Abstract
Aging leads to a number of physiological alterations, specifically changes in circulating hormone levels, increases in fat deposition, decreases in metabolism, changes in inflammatory responses, and reductions in growth factors. These progressive changes in physiology and metabolism are exacerbated by modern culture and Western diet and give rise to diseases such as obesity, metabolic syndrome, and type 2 (non–insulin-dependent) diabetes (T2D). These age and lifestyle-related metabolic diseases are often accompanied by insulin and leptin resistance, as well as aberrant amylin production and signaling. Many of these alterations in hormone production and signaling are directly influenced by an increase in both oxidative stress and inflammation. Importantly, changes in hormone production and signaling have direct effects on brain function and the development of age-related neurologic disorders. Therefore, this review aims to present evidence on the effects that diet and metabolic disease have on age-related cognitive decline and the development of cognitive diseases, particularly Alzheimer disease. This review will focus on the metabolic hormones insulin, leptin, and amylin and their role in cognitive decline, as well as the therapeutic potential of these hormones in treating cognitive disease. Future investigations targeting the long-term effects of insulin and leptin treatment may reveal evidence to reduce risk of cognitive decline and Alzheimer disease.
Keywords: Diabetes, Obesity, Alzheimer disease, Insulin, Leptin, Amylin
1. Introduction
Technological, medical, agricultural, and cultural advances have led to decreases in cost and increases in the availability and accessibility of medical care and food. Although these advances have largely increased the overall quality of life, they have also indirectly led to increases in the prevalence of age- and lifestyle-related diseases. To this end, obesity rates have steadily increased in the United States over the past 50 years; an estimated 31.5% and 13.4% of the population was overweight or obese in the 1960s, respectively, whereas 34.7% and 36% of US citizens were considered overweight or obese, respectively, as of 2012 [1,2]. The average American meal in the 1960s contained approximately half the energy load that the average meal consists of today [3,4]. Although many studies have evaluated the macronutrient content of various diets and their correlation to fat deposition, a high profile study published in the New England Journal of Medicine showed that adherence to energy restriction was the most important factor when dieting. The macronutrient content of a diet is less important than a person’s ability to maintain energy restriction over time [5], suggesting that positive energy balance is the greatest contributing factor to weight gain. This fact, coupled with a simultaneous decrease in physical activity, has led to a state of chronic positive energy balance for most Americans [6]. Collectively, this amounts to more than 65% of the US population being classified as either overweight or obese.
Obesity was officially recognized as a disease in 2013 by the American Medical Association and must be treated as such by medical professionals and insurance companies in the United States [7,8]. Overweight and obese individuals have a significantly higher risk of developing other conditions, namely, cardiovascular diseases such as heart disease, coronary artery disease, urolithiasis [9], metabolic syndrome [10], and type 2 (non–insulin-dependent) diabetes (T2D) [11]. Together, obesity, metabolic disorder, and T2D have emerged as some of the most prominent and most expensive health concerns in the world [1,12].
Importantly, lifestyle-related metabolic diseases are strong contributing factors to the development of age-related cognitive diseases, such as Alzheimer disease (AD) [13–21]. Although poor diet and lack of exercise can give rise to obesity, fat deposition can also increase during aging as metabolic function decreases. Baby boomers, being the largest aging population in the United States [22,23], also have the highest obesity rates of all age groups, approximately 40% [12]. Thus, aging and lifestyle-related metabolic disease likely synergize with one another, vastly increasing the rate and incidence of age-associated cognitive decline and AD development.
The mechanisms that underlie both metabolic and age-related neurologic diseases such as AD and how these mechanisms synergize to speed up development of AD are not fully understood; it is certain, however, that both aging and metabolic disease are accompanied by similar changes in oxidative stress (OS) and inflammation. Regardless of etiology, metabolic dysfunction is strongly associated with aspects of cognitive decline and the development of cognitive diseases. To this end, this review will explore the effects of metabolic disease and metabolic hormone dysregulation on mechanism underlying age-related cognitive decline and AD. This review does not focus on specific macronutrients or diets, but instead on consequences and risk factors associated with the obese state and diabetes and their contribution to brain aging/cognitive decline. Articles selected for this review were found using PubMed, published in the last 5 years, and highly cited studies from the fields of endocrinology and neuroscience were of greatest interest. The publicly available clinical trials database, ClinicalTrials.gov, was used to search for studies involving the hormones insulin, leptin, and amylin with regard to cognitive and metabolic disease.
2. Mechanisms of cognitive aging
2.1. Oxidative stress
There are a number of synonymous physiological changes that occur both in the periphery and in the brain during aging. One critical aspect associated with both normal aging and dysregulated metabolism is increased OS [24–26]. Oxidative stress is a direct result of an increased production of reactive oxygen species (ROS). Reactive oxygen species are small, chemically reactive molecules containing oxygen that are naturally produced during cellular metabolism and are tightly regulated within the cell by antioxidants and enzymes [27]. Although ROS hold a number of functions within the cell, an overproduction of ROS results in a disruption of normal cellular functioning. For example, excessive ROS in a system causes damage to proteins, lipids, and nucleic acids, eventually leading to diseases such as diabetes, cancer, chronic inflammation, cardiovascular diseases, aging, and a number of degenerative diseases [24–26,28].
Extensive evidence shows that there is increased OS in the normal aging brain [24–26]. This is mainly due to the fact that the brain requires high levels of oxygen to function, while having high levels of iron and ascorbate, high levels of unsaturated fatty acids, and low antioxidant capabilities [24]. Together, these physiological characteristics of the brain leave neural tissue susceptible to OS and related damage. Thus, aberrant ROS production resulting from age-related losses in endogenous free radical defenses [26], exposure to excess environmental OS, [29] and diets poor in antioxidants [28] lead to increased oxidative damage. This damage then has direct repercussions on protein structure and function, causes damage to DNA, and facilitates inflammation [30].
Importantly, age-related changes in metabolism, specifically increased adiposity and hormone resistance, also lead to increases in OS [31,32]. For example, aberrant insulin signaling results in mitochondrial dysfunction and increased ROS production [33–35]. More specifically, ATP production, chemical gradients within the mitochondria, and mitochondrial fission were altered in diabetic and obese rodents [33,34]. Similarly, excessive amylin signaling is associated with increased ROS production, which further facilitates OS, apoptosis, and decreased insulin production within the pancreas [36,37]. This OS facilitates hormone resistance [32,38] and diffuse cellular dysfunction both centrally and peripherally [39,40]. As such, the relationship between OS and hormone resistance is highly dynamic: hormone resistance gives rise to OS, but OS can also contribute to the initial or further hormone resistance. To this end, diets high in fat and low in antioxidants are main contributors to excessive OS production and thus the development of metabolic diseases and related cognitive impairments. As previously mentioned, OS gives rise to cellular damage and dysfunction, but it also results in increased inflammation, a second hallmark of aging and metabolic disease.
