Abstract
Folate and other B vitamins are essential co-factors of one-carbon metabolism, and genetic variants, such as polymorphisms, can alter the metabolism. Furthermore, the adoption of food fortification with folic acid showed a decrease of homocysteine concentration. The aim of this study was to investigate the frequencies of the polymorphisms of enzymes and carrier proteins involved in one-carbon metabolism, and to evaluate homocysteine concentrations in the presence of these genetic variants in a population exposed to mandatory food fortification with folic acid. Using data from a population-based cross-sectional study in São Paulo, Brazil, the study population comprised 750 participants above 12 years of age of both genders. A linear regression model was used to evaluate the homocysteine concentrations according to genetic variants and folate level. The results showed that the minor allelic frequencies were 0.33 for MTHFR (rs1801133), 0.24 for MTHFR (rs1801131), 0.19 for MTR (rs1805087), 0.42 for MTRR (rs1801394), 0.46 for RFC1 (rs1051266), and 0.47 for DHFR (19-bp deletion). The genetic variants of MTHFR 677C>T, MTRR 66A>G and RFC-1 80G>A were different according to race. The homocysteine concentrations increased in the CT and TT compared to CC genotypes of polymorphism MTHFR 677C>T in all populations, and differences between the homocysteine concentrations according to the genotypes of MTHFR 677C>T were observed regardless of folate level.
Keywords: one-carbon metabolism, folic acid fortification, genetic variants, homocysteine, polymorphisms
1. Introduction
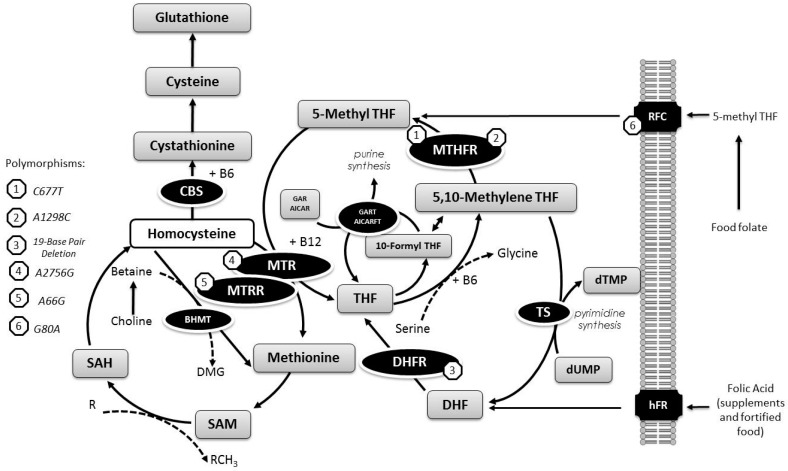
One-carbon metabolism plays an important role in complex and essential metabolic pathways, as hundreds of intracellular transmethylation reactions, including DNA methylation and DNA synthesis, have been implicated in carcinogenesis [1] and processes closely associated with homocysteine (Hcy) metabolism [2]. Vitamins, particularly folate (B9) and other B vitamins, such as B6 and B12, are essential co-factors of one-carbon metabolism (Figure 1) [3].
Figure 1.
Overview of one-carbon metabolism considering metabolites, enzymes, coenzymes, and genetic variants. Abbreviations: methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR), methionine synthase reductase (MTRR), reduced folate carrier (RFC), dihydrofolate reductase (DHFR), tetrahydrofolate (THF), cystathionine β-synthase (CBS), dihydrofolate (DHF), human folate receptor (hFR), S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), thymidylate synthase (TS), deoxyuridine monophosphate (dUMP), deoxythymidine monophosphate (dTMP), Betaine--Homocysteine S-Methyltransferase (BHMT), dimethyglycine (DMG), glycinamide ribonucleotide (GAR), 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), glycinamide ribonucleotide transformylase (GART), 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase (AICARFT).
Studies have shown that the elevation of homocysteine levels is an important risk marker for the occurrence of adverse events, such as dementia, Alzheimer’s disease, bone fractures, cancers, and particularly cardiovascular diseases [4,5]. Environmental and genetic factors modify homocysteine concentrations, for example, polymorphisms can alter metabolism, generating homocysteine accumulation [6]. However, increases in the levels of some vitamins, particularly folate and other vitamins, such as B6 and B12, modulates metabolic functions [5], consequently facilitating the remethylation of homocysteine, thereby hindering increases in the concentrations of this metabolite [6]. As a result, an important reduction of homocysteine concentration in some populations was observed after the adoption of food fortification with folic acid (synthetic form of folate) in several countries [7,8].
