INTRODUCTION
Williams syndrome (WS) [OMIM #194050] is a distinct genetic disorder caused by a chromosomal 7q11.23 microdeletion of ~ 1.5 million base pairs. The resulting loss of approximately 25 coding genes leads to a broad but well-characterized array of medical problems [Pober 2010]. However, limited data exist on the body composition of individuals with WS. Stagi et al. recently report decreased bone mineral density (BMD) in a cohort of children and young adults [Stagi and others 2016], and another report shows decreased bone density in a small sample of adults over the age of 30 [Cherniske and others 2004], but this observation has yet to be reproduced in a larger cohort of adults. Likewise, only two small studies assessing WS children and adults, respectively, describe body proportions from standard anthropometric measurements. Both these studies report diminished fat stores in their subjects with Williams syndrome [Kaplan and others 1998; Nogueira and others 2011]. Our clinical observations suggest that the classic infant and child WS phenotype, described as “failure to thrive” or “FTT” with low body weight and height, does not necessarily persist into adulthood. Rather, a subset of adults accumulate fat in a distinct distribution developing a phenotype that resembles lipedema [Cherniske and others 2004].
To broaden the described age range and confirm the previous reports of decreased bone density in adults with WS as well as our clinical impressions of a tendency for lipedema-like fat accumulation in adulthood, we chose to perform screening studies of bone mineral density and body composition on a wide age range sample of WS children and adults attending the 2014 Williams Syndrome Association Family Convention. We observed that bone mineral density was reduced in WS compared to sibling controls across the lifespan, and that adiposity, particularly in the lower extremities, is lower than controls in childhood, as expected, but is increased compared to controls in both men and women with WS in later adulthood.
METHODS
Individuals with Williams Syndrome and Siblings
This study was conducted at the July 1–5, 2014 Williams Syndrome Family Convention in Anaheim, CA. Families of individuals with WS learned of the study through announcements from the sponsoring organization (the non-profit parent support group, the Williams Syndrome Association), by being members of the Williams Syndrome Patient & Clinical Research Registry (www.williams-syndrome.org/registry), or by seeing a study flyer and “walking-in” during the Convention. Verbal consent was obtained from the parents/guardians of individuals with WS, as was verbal assent from the individual with WS, after each reviewed a developmentally appropriate fact sheet. Siblings of individuals with WS also provided verbal consent or assent (with verbal parent consent) as appropriate for age. This study was approved by the Institutional Review Board at Massachusetts General Hospital as a minimal risk study which did not authorize collecting either identifying information or biological samples from the participants.
Fifty-eight individuals with WS and twenty-six sibling controls participated in the study. Subjects met the following inclusion criteria: ages 7–70 years old; a parent or guardian available to provide medical history and review details of the study with the individual with WS (WS group) and with minors (WS group and sibling controls); and a parent- or guardian-reported diagnosis of WS (WS group) clinically confirmed by a study physician. Data from three subjects were excluded from all analyses due to the presence of conditions thought to substantially confound measures of body composition and bone density (Figure 1): one WS subject was in a wheel-chair, one WS subject was total parental nutrition-dependent, and one sibling control had psuedoxanthoma elasticum. In addition, the healthy sib control of the wheel-chair bound WS subject was also excluded. Thus, the final sample size of our series consisted of 56 WS participants and 24 sib participants (Figure 1).
Figure 1.
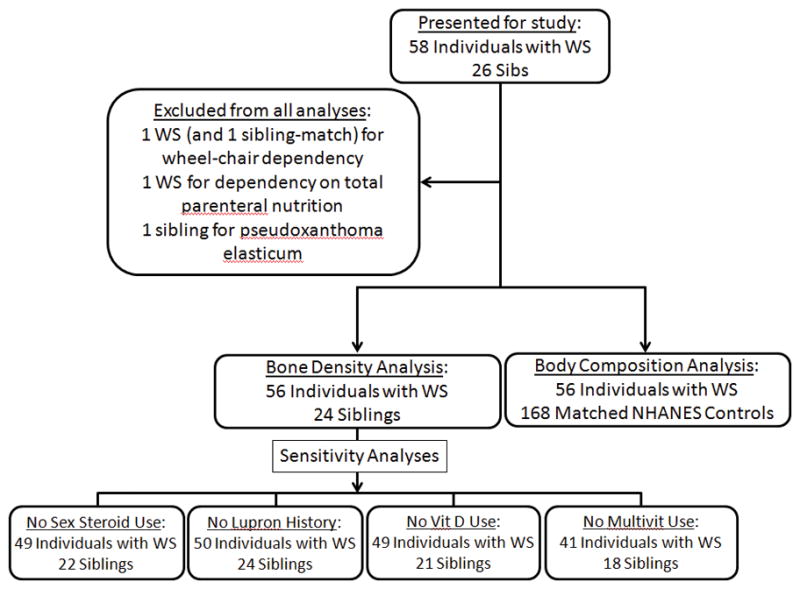
Flowchart showing inclusion of individuals with WS, sibling controls, and NHANES controls in different study analyses.