2.2. Inflammation
Many neurodegenerative diseases are multifactoral in nature. As such, cellular damage and dysfunction resulting from normal age-related and/or disease state induced OS can result in genotypic and phenotypic changes, one of which is inflammation.
Aging and metabolic dysfunction are both responsible for producing OS and cellular dysfunction, 2 phenomena known to activate the immune system [30]. Adiposity is also positively correlated with secretion of proinflammatory cytokines [41–43]. In turn, adipocyte-produced proinflammatory cytokines facilitate insulin resistance and further metabolic dysfunction. high-fat diets have also been shown to increase inflammation both in the periphery and in the brain [44] while also reducing insulin sensitivity [45,46]. Taken together, normal aging, diet, and metabolic disease contribute to inflammatory processes that further facilitate cognitive decline and the propensity to develop cognitive disease states.
Alzheimer disease in particular is a well-established multifactorial disease that is strongly associated with inflammation, OS [31,32], and changes in protein processing [47]. Metabolic hormone dysfunction is strongly implicated in age-related cognitive decline and AD; as such, the remainder of this review will explore how changes in important specific metabolic hormones due to aging, obesity, and T2D affect the brain and cognition and how these hormones may serve as treatment strategies to prevent or slow down AD development.
3. Metabolic dysregulation links to cognitive decline and AD development
Alterations in peripheral metabolic function that stem from a sedentary lifestyle have profound effects on brain function and development of AD, the most common age-related type of neurodegenerative disease and dementia [48]. Alzheimer disease is clinically marked by changes in mood, aggression, loss of appetite, and loss of the ability to form new memories as well as to recall old memories. [49]. Alzheimer disease pathology is characterized by the development of 2 distinct pathological hallmarks: extracellular senile amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs) composed of hyper phosphorylated tau proteins [47,50]. These specific pathological phenomena accumulate within the brain and materialize as cellular dysfunction and eventual neuronal loss, ultimately externalizing as the clinical symptoms of AD [47].
It is still unclear whether pathology drives OS production and inflammation directly or if pathology is driven by age-related changes in metabolism. However, it is known that OS and mitochondrial dysfunction are intimately associated with neurodegeneration and AD [51–53]. Improper processing of the synaptic protein amyloid precursor protein due to mutations of unknown age-related mechanisms leads to the production of Aβ; soluble Aβ proteins tend to aggregate into toxic Aβ fibrils. The other histologic hallmark of AD pathology is associated with the protein tau. Tau is a microtubule-associating protein, primarily responsible for binding to and stabilizing the cytoskeletal aspects of neuronal processes. Hyper phosphorylated tau is no longer able to perform its primary function and also begins to aggregate, forming disruptive and toxic NFTs. Together, and perhaps synergistically with accumulated age-related, metabolic damage, these Aβ plaques and NFTs disrupt normal neuronal functioning by inducing OS, inflammation, and mitochondria dysfunction [35]. These then disrupt physiological signaling and organelle trafficking and destabilize neurites, ultimately leading to large-scale neuronal loss [54].
Mounting evidence suggests that midlife obesity contributes an increased risk for age-related cognitive decline [18–20]; however, conflicting evidence exists with regard to the direct relationship between obesity and risk for dementia [17–20,55–57]. However, the relationship between T2D, cognitive decline, and risk for developing AD is significantly stronger.
To this end, several cellular parallels between T2D, neuronal dysfunction, and AD development exist [35,58]. These range from neuronal loss of insulin signaling and general losses in brain energy usage [59,60] to the involvement and loss of insulin-related mechanisms in the regulation and clearance of AD pathology [61]. Interestingly, amylin, another β cell–produced pancreatic hormone, tends to aggregate and cause cellular dysfunction within the pancreas, similar to Aβ in the AD brain [62,63]. For example, amylin-derived amyloid fibrils cause similar disruptions in ionic gradients and cell survival in brain as in the pancreas [40,64]. As such, the regulation of proper brain metabolism and function is not limited to optimal insulin function and regulation. Rather, like in the periphery, it extends to several metabolic hormones that in concert with insulin are critical for metabolic homeostasis and proper energy production and consumption. Here, we explore the role of the metabolic hormones insulin, amylin, and leptin and how their dysfunction contributes to the development of age-related cognitive disease and AD development.
3.1. Insulin
Changes in body composition, nutrient availability, physical activity, and age contribute to changes in both insulin production and insulin sensitivity [6,65–70]; each of these factors can contribute to the development of obesity and related metabolic disorders, including T2D. Changes in lean mass and fat mass are closely linked to insulin production and resistance [71], and fluctuations in insulin signaling resulting from adiposity commonly contribute to the development of metabolic syndrome and progress further toward T2D.
Type 2 diabetes is characterized by hyperinsulinemia and postprandial hyperglycemia stemming from insulin resistance [72]. Hyperinsulinemia is strongly linked to decreased insulin signaling within the brain. Insulin receptors (IRSs) mediate insulin transport across the blood-brain barrier (BBB) and these receptors are saturable [73–75]. Although acute hyperinsulinemia increases transport across the BBB, chronic hyperinsulinemia leads to down-regulation of IRSs and thus decreases in insulin transport across the BBB [76]; this results in aberrant insulin signaling within the brain and brain hypometabolism.
Disruptions in insulin signaling and resultant brain hypometabolism are strongly associated with AD [77,78] and cognitive impairment. Insulin receptors are expressed within the cerebral cortex, hippocampus, hypothalamus, cerebellum, and olfactory bulb [79]. Human studies show that AD patients often have reduced IRS sensitivity [59,60], decreased IRS phosphorylation and subsequent downstream signaling, and decreased insulin and insulin-like growth factor receptor expression within the brain [60,80].
Alterations in insulin signaling pathways are intimately associated with OS and mitochondrial dysfunction [35,70]. Insulin-related mitochondrial dysfunction is common in both T2D and obese patients [33–35]; both human and rodent studies of T2D show higher levels of mitochondrial fission within the liver, muscle, and brain. This change in mitochondrial morphology has been attributed to mitochondrial fragmentation, impaired ATP production, and increased OS [81]. These downstream insulin-induced changes in mitochondrial function and morphology ultimately lead to impaired glucose uptake and OS [38]; this aspect has been validated in vivo, where obese adults subjected to exercise training show increased insulin sensitivity and decreased mitochondrial fragmentation in skeletal muscle.
