Visual Abstract
Key Words: cardiomyopathy, catecholamine, gene therapy, heart failure
Abbreviations and Acronyms: AAV, adeno-associated virus; AC, adenylyl cyclase; AC6mut, AC6 mutant, contains an amino acid substitution that reduces its catalytic activity; Ad5, adenovirus-5; AKAP, A-kinase-anchoring protein; ATP, adenosine triphosphate; AV, atrioventricular; βAR, β-adrenergic receptor; C1C2, a fusion of the C1 and C2 cytoplasmic domains of AC; cAMP, 3′,5′-cyclic adenosine monophosphate; CREB, cAMP response element binding protein; CryAB, αB-crystallin; HF, heart failure; Iso, isoproterenol; LV, left ventricle, left ventricular; MDM2, murine double mutant 2; PHLPP2, PH domain leucine-rich protein phosphatase 2; PI3K, phosphatidylinositide 3-kinase; PLB, phospholamban; P-Rex2, phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 2; RGAS, regulator of G protein signaling; SERCA2a, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
Highlights
-
•
Cardiac-targeted expression of C1C2 reduces cAMP production yet mice maintain normal cardiac function through increased Ca2+ handling.
-
•
Sustained isoproterenol infusion reduces heart function in normal mice, but improves heart function in mice with increased cardiac C1C2 expression.
-
•
Reduced cardiac cAMP generation and resistance to catecholamine cardiomyopathy are attractive features of this potential heart failure therapeutic.
-
•
Removing the large transmembrane domains of AC6 and fusing the two intracellular domains provides a small molecule, C1C2, that replicates many of the beneficial effects of AC6, but is sufficiently small to be expressed in an AAV vector for gene transfer.
Summary
Transgenic mice with cardiac-directed C1C2, a fusion protein of the intracellular C1 and C2 segments of adenylyl cyclase type 6, had normal left ventricular (LV) function, but diminished cAMP generation. Cardiac myocytes from C1C2 mice showed increased Ca2+ release. Mice underwent continuous isoproterenol infusion to stress the heart. In C1C2 mice, sustained isoproterenol infusion increased rather than decreased LV function. LV SERCA2a and Ca2+ release were increased. Reduced cAMP generation and resistance to catecholamine cardiomyopathy are attractive features of this potential heart failure therapeutic.
Adenylyl cyclase (AC) is a transmembrane protein in cardiac myocytes and other cells, the effector molecule for β-adrenergic receptor (βAR) and other G protein-coupled receptors, which regulates the conversion of adenosine triphosphate (ATP) to 3′,5′-cyclic adenosine monophosphate (cAMP) and thereby initiates a variety of intracellular signaling cascades that influence heart function and additional physiological events.
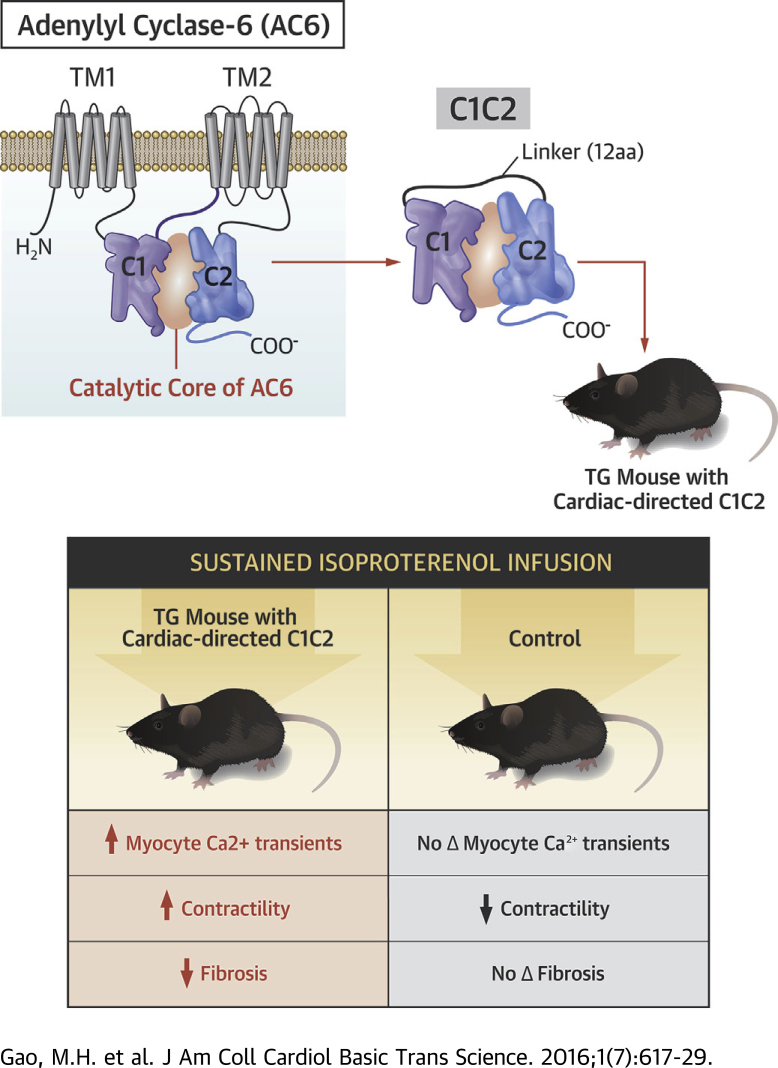
There are 9 membrane-bound isoforms of mammalian ACs, each consisting of 2 transmembrane domains and 2 cytoplasmic domains (C1 and C2). The C1 and C2 domains form the catalytic core of AC (Figure 1A). When expressed as a fusion protein, C1C2 is soluble and retains forskolin-stimulated catalytic activity (1). C1C2 contains binding sites for Gαs, Gαi, forskolin, ATP, Mg2+, the regulator of G protein signaling (RGΑS2), protein associated with Myc (PAM), Snapin, Ric8a, A-kinase-anchoring protein (AKAP79), protein kinase C, PH domain leucine-rich protein phosphatase 2 (PHLPP2) and phosphorylation and dephosphorylation sites for protein kinase A. Interactions of these factors alters the conformation of C1C2 and regulates AC activity (2).
Figure 1.