National Health and Nutrition Examination Survey (NHANES) Controls
NHANES data from the 2003–2004 survey were utilized to provide a larger control population for analysis of anthropometric measurements. This survey was chosen as the most recent one with data on both anthropometrics of interest as well as percent body fat by bioelectric impedance analysis. Pregnant individuals and those whose pregnancy status could not be confirmed were excluded from the data set. One-to-three matching was performed using controls randomly selected from the NHANES dataset. Matching was performed by SAS on gender, race, and age (within 2 years) using a published SAS macro (Paper 061-2010 SAS Global Forum 2010).
Study Assessments
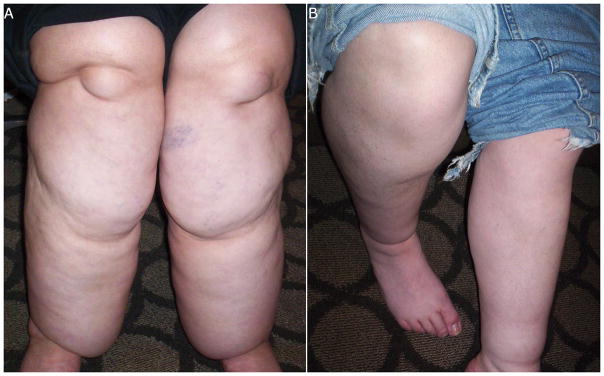
The following procedures were performed at the Convention: brief physical examination by a study physician with particular expertise in WS (BRP and TLS); measurement of height and weight; measurement of anthropometrics including neck, mid-upper arm, waist, hip, and mid-thigh circumferences measured in triplicate; bioelectrical impedance analysis (BIA); and determination of phalangeal bone density (Accudxa, see below). In addition, photographs were taken of an individual’s legs if more than mild lipedema was observed by a member of the study team and the individual consented to be photographed. Presence of mild or moderate-to-severe lipedema was subjectively determined by the study physician who performed the physical examinations for the study. Moderate-to-severe lipedema was noted if an individual had significantly increased fat accumulation in the lower and/or upper extremities that included “draping” of fat as well as “cuffing” at the ankles with sparing of the feet (see Figure 2A). Mild lipedema was noted if an individual had fat accumulation in the lower and/or upper extremities that was out of proportion to overall body fat and had a non-homogeneous distribution, including mild “cuffing” at the ankles and sparing of the feet (see Figure 2B). A parent/guardian for each WS participant was asked to complete a medical and surgical history questionnaire. Adult siblings completed their own questionnaire.
Figure 2.
Lipedema-like phenotype in an adult male and female with WS. (A) Severe lower extremity fat accumulation in a female with WS, >50yo with BMI > 40kg/m2 and (B) mild lower extremity fat accumulation in a male with WS, aged between 30–40yo with BMI between 30–40kg/m2.
In order to facilitate adjustment for stature in the analyses of this combined pediatric-adult cohort, height z-scores (accounting for age and gender) were calculated for each participant using the 2000 Centers for Disease Control (CDC) standards. For adults, z-scores were calculated using the available standards for adult height, i.e., those of 19-year-olds.
The UM-081 Tanita™ Bioelectrical Impedance Analysis (BIA) scale (Tanita Corporation of America, Inc., Arlington Heights, IL) was used to measure total body fat percentage, total body water percentage, and weight.
The AccuDXA2®, model 7200 (Lone Oak Medical Technologies™, Doylestown, PA) was used to measure bone mineral density (BMD) of the middle phalanx of the non-dominant hand. Short-term reproducibility of this measurement is 1–2% [Fiter and others 2001; Patel and others 2010]. In adults, middle phalangeal BMD and t-score measurements show moderate correlation (r = 0.59–0.66) with measurements at the lumbar spine and hip [Fiter and others 2001; Patel and others 2010], and have been validated as a predictor of hip fracture [Mussolino and others 1997]. Z-scores for phalangeal BMD are provided by AccuDXA for adults 19 years and older, but normative data are not currently available for children.