Type 2 diabetes clearly has effects on insulin production and signaling, but many with T2D are also obese. Decreased insulin sensitivity results in poor glucose utilization, which leads to increased food intake and fat deposition. Consequently, T2D patients have substantially more white adipose tissue, which produces and releases a wide array of substances, including the inflammatory cytokines tumor necrosis factor α and interleukin 6, fatty acids, glycerol, and the metabolic hormones leptin and adiponectin [41–43]. As fat mass increases, so too does the production and secretion of many of these compounds. Inflammatory cytokines and free fatty acids decrease insulin sensitivity [41,69,82,83]. Studies have also shown that high-fat diet–induced adiposity and subsequent macrophage activation precede insulin resistance in rodents [84,85]. Thus, chronic high-fat diets are linked to inflammation, insulin resistance, and neuronal loss within the hypothalamus [44,86]. This demonstrates a potential link between high fat intake and diabetes as well as diet-induced inflammation and subsequent neurodegeneration.
Insulin resistance, aberrant insulin signaling, and related pathology can also arise from impaired insulin degradation and clearance. Insulin is cleared through its degradation by insulin degrading enzyme (IDE). Insulin degrading enzyme is present in peroxisomes, endosomes, and cytosol as we all as on the cell surface of expressing tissues [87–90]. Insulin degradation takes place mostly in the liver and kidneys, but IDE is expressed in most other tissues, including the brain [91]. Interestingly, IDE degrades several other proteins that have the propensity to form amyloid fibrils, including glucagon, amylin, calcitonin, and Aβ [92,93]. Mutations in the gene coding for IDE have been linked to the development of both AD and T2D in humans and rats [94–96]. In addition, IDE messenger RNA levels within the hippocampus of AD patients are reduced, suggesting that the hippocampus is less capable of clearing Aβ [61]. Regardless of etiology, insulin resistance ultimately leads to overall dysfunction in brain metabolism, brain aging, and an increase in AD pathology [97].
Insulin therapies are obviously useful and essential for the treatment of diabetes, but studies using intranasal insulin have also shown great promise in treating and preventing AD-related symptoms and pathology. Intranasal insulin has been used in both preclinical and clinical settings and demonstrates marked improvements in cognition [98–100]. Animal studies showed that intranasal insulin is able to cross the cribriform plate, readily disperses throughout the brain, and is capable of rescuing learning and memory deficits in a rodent model of AD [98]. Importantly, a clinical study using intranasal insulin on AD and mild cognitive impairment patients demonstrated a delay in memory-related deficits as well as increased glucose uptake in frontal and parietotemporal cortices via positron emission tomography scan [99]. Insulin therapies have proved less effective in AD patients positive for Apoε4, but these individuals had positive results when treated with fast acting insulin [100]. Although the results thus far are promising, it remains unclear whether chronic central nervous system (CNS) administration of insulin will eventually lead to insulin resistance within the brain as it does in the periphery. Therefore, it is important to also study the therapeutic relationship of co-administration of insulin and other hormones that modulate insulin sensitivity and function such as leptin and amylin.
3.2. Leptin
Leptin is produced by white adipocytes and is primarily characterized as the regulator of long-term energy balance [101]. Fat mass is positively correlated with leptin production [102]; obese states are thus characterized by hyperleptinemia, which like insulin gives rise to leptin resistance [103]. Under normal conditions, leptin signals through its receptor, Ob-R, to modulate a wide array of functions throughout the body. For example, Ob-Ra, the short isoform, is thought to facilitate leptin transport across the BBB [104], whereas Ob-Rb, the long isoform, possesses the intracellular domain necessary for signal transduction [105,106] and is highly expressed in the hypothalamus, particularly within the arcuate nucleus. Leptin signaling within the arcuate nucleus leads to reductions in energy in take, increases in energy expenditure, and overall satiety. This relationship, however, is disrupted during aging and in obese states due to leptin resistance [103,107]. There are many proposed mechanisms for leptin resistance, including deficits in receptor capabilities or downstream signaling, increased expression of Ob-R inhibitors [103], alterations in leptin transport across the BBB [108], and decreased plasma membrane Ob-R expression [109].
Leptin signaling is traditionally associated with the hypothalamus, but has also been identified within the hippocampus and cerebral cortex, 2 regions heavily implicated in AD [104,105,110,111]. Within the brain, leptin is known to be both neurotrophic, and neuroprotective and to (1) facilitate neurite outgrowth in primary hippocampal and cortical neurons [112,113], (2) promote adult neurogenesis within the hippocampus [114], (3) increase cell survival in hypothalamic neurons [115], and (4) attenuate cell death in the face of ischemia [116,117], OS [32], cytoxicity [118], and other stress events [119–121]. Leptin also increases long-term potentiation (LTP) within the hippocampus [122]. Thus, leptin signaling is an essential component of the healthy brain.
A relationship exists between body weight and dementia. Strong evidence suggests that midlife obesity is a significant risk factor for the development of AD and other neurodegenerative diseases [13–21]. An interesting paradox exists, however, between leptin and AD incidence. A large-scale human study revealed that serum leptin levels negatively correlate with AD incidence, even when correcting for typical obesity measures (body mass index, hip-to-waist ratio, and vascular risks) [123]. Separate studies have also shown that low serum leptin levels are correlated with an increased risk for the development dementia [124,125]. Interestingly, when leptin levels were measured in the hippocampus and cerebrospinal fluid, the opposite was observed. Contrary to peripheral levels, CNS levels were increased in AD brains compared with controls. This aspect was also accompanied by decreased Ob-R expression compared with age-matched control [126]. Together, these data suggest that the AD brain may become leptin resistant.
In vitro studies showed that leptin treatments decrease both Aβ production and tau phosphorylation [127–129]. Transgenic rodent models of AD have been widely used to confer these human findings. A number of studies conducted on several different transgenic AD models, by different laboratories, have all demonstrated that diet-induced obesity leads to an increase in cerebral Aβ pathology [130–134]. These aspects have been replicated in vivo. To this end, chronic leptin treatment in a transgenic rodent model of AD also showed marked reductions in both Aβ and tau pathology while also improving learning and memory [135].
Leptin dysfunction in AD is hypothesized to be due to leptin resistance. Given that treating obesity with leptin administration is largely ineffective due to leptin resistance, it seems unlikely that AD will respond to leptin therapy when considering leptin resistance as a factor in AD. It is also important to note that the rodent models of AD used in the previously mentioned studies are not leptin resistant. Collectively, leptin research and treatments are promising with regard to cognitive disease; however, similar problems exist with leptin to that with insulin. That is, CNS leptin treatment may be therapeutic but chronic treatment may lead to similar types of resistance. As such, treatments that modulate leptin sensitivity may prove to be significantly more effective than solely replacing or supplementing leptin.