C1C2 Design, Expression, Cellular Distribution, Activity, and Intracellular Signaling
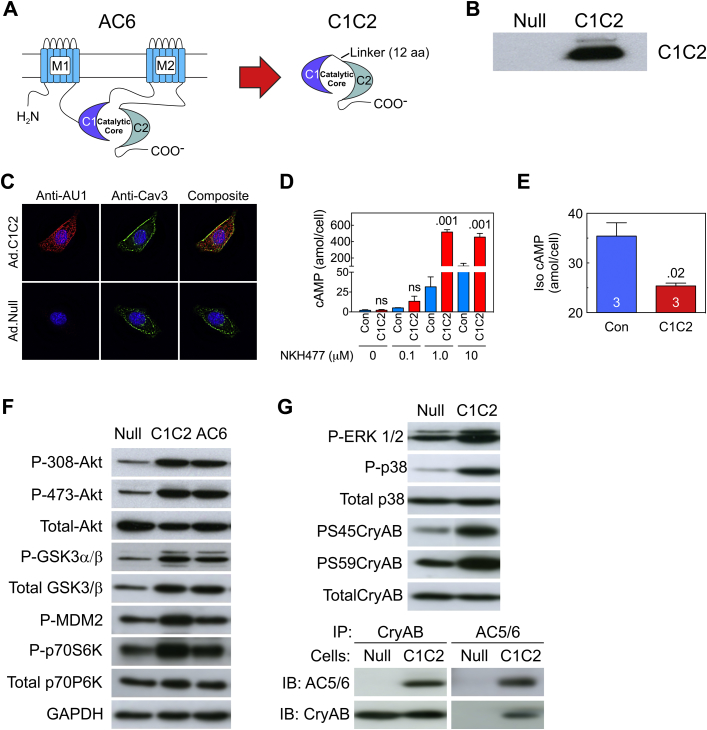
(A) C1C2 construct that forms the catalytic core. M1 and M2, transmembrane domains of AC6; C1 and C2, cytoplasmic domains of AC6; Linker, 12 amino acids. (B) C1C2 protein was detected in immunoblotting using an anti-AU1 tag antibody in NRCM after gene transfer with Ad5.C1C2 (200 vp/cell). (C) Double-immunofluorescence staining of C1C2 protein in NRCM using anti-AU1 antibody (red); anti-caveolin 3 (Cav-3) antibody (green). Yellow indicates co-localization of C1C2 with caveolin. (D, E) NRCM underwent Ad5.C1C2 gene transfer and the amount of cAMP production in response to NKH477, a forskolin analog (D) or 10 μM Iso (10 min) (E). Cardiac myocytes expressing C1C2 showed increased catalytic activity in a dose-dependent manner after stimulation with NKH477 (D) (activates AC) but reduced cAMP activity in response to βAR (Iso) stimulation (E). Results were confirmed in 3 separate experiments. A representative experiment is shown with triplicate samples. Bars denote mean ± SE; p values are from Student′s t-test (unpaired, 2-tailed). (F) Immunodetection of molecules in the Akt signaling pathway indicate that C1C2 and AC6 expression were associated with similar increases in phosphorylation of Akt, GSK3α/β, MDM2, and p70S6k, suggesting that the effect does not require AC6-mediated cAMP production. (G) C1C2 expression was associated with phosphorylation of ERK1/2, p38 MAPK, and αB-crystallin (CryAB, upper). Interaction of CryAB and C1C2 detected by co-immunoprecipitation and immunoblotting (lower). In A to E, Con denotes transgene negative mice. Iso = isoproterenol; NRCM = neonatal rat cardiac myocytes.
We have published a series of papers indicating that increased cardiac expression of AC type 6 (AC6), a dominant AC isoform expressed in mammalian cardiac myocytes (3), has protean beneficial effects on the failing left ventricle (LV). These effects include: 1) increased survival in genetically-induced cardiomyopathy (4) and in acute myocardial infarction (5); 2) reduced action potential duration (6), facilitated atrioventricular (AV) conduction (7), and reduced AV block (5); 3) reductions in both LV dilation and pathological hypertrophy 4, 8; 4) beneficial effects on Ca2+ handling via altered activity of SERCA2a and phospholamban (PLB) 9, 10; and 5) increased cardiac troponin I phosphorylation (11).
These beneficial effects, consistent in several species and models, appear in large part to not depend upon increased cAMP generation. A phase 2 randomized clinical trial in patients with symptomatic heart failure (HF) and reduced ejection fractions showed that intracoronary AC6 gene transfer appears to be safe and potentially effective, and not associated with increased cardiac arrhythmias (12). Even so, there may be advantages in selecting a transgene that attenuates βAR responsiveness when treating HF.
We subsequently generated a catalytically inactive AC6 mutant (AC6mut) molecule by replacing Ala with Asp at position 426 in AC6's catalytic core. This AC6mut is catalytically inactive (does not generate cAMP) but retains the cellular distribution pattern and favorable signaling effects associated with normal AC6, thereby providing compelling evidence that the beneficial effects of AC6 do not require increased cAMP generation (13). AC6mut seemed an ideal candidate for the treatment of HF, retaining the beneficial effects of the parent AC6 while circumventing the potential deleterious effects of sustained cAMP generation. However, a shortcoming of both AC6mut and AC6 is that the molecules are too large to insert into an adeno-associated virus (AAV) with regulated expression, therefore only constitutive expression would be possible, a potential limitation.
Eliminating the amino terminus and the 2 transmembrane domains of AC6 and subsequently fusing the 2 cytoplasmic domains (C1 and C2) with a 12-amino acid linker yields a C1C2 protein (Figure 1A). C1C2 has an intact catalytic domain but is disengaged from membrane-associated βARs and is therefore less responsive to βAR stimulation in intact cells. C1C2 is sufficiently small to be inserted in an AAV vector with a regulated expression cassette, enabling turning off transgene expression when desired. Many of the beneficial effects of AC6 that we have described are independent of cAMP generation and appear, instead, to involve intracellular AC6-protein interactions 10, 13. We speculated that elimination of the transmembrane regions of AC6 (Figure 1A) would facilitate intracellular distribution and would include the region of the AC6 molecule (C1C2) most likely engaged in protein-protein interactions (14). Initial tests using adenovirus-mediated C1C2 gene transfer in cultured cardiac myocytes confirmed our speculations vis-à-vis C1C2’s beneficial effects on signaling and reduced cAMP generation, setting the stage for development of gene transfer of C1C2 as a potential therapy for HF. The goal of the current study was to perform mechanistic and translational studies of C1C2. Our hypothesis was that cardiac-directed expression of the cytoplasmic domains of AC6 would have beneficial effects on the heart.
Methods
Generation of C1C2 fusion protein
C1C2 peptide was generated by ligating the cytoplasmic domain 1 (C1, amino acids 349 - 576) with cytoplasmic domain 2 (C2, amino acids 939 - 1157) of mouse adenylyl cyclase type 6 (AC6) using a linker (12 amino acids: AAAGGIPPAAAM) as illustrated in Figure 1A. The C-terminus of C1C2 was linked with an AU1 epitope tag (DTYRYI) to facilitate detection.
Generation of C1C2 transgenic mice
Animals were used in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and approved by the Institutional Animal Care and Use Committee of VA San Diego Healthcare System. To generate mice with cardiac-directed expression of C1C2, the murine C1C2 cDNA with an AU1 tag at the C terminus was subcloned between the α-myosin heavy chain promoter and SV40 poly(A). A 7.1-kb fragment containing the expression cassette was used for pronuclear injection, carried out in the transgenic mouse facility at University of California, San Diego (inbred C57BL/6). Founder mice were identified by polymerase chain reaction (PCR) of genomic DNA prepared from tail tips. The C1C2 gene was detected using a primer homologous to the α-MHC promoter (forward: 5'-CACATAGAAGCCTAGCCCACACC-3′) and another primer in the C1 region (5'-GTTAGCCAGGGTCACATCGT-3′). C1C2 mRNA was detected in reverse transcription quantitative PCR, using the forward primer 5'-TGGGCCTCTCTACTCTGCAT-3′ and the reverse primer 5'-TGGATGTAACCTCGGGTCTC-3′, enabling quantification of fold increase of C1C2 mRNA relative to that of endogenous AC6 mRNA. Founder animals were crossbred with normal mice of the same strain, and selected animals were used for analysis of cardiac transgene expression. We documented variable transgene mRNA expression but similar levels of C1C2 protein expression in 2 lines and used the line with a 23-fold increase in C1C2 mRNA expression (vs. endogenous AC6) in this study. C1C2 protein was detected using anti-AU1 antibody, which has a low background in LV homogenates from transgene negative mice. C1C2 protein was 13-fold increased relative to that of the AU1 signal in transgene negative mice. The study included 114 mice (68 male, 46 female), 4.4 ± 0.1 months of age, weighing 23.9 ± 0.6 g.
