 2 and 3 were participants in an IRB-approved open-label non-controlled prospective pilot study conducted at the National Eye Institute, NIH (ClinicalTrials.gov Identifier: NCT00673816).13 This study aimed to enroll five patients with ocular VHL disease. However, enrollment was limited due to stringent inclusion criteria, and the study was terminated early after enrolling only two patients. The off-label use of sunitinib malate for ocular VHL lesions was discussed with all participants. Participants received sunitinib malate therapy with each cycle consisting of 50 mg oral sunitinib once daily for four weeks followed by a two-week rest period. Routine systemic evaluation and laboratory testing was conducted throughout the trial to assure safety. The study aimed to complete six cycles of therapy (nine months) for each patient. As discussed below, both patients stopped the trial early because of adverse effects.
Results
Patient 1
A 32 year-old woman with genetically confirmed VHL disease started systemic sunitinib therapy through her oncologist for metastatic pancreatic neuroendocrine and kidney tumors. She had a history of progressive RCH in her left eye that had resulted in blindness and subsequent enucleation approximately 20 years prior. In her right eye, she had an exudative juxtapapillary RCH. Systemically, her VHL disease also involved CNS hemangioblastomas requiring multiple craniotomies, and a pancreatic tumor requiring pancreaticoduodenectomy as well as radiation therapy to the remaining pancreatic tissue.
Four years prior to starting sunitinib, visual acuity in her right eye measured at 20/63. At that time, the juxtapapillary RCH OD was approximately two disc diameters in size on the supratemporal aspect of the optic nerve and was associated with significant surrounding retinal vascular proliferation and worsening exudation and macular lipid accumulation. She received six intravitreal injections of pegaptanib as part of a clinical trial6 as well as 1 dose of intravitreal triamcinolone over a three-year period. One year prior to starting sunitinib, she received external beam radiation therapy to this lesion. These treatments had not significantly altered the size of the lesion. During this period, she also developed epiretinal fibrosis around the RCH and a lamellar hole in the nasal macula (Fig. 1A). Approximately two months prior to starting sunitinib, visual acuity in her right eye measured 20/125.
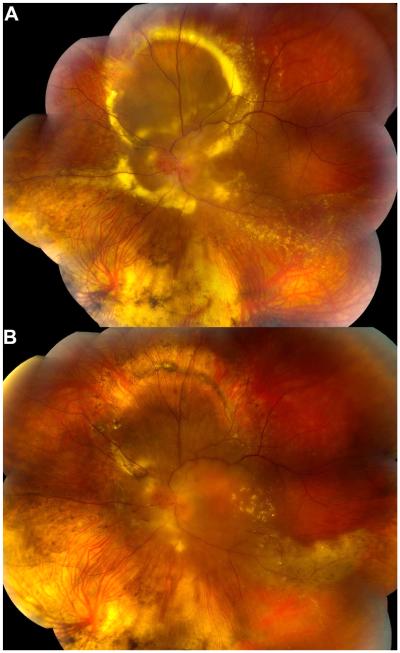
Figure 1.
Color fundus photographs of the right eye of a 32 year-old woman with advanced VHL-associated juxtapapillary RCH. A) Approximately two months prior to starting sunitinib therapy. Note the large vascular optic nerve RCH with surrounding fibrovascular proliferation and nasal macular lamellar hole. B) Photographs at the indicated times after starting sunitinib therapy. She underwent vitrectomy with membrane peeling shortly after the 1 year photo. Note the decrease in tumor vascularity with time. Visual acuity remained stable at 20/125 over this time period.
The patient was initially started on sunitinib 50mg/day orally in cycles of 4 weeks followed by a two-week rest period according to the standard chemotherapy regimen. However, she developed nausea, fatigue, jaundice, and muscle aches associated with treatment that led to a dose reduction to 25mg/day. Approximately one year after starting sunitinib malate, she had repeat vitrectomy performed for macular traction. During the subsequent eight years of sunitinib therapy, her right eye juxtapapillary RCH became progressively less vascular with reduction in the surrounding retinal elevation (Fig. 1B). She maintained 20/125 visual acuity in this eye without additional ocular treatment. After receiving approximately nine years of treatment, her oncologist stopped sunitinib malate and started everolimus for enlarging hepatic metastases.