One promising avenue to address the above issue is to study hormones that increase the sensitivity of leptin and insulin. Leptin is known to synergize with the pancreatic hormone amylin. Amylin has been shown to increase leptin sensitivity in the obese state, inducing synergistic effects on weight loss [136,137]. A study has also shown that both amylin and leptin are able to reduce food intake in a histamine receptor-1–mediated fashion [138]. This reduction in food intake was lost in histamine receptor-1 knockout mice, suggesting that both hormones somehow work through this receptor within the hypothalamus to reduce feeding. As such, sufficient evidence exists to suggest that the administration of amylin alone or together with leptin may have a combined effect on physiology in the brain.
3.3. Amylin
Amylin is an amyloid protein produced by β-islets of the pancreas that is co-secreted with insulin. Amylin is secreted at a constant basal level with a postprandial surge [139,140]. Amylin is co-secreted with insulin at a 15:1 molar ratio (insulin/amylin) [141] and works in conjunction with insulin to reduce blood glucose by inhibiting glucagon secretion [142] and slowing gastric and intestinal emptying [143]. As such, these effects of amylin help to reduce the systemic demand for insulin by reducing gastrointestinal absorption, hindering glycogen breakdown, and glucose release from the liver [144].
Although amylin signaling has clear homeostatic function, assisting in the regulation of blood glucose, insulin demand, and food intake, amylin signaling is also linked to disease states, particularly T2D and AD [62–64,145–147]. Because amylin is co-secreted with insulin, diet- or T2D-induced hyperinsulinemia also leads to hyperamylinemia. As previously mentioned, amylin is an amyloid that has the propensity to self-aggregate [92,93]. Similar to Aβ in AD, the overproduction of amylin in T2D or states of insulin resistance causes amylin to aggregate, forming toxic and disruptive amyloid fibrils within the pancreas [146,147].
More than 95% of patients with T2D have identifiable amyloid fibrils within the pancreas, specifically localized around β-islets [62,63]. Amylin-derived amyloid fibrils near β cells are associated with unregulated Ca2+ influx, a phenomenon that has cytotoxic and deleterious effects on β-cell function [40]. Both amylin and Aβ-derived amyloid fibrils are closely associated with aberrant amylin signaling, OS, and resulting pathology seen in both T2D and AD [64,148]. To this end, amyloid fibrils composed of amylin or Aβ have synonymous toxic effects in the CNS and the periphery. Importantly, amylin is BBB permeable and is capable of aggregating within the brain and are suggested to seed further Aβ aggregation [58]. To this end, high doses of amylin may also have a toxic function in some areas of the brain.
Studies suggest that Aβ and human amylin may signal similarly through its endogenous receptor, AMY, to induce toxicity within the hippocampus [149]. Amylin signaling, like Aβ, has been shown to be involved in LTP in the brain, a process intimately associated with synapse stabilization, learning, and memory [64,145]. Evidence suggests that high doses of amylin and Aβ signal through the AMY within the hippocampus, causing deficits in LTP [150]. These reductions in hippocampal LTP lead to destabilization of hippocampal synapses and ultimately to neuronal loss.
Amyloid fibrils are also thought to be involved in aberrant OS seen in AD and diabetes. Both Aβ and human amylin have been shown to produce ROS in vitro, and this OS is exacerbated further in the presence of redox metals [37]. It is hypothesized that amyloid fibrils form metalo-peptide complexes that facilitate OS and cell death [39]. However, conflicting evidence exists demonstrating that the formation of these complexes by Aβ and amylin may actually prevent further ROS production and reduce OS in vitro [151,152].
Because of human amylin’s propensity to self-aggregate, the toxic effect of aggregation, and the net loss of circulating amylin, a synthetic analogue that does not aggregate, pramlintide (PRAM) was developed and is widely used to replace physiological amylin in T2D. Pramlintide structure closely resembles rodent amylin but does not aggregate like human amylin and Aβ [153].
Pramlintide differs from physiological amylin by 3 key amino acids that keep the peptide from aggregating while conserving most of amylin’s biological activity [154,155]. To this end, PRAM has been used in a number of studies to treat obesity, T2D, and AD. Used as a treatment for obesity, PRAM works to increases leptin sensitivity, leading to a large reduction in food intake that ultimately results in weight loss [136,137]. Pramlintide is also used to treat T2D and helps to better regulate blood glucose levels and insulin demand [156]. Treatment with PRAM also ameliorates Aβ-induced OS in the brain [157]. Mechanistically, it is hypothesized that PRAM interacts with the antioxidizing enzyme glutathione to reduce OS [158]. In vivo studies have demonstrated PRAM’s ability to ameliorate both inflammation and OS in a rodent model of AD [159]. Treatment also rescues deficits in LTP induced by Aβ and amylin and even ameliorates these effects in the presence of human amylin and Aβ [64]. This evidence works in concert with the human studies showing that PRAM treatment reduced OS [160].
At this juncture, it is unclear how exactly PRAM implements its therapeutic effects. Under normal conditions, amylin signaling reduces food intake, improves glycemic control, and increases insulin and leptin sensitivity. In disease states, however, both human amylin and Aβ aggregate, forming toxic plaques and causing OS, which ultimately results in a loss of amylin function and signaling. Kimura et al [64] hypothesize that PRAM effectively works as an AMY antagonist, blocking the toxic function of both amylin and Aβ in the hippocampus. Alternatively, PRAM may work by replacing the lost native function of these proteins. Additional work is necessary to determine the nature of PRAM’s interaction with AMY and its direct effects on the CNS and periphery. Nevertheless, the treatment of both T2D and AD with none aggregating forms of amylin holds great therapeutic potential.
4. Future research
A great deal of research has been conducted in the clinical setting using insulin as a therapy for both metabolic and cognitive diseases [99,100]. Few studies, however, have used leptin or PRAM as a therapy for metabolic disorders, and to date, no clinical studies have been conducted using PRAM and leptin as a treatment for cognitive decline. Because hormone resistance correlates with serum hormone concentrations, the potential development of hormone resistance within the CNS remains highly relevant. Thus, it is necessary to study the long-term effects of CNS hormone treatment with both insulin and leptin. Fortunately, native human amylin and PRAM increase both insulin and leptin sensitivity [138,144]. Pramlintide enhances insulin function indirectly by reducing the body’s overall demand for insulin [144]. It also synergizes with leptin, increasing leptin sensitivity in the obese state [136,137]. Because of its ability to enhance the function of other essential metabolic hormones, PRAM holds high therapeutic promise as a treatment for a wide variety of metabolic and cognitive diseases. Based on the evidence presented, studies are warranted using combination therapies of insulin and PRAM as well as leptin and PRAM to prevent or treat cognitive decline, but particularly AD.
Importantly, many studies conducted using intranasal insulin have treated individuals that have already shown evidence of cognitive decline [98–100]. Further research is also warranted to determine whether CNS intervention with metabolic hormones will help to reduce the risk of cognitive decline in high-risk individuals (chronically obese or diabetic individuals). These studies will greatly increase our understanding of the effects of metabolic hormones on cognition and their potential application as a therapy for cognitive decline.