Echocardiography
Echocardiography was performed prior to isoproterenol (Iso) infusion and on day 6 or 7 of sustained Iso infusion, using methods previously described (15). Anesthesia was induced with 5% isoflurane (at a flow rate of 1 L/min oxygen) and maintained with 1% isoflurane in oxygen.
LV systolic and diastolic function
Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg), and a 1.4-F micromanometer catheter (Millar Instruments, Houston, Texas) was advanced via the right carotid artery across the aortic valve and into the LV cavity. Left ventricular pressure was recorded and stored digitally for processing (IOX1.8; Emka Technologies, Christchurch, Virginia) as previously reported (11). Data were acquired and analyzed without knowledge of group identity. Subsequently, tissue samples were obtained.
Cardiac myocyte isolation
Cardiac myocytes were isolated as previously described 15, 16.
Ca2+ transients
Cytosolic Ca2+ transients (Indo-1) were measured from cardiac myocytes isolated from transgenic mice as previously described 13, 17.
Cyclic AMP measurement and PKA activity assay
Isolated cardiac myocytes were stimulated with Iso (10 μM, 10 min) or the water-soluble forskolin analog NKH477 (10 μM, 10 min). Cyclic AMP was measured using the cAMP Biotrak EIA (GE Healthcare, Chalfont, United Kingdom) as previously reported (15). PKA activity was determined as previously described (17).
Immunofluorescence
Isolated cardiac myocytes were attached to laminin-coated 2-well chamber slides for 1 h, washed, fixed (10% formalin, 15 min, 23°C), blocked with normal goat serum (1 h), and incubated (4°C, overnight) with the following antibodies: anti-AU1 antibody (1:300 dilution; for detecting C1C2 transgene protein; Fitzgerald, Atlanta, Georgia) and anti-Cav3 antibody (1:100 dilution, for detecting caveolae; BD Pharmingen, San Jose, California). Cardiac myocytes were washed with phosphate-buffered saline and then incubated with secondary antibodies (Alexa Fluor 488 or 594 conjugated; 1:1,000 dilution) for 1 h. To identify the nucleus, cells were incubated with Hoechst dye (1:1,000 dilution, 20 min). Then, cardiac myocytes were imaged as previously described (18).
Isoproterenol infusion
Osmotic minipumps (Alzet; DuRECT Corp., Cupertino, California) were filled with Iso or saline. The 7-day continuous infusion delivered 60 mg/d/kg as previously described (19).
RT-PCR
Primers for detecting phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 2 (P-Rex2) included forward primer 5′-ATCATGTGCAGCAGTGGTGT and reverse primer 5′-CCTTGGAGCTGACTGAGGAG. The absence of suitable AC type-specific antibodies precluded quantitative assessment of LV AC isoform protein content. Instead, we used quantitative real-time RT-PCR to determine whether cardiac-directed C1C2 expression altered the cardiac expression of other AC isoforms (AC3, AC4, AC5, AC6, AC7, AC8, and AC9). Details of PCR conditions and primer sequences can be found in our previous studies (20).
Microarray, antibodies
Expression of adenylyl cyclase-related proteins were analyzed using the Agilent gene expression microarray technique (PhalanxBio Inc., San Diego, California). C1C2 protein was detected by anti-AU1 antibody (1:2,000 dilution: Fitzgerald) or anti-AC5/6 antibody (1:200 dilution; Santa Cruz Biotechnology, Dallas, Texas). Additional antibodies included GAPDH (1:20,000 dilution; Fitzgerald); cMLCK (1:1,000 dilution; Abgent, San Diego, California); MLC2v (1:1,000 dilution; Synaptic Systems, Gottingen, Germany), PKA catalytic subunit (1:1,000 dilution; BD Transduction, San Jose, California); p-ERK1/2, p38, p-PKARII α and β (1:200 dilution; Santa Cruz Biotechnology); PLB (1:5,000 dilution; Affinity Bioreagents, Rockford, Illinois); phospho-16-PLB (1:3,000 dilution; Badrilla, Leeds, United Kingdom); S100A1 (1:1,000 dilution; Acris); SERCA2a antibody (1:1,000 dilution; Enzo, Exeter, United Kingdom); phospho-αB-crystallin (CryAB) and total CryAB antibodies (1:1,000 dilution; Enzo); P-308-Akt, P-473-Akt, T-Akt, p-GSK3a/b, p-MDM2, P-p70S6K, T-p70S6K, and phospho-S22/23 troponin I (TnI) (1:1,000 dilution each; Cell Signaling, Danvers, Massachusetts); vinculin (1:100,1000 dilution; Sigma, Darmstadt, Germany).
Necropsy
Body, liver, lung, and LV weight (including interventricular septum) were recorded and a short axis midwall LV ring was fixed in formalin and embedded in paraffin. The remaining LV was quickly frozen in liquid nitrogen and stored at −80°C.
Histology
Transmural sections of the LV were formalin-fixed and paraffin-embedded. Sections (5 μm) were mounted and counterstained with hematoxylin and eosin and with Masson's trichrome. For quantitative assessment of LV fibrosis, images of a short-axis midwall LV ring stained with Picro Sirius red stain were obtained by using a NanoZoomer digital slide scanner (Hamamatsu, Hamamatsu, Japan). Blinded analysis of the degree of fibrosis was conducted using ImageJ software (U.S. National Institutes of Health, Bethesda, Maryland).
Statistical analysis
Data are mean ± SE. Between-group comparisons were made using Student t-test (unpaired, 2-tailed). Interactions of C1C2 expression and Iso infusion on outcomes were tested for statistical significance using 2-way ANOVA followed by Bonferroni t-test. The null hypothesis was rejected when p < 0.05.
Results
C1C2 gene transfer in cardiac myocytes
Location and AC activity
Anti-AU1 antibody was used to detect C1C2 expression in neonatal rat cardiac myocytes after Ad5.C1C2 infection (200 vp/cell) (Figure 1B). Intracellular C1C2 transgene protein was detected in the cytosol and plasma membrane/caveolae (Figure 1C) and exhibited dose-dependent augmentation in AC activity when stimulated with the water soluble forskolin analog NKH477 (Figure 1D). Ad5-mediated C1C2 expression in neonatal rat cardiac myocytes reduced net Iso-stimulated cAMP generation (Figure 1E), which was also seen in LV samples from control and C1C2 mice (Figure 2B). In contrast, we did not see increased NKH477-stimulated cAMP in LV homogenates from C1C2 TG mice (Figure 2B) as we saw after C1C2 gene transfer (Figure 1D), which may reflect differences in amounts of C1C2 expressed (Ad5-mediated vs. transgenic line), species (rat vs. mice), or age (neonatal vs. adult cardiac myocytes). Although we saw reduced Iso-stimulated cAMP generation in cardiac myocytes from C1C2 mice compared to control (Figure 2B), this decrement was not sufficient to reduce PKA activity (Figure 2C).