Patient 2
A 40 year-old woman presented to her ophthalmologist with a large juxtapapillary RCH in her left eye and was subsequently diagnosed with VHL disease by genetic testing. Her right eye had no evidence of retinal hemangioblastoma with excellent vision at 20/16. Her visual acuity was 20/50 in the left eye, and examination of the left eye revealed a large juxtapapillary RCH approximately two disc diameters in size on the supranasal aspect of the optic nerve with significant retinal edema and exudate involving the fovea (Fig. 2A). She was treated with multiple intravitreal injections of bevacizumab (3 injections) and ranibizumab (11 injections) over a period of 2 years, as well as external beam radiation therapy. Despite these treatments, exudation from the RCH resulted in an exudative retinal detachment and decline in vision to 20/640 over the course of four years following presentation.
Figure 2.
Color fundus photographs of the right eye of a 40 year-old woman with advanced VHL-associated juxtapapillary RCH. A) Approximately three months prior to starting sunitinib therapy. Note the large vascular optic nerve RCH with surrounding retinal edema and lipid exudate. B) Five months after starting sunitinib therapy. Note the decreased peripapillary retinal edema and lipid exudate especially superior to the optic nerve. Visually significant macular edema remained.
At this point, the patient was enrolled in the clinical trial NCT00673816 and was started on systemic sunitinib therapy. Each cycle of treatment consisted of 50mg oral sunitinib daily for four weeks, followed by a two week rest period according to the standard chemotherapy regimen. After the first treatment cycle, she was noted to have progressively elevated thyroid stimulating hormone (TSH) levels. She was started on levothyroxine with normalization of her thyroid function labs. During the third cycle of sunitinib treatment, she developed acute thyroiditis, which resolved without treatment. As these adverse events were judged to be possibly related to study treatment, sunitinib therapy was stopped three weeks into her third treatment cycle (approximately five months of treatment). No further abnormalities in her thyroid function tests were noted after discontinuation of sunitinib malate.
Three months after starting sunitinib (after two cycles of treatment), her visual acuity OS measured 20/640. The peripapillary retinal edema and lipid were reduced from baseline, however, lipid exudate in the temporal macula increased. When treatment was terminated at 5 months post-initiation, visual acuity OS measured 20/640 and further reduction in peripapillary retinal edema and lipid exudate was noted relative to her examination at 3 months post-treatment (Fig. 2B). However, the size of the juxtapapillary RCH was unchanged from baseline and significant residual edema remained in the macula. Examination approximately five years later, during which no additional retinal treatment was administered, visual acuity OS was found to be stable at 20/640, and the juxtapapillary RCH was observed to be unchanged in size, indicating a lack of significant lesion progression following sunitinib cessation.
Patient 3
A 52 year-old woman with a VHL-related juxtapapillary RCH and longstanding light perception vision in her left eye due to prior increased intracranial pressure was enrolled in the clinical trial NCT00673816 and started on sunitinib in an attempt to halt progression of RCH-associated complications. The juxtapapillary RCH was approximately two disc diameters in size on the infratemporal aspect of the optic nerve and was associated with significant macular edema, epiretinal membrane, temporal macular fibrosis, and lipid exudate throughout the posterior pole that was progressing despite two prior PDT treatments (Fig 3A&B).