Although a great deal of research has been conducted on metabolic disease and its influence on cognitive disease, little is known about the reverse. Recent evidence suggests that AD may, in fact, contribute to the development of diabetes. Global knockout of β-secretase 1, a key enzyme involved in Aβ production, has been shown to protect against diet-induced obesity and diabetes [161]. Furthermore, β-secretase 1 expression exclusively in the CNS facilitates hypothalamic dysfunction, impaired glucose tolerance, and glycogen storage, as well as a fatty liver phenotype [162]. To this end, further investigation is warranted to discern the exact nature of the relationship between AD and metabolic dysfunction.
5. Conclusions
There is a clear association between diet, metabolic dysfunction, aging, and cognitive decline. Metabolic hormone function and production are highly intertwined; affecting one metabolic hormone often affects others. As such, disease states that arise from hormone deficiencies or resistances brought on by age or lifestyle often result in large-scale metabolic dysfunction. Diet-induced obesity has profound effects on the hormones insulin, leptin, and amylin, each of which also plays a role in cognition [64,99,123]. Thus, aberrant insulin, leptin, and amylin signaling that stems from poor diet, aging, or metabolic disease can result in cognitive decline and contribute to the development of AD [60,126,159].
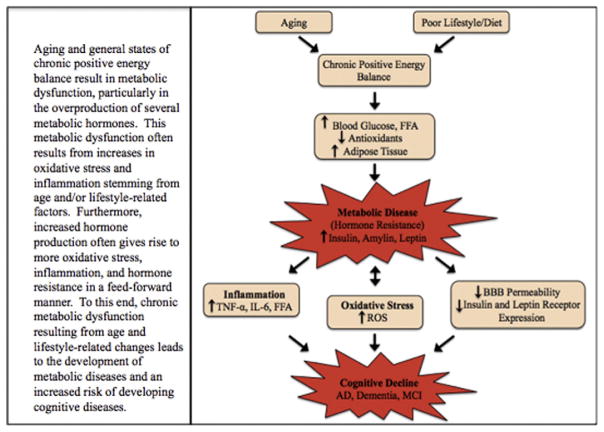
Aging and lifestyle factors such as diet and exercise have a substantial influence over metabolic function and metabolic hormone production [28,30,38]. Age and lifestyle-related changes in metabolism are often a result of increases in OS and inflammation [24,30,41,44], but aberrant hormone production and signaling also result in further OS and inflammation in a feed-forward fashion (Fig. 1) [35,36,84]. As such, OS and inflammation appear to be quintessential factors facilitating metabolic dysfunction and cognitive decline. Therefore, therapies that aim to return these metabolic systems to a homeostatic state hold the highest therapeutic potential for the subsequent treatment of both metabolic and cognitive diseases.
Fig. 1.
Aging and general states of chronic positive energy balance result in metabolic dysfunction, particularly in the overproduction of several metabolic hormones. This metabolic dysfunction often results from increases in oxidative stress and inflammation stemming from age and/or lifestyle-related factors. Furthermore, increased hormone production often gives rise to more oxidative stress, inflammation, and hormone resistance in a feed-forward manner. To this end, chronic metabolic dysfunction resulting from age and lifestyle-related changes leads to the development of metabolic diseases and an increased risk of developing cognitive diseases.
Insulin therapies are essential for the treatment of diabetes and metabolic syndrome and have emerged as promising therapies for cognitive decline [98–100]. Although leptin therapy is not effective in treating obesity due to resistance, it shows promise as a therapy for AD [127–129,135]. In addition, evidence suggests that overproduction of human amylin is likely more toxic than therapeutic due to its propensity to self-aggregate and cause cellular distress [40,62,63,145]. As such, PRAM has emerged as a promising alternative in replacing the native therapeutic function of human amylin and thus the treatment of both metabolic and cognitive disease [64,157–160]. Regardless of hormone intervention, proper diet and exercise can drastically improve cardiovascular health, insulin sensitivity, and the lean mass/fat mass ratio [6,38,67] and thus reduce the risk of developing both metabolic and cognitive diseases. A great deal of controversy still exists with regard to various diets and their efficacy in reducing fat mass. At this juncture, it appears that commitment to energy restriction is the most relevant aspect of dieting, regardless of dietary content [5]. As such, properly balanced diets, rich in antioxidants, coupled with regular exercise throughout life can vastly improve cardiovascular and metabolic health, ultimately deterring the risk of cognitive decline. Should disease states arise, however, hormone therapies show promise in treating and preventing metabolic-related cognitive decline.
Acknowledgments
The authors thank Jeffrey A. Blair and Sabina Bhatta for their assistance in proofreading, editing, and formatting this review. This work was supported by the National Institutes of Aging (Grant No. 1R15AG050292) and the Alzheimer’s Association (Grant No. NPSPAD 247219).