Statistical analyses
SAS 9.4 (SAS institute, Cary, NC) was used for all statistical analyses. Sample sizes for individuals with WS, Sibling Controls, and NHANES Controls in each analysis are shown in Figure 1. Because both siblings and NHANES controls were paired to individuals with WS, comparisons between groups were performed with mixed effects modeling using restricted maximum likelihood (REML), with matched-pairing or sibship entered as a random effect and other variables of interest entered as fixed effects. Due to the normal pattern of bone mineral accrual across age groups (with a steep increase during childhood and slow decline during adulthood), age was modeled using squared and cubic terms (i.e., age, age2, and age3), all of which had a highly statistically significant association with BMD. Anthropometric comparisons were performed separately for children less than 18 years old, adults between 18–30 years old, and adults >30 years old. Age x WS status interaction was also investigated. A pre-determined alpha of 0.05 was used to determine statistical significance.
RESULTS
Data are presented from 56 individuals with Williams syndrome (WS), aged 7–65yo, along with 24 sibling controls, aged 7–63yo. (There were a total of 125 individuals with WS over 7 years of age whose families attended the convention and expressed interest in participating in research; thus our sample represents 45% of attendees with WS intending to engage in research at the convention.) One-hundred and sixty eight age-, gender-, and race/ethnicity-matched controls were randomly selected from the NHANES 2003–2004 database. Demographic and clinical characteristics of these groups are shown in Table 1.
Table 1.
Baseline Characteristics of Individuals with Williams Syndrome, Siblings, and NHANES Matched Controls
| Williams Syndrome (N = 56) | Siblings (N = 24) | Matched Controls (N = 162) | |
|---|---|---|---|
| Sex (% Male / % Female) | 39.3 / 60.7 | 45.8 / 54.2 | 39.3 / 60.7 |
| Age (years) | 21.3 ± 12.4 | 20.2 ± 14.6 | 21.0 ± 12.5 |
| Race/Ethnicity (N (%)) | |||
| Non-Hispanic White | 46 (82.1%) | 19 (79.2%) | 138 (82.1%) |
| Hispanic | 5 (8.9%) | 2 (8.3%) | 15 (8.9%) |
| Other | 5 (8.9%) | 3 (12.5%) | 15 (8.9%) |
| Age at Menarche (years, females) | 11.3 ± 1.6 | 12.7 ± 1.7 | Not Available |
Data are mean ± standard deviation (SD) unless otherwise noted.
Phalangeal Bone Mineral Density
In comparison of WS vs. sibling controls, accounting for sibship, individuals with WS had significantly lower phalangeal BMD than sibling controls (0.426±0.11 vs. 0.483±0.11 g/cm2, mean±SD of WS vs. sibling control, p = 0.004). Further controlling for age and gender, WS remained associated with significantly reduced phalangeal BMD (p < 0.0001), with an effect size of −0.07 g/cm2 (95% CI −0.1, −0.04). This association persisted in separate sensitivity analyses removing subjects with 1) use of Vitamin D supplementation, 2) multivitamin use, 3) current use of sex steroid medications, including post-menopausal hormone replacement therapy, or 4) known history of lupron treatment (data not shown, see Figure 1 for sample sizes).
The association between WS and decreased BMD was attenuated when further adjusting for height z-score (effect size of WS −0.03 g/cm2 [95% CI −0.07, 0.00], p=0.08). Additional adjustment for body mass index (BMI) did not substantially change the relationship. There was not a significant interaction between WS and gender (p=0.57), suggesting that the association between WS and lower BMD was relatively similar in both sexes.
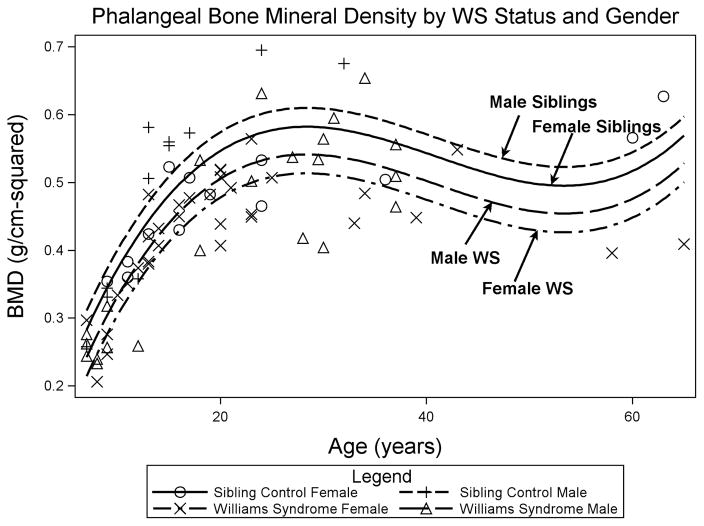
To depict differences in bone mineral density graphically, a multivariable model was constructed as a complement to the analyses above. This model used phalangeal BMD as the dependent variable and age (age, age2, and age3 as described in methods), gender, and WS as independent variables. WS was associated with decreased BMD for both males and females (p < 0.0001, Figure 3).