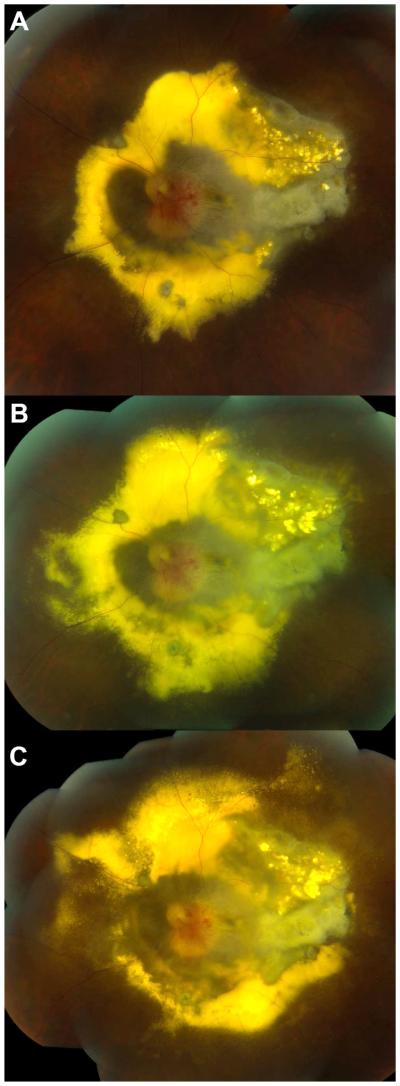
Figure 3.
Color fundus photographs of the right eye of a 52 year-old woman with advanced VHL-associated juxtapapillary RCH. A) Approximately six months prior to starting sunitinib therapy. Note the large optic nerve RCH with surrounding retinal edema and lipid exudate. B) Increase in lipid exudate at the time sunitinib therapy was initiated. C) Four months after receiving a discontinuous and truncated course of sunitinib therapy. No improvement in RCH size or amount of lipid exudation was observed.
Systemic sunitinib was initiated with planned treatment cycles of 50 mg orally per day for four weeks followed by a two-week rest period according to the standard chemotherapy regimen. However, laboratory testing revealed thrombocytopenia on day 12 of treatment in the first cycle, and the treatment phase of the cycle was terminated early at this point. She also developed hypertension requiring anti-hypertensive medication. These adverse events were judged to be possibly related to sunitinib. Her platelet count returned to normal within two months of stopping the medication. For the second cycle, the sunitinib dose was reduced to 37.5mg per day. After 14 days of treatment, she developed epistaxis, and the treatment cycle was again stopped early. She was able to successfully complete the third cycle at a sunitinib dose of 37.5mg per day; however, reductions in both white blood cell and platelet counts precluded further treatment and the patient was terminated from the study approximately four months after initiating sunitinib. Her vision remained in the range of hand motions to light perception, and no improvements in RCH size or lipid exudation were observed during sunitinib therapy (Fig. 3C).
Discussion
Successful treatment of advanced VHL lesions, especially exudative juxtapapillary RCH, remains a challenge. Elevated levels of VEGF have been detected in the ocular fluids and in pathological specimens of eyes with VHL-associated RCH.10, 14 Given the proposed role for VEGF in the pathogenesis of VHL-associated RCH, blockade of the VEGF signaling axis has been attempted in the treatment of VHL-associated ocular lesions, including advanced juxtapapillary RCH. Two small, non-controlled, prospective clinical trials have been conducted investigating the use of pegaptanib, a pegylated aptamer that specifically binds VEGF165, and ranibizumab, an anti-VEGF antibody fragment, in the treatment of VHL-associated RCH.6, 7 Results from these trials are variable but suggest that VEGF blockade may reduce exudation from RCH in some eyes without any evidence for regression of RCH.