Figure 2.
C1C2 Transgenic Mice
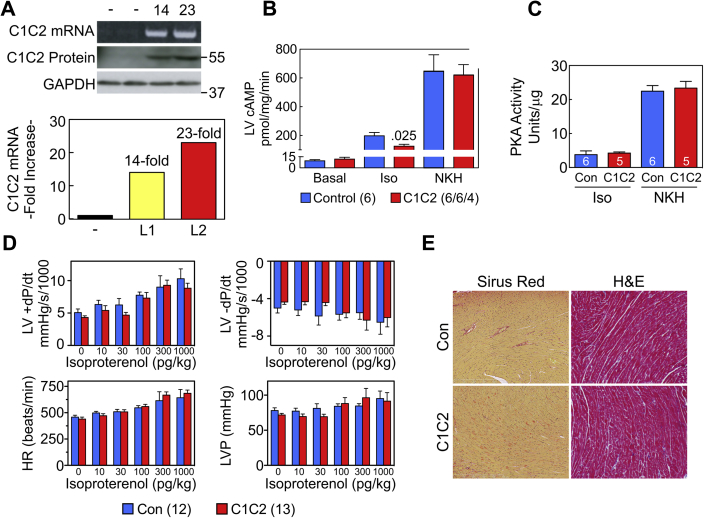
(A) Increased C1C2 mRNA and protein were documented from 2 founder lines (L1, L2) that showed different expression levels of mRNA but similar levels of protein. (B) Transmural LV samples underwent stimulation with Iso (10 μM, 10 min) or NKH477 (AC activator; 10 μM, 10 min). Net Iso cAMP production was reduced in LV from C1C2 mice. Basal and AC-dependent cAMP showed no group differences. (C) PKA activity was unchanged by increased C1C2 expression. (D) Despite diminished Iso-stimulated cAMP production in LV from C1C2 mice, there were no group differences (C1C2 vs. Con) in LV peak +dP/dt, LV peak −dP/dt, HR, or LVP through a wide range of Iso doses. (E) Histological inspection of transmural LV samples stained with H&E and Masson’s trichrome showed normal cardiac histology without fibrosis in C1C2 mice at 8 months of age. (B to E) Con denotes transgenic negative mice. H&E, hematoxylin and eosin; HR = heart rate; Iso = isoproterenol; LV = left ventricle; LVP = LV developed pressure.
Akt signaling
Adenovirus-mediated gene transfer of C1C2 or AC6 into cultured cardiac myocytes was performed to compare signaling events evoked by C1C2 versus AC6. Both C1C2 and AC6 expression activated PI3K/Akt signaling pathways, increased phosphorylation of Akt at Ser308 and Thr473, and increased phosphorylation of the downstream Akt target proteins GΑSK3α/β, murine double mutant 2 (MDM2), and p70S6K (Figure 1F). Finally, C1C2 expression was associated with increased phosphorylation of ERK1/2, p38, and CryAB (αB-crystallin) at both S45 and S59 sites (Figure 1G, upper). C1C2 protein was immunoprecipitated by anti-CryAB antibody and CryAB protein was immunoprecipitated by anti-AC5/6 antibody (for pull-down and detecting C1C2) (Figure 1G, lower), indicating that C1C2 interacts with CryAB in cardiac myocytes.
C1C2 transgenic lines
Cardiac-directed expression of C1C2 increased LV C1C2 mRNA: one line 14-fold greater than endogenous AC6 mRNA, a second line 23-fold greater (Figure 2A, lower). However, LV C1C2 protein, detected by AU1 antibody, was increased similar amounts in both lines (Figure 2A, upper).
LV systolic and diastolic function
Echocardiography
C1C2 mice had normal LV dimensions and fractional shortening prior to 7 days of Iso infusion (Table 1, No Iso). Fractional shortening was reduced after 7 days of Iso infusion with no between-group difference (Table 1, 7 days of Iso).
Table 1.
Echocardiography
| No Iso Infusion |
7-d Iso Infusion |
p Value |
|||||
|---|---|---|---|---|---|---|---|
| Con (28) | C1C2 (29) | Con (6) | C1C2 (6) | Interaction | Iso | Gene | |
| Heart rate, beats/min | 530 ± 10 | 521 ± 17 | 508 ± 7 | 499 ± 3 | 0.99 | 0.31 | 0.68 |
| End-diastolic dimension, mm | 3.9 ± 0.1 | 4.0 ± 0.1 | 4.0 ± 0.2 | 3.9 ± 0.1 | 0.56 | 0.91 | 0.88 |
| End-systolic dimension, mm | 2.5 ± 0.1 | 2.6 ± 0.1 | 2.7 ± 0.2 | 2.7 ± 0.1 | 0.68 | 0.25 | 0.90 |
| % Fractional shortening | 38 ± 2 | 36 ± 2 | 32 ± 2 | 31 ± 2 | 0.85 | 0.04 | 0.70 |
Values are mean ± SE; (group size), p values are from 2-way ANOVA.
7-day Iso = 7 days continuous isoproterenol infusion; C1C2 = transgenic mice with cardiac-directed C1C2 expression; Con = transgene-negative sibling mice used as controls.
LV function prior to sustained isoproterenol infusion
In vivo assessment of rates of LV pressure development (+dP/dt) and decline (−dP/dt) showed no group differences in peak +dP/dt (control [Con] mice: 5,070 ± 553 mm Hg/s, n = 12; C1C2 mice: 4,319 ± 253 mm Hg/s, n = 13; p = 0.22) or in peak −dP/dt (Con: −5,003 ± 527 mm Hg/s, n = 12; C1C2 mice: −4,362 ± 248 mm Hg/s, n = 13; p = 0.27) (Table 2). In addition, there were no differences in LV peak +dP/dt, LV peak −dP/dt, LV developed pressure or heart rate through a wide range of brief graded doses of infused Iso (Figure 2D). No baseline between-group differences were seen in LV developed pressure or heart rate (Table 2, No Iso).
Table 2.
LV Pressure Development and Decline
| No Iso Infusion |
7-d Iso Infusion |
p Value |
|||||
|---|---|---|---|---|---|---|---|
| Con (12) | C1C2 (13) | Con (10) | C1C2 (8) | Interaction | Iso | Gene | |
| LV peak +dP/dt, mm Hg/s | 5,070 ± 553 | 4,319 ± 253 | 3,911 ± 406 | 5,298 ± 174∗ | 0.012 | 0.83 | 0.44 |
| LV peak −dP/dt, mm Hg/s | -5,003 ± 527 | -4,362 ± 248 | -3,485 ± 321 | -5,524 ± 149† | <0.001 | 0.64 | 0.07 |
| LV pressure, mm Hg | 78 ± 4 | 72 ± 2 | 62 ± 3‡ | 80 ± 1§ | <0.001 | 0.05 | 0.19 |
| Heart Rate, beats/min | 458 ± 19 | 439 ± 19 | 447 ± 10 | 481 ± 17 | 0.16 | 0.40 | 0.68 |
Values are mean ± SE, (group size). There were no baseline between-group differences (Con vs. C1C2) in LV peak +dP/dt (p = 0.22), LV peak −dP/dt (p = 0.27), LVP (p = 0.18) or heart rate (p = 0.49).