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer disease
- AMY
amylin receptor
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- CNS
central nervous system
- IDE
insulin degrading enzyme
- IRS
insulin receptor substrate protein
- LTP
long-term potentiation
- NFT
neurofibrillary tangle
- ROS
reactive oxygen species
- OS
oxidative stress
- PRAM
pramlintide
- T2D
type 2 diabetes
References
- 1.Fryar CD, Carroll MD, Ogden CL. C.f.D. Control, editor. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, 1960–1962 Through 2011–2012. 2014. pp. 1–6. [Google Scholar]
- 2.Yang L, Colditz GA. Prevalence of Overweight and Obesity in the United States, 2007–2012. JAMA Intern Med. 2015;175(8):1412–3. doi: 10.1001/jamainternmed.2015.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ineichen B. Fast food nation: What the All-American meal is doing to the world. J Adv Nurs. 2001;36(2):322. [Google Scholar]
- 4.Brown WV, Carson JAS, Johnson RK, Kris-Etherton P. JCL Roundtable: Fast Food and the American Diet. J Clin Lipidol. 2015;9(1):3–10. doi: 10.1016/j.jacl.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanningham-Foster L, Nysse LJ, Levine JA. Labor saved, calories lost: the energetic impact of domestic labor-saving devices. Obes Res. 2003;11(10):1178–81. doi: 10.1038/oby.2003.162. [DOI] [PubMed] [Google Scholar]
- 7.Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, et al. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet. 2015;385(9986):2521–33. doi: 10.1016/S0140-6736(14)61748-7. [DOI] [PubMed] [Google Scholar]
- 8.Puhl RM, Liu S. A national survey of public views about the classification of obesity as a disease. Obesity. 2015;23(6):1288–95. doi: 10.1002/oby.21068. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. 2014;66(4):724–9. doi: 10.1016/j.eururo.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–4. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 11.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States–no statistically significant change since 2003–2004. NCHS Data Brief. 2007;(1):1–8. [PubMed] [Google Scholar]
- 13.Ashrafian H, Harling L, Darzi A, Athanasiou T. Neurodegenerative disease and obesity: what is the role of weight loss and bariatric interventions? Metab Brain Dis. 2013;28(3):341–53. doi: 10.1007/s11011-013-9412-4. [DOI] [PubMed] [Google Scholar]
- 14.Hedström AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler J. 2012;18(9):1334–6. doi: 10.1177/1352458512436596. [DOI] [PubMed] [Google Scholar]
- 15.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 16.Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548–52. doi: 10.1212/WNL.0b013e31828154f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafson D, Bäckman K, Waern M, Östling S, Guo X, Zandi P, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73(19):1559–66. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitmer R, Gustafson D, Barrett-Connor E, Haan M, Gunderson E, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 19.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360–5. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmer RA, Gunderson EP, Quesenberry CP, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4(2):103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 21.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 22.Hetzel L. 65 Years and Over Population: 2000: Census 2000 Brief. DIANE Publishing; 2008. [Google Scholar]
- 23.Meyer J. Age: 2000: census 2000 brief. DIANE Publishing; 2008. [Google Scholar]
- 24.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Exp Biol Med. 1999;222(3):236–45. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 25.Floyd RA, Carney JM. Age influence on oxidative events during brain ischemia/reperfusion. Arch Gerontol Geriatr. 1991;12(2):155–77. doi: 10.1016/0167-4943(91)90025-l. [DOI] [PubMed] [Google Scholar]
- 26.Floyd RA, Carney JM. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32(S1):S22–7. doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- 27.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74(1):139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 28.Fang Y-Z, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18(10):872–9. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 29.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959–75. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 30.Floyd RA, Hensley K. Oxidative stress in brain aging: implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23(5):795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 31.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are Oxidative Stress–Activated Signaling Pathways Mediators of Insulin Resistance and β-Cell Dysfunction? Diabetes. 2003;52(1):1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283(3):1754–63. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- 33.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48(2):282–9. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 34.Holmström MH, Iglesias-Gutierrez E, Zierath JR, Garcia-Roves PM. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab. 2012;302(6):E731–9. doi: 10.1152/ajpendo.00159.2011. [DOI] [PubMed] [Google Scholar]
- 35.Butterfield DA, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2014;1842(9):1693–706. doi: 10.1016/j.bbadis.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zraika S, Hull R, Udayasankar J, Aston-Mourney K, Subramanian S, Kisilevsky R, et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52(4):626–35. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inestrosa N, Alvarez A, Godoy J, Reyes A, De Ferrari G. Acetylcholinesterase–amyloid-β-peptide interaction and Wnt signaling involvement in Aβ neurotoxicity. Acta Neurol Scand. 2000;102(s176):53–9. doi: 10.1034/j.1600-0404.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- 38.Fealy CE, Mulya A, Lai N, Kirwan JP. Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. J Appl Physiol. 2014;117(3):239–45. doi: 10.1152/japplphysiol.01064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiménez-Palomares M, Ramos-Rodríguez JJ, López-Acosta JF, Pacheco-Herrero M, Lechuga-Sancho AM, Perdomo G, et al. Increased Aβ production prompts the onset of glucose intolerance and insulin resistance. Am J Physiol Endocrinol Metab. 2012;302(11):E1373–80. doi: 10.1152/ajpendo.00500.2011. [DOI] [PubMed] [Google Scholar]
- 40.Kawahara M, Kuroda Y, Arispe N, Rojas E. Alzheimer’s β-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem. 2000;275(19):14077–83. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- 41.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherer PE. Adipose tissue from lipid storage compartment to endocrine organ. Diabetes. 2006;55(6):1537–45. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 43.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296(5):E1003–12. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verchere CB, D’Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci. 1996;93(8):3492–6. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Höppener J, Oosterwijk C, Nieuwenhuis M, Posthuma G, Thijssen J, Vroom TM, et al. Extensive islet amyloid formation is induced by development of type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia. 1999;42(4):427–34. doi: 10.1007/s001250051175. [DOI] [PubMed] [Google Scholar]
- 47.LaFerla FM, Oddo S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med. 2005;11(4):170–6. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Alzheimer’s A. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Förstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288–90. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- 50.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122(3):1131–5. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 51.Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J Alzheimers Dis. 2009;16(4):741–61. doi: 10.3233/JAD-2009-0972. [DOI] [PubMed] [Google Scholar]
- 52.Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, Hirai K, et al. Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis. 2006;9(2):147–53. doi: 10.3233/jad-2006-9207. [DOI] [PubMed] [Google Scholar]
- 53.Clark TA, Lee HP, Rolston RK, Zhu X, Marlatt MW, Castellani RJ, et al. Oxidative stress and its implications for future treatments and management of Alzheimer disease. Int J Biomed Sci. 2010;6(3):225. [PMC free article] [PubMed] [Google Scholar]
- 54.Wenk GL. Neuropathologic changes in Alzheimer’s disease: potential targets for treatment. J Clin Psychiatry. 2006;67:3. [PubMed] [Google Scholar]