Figure 3.
Decreased Phalangeal BMD in WS vs Gender-matched Siblings. Phalangeal bone mineral density by age in WS females (X), sibling females (O), WS males (Δ), and sibling males (+). Model was fit using age (p<0.0001), age2 (p<0.0001), age3 (p<0.0001), gender (p=0.06), and WS (p<0.0001).
Phalangeal BMD z-scores were available for 28 individuals with WS and 8 sibling controls who were over 19yo and of a race/ethnicity with normative data available for AccuDXA. Z-scores were significantly lower in those with WS (−0.82±1.2 mean±SD) compared to the group of sibling controls (0.55±1.01 mean±SD, p-value 0.004 for comparison). This difference persisted when controlling for adult height (p-value = 0.04 for effect of WS on z-score adjusting for height). The distribution of Z-scores for adults with WS and sibling controls is shown in Figure 4.
Figure 4.
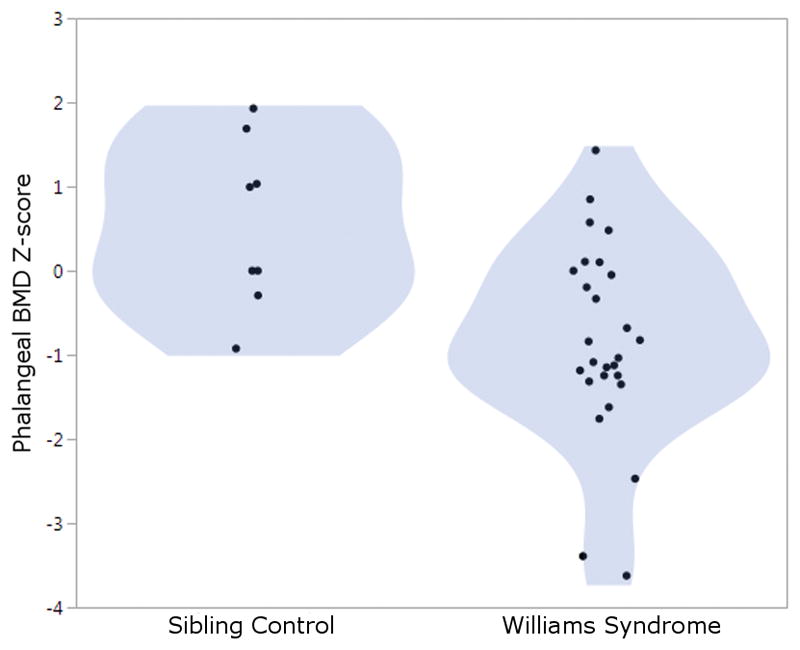
Decreased Phalangeal BMD Z-score in WS Adults vs Siblings. Phalangeal BMD z-scores for individuals with Williams Syndrome compared to Sibling Controls. The shaded area represents the density of distribution. P = 0.0046 for comparison between WS and controls.
Body Composition
Anthropometric measurements by age group are shown in Table 2. As expected, all WS individuals were shorter than NHANES controls, and children with WS tended to have smaller circumferential measurements than controls, with significant differences at the midarm and waist. Young adults with WS were similar to controls with regard to BMI and anthropometric measurements. Older adults with WS, however, were heavier than controls (BMI 33.6±8.3 vs. 27.55.3 cm, WS vs. control, p=0.004) and had a larger thigh circumference (61.3±8.6 vs. 54.7±5.6cm, p=0.002).
Table 2.