LV = left ventricle; other abbreviations as in Table 1.
p = 0.026 vs. C1C2 No Iso; †p = 0.006 vs. C1C2 No Iso; ‡p = 0.012 vs. Con No Iso; §p = 0.016 vs. C1C2 No Iso (post hoc within-group comparisons using Student’s t-tests with Bonferroni correction for multiple testing).
LV function after 7 days of continuous isoproterenol infusion
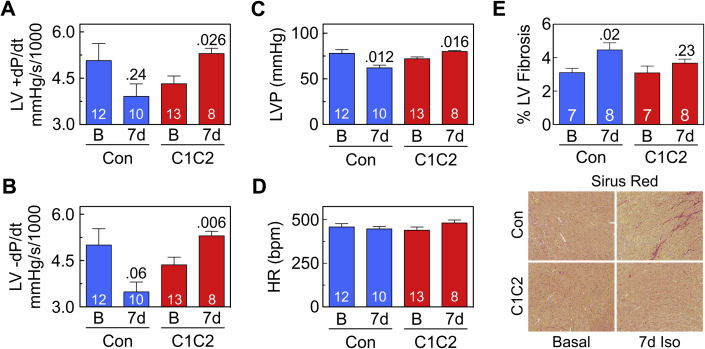
The response to sustained Iso infusion was critically influenced by the presence of C1C2 for LV peak +dP/dt (p = 0.012), LV peak −dP/dt (p < 0.001), and LV developed pressure (p < 0.001), with no such interaction with heart rate (Table 2). In Con mice, 7 days of continuous Iso infusion tended to reduce LV peak +dP/dt (23% reduction; p = 0.24) and LV peak −dP/dt (30% reduction; p = 0.06) (Figures 3A and 3B, Table 2). In contrast, continuous Iso infusion showed a directionally opposite effect on LV function in mice with cardiac-directed C1C2 expression, with increases in both LV peak +dP/dt (23% increase, p = 0.026) and LV peak −dP/dt (27% increase; p = 0.006) (Figures 3A and 3B, Table 2). In addition, Con mice showed reduced (p = 0.012) but C1C2 mice showed increased LV developed pressure (p = 0.016) (Figure 3C) at similar heart rates (Figure 3D).
Figure 3.
C1C2 Expression Prevents Deleterious Effects of Continuous Isoproterenol Infusion on LV Function
(A, B) In Con, 7 days of continuous Iso infusion tended to reduce LV peak +dP/dt (A) and LV peak −dP/dt (B). In contrast, in C1C2 mice, 7 days of Iso infusion increased LV peak +dP/dt (p = 0.026) (A) and peak −dP/dt (p = 0.006) (B). No between-group differences were seen in pre-Iso LV +dP/dt (p = 0.22) or LV −dP/dt (p = 0.27). (C) Similarly, continuous Iso infusion was associated with reduced LV developed pressure in Con mice (p = 0.012) but with increased LV developed pressure in C1C2 mice (p = 0.016). (D) Heart rate showed no group differences within or between groups. (E) Histological analysis of fixed transmural LV samples stained with Picro Sirius red showed increased LV fibrosis after 7 days (7d) of continuous Iso infusion in Con mice only (p = 0.02), but a between-group difference in fibrosis at 7 days was not seen. Bars = mean values; SE = error bars; values in bars = numbers of mice. Two-way ANOVA showed significant interaction of C1C2 on LV peak +dP/dt (p = 0.012), LV peak −dP/dt (p = 0.0009), and LVP (p = 0.0003); numbers above bars indicate p values from within-group post hoc comparisons (Student t-test, unpaired, 2-tailed, Bonferroni correction). Con = transgene negative mice; other abbreviations as in Figures 1 and 2.
Necropsy
Table 3 shows that sustained Iso infusion was associated with similar increases in LV and liver weight in both groups.
Table 3.
Necropsy
| No Iso Infusion |
7-d Iso Infusion |
p Value |
|||||
|---|---|---|---|---|---|---|---|
| Con (11) | C1C2 (14) | Con (8) | C1C2 (8) | Interaction | Iso | Gene | |
| BW, g | 27.2 ± 3.0 | 28.2 ± 2.5 | 29.5 ± 1.1 | 26.8 ± 0.8 | 0.47 | 0.86 | 0.74 |
| LV, mg | 92 ± 8 | 95 ± 8 | 118 ± 6 | 102 ± 4 | 0.24 | 0.044 | 0.42 |
| LV/BW, mg/g | 3.5 ± 0.1 | 3.4 ± 0.1 | 4.0 ± 0.1 | 3.8 ± 0.1 | 0.65 | 0.0002 | 0.17 |
| Liver/BW, mg/g | 46.0 ± 1.9 | 48.6 ± 2.9 | 57.3 ± 1.7 | 59.3 ± 1.0 | 0.91 | <0.0001 | 0.37 |
| Lung/BW, mg/g | 5.8 ± 0.4 | 6.2 ± 0.4 | 6.2 ± 0.2 | 6.3 ± 0.1 | 0.70 | 0.52 | 0.52 |
Histology
Histological inspection showed normal morphology without group differences in fibrosis of C1C2 mice compared to control mice at 8 months of age (Figure 2E). LV samples showed increased LV fibrosis (p = 0.02) in control mice (but not C1C2 mice) after 7 days of continuous Iso infusion (Figure 3E).
Cyclic AMP generation and PKA activity
Before sustained Iso infusion, transmural LV samples showed no group differences (C1C2 vs. control mice) in basal cAMP, but Iso-stimulated cAMP production was reduced in LV samples from C1C2 mice (Figure 2B). Stimulation of cAMP by NKH477, a forskolin analog that directly activates AC, showed no group differences (Figure 2B). LV PKA activity in response to brief Iso or AC stimulation with NKH477 showed no group differences (Figure 2B). Cardiac-directed expression of C1C2 did not alter mRNA expression of AC3, AC4, AC5, AC6, AC7, AC8, or AC9. AC isoform expression ranged from a 14% increase (AC6) to a 13% decrease (AC7), but none was statistically significant.