- 55.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 56.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth W, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central obesity in the elderly is related to late onset Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2012;26(2):101. doi: 10.1097/WAD.0b013e318222f0d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oskarsson ME, Paulsson JF, Schultz SW, Ingelsson M, Westermark P, Westermark GT. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol. 2015;185(3):834–46. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247–68. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 60.Talbot K, Wang H-Y, Kazi H, Han L-Y, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, et al. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-ε4 allele. Am J Pathol. 2003;162(1):313–9. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson K, O’Brien T, Jordan K, Westermark P. Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol. 1989;135(2):245. [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson KH, O’Brien TD, Betsholtz C, Westermark P. Islet amyloid, islet-amyloid polypeptide, and diabetes mellitus. N Engl J Med. 1989;321(8):513–8. doi: 10.1056/NEJM198908243210806. [DOI] [PubMed] [Google Scholar]
- 64.Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH. Pramlintide Antagonizes Beta Amyloid (Aβ)-and Human Amylin-Induced Depression of Hippocampal Long-Term Potentiation. Mol Neurobiol. 2016:1–7. doi: 10.1007/s12035-016-9684-x. [DOI] [PubMed] [Google Scholar]
- 65.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats: relationship to muscle triglyceride and ω-3 fatty acids in muscle phospholipid. Diabetes. 1991;40(2):280–9. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 66.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat–fed rats. Diabetes. 1991;40(11):1397–403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 67.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity. 2011;19(2):402–8. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan AS. Insulin resistance with aging. Sports Med. 2000;30(5):327–46. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- 69.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46(1):3–10. [PubMed] [Google Scholar]
- 70.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 72.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci. 2003;100(7):4162–7. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18(9):1423–9. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 74.Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides. 1997;18(8):1257–62. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 75.Baura GD, Foster DM, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92(4):1824–30. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallum B, Taborsky G, Jr, Porte D, Jr, Figlewicz D, Jacobson L, Beard J, et al. Cerebrospinal Fluid Insulin Levels Increase During Intravenous Insulin Infusions in Man*. J Clin Endocrinol Metab. 1987;64(1):190–4. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 77.Hoyer S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm. 1998;105(4–5):415–22. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 78.Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3(1):1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- 79.Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16(2):59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 81.Jheng H-F, Tsai P-J, Guo S-M, Kuo L-H, Chang C-S, Su I-J, et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32(2):309–19. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 83.Reaven GM, Hollenbeck C, Jeng C-Y, Wu MS, Chen Y-DI. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37(8):1020–4. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 84.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 86.Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4(4):e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential 1. Endocr Rev. 1998;19(5):608–24. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 88.Goldfine ID, Williams JA, Bailey AC, Wong K, Iwamoto Y, Yokono K, et al. Degradation of insulin by isolated mouse pancreatic acini: evidence for cell surface protease activity. Diabetes. 1984;33(1):64–72. doi: 10.2337/diab.33.1.64. [DOI] [PubMed] [Google Scholar]
- 89.Seta KA, Roth RA. Overexpression of insulin degrading enzyme: cellular localization and effects on insulin signaling. Biochem Biophys Res Commun. 1997;231(1):167–71. doi: 10.1006/bbrc.1997.6066. [DOI] [PubMed] [Google Scholar]
- 90.Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, et al. Neurons regulate extracellular levels of amyloid β-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20(5):1657–65. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lynch JA, George AM, Eisenhauer PB, Conn K, Gao W, Carreras I, et al. Insulin degrading enzyme is localized predominantly at the cell surface of polarized and unpolarized human cerebrovascular endothelial cell cultures. J Neurosci Res. 2006;83(7):1262–70. doi: 10.1002/jnr.20809. [DOI] [PubMed] [Google Scholar]
- 92.Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000;275(47):36621–5. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- 93.Kurochkin IV. Insulin-degrading enzyme: embarking on amyloid destruction. Trends Biochem Sci. 2001;26(7):421–5. doi: 10.1016/s0968-0004(01)01876-x. [DOI] [PubMed] [Google Scholar]
- 94.Karamohamed S, Demissie S, Volcjak J, Liu C, Heard-Costa N, Liu J, et al. Polymorphisms in the insulin-degrading enzyme gene are associated with type 2 diabetes in men from the NHLBI Framingham Heart Study. Diabetes. 2003;52(6):1562–7. doi: 10.2337/diabetes.52.6.1562. [DOI] [PubMed] [Google Scholar]
- 95.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 96.Marlowe L, Peila R, Benke KS, Hardy J, White LR, Launer LJ, et al. Insulin-degrading enzyme haplotypes affect insulin levels but not dementia risk. Neurodegener Dis. 2007;3(6):320–6. doi: 10.1159/000097300. [DOI] [PubMed] [Google Scholar]
- 97.Giordano V, Peluso G, Iannuccelli M, Benatti P, Nicolai R, Calvani M. Systemic and brain metabolic dysfunction as a new paradigm for approaching Alzheimer’s dementia. Neurochem Res. 2007;32(4–5):555–67. doi: 10.1007/s11064-006-9125-8. [DOI] [PubMed] [Google Scholar]
- 98.Salameh TS, Bullock KM, Hujoel IA, Niehoff ML, Wolden-Hanson T, Kim J, et al. Central nervous system delivery of intranasal insulin: mechanisms of uptake and effects on cognition. J Alzheimers Dis. 2015;47(3):715–28. doi: 10.3233/JAD-150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosenbloom MH, Barclay TR, Pyle M, Owens BL, Cagan AB, Anderson CP, et al. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild–moderate Alzheimer’s disease. CNS Drugs. 2014;28(12):1185–9. doi: 10.1007/s40263-014-0214-y. [DOI] [PubMed] [Google Scholar]
- 101.Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat Rev Endocrinol. 2006;2(6):318–27. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- 102.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactiveleptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 103.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 104.Bjørbæk C, Elmquist JK, Michl P, Ahima RS, Van Bueren A, McCall AL, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139(8):3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 105.Elmquist JK, Bjørbæk C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–47. [PubMed] [Google Scholar]
- 106.Fei H, Okano HJ, Li C, Lee G-H, Zhao C, Darnell R, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci. 1997;94(13):7001–5. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51(4):1016–21. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- 108.Münzberg H. Differential leptin access into the brain—a hierarchical organization of hypothalamic leptin target sites? Physiol Behav. 2008;94(5):664–9. doi: 10.1016/j.physbeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 109.Couturier C, Sarkis C, Séron K, Belouzard S, Chen P, Lenain A, et al. Silencing of OB-RGRP in mouse hypothalamic arcuate nucleus increases leptin receptor signaling and prevents diet-induced obesity. Proc Natl Acad Sci. 2007;104(49):19476–81. doi: 10.1073/pnas.0706671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tezapsidis N, Johnston JM, Smith MA, Ashford JW, Casadesus G, Robakis NK, et al. Leptin: a novel therapeutic strategy for Alzheimer’s disease. J Alzheimers Dis. 2009;16(4):731–40. doi: 10.3233/JAD-2009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71(3):187–95. doi: 10.1159/000054536. [DOI] [PubMed] [Google Scholar]
- 112.O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35(4):559–72. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, et al. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3β. J Biol Chem. 2006;281(18):12950–8. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]