Anthropometric Characteristics in Williams Syndrome and Matched Controls by Age Group
| Age < 18y | Ages 18–30y | Age > 30y | ||||
|---|---|---|---|---|---|---|
| Williams Syndrome (N=24) |
Matched Controls (N=77) |
Williams Syndrome (N=21) |
Matched Controls (N=56) |
Williams Syndrome (N=11) |
Matched Controls (N=35) |
|
| Height (cm) | 135.8 ± 15.4† | 146.1 ± 18.8† | 157.3 ± 8.2†† | 169.3 ± 8.2†† | 159.0 ± 10.2†† | 172.5 ± 9.9†† |
| Weight (kg) | 35.2 ± 13.8† | 45.3 ± 17.3† | 60.4 ± 19.3† | 66.8 ± 13.9† | 85.1 ± 23.1 | 82.0 ± 16.7 |
| BMI (kg/m2) | 18.3 ± 3.8† | 20.5 ± 4.6† | 24.2 ± 7.4 | 23.2 ± 3.7 | 33.6 ± 8.3† | 27.5 ± 5.3† |
| Percent body fat (%)** | 20.7 ± 7.7†† | 31.3 ± 9.0†† | 24.8 ± 16.7 | 28.2 ± 8.7 | 30.8 ± 7.2 | 28.8 ± 9.6 |
| Mid-arm circumference (cm) | 21.0 ± 5.9† | 24.6 ± 4.7† | 27.6 ± 6.2 | 29.0 ± 3.9 | 32.6 ± 4.4 | 32.9 ± 3.6 |
| Waist circumference (cm) | 63.2 ± 10.2† | 71.2 ± 13.1† | 82.1 ± 17.9 | 83.0 ± 11.6 | 101.0 ± 21.8 | 95.9 ± 13.9 |
| Thigh circumference (cm)* | 41.1 ± 8.5† | 46.8 ± 6.3† | 51.4 ± 7.5 | 50.6 ± 4.5 | 61.3 ± 8.6† | 54.7 ± 5.6† |
Values are mean±standard deviation. BMI: Body mass index.
Thigh circumference measurements available only for 23 WS and 60 controls age <18y.
Percent body fat available for 21 WS and 56 controls age <18y, 20 WS and 46 controls ages 18–30y, and 10 WS and 26 controls age >30y.
p < 0.05 for comparision between Williams Syndrome and Controls using mixed modeling with pair-match as a random effect.
p < 0.001 for comparison between Williams Syndrome and Controls using mixed modeling with pair-match as a random effect.
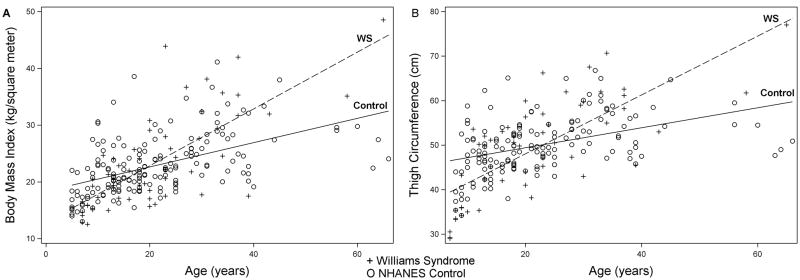
Modeling of both BMI and thigh circumference by age showed a significant interaction between WS and age, such that individuals with WS begin with lower-than-average BMI and thigh circumference and develop higher-than-average BMI and thigh-circumference in adulthood (Figure 5).
Figure 5.
Interaction Between WS and Age on BMI and Thigh Circumference. BMI (3A) and thigh circumference (3B) by age in WS (+) and NHANES controls (O). P < 0.0001 for the interaction between age and Williams Syndrome in both models, indicating greater increase in BMI and thigh circumference with increasing age in WS compared to controls.
Eight individuals with WS were noted to have at least a mild degree of fat accumulation in the lower extremities with an appearance consistent with lipedema. Analysis by age category demonstrated this finding in 6/11 >30 year olds, 1/19 18–30 year olds, and 1/24 children under 18. The youngest individual in whom this was noted was a 17 year old female, whereas the oldest was a 65 year old female. Three individuals were males, and five were females; seven were self-described as white; and one as mixed race. BMIs of these individuals ranged from 24.6–48.6kg/m2. Four of these individuals had a total of five siblings, none of whom were noted to have lipedema. Five WS individuals had moderate-to-severe lower extremity lipedema, and two of these individuals also had lipedema in the upper extremities. Two subjects with this lower-extremity fat accumulation are shown in Figure 2.
DISCUSSION
Our data provide further information about bone mineral density and body composition across the age span in individuals with WS. Results from phalangeal AccuDXA scanning indicate decreased BMD in both children and adults with WS compared to controls. In terms of body composition, individuals with WS demonstrate a crossing interaction of BMI x age compared to healthy controls. Specifically, young WS children tend to have lower BMIs, while WS adults tend to have higher BMIs, than age-gender-race matched population controls. Thigh circumference demonstrates the same trend. Physical examination findings show a relative increase in girth of the lower half of the body in several adults (~25% of those >18 years of age), most resembling the phenotype lipedema. The significance of these observations and their potential limitations are further discussed below.