Ca2+ transient and Ca2+ handling proteins
Before sustained isoproterenol infusion
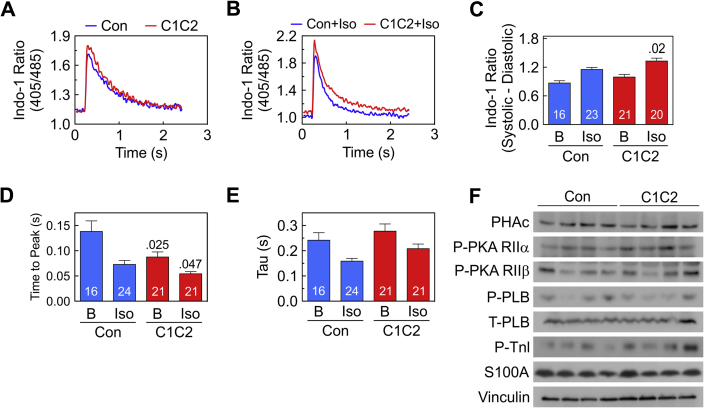
Basal Ca2+ released during contraction (systolic-diastolic Ca2+) was not affected by C1C2 expression (Figure 4A), but peak systolic Ca2+ transient amplitude during brief Iso stimulation was increased (p = 0.02) (Figures 4B and 4C).
Figure 4.
Cytosolic Ca2+ Transients, Contractile Proteins and Signaling Molecules Before Continuous Iso Infusion
(A, B) Representative Indo-1 Ca2+ recordings from cardiac myocytes from C1C2 and Con mice showing unstimulated (A) and brief Iso-stimulated Ca2+ transients (Iso; 10 μM) (B). (C) Summary data of unstimulated (basal) and Iso-stimulated Ca2+ release (systolic-diastolic Ca2+) show no group difference before stimulation (B). However, Ca2+ release in the presence of Iso was increased in cardiac myocytes from both groups and was higher in cardiac myocytes from C1C2 than Con mice (p = 0.02). (D) Time to peak Ca2+ release in the presence of Iso was decreased in cardiac myocytes from both groups and was lower in cardiac myocytes from C1C2 than in Con mice before (p = 0.025) and after (p = 0.047) brief Iso stimulation. (E) Tau was reduced by Iso in both groups, but no between-group differences were seen. (F) LV expression and phosphorylation of signaling proteins that modulate Ca2+ handling (PKA, PLB, TnI, S100A1) showed no group differences. (C to E) Bars = mean values; error bars = SE; numbers in bars = number of cardiac myocytes; values above bars = p values from Student′s t-test (unpaired, 2-tailed). Abbreviations as in Figures 1, 2, and 3.
C1C2 expression reduced time to peak Ca2+ release before (p = 0.025) and during brief Iso stimulation (p = 0.047) (Figure 4D). Finally, Ca2+ decline time (t ½, Tau) was reduced in both groups similarly after brief Iso stimulation (Figure 4E).
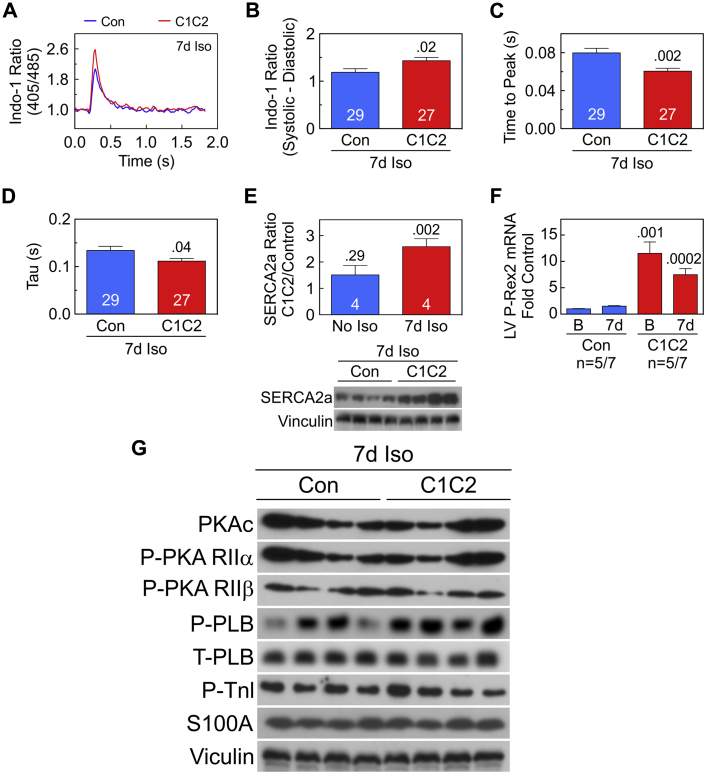
After sustained isoproterenol infusion
After 7 days of Iso infusion, C1C2 expression was associated with increased peak Ca2+ release (Figures 5A and 5B) and more rapid Ca2+ rise and decline (Figures 5C and 5D). The LV SERCA2a protein expression showed no group differences prior to sustained Iso infusion. However, after 7 days of Iso infusion, LV samples from C1C2 mice showed a 2.6-fold increase in SERCA2a protein content compared to control mice (p = 0.002) (Figure 5E). In vivo expression of C1C2 increased expression of P-Rex2 mRNA by 11.5-fold (p = 0.001) (Figure 5F), which remained 7.5-fold increased versus control after sustained Iso infusion (p = 0.0002) (Figure 5F). Expression or phosphorylation of other signaling proteins that modulate intracellular Ca2+ transients (S100A1, PLB, TnI) were not altered in C1C2 LV either before (Figure 4F) or after 7 days of Iso (Figure 5G).
Figure 5.
Ca2+ Transients and Ca2+ Handling Proteins After 7 Days of Continuous Iso Infusion
(A–D) Ca2+ transients. (A) Representative Indo-1 Ca2+ recordings from cardiac myocytes from C1C2 and Con mice after 7 days of Iso infusion. (B) Basal Ca2+ release (systolic-diastolic Ca2+) show increased Ca2+ release in C1C2 cardiac myocytes (p = 0.02). (C) Time to peak Ca2+ transient was reduced in C1C2 cardiac myocytes (p = 0.002). (D) Time to Ca2+ decline (Tau) was reduced in cardiac myocytes from C1C2 mice (p = 0.04). (E) LV SERCA2a content was 2.6-fold greater in C1C2 than in Con mice after 7 days of Iso infusion (p = 0.002; immunoblot below bar graph), although before Iso infusion, the fold difference (C1C2 vs. Con) was not significant (p = 0.29). (F) Cardiac-directed C1C2 expression was associated with an 11.5-fold increase in LV P-Rex2 mRNA before (p = 0.001) and 7.5-fold after 7 days of Iso infusion (p = 0.002) vs. Con. (G) No group differences in expression of PKA, PLB, S100A1, or vinculin were seen. Phosphorylation of PKA, PLB, and TnI also showed no group differences. Bars = mean values; SE = error bars; number in bars = numbers of cells (B to D) or mice (E, F); numbers above bars = p values from Student t-test (unpaired, 2-tailed). Abbreviations as in Figures 1, 2, 3, 4, and 5.
Discussion
The most important finding of this study is that, in mice with cardiac-directed expression of C1C2, sustained Iso infusion increases LV function. Seven days of continuous Iso infusion increased Ca2+ release, reduced time to peak Ca2+ release, and reduced rate of Ca2+ decline (Tau) in cardiac myocytes from C1C2 mice, and LV SERCA2a content from C1C2 mice was increased. These favorable alterations in Ca2+ handling provide mechanistic underpinnings for this interesting and unexpected LV response to catecholamine infusion.