- 114.Garza JC, Guo M, Zhang W, Lu X-Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283(26):18238–47. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 116.Zhang F, Chen J. Leptin protects hippocampal CA1 neurons against ischemic injury. J Neurochem. 2008;107(2):578–87. doi: 10.1111/j.1471-4159.2008.05645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38(8):2329–36. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- 118.Shanley LJ, O’Malley D, Irving A, Ashford M, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545(3):933–44. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Doherty G, Oldreive C, Harvey J. Neuroprotective actions of leptin on central and peripheral neurons in vitro. Neuroscience. 2008;154(4):1297–307. doi: 10.1016/j.neuroscience.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 120.Valerio A, Dossena M, Bertolotti P, Boroni F, Sarnico I, Faraco G, et al. Leptin Is Induced in the Ischemic Cerebral Cortex and Exerts Neuroprotection Through NF-κB/c-Rel–Dependent Transcription. Stroke. 2009;40(2):610–7. doi: 10.1161/STROKEAHA.108.528588. [DOI] [PubMed] [Google Scholar]
- 121.Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, et al. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282(47):34479–91. doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- 122.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21(24):RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302(23):2565–72. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: findings from the Health ABC Study. Neurobiol Aging. 2009;30(9):1483–9. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bigalke B, Schreitmüller B, Sopova K, Paul A, Stransky E, Gawaz M, et al. Adipocytokines and CD34+ progenitor cells in Alzheimer’s disease. PLoS One. 2011;6(5):e20286. doi: 10.1371/journal.pone.0020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, et al. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128(1):162–72. doi: 10.1111/jnc.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, et al. Leptin inhibits glycogen synthase kinase-3β to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009;455(3):191–4. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun. 2009;380(1):98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, et al. Leptin reduces Alzheimer’s disease-related tau phosphorylation in neuronal cells. Biochem Biophys Res Commun. 2008;376(3):536–41. doi: 10.1016/j.bbrc.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Halagappa VKM, Guo Z, Pearson M, Matsuoka Y, Cutler RG, LaFerla FM, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26(1):212–20. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 131.Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci Lett. 2009;464(3):184–7. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, et al. Caloric restriction attenuates Aβ-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26(7):995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 133.Van Der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab. 2005;2(1):1. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, et al. Caloric restriction attenuates β-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;19(6):659–61. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- 135.Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1155–67. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci. 2008;105(20):7257–62. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149(11):5679–87. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- 138.Mollet A, Lutz T, Meier S, Riediger T, Rushing P, Scharrer E. Histamine H1 receptors mediate the anorectic action of the pancreatic hormone amylin. Am J Phys Regul Integr Comp Phys. 2001;281(5):R1442–8. doi: 10.1152/ajpregu.2001.281.5.R1442. [DOI] [PubMed] [Google Scholar]
- 139.Boyle CN, Rossier MM, Lutz TA. Influence of high-fat feeding, diet-induced obesity, and hyperamylinemia on the sensitivity to acute amylin. Physiol Behav. 2011;104(1):20–8. doi: 10.1016/j.physbeh.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 140.Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89(4):465–71. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 141.Hwang JJ, Chan JL, Ntali G, Malkova D, Mantzoros CS. Leptin does not directly regulate the pancreatic hormones amylin and pancreatic polypeptide Interventional studies in humans. Diabetes Care. 2008;31(5):945–51. doi: 10.2337/dc07-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gedulin BR, Rink TJ, Young AA. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46(1):67–70. doi: 10.1016/s0026-0495(97)90170-0. [DOI] [PubMed] [Google Scholar]
- 143.Young A. Inhibition of gastric emptying. Adv Pharmacol. 2005;52:99–121. doi: 10.1016/S1054-3589(05)52006-4. [DOI] [PubMed] [Google Scholar]
- 144.Ratner R, Dickey R, Fineman M, Maggs D, Shen L, Strobel S, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004;21(11):1204–12. doi: 10.1111/j.1464-5491.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 145.Kimura R, MacTavish D, Yang J, Westaway D, Jhamandas JH. Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci. 2012;32(48):17401–6. doi: 10.1523/JNEUROSCI.3028-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89(8):3629–43. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 147.Kapurniotu A, Schmauder A, Tenidis K. Structure-based design and study of non-amyloidogenic, double N-methylated IAPP amyloid core sequences as inhibitors of IAPP amyloid formation and cytotoxicity. J Mol Biol. 2002;315(3):339–50. doi: 10.1006/jmbi.2001.5244. [DOI] [PubMed] [Google Scholar]
- 148.Gotz J, Lim Y-A, Eckert A. Lessons from two prevalent amyloidoses—what amylin and Aβ have in common. Front Aging Neurosci. 2013;5(38):1–9. doi: 10.3389/fnagi.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mayer A-PT, Durward A, Turner C, Skellett S, Dalton N, Tibby SM, et al. Amylin is associated with delayed gastric emptying in critically ill children. Intensive Care Med. 2002;28(3):336–40. doi: 10.1007/s00134-002-1224-7. [DOI] [PubMed] [Google Scholar]
- 150.Fu W, Ruangkittisakul A, MacTavish D, Shi JY, Ballanyi K, Jhamandas JH. Amyloid β (Aβ) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J Biol Chem. 2012;287(22):18820–30. doi: 10.1074/jbc.M111.331181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, et al. Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395(6703):698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- 152.Fraser PE, Yang D-S, Yu G, Lévesque L, Nishimura M, Arawaka S, et al. Presenilin structure, function and role in Alzheimer disease. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2000;1502(1):1–15. doi: 10.1016/s0925-4439(00)00028-4. [DOI] [PubMed] [Google Scholar]
- 153.Fox A, Snollaerts T, Errecart Casanova C, Calciano A, Nogaj LA, Moffet DA. Selection for nonamyloidogenic mutants of islet amyloid polypeptide (IAPP) identifies an extended region for amyloidogenicity. Biochemistry. 2010;49(36):7783–9. doi: 10.1021/bi100337p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Janes S, Gaeta L, Beaumont K, Beeley K, Rink T. The selection of pramlintide for clinical evaluation. Diabetes. 1996;45(Suppl 2):235A. [Google Scholar]
- 155.Young AA, Vine W, Gedulin BR, Pittner R, Janes S, Gaeta LS, et al. Preclinical pharmacology of pramlintide in the rat: comparisons with human and rat amylin. Drug Dev Res. 1996;37(4):231–48. [Google Scholar]
- 156.Hollander PA, Levy P, Fineman MS, Maggs DG, Shen LZ, Strobel SA, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes a 1-year randomized controlled trial. Diabetes Care. 2003;26(3):784–90. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]
- 157.Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, et al. Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci. 1995;92(6):1989–93. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maguschak KA, Ressler KJ. β-catenin is required for memory consolidation. Nat Neurosci. 2008;11(11):1319–26. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, et al. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging. 2014;35(4):793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 160.Ceriello A, Piconi L, Quagliaro L, Wang Y, Schnabel CA, Ruggles JA, et al. Effects of pramlintide on postprandial glucose excursions and measures of oxidative stress in patients with type 1 diabetes. Diabetes Care. 2005;28(3):632–7. doi: 10.2337/diacare.28.3.632. [DOI] [PubMed] [Google Scholar]
- 161.Meakin PJ, Harper AJ, Hamilton DL, Gallagher J, McNeilly AD, Burgess LA, et al. Reduction in BACE1 decreases body weight, protects against diet-induced obesity and enhances insulin sensitivity in mice. Biochem J. 2012;441(1):285–96. doi: 10.1042/BJ20110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Plucinska K, Dekeryte R, Koss D, Shearer K, Mody N, Whitfield PD, et al. Neuronal human BACE1 knockin induces systemic diabetes in mice. Diabetologia. 2016;59(7):1513–23. doi: 10.1007/s00125-016-3960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]