Phalangeal Bone Mineral Density
The finding of reduced phalangeal BMD in both males and females with WS is consonant with previous observations from conventional dual-energy X-ray absorptiometry (DXA) performed on a small cohort of WS adults [Cherniske and others 2004] as well as a recent report of reduced bone mineralization in children and young adults assessed by phalangeal ultrasound [Stagi and others 2016]. The data herein is collected across the lifespan, and raises the possibility of a deficit in accrual of BMD, since significant reductions are noted even in childhood. Adjusting for the fact that children and adults with WS are shorter than comparably aged individuals in the general population attenuates but does not eliminate the relationship between WS and reduced BMD. Several potential risk factors, some of which are specific to WS, could contribute to low BMD. For instance, parents of individuals with WS report that life-long calcium and vitamin D restricted diets often continued even when hypercalcemia was never (or not recently) documented (BRP, personal observations), while one of the genes typically deleted in WS, FZD9, has been associated with bone loss in knockout mice ([Albers and others 2011]. Factors not specific to WS are also possible, since decreased BMD has been noted in other developmental disability syndromes such as Down, Prader-Willi, Turner and diGeorge, among others. Potential risk factors that cut across one or more of these disorders, and possibly extend to WS, include hypotonia, reduced physical activity, celiac disease, and perturbations in estrogen and/or testosterone ratios [Stagi and others 2015].
Body Composition
“Failure to thrive” and small body size have historically been associated with WS. These descriptions stem from early case reports [Fanconi and others 1952; Russell and Young 1954] and publication of WS specific growth curves [Martin and others 2007; Morris and others 1988].
Our results confirm decreased BMI and percent body fat among individuals with WS under 18 years of age [Kaplan and others 1998; Nogueira and others 2011]. The limited adult data (3 adults described by Kaplan et al. [Kaplan and others 1998] suggest that lower BMI and % body fat persists into adulthood. In contrast, our data show that, compared to NHANES controls, adults with WS have higher BMI and increased lower extremity fat, as assessed by thigh circumference (Table 2). The interaction between WS and age in predicting BMI and thigh circumference (Fig 3) indicate a greater gain of weight, on average, over time especially in the lower half of the body compared to controls.
Further, a subset of adults with WS demonstrates a clinical phenotype similar to lipedema (Figure 2). The current observations confirm the initial report of this phenotype in 20% of adults at least 30 years of age [Cherniske and others 2004]. Lipedema [OMIM %614103] is a rare though increasingly recognized disorder in the general population. It consists of slowly progressive symmetric deposition of adipose tissue, primarily in the buttock and hip region but extending down the leg with sparing of the feet. Pitting is minimal, but pain, easy bruising, and tenderness are common. Arm involvement accompanies leg involvement in ~30% of cases. Lipedema typically develops after puberty and predominantly occurs in females. Divergent prevalence estimates exist due to various factors, such as whether patients were identified in the general population or in a specialty clinic [Child and others 2010; Fife and others 2010; Forner-Cordero and others 2012]. Currently, the etiology of lipedema is unknown, but familial clustering is noted and mechanistic hypotheses include impaired estrogen signaling and microangiopathy due to endothelial barrier dysfunction [Szel and others 2014]. Several aspects of the lipedema-like phenotype seen in WS merit special mention because they are distinct from general population lipedema. These include: a seemingly higher prevalence (e.g., a frequency of ~20% has not been reported in any other population of adults); the occurrence of affected WS males, which starkly contrasts to the preponderance of affected females in the general population; and finally, the absence of pain, tenderness and easy bruising of the involved areas in persons with WS. The latter observation is based on our examination of subjects participating in this study and also WS patients and research subjects seen in our practices. Given these differences, it will be important for future studies to assess the lower extremities of adults with WS by DXA or other imaging to confirm that excess adipose tissue is indeed responsible for the apparent lipedema and to elucidate any characteristics of this tissue that may differentiate it from typical subcutaneous adipose tissue. Future studies should also determine if this phenotype is associated with metabolic abnormalities, in light of the relatively high prevalence of impaired glucose tolerance and type 2 diabetes mellitus seen in WS [Cherniske and others 2004; Masserini and others 2011; Stagi and others 2014].