LV function
There were 2 lines of evidence for C1C2's favorable effects on LV function. First, despite reduced Iso-stimulated cAMP generation in LV from C1C2 transgenic mice, LV systolic and diastolic function were preserved through a wide range of Iso concentrations (Figure 2D). This paradox reflects effects on elements that increase contractile performance independent of increases in cAMP generation. For example, we saw increased Iso-stimulated Ca2+ release (Figure 4C) and increased expression of SERCA2a (Figure 5E), events also seen with alterations in AC6 content in previous studies 20, 21, 22. The current data indicate that increased cAMP is not a requirement for beneficial alterations in Ca2+ handling. Second, the C1C2 line not only resisted Iso-induced cardiomyopathy, but LV systolic and diastolic function was increased. After the 7-day Iso infusion, C1C2 mice showed increases in LV peak +dP/dt and peak −dP/dt (Figures 3A and 3B, Table 2). In normal animals, the stress of 7 days of Iso was associated with a propensity for both measurements to decline. The beneficial physiological changes seen in C1C2 mice were associated with increases in rates of Ca2+ release and decline in cardiac myocytes from C1C2 mice (Figures 5A to 5D). Also seen was a 2.6-fold increase in LV SERCA2a content (Figure 5E), which likely was of mechanistic importance in the enhancement of Ca2+ handling. Indeed, other studies have reported that cardiac-directed expression of SERCA2a increases Ca2+ transient amplitude (23). If these effects were also to occur in clinical settings, one would predict protection of the heart against sustained catecholamine stimulation, a common feature of clinical HF, and one thought to be responsible, at least in part, for the inexorable decline of cardiac function frequently seen in HF.
However, increased Ca2+ uptake and release were seen in cardiac myocytes from C1C2 animals prior to 7 days of Iso infusion (Figures 4C and 4D), even though LV SERCA2a content was unchanged (Figure 5E, left). However, there were no group differences in Tau before (Figure 4E) but improved Tau after 7 days of Iso infusion (Figure 5D), which correlated well with LV SERCA2a content, which had increased after Iso infusion (Figure 5E, right). C1C2 expression is associated with changes in multiple intracellular events aside from SERCA2a, including an 11.5-fold increase in LV expression of P-Rex2 (Figure 5F). P-Rex2 inhibits the activity of phosphatase and tensin homolog (PTEN), which increases Akt activation (24). PTEN deletion increases cardiac L-type Ca2+ currents via increased Akt activation (25). Increased P-Rex2 expression and subsequent Akt activation would be predicted to increase Ca2+ uptake and release in cardiac myocytes. C1C2 gene transfer activated Akt in cultured cardiac myocytes (Figure 1F), supporting this idea. However, LV homogenates showed no group differences in Akt activation (data not shown). What one sees in cultured cell studies does not always reflect what one sees in LV homogenates, so linking Akt activation with Ca2+ handling will require additional studies.
Why was the increase in LV peak +dP/dt in the C1C2 mice after sustained Iso infusion (Figure 3A) not also seen in LV fractional shortening in the echocardiographic studies (Table 1)? Fractional shortening is an ejection-phase index of LV contractile function, and is therefore susceptible to alterations in afterload, an effect that does not alter LV peak +dP/dt, which occurs prior to aortic valve opening. Therefore, peak +dP/dt is a more reliable measurement of LV contractility (26). The C1C2 group showed higher LV pressure (afterload) after sustained Iso infusion (Figure 3C), which would be predicted to lower fractional shortening. The beneficial effects of C1C2 on LV SERCA2a expression (Figure 5E) and Ca2+ release (Figures 5A to 5D) provide plausible underpinnings for the beneficial effects of C1C2 on LV contractile function.
Cardiac cAMP generation
C1C2 expression reduced Iso-stimulated cAMP generation in LV homogenates and in cardiac myocytes, but did not reduce LV AC6 expression, or the expression of AC types 3-9. Absent the transmembrane domains of AC6, C1C2 distribution was predominantly cytoplasmic and less efficiently recruited for participation in cAMP generation following βAR stimulation. Our data indicate that C1C2 may act as a dominant negative mutant by interacting with Gαs to reduce the effects of Gαs on endogenous AC responsiveness. Such protection against cAMP generation while fostering increased Ca2+ handling and more efficient myofilament activation constitutes an appealing profile for a HF therapeutic drug.
Typically, LV cAMP generating capacity is tightly linked with LV contractile function. In severe HF, LV cAMP production is 50% reduced, and LV contractile function is similarly reduced 3, 8. Targeted deletion of AC6 is associated with a 60% reduction in LV cAMP generating capacity, and a proportional reduction in LV contractile function (20). In contrast, C1C2 expression was associated with preserved LV function in response to sustained βAR stimulation despite reduction in cAMP generation. In the present study, C1C2 had favorable effects on SERCA2a expression and Ca2+ release, effects that may have increased LV function, counterbalancing decreased cAMP generation. C1C2 expression did not increase phosphorylation of PLB, troponin I (Figures 4F and 5G) or CREB protein (data not shown).
C1C2 and intracellular signaling pathways
C1C2 influences other intracellular signaling pathways such as CryAB, Akt, ERK1/2 and p38 MAPK pathways (Figures 1F and 1G). We do not know the precise pathway by which C1C2 affects intracellular signaling and protein expression and phosphorylation. C1C2 interacts with G-protein coupled receptors, Gαs, Gβγ and A-kinase anchoring proteins (AKAPs), and could thereby influence intracellular signaling. Previous studies indicate that the C1C2 portion of AC6 enables AC6 targeting to lipid rafts (14).
C1C2 expression was associated with increased LV P-Rex2 expression detected by gene array and confirmed by RT-PCR (Figure 5F). P-Rex2 inhibits the activity of PTEN and increases Akt activation (24). Activation of Akt signaling pathways by C1C2 was detected in cultured cardiac myocytes after Ad5.C1C2 gene transfer (Figure 1F), which was quantitatively similar to effects seen with AC6. In previous studies we established that this is one of the mechanisms by which AC6 expression has beneficial effects on cardiac function (21). C1C2 mimics this property of AC6, confirming the mechanism does not require increased LV cAMP.
C1C2 expression increased phosphorylation of CryAB at S45 and S59 sites and C1C2 protein, based on co-immunoprecipitation, is physically associated with CryAB (Figure 1G). CryAB, a member of the small heat shock protein (HSPs) family, is expressed at high levels in cardiac myocytes, and its expression protects against ischemia-reperfusion injury (27). The role of CryAB-C1C2 association will require additional studies, but it underscores the importance that protein-protein interactions play in mechanisms by which C1C2 affects cardiac function. The study of C1C2-interacting proteins is a focus of study in our laboratory.
Study limitations
Because of the complexity of multiple signaling pathways that are relevant in the failing heart, no single study can establish precise molecular pathways for every favorable adaptation. Despite this generic limitation, it is encouraging that cardiac-directed expression of C1C2 has a favorable impact on function of the intact heart in the setting of conditions that mimic aspects of clinical heart failure. The present study examines Iso-stimulated cardiomyopathy only at 7 days, and it remains to be seen whether these protective effects of C1C2 will persist for longer periods and in other models of heart failure.