In addition to VEGF, other pro-angiogenic cytokines, such as PDGF, have been implicated in VHL disease tumorigenesis.9 VEGF and PDGF mediate their function through binding to RTK. TKI, like sunitinib malate, block these receptors and subsequent downstream signaling cascades. Two patients with VHL-associated RCH treated with semaxanib, a TKI specific for VEGF receptor-2, have been reported.15, 16 While neither experienced a decrease in the size of RCH, one observed reduction in associated macular edema15 and the other an improvement in visual function measured using Humphrey visual field parameters and contrast sensitivity.16
Sunitinib is a multi-targeted TKI that blocks receptors for VEGF, PDGF, and several other growth factors.12 Sunitinib is approved by the United States Food and Drug Administration for the treatment of refractory gastrointestinal stromal tumors, advanced renal cell carcinoma, and pancreatic neuroendocrine tumors.17 We hypothesized that blockade of PDGF signaling in addition to that of VEFG might enhance the efficacy of treating VHL-associated RCH. All of the patients in this report had progressive disease attributable to RCH prior to starting sunitinib. Patient 1, who was treated with systemic sunitinib for approximately 9 years, showed a reduction in the vascularity of her juxtapapillary RCH and retinal edema. However, no change in the size of the RCH was observed. Of note, the patient also received external beam radiation to her juxtapapillary RCH about one year prior to starting sunitinib therapy, and this may have had ongoing beneficial effects on tumor vascularity and leakage throughout her treatment with sunitinib. Improvement in parapapillary fibrovascular proliferation was also observed but was likely a result of vitrectomy surgery with membrane peeling. Patient 2, who was treated with sunitinib for approximately five months, showed a reduction in peripapillary retinal edema and lipid exudate. As in patient 1, prior external beam radiation therapy may have contributed to these mild improvements. The size of her RCH did not change, and she continued to have visually significant macular edema. Moreover, this patient had to discontinue treatment prematurely due to adverse effects. Patient 3, who had several treatment interruptions and had to discontinue treatment prematurely due to adverse effects, did not manifest any observable benefits. Visual acuity remained stable during treatment in all three patients; however tumor regression was not observed. These cases suggest a possible role for sunitinib therapy in reducing the exudative effects of RCH, but sunitinib had little apparent effect on inducing tumor regression. These findings are in accord with those of a previous small phase II trial of 15 patients with VHL disease, nine of whom had RCH, in which systemic sunitinib therapy did not reduce the size of RCH.18
Systemic sunitinib therapy has been associated with several adverse effects, including but not limited to hepatotoxicity, left ventricular dysfunction, hypertension, hemorrhagic events, and thyroid dysfunction.19 Patient 2 developed overt thyroiditis and hypothyroidism after starting sunitinib. Her thyroiditis resolved without treatment, and her thyroid hormone levels normalized after stopping sunitinib. Patient 3 in our series experienced hypertension that required treatment as well as thrombocytopenia that ultimately required cessation of sunitinib. The use of an alternative TKI, sorafenib, which also inhibits VEGFR, has been reported in two patients with neovascular age-related macular degeneration (nvAMD), another ocular pathology driven by VEGF.20 In this report, two patients were treated with 200 mg sorafenib three times per week for 1-2 months. The authors report possible improvements in intraretinal fluid on OCT with this therapy. One patient developed acral dermatitis that subsequently resolved. No other side effects were reported. Perhaps alternative TKI or reduced doses of sunitinib may reduce adverse effects in patients with VHL.
RCH associated with VHL disease may cause significant vision loss, and these lesions and their sequelae remain difficult to treat, especially when large or juxtapapillary in location. Intravitreal anti-VEGF therapy has shown occasional reduction in exudation in some patients without a beneficial effect on RCH regression.6, 7 Multi-targeted TKI, such as sunitinib malate, block receptors for several cytokines, including VEGF and PDGF, involved in the pathogenesis of VHL disease and have been useful in treating VHL-associated renal cell carcinoma.12 In the current report of three patients with advanced VHL-associated RCH treated with systemic sunitinib malate, improvement in retinal edema was observed in two patients, and none of the patients lost vision during sunitinib therapy. However, treatment with sunitinib malate did not reduce the size of RCH or improve visual acuity. Moreover, systemic adverse effects led to dose reduction in the patient 1 and premature discontinuation of sunitinib malate in patients 2 and 3. These results suggest that systemic sunitinib may be useful in reducing exudation from advanced VHL-associated RCH for cases in which local ablative therapies are not feasible or have failed, however, the severe systemic adverse effects limit the use of this drug. Further studies are required to determine the safety and efficacy of systemic sunitinib treatment perhaps at a lower dose or with a different treatment strategy. It may be important to evaluate other drugs in this class or other drugs that may affect multiple targets in the tumors in patients with advanced or refractory VHL-associated RCH to find a safer and more effective treatment. Given these well-known severe systemic adverse effects, studies of intraocular delivery of drugs that block VEGF and PDGF in eyes with age-related macular degeneration may also be translated for testing in eyes with advanced VHL ocular lesions in the future.