Strengths and Limitations
The strengths of this study are the relatively large sample and broad age range of the cohort. The latter allows us to explore development al trajectories relating to BMI and bone density, for which little WS specific data currently exist. Recruitment of WS individuals and available siblings as their controls is also a strength, as it serves to maximize environmental similarities (eg, WS individual and his/her sib were likely raised in the same household) while at the same time minimize genetic variation on traits such as BMI and bone density. The study also has several limitations. The subjects are a convenience sample who self-selected to take part in a project entitled “Sugar and Fat”. Participation may have been of particular interest to those with body composition abnormalities. The definition of lipedema was subjective, as there is no laboratory or imaging standard; accordingly, the extent of lipedema was based on clinical impression by the two study physicians. The diagnosis of WS was reported by participants and clinically confirmed in each subject by an experienced examiner, but the absence of biological samples precluded molecular confirmation. In terms of the BMD findings, phalangeal bone density has not previously been employed in children but correlates with standard DXA results in adults [Fiter and others 2001; Patel and others 2010]. Though there is no pediatric control cohort for phalangeal BMD, the available Z-scores in adults, the observations made throughout the age-span using the sibling controls, plus the recent findings by Stagi et al. [Stagi and others 2016] suggest that decreased BMD is common in WS. The current findings are also consistent with results from standard DXA scanning performed in a separate adult cohort [Cherniske and others 2004]. However, studies using conventional DXA scanning, which has well established norms in children, will be required for confirmation. Further studies will also need to address several other issues. For instance, the children in our study were not Tanner staged. Even so, the fact that puberty tends to occur earlier in persons with WS than controls [Cherniske and others 1999; Partsch and others 2002] may very well accentuate the BMD differences we observed controlling for age (as BMD should be relatively higher at a younger chronological age in persons with WS). Dietary history should also be sought in future studies. In our design, sib controls served as a proxy for environmental “sameness”. Presumably, siblings were raised in same household as their family member with WS; however, some parents report maintaining their child with WS on a calcium and vitamin D restricted diet (BRP, personal observations). Accordingly, it will be important to clarify if individuals with WS have different dietary patterns, especially in the intake of calcium and vitamin D which might contribute to reduced BMD. Additionally, levels of physical activity can impact BMD and could not be controlled for in our analyses. We attempted to collect activity information by self-report; review of these data deemed them unreliable. Finally, it will be important to determine if reduced BMD conveys a higher fracture risk, as this would impact clinical management across the WS lifespan.
In summary, this work adds to existing knowledge on bone density and body composition in children and adults with WS. Given that low BMD is present in WS starting in childhood, our data suggest this may result from abnormal accrual rather than accelerated loss. The reduction in BMD compared to sibling controls confirms the previous report of abnormal BMD as yet another characteristic of WS. In terms of body composition, we observed a WS-age interaction on BMI and thigh circumference and, further, a patient subset have a lower extremity phenotype resembling lipedema. Future work that includes detailed imaging and metabolic studies is needed to characterize these features in greater detail and elucidate underlying mechanisms.
Acknowledgments
We would like to thank the individuals and families who volunteered for this study as well as the Williams Syndrome Association (WSA) for facilitating the conduct of research during the WSA Convention. We also thank Dr Lynn Copes (Department of Medical Sciences, Frank H Netter School of Medicine, Quinnipiac University, North Haven CT.) for kindly allowing us to use her AccuDXA2® device.
Funding Source:
The cost of this work was supported, in part, by funds from the Department of Medical Sciences, Frank H Netter School of Medicine, Quinnipiac University, North Haven CT, USA.
Footnotes
Conflicts of Interest: None of the authors has any conflict of interested relevant to the content of this manuscript.