Conclusions
Increased expression of C1C2 in cardiac myocytes and heart mimics some of the beneficial cardiac effects of AC6. C1C2 enhances Ca2+ handling, which is of mechanistic importance in its beneficial effects. Sustained Iso infusion decreases LV function in control mice, as expected, but increases LV function in C1C2 mice. If these benefits of C1C2 expression translate to clinical settings, one would predict protection of the heart against catecholamine stimulation, a common feature in clinical HF. Finally, C1C2 is sufficiently small to be inserted in an AAV vector with a regulated expression system. It will be interesting to determine whether transgene delivery of C1C2 via AAV can increase function of the failing heart.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Heart failure is one of the most frequent reasons for emergent hospital admission, and it carries a dire prognosis despite recent advances in device and drug therapy. New strategies to treat heart failure urgently are needed. Data from the current study indicate that cardiac expression of C1C2 may prevent the deleterious effects of sustained catecholamine stimulation, which contributes to heart failure.
TRANSLATIONAL OUTLOOK: The present paper is an important initial step in translating C1C2 cardiac gene transfer to clinical applications. A potential obstacle to translation is whether one can attain sufficient cardiac expression of C1C2 to attain an effect after intracoronary delivery of a virus vector encoding C1C2. We recently showed in an initial Phase 2 randomized clinical trial that intracoronary delivery of a virus vector encoding human adenylyl cyclase type 6 was effective in human subjects with symptomatic heart failure (12), suggesting that sufficient cardiac transgene expression can be achieved by this route of administration. Ongoing research in our laboratory has identified adeno-associated virus type (AAV) is a suitable vector. Intracoronary delivery of AAV encoding C1C2 will be tested in murine heart failure with reduced ejection fraction, and, given the favorable effects of C1C2 on LV diastolic function (Tau, peak −dP/dt), we also will test C1C2 gene transfer in murine HF with preserved ejection fraction.
Footnotes
This work was supported by U.S. National Institutes of Health grants P01 HL66941 and HL088426, and U.S. Department of Veteran Affairs Merit grants 1101BX001515 and 5101BX001121. Dr. Hammond is a co-founder, board member, and unpaid consultant for Renova Therapeutics, which had no involvement or licenses related to this work and played no role in the studies. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Tang W.J., Gilman A.G. Construction of a soluble adenylyl cyclase activated by Gs alpha and forskolin. Science. 1995;268:1769–1772. doi: 10.1126/science.7792604. [DOI] [PubMed] [Google Scholar]
- 2.Sunahara R.K., Dessauer C.W., Whisnant R.E., Kleuss C., Gilman A.G. Interaction of Gsα with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem. 1997;272:22265–22271. doi: 10.1074/jbc.272.35.22265. [DOI] [PubMed] [Google Scholar]
- 3.Ping P., Anzai T., Gao M., Hammond H.K. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am J Physiol. 1997;273:H707–H717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- 4.Roth D.M., Bayat H., Drumm J.D. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T., Tang T., Lai N.C. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114:388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 6.Timofeyev V., He Y., Tuteja D. Cardiac-directed expression of adenylyl cyclase reverses electrical remodeling in cardiomyopathy. J Mol Cell Cardiol. 2006;41:170–181. doi: 10.1016/j.yjmcc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Sastry A., Arnold E., Gurji H. Cardiac-directed expression of adenylyl cyclase VI facilitates atrioventricular nodal conduction. J Am Coll Cardiol. 2006;48:559–565. doi: 10.1016/j.jacc.2006.01.082. [DOI] [PubMed] [Google Scholar]
- 8.Lai N.C., Roth D.M., Gao M.H. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110:330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 9.Tang T., Gao M.H., Roth D.M., Guo T., Hammond H.K. Adenylyl cyclase type VI corrects cardiac sarcoplasmic reticulum calcium uptake defects in cardiomyopathy. Am J Physiol Heart Circ Physiol. 2004;287:H1906–H1912. doi: 10.1152/ajpheart.00356.2004. [DOI] [PubMed] [Google Scholar]
- 10.Gao M.H., Tang T., Guo T., Sun S.Q., Feramisco J.R., Hammond H.K. Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J Biol Chem. 2004;279:38797–38802. doi: 10.1074/jbc.M405701200. [DOI] [PubMed] [Google Scholar]
- 11.Lai N.C., Tang T., Gao M.H. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51:1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond H.K., Penny W.F., Traverse J.H. Intracoronary gene transfer of adenylyl cyclase 6 in patients with heart failure: a randomized clinical trial. JAMA Cardiol. 2016;1(2):163–171. doi: 10.1001/jamacardio.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao M.H., Lai N.C., Tang T. Preserved cardiac function despite marked impairment of cAMP generation. PLoS One. 2013;8:e72151. doi: 10.1371/journal.pone.0072151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thangavel M., Liu X., Sun S.Q., Kaminsky J., Ostrom R.S. The C1 and C2 domains target human type 6 adenylyl cyclase to lipid rafts and caveolae. Cell Signal. 2009;21:301–308. doi: 10.1016/j.cellsig.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao M.H., Lai N.C., Roth D.M. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99:1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 16.O'Connell T.D., Rodrigo M.C., Simpson P.C. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 17.Gao M.H., Lai N.C., Miyanohara A. Intravenous adeno-associated virus serotype 8 encoding urocortin-2 provides sustained augmentation of left ventricular function in mice. Hum Gene Ther. 2013;24:777–785. doi: 10.1089/hum.2013.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao M.H., Tang T., Miyanohara A., Feramisco J.R., Hammond H.K. Beta(1)-adrenergic receptor vs. adenylyl cyclase 6 expression in cardiac myocytes: differences in transgene localization and intracellular signaling. Cell Signal. 2010;22:584–589. doi: 10.1016/j.cellsig.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang T., Lai N.C., Wright A.T. Adenylyl cyclase 6 deletion increases mortality during sustained beta-adrenergic receptor stimulation. J Mol Cell Cardiol. 2013;60:60–67. doi: 10.1016/j.yjmcc.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang T., Gao M.H., Lai N.C. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 21.Gao M.H., Tang T., Guo T. Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J Biol Chem. 2008;283:33527–33535. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang T., Hammond H.K., Firth A. Adenylyl cyclase 6 improves calcium uptake and left ventricular function in aged hearts. J Am Coll Cardiol. 2011;57:1846–1855. doi: 10.1016/j.jacc.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker D.L., Hashimoto K., Grupp I.L. Targeted overexpression of the sarcoplasmic reticulum Ca2+ATPase increases cardiac contractility in transgenic mouse hearts. Circ Res. 1998;83:1205–1214. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- 24.Fine B., Hodakoski C., Koujak S. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science. 2009;325:1261–1265. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun H., Kerfant B.G., Zhao D. Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kalpha/PKB signaling. Circ Res. 2006;98:1390–1397. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- 26.Kass D.A., Maughan W.L., Guo Z.M., Kono A., Sunagawa K., Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- 27.Velotta J.B., Kimura N., Chang S.H. Alpha-B-crystallin improves murine cardiac function and attenuates apoptosis in human endothelial cells exposed to ischemia-reperfusion. Ann Thorac Surg. 2011;91:1907–1913. doi: 10.1016/j.athoracsur.2011.02.072. [DOI] [PubMed] [Google Scholar]