Acknowledgments
Financial support: This work was supported by the National Eye Institute Intramural Research Program, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflicting relationship exists for any author.
References
- 1.Lonser RR, Glenn GM, Walther M, et al. 2003;361(9374):2059–67. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 2.Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001;46(2):117–42. doi: 10.1016/s0039-6257(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 3.Chew EY. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495–511. [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AD, Nouri M, Shields CL, et al. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109(10):1799–806. doi: 10.1016/s0161-6420(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 5.Sachdeva R, Dadgostar H, Kaiser PK, et al. Verteporfin photodynamic therapy of six eyes with retinal capillary haemangioma. Acta Ophthalmol. 2010;88(8):e334–40. doi: 10.1111/j.1755-3768.2010.02008.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahr SS, Cusick M, Rodriguez-Coleman H, et al. Intravitreal anti-vascular endothelial growth factor therapy with pegaptanib for advanced von Hippel-Lindau disease of the retina. Retina. 2007;27(2):150–8. doi: 10.1097/IAE.0b013e318030a290. [DOI] [PubMed] [Google Scholar]
- 7.Wong WT, Liang KJ, Hammel K, et al. Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease. Ophthalmology. 2008;115(11):1957–64. doi: 10.1016/j.ophtha.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohh M, Park CW, Ivan M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2(7):423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 9.Kaelin WG., Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2(9):673–82. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 10.Chan CC, Vortmeyer AO, Chew EY, et al. VHL gene deletion and enhanced VEGF gene expression detected in the stromal cells of retinal angioma. Arch Ophthalmol. 1999;117(5):625–30. doi: 10.1001/archopht.117.5.625. [DOI] [PubMed] [Google Scholar]
- 11.Chan CC, Lee YS, Zhuang Z, et al. Von Hippel-Lindau gene deletion and expression of hypoxia-inducible factor and ubiquitin in optic nerve hemangioma. Trans Am Ophthalmol Soc. 2004;102:75–9. discussion 9-81. [PMC free article] [PubMed] [Google Scholar]
- 12.Rini BI. Sunitinib. Expert Opin Pharmacother. 2007;8(14):2359–69. doi: 10.1517/14656566.8.14.2359. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs-El N, Chew EY, Srinivasan R, et al. Systemic Sunitinib Malate in the Treatment of Optic Nerve Hemangioblastomas. Investigative Ophthalmology & Visual Science. 2012;53 ARVO E-Abstract 4940. [Google Scholar]
- 14.Los M, Aarsman CJ, Terpstra L, et al. Elevated ocular levels of vascular endothelial growth factor in patients with von Hippel-Lindau disease. Ann Oncol. 1997;8(10):1015–22. doi: 10.1023/a:1008213320642. [DOI] [PubMed] [Google Scholar]
- 15.Girmens JF, Erginay A, Massin P, et al. Treatment of von Hippel-Lindau retinal hemangioblastoma by the vascular endothelial growth factor receptor inhibitor SU5416 is more effective for associated macular edema than for hemangioblastomas. Am J Ophthalmol. 2003;136(1):194–6. doi: 10.1016/s0002-9394(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 16.Aiello LP, George DJ, Cahill MT, et al. Rapid and durable recovery of visual function in a patient with von hippel-lindau syndrome after systemic therapy with vascular endothelial growth factor receptor inhibitor su5416. Ophthalmology. 2002;109(9):1745–51. doi: 10.1016/s0161-6420(02)01159-4. [DOI] [PubMed] [Google Scholar]
- 17.Rock EP, Goodman V, Jiang JX, et al. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12(1):107–13. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- 18.Jonasch E, McCutcheon IE, Waguespack SG, et al. Pilot trial of sunitinib therapy in patients with von Hippel–Lindau disease. Ann Oncol. 2011;22(12):2661–6. doi: 10.1093/annonc/mdr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.2011. Sutent Prescribing Information.
- 20.Diago T, Pulido JS, Molina JR, et al. Ranibizumab combined with low-dose sorafenib for exudative age-related macular degeneration. Mayo Clin Proc. 2008;83(2):231–4. doi: 10.1111/j.1600-0420.2007.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]