References
- Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A. 2004;131(3):255–264. doi: 10.1002/ajmg.a.30400. [DOI] [PubMed] [Google Scholar]
- Cherniske EM, Sadler LS, Schwartz D, Carpenter TO, Pober BR. Early puberty in Williams syndrome. Clin Dysmorphol. 1999;8(2):117–121. [PubMed] [Google Scholar]
- Child AH, Gordon KD, Sharpe P, Brice G, Ostergaard P, Jeffery S, Mortimer PS. Lipedema: an inherited condition. Am J Med Genet A. 2010;152A(4):970–976. doi: 10.1002/ajmg.a.33313. [DOI] [PubMed] [Google Scholar]
- Fanconi G, Girardet P, Schlesinger B, Butler N, Black J. Chronic hyperglycemia, combined with osteosclerosis, hyperazotemia, nanism and congenital malformations. Helv Paediatr Acta. 1952;7(4):314–349. [PubMed] [Google Scholar]
- Fife CE, Maus EA, Carter MJ. Lipedema: a frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv Skin Wound Care. 2010;23(2):81–92. doi: 10.1097/01.ASW.0000363503.92360.91. quiz 93–84. [DOI] [PubMed] [Google Scholar]
- Fiter J, Nolla JM, Gomez-Vaquero C, Martinez-Aguila D, Valverde J, Roig-Escofet D. A comparative study of computed digital absorptiometry and conventional dual-energy X-ray absorptiometry in postmenopausal women. Osteoporos Int. 2001;12(7):565–569. doi: 10.1007/s001980170078. [DOI] [PubMed] [Google Scholar]
- Forner-Cordero I, Szolnoky G, Forner-Cordero A, Kemeny L. Lipedema: an overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome - systematic review. Clin Obes. 2012;2(3–4):86–95. doi: 10.1111/j.1758-8111.2012.00045.x. [DOI] [PubMed] [Google Scholar]
- Kaplan AS, Stallings VA, Zemel BS, Green KA, Kaplan P. Body composition, energy expenditure, and energy intake in patients with Williams syndrome. J Pediatr. 1998;132(2):223–227. doi: 10.1016/s0022-3476(98)70435-4. [DOI] [PubMed] [Google Scholar]
- Martin ND, Smith WR, Cole TJ, Preece MA. New height, weight and head circumference charts for British children with Williams syndrome. Arch Dis Child. 2007;92(7):598–601. doi: 10.1136/adc.2006.107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserini B, Bedeschi MF, Bianchi V, Lunati ME, Lalatta F, Beck-Peccoz P, Orsi E. High prevalence of impaired glucose metabolism in young adult patients with Williams syndrome. European Congress of Endocrinology; 2011; Rotterdam, The Netherlands. 2011. [Google Scholar]
- Morris CA, Demsey SA, Leonard CO, Dilts C, Blackburn BL. Natural history of Williams syndrome: physical characteristics. J Pediatr. 1988;113(2):318–326. doi: 10.1016/s0022-3476(88)80272-5. [DOI] [PubMed] [Google Scholar]
- Mussolino ME, Looker AC, Madans JH, Edelstein D, Walker RE, Lydick E, Epstein RS, Yates AJ. Phalangeal bone density and hip fracture risk. Arch Intern Med. 1997;157(4):433–438. [PubMed] [Google Scholar]
- Nogueira RJ, Zimmerman LF, Moreno YM, Comparini CR, Viana DV, Vieira TA, Steiner CE, Gil-da-Silva-Lopes VL. Anthropometric and body-mass composition suggests an intrinsic feature in Williams-Beuren syndrome. Rev Assoc Med Bras. 2011;57(6):681–685. doi: 10.1590/s0104-42302011000600016. [DOI] [PubMed] [Google Scholar]
- Partsch CJ, Japing I, Siebert R, Gosch A, Wessel A, Sippell WG, Pankau R. Central precocious puberty in girls with Williams syndrome. J Pediatr. 2002;141(3):441–444. doi: 10.1067/mpd.2002.127280. [DOI] [PubMed] [Google Scholar]
- Patel R, Blake GM, Panayiotou E, Fogelman I. Clinical evaluation of a phalangeal bone mineral density assessment system. J Clin Densitom. 2010;13(3):292–300. doi: 10.1016/j.jocd.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362(3):239–252. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- Russell A, Young WF. Severe idiopathic infantile hypercalcaemia; long-term response of 2 cases to low calcium diet. Proc R Soc Med. 1954;47(12):1036–1040. doi: 10.1177/003591575404701204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagi S, Iurato C, Lapi E, Cavalli L, Brandi ML, de Martino M. Bone status in genetic syndromes: a review. Hormones (Athens) 2015;14(1):19–31. doi: 10.1007/BF03401378. [DOI] [PubMed] [Google Scholar]
- Stagi S, Lapi E, Cecchi C, Chiarelli F, D’Avanzo MG, Seminara S, de Martino M. Williams-beuren syndrome is a genetic disorder associated with impaired glucose tolerance and diabetes in childhood and adolescence: new insights from a longitudinal study. Horm Res Paediatr. 2014;82(1):38–43. doi: 10.1159/000360476. [DOI] [PubMed] [Google Scholar]
- Stagi S, Manoni C, Scalini P, Chiarelli F, Verrotti A, Cecchi C, Lapi E, Giglio S, Romano S, de Martino M. Bone mineral status and metabolism in patients with Williams-Beuren syndrome. Hormones (Athens) 2016 doi: 10.14310/horm.2002.1683. [DOI] [PubMed] [Google Scholar]
- Szel E, Kemeny L, Groma G, Szolnoky G. Pathophysiological dilemmas of lipedema. Med Hypotheses. 2014;83(5):599–606. doi: 10.1016/j.mehy.2014.08.011. [DOI] [PubMed] [Google Scholar]