This observation highlights the importance of adequate nutrient intake, knowledge concerning genetic variant frequencies, and evaluation of country conditions in which the mandatory policy is to fortify foods with folic acid, as has occurred in Brazil since 2002. Furthermore, despite the accumulated evidence, there are gaps in the knowledge of the frequencies of genetic variants in several primarily healthy populations, and the impact of individual responses to diseases remains unknown. The objective of the present study was to investigate the frequency of the genetic variants involved in one-carbon metabolism and to evaluate homocysteine concentrations in accordance with the presence of these genetic variants considering the folate plasma level.
2. Materials and Methods
2.1. Study and Sample Population
We used the data from ‘Health Survey of Sao Paulo’ (ISA-Capital), a population-based and cross-sectional household health survey conducted in 2008 [9]. The study population comprised residents of private households in the urban area of São Paulo, Brazil. This complex probabilistic sample was obtained using conglomerates in two stages: census and household sectors (data from National Survey of Households 2005, IBGE). Six sample domains, comprising adolescents (12 to 19 years of age), adults (20 to 59 years of age), and elderly (60 years of age and older) individuals of both genders, were considered, and a total of 2691 participants were included to facilitate general data collection. Among these participants, blood samples and blood pressure and anthropometric measurements were obtained from 750 individuals. Other details of the sampling have been previously published [10].
In the present study, we considered all participants aged 12 years or older at the time of collection. A total of 750 individuals (158 adolescents, 303 adults, and 289 elderly) responded to a social-economic survey and underwent anthropometric measurement, and subsequently, blood samples were collected for genotyping.
The Ethical and Research Committee of the School of Public Health University of São Paulo (Protocol number 2001) approved this study, and informed consent was obtained from all participants.
2.2. Data Collection and Processing
In 2008, household information was obtained from randomly selected residents using structured questionnaires applied by previously trained interviewers. Demographic, social-economic, lifestyle, referred morbidity, family-history diseases, supplementation, medicine, and diet information were collected. In a subsequent home visit, blood samples were collected and anthropometric measurements were recorded.
2.3. Diet
Two 24-h recalls (24hR) were performed as a dietary survey. Food consumption described in both recalls was converted into energy and nutrient values using the Nutrition Data System for Research (NDSR, version 2007, Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN, USA). This software is used to calculate the folate quantity in three different ways: (1) natural folate—the vitamin naturally present in food; (2) synthetic folate (folic acid)—vitamin added to fortified food and dietary supplements; and (3) dietary folate equivalents (DFE)—sum of the dietary quantity of natural folate and synthetic folate, considering the difference in the bioavailability of both forms. Additionally, synthetic folate values and, consequently, the DFE values were corrected, considering the mandatory fortification of wheat and corn flour in Brazil since 2004. Moreover, the Multiple Source Method (MSM) was used to measure folate intake to estimate the usual consumption distribution of nutrients, mitigating the effects of intra-individual variation when at least two dietary measurements per individual are available [11].
2.4. Anthropometric Measures
Weight and height were measured and the anthropometric data were used to calculate Body Mass Index (BMI), and the individuals were classified, according to the BMI cut-off points of the World Health Organization (WHO) [12], as underweight—BMI < 18.5 kg/m2; eutrophic—BMI 18.5–24.9 kg/m2, and overweight—BMI ≥ 25 kg/m2. A previously trained nurse technician performed all measurements.
2.5. Blood Collection
A trained nurse technician performed blood collection at home using disposable needles and syringes according to the standardized procedures described in the Guidelines for the Assessment of Food Consumption in population studies which reported an experience of the Health Survey in São Paulo [9]. The blood samples were collected through venipuncture after 12-h fasting.
Approximately 20 mL of blood were collected in tubes containing EDTA (ethylenediaminetetraacetic acid) and plastic serum tubes which had spray-coated silica through venipuncture. The tubes were stored in Styrofoam packages with recyclable ice packs and were transported to the Laboratory of Human Nutrition at School of Public Health, followed by centrifugation and processing into aliquots of serum and plasma, and storage at −80 °C.
2.6. Biochemical Analysis
The folate dosage was determined through High-Performance Liquid Chromatography (HPLC) with electrochemical detection [13]. The B6 concentrations were analyzed by HPLC with fluorometric detection using the ImmunDiagnostik AG® HPLC-Analytik system [14]. Vitamin B12 concentrations were determined by a chemiluminescence immunoassay with paramagnetic particles for the quantitative determination of vitamin levels in human serum and plasma using the Access Immunoassay System® (Beckman Coulter, Inc., Galway, Ireland). The immunoassay method of chemiluminescence microparticles using the ARCHITECT Homocysteine Reagent Kit (Abbott Diagnostics Division, Abbott Park, IL, USA) was used to analyze the plasma concentrations of homocysteine. All tests were performed according to the manufacturer’s instructions.
Mean intra-assay coefficients of variation (CVs) for B6, B12, folate, and homocysteine, respectively, were 7.2%, 7.0%, 2.0%, and 2.3%, and mean inter-assay CVs were 5.9%, 8.5%, 3.4%, and 4.0%, respectively.
2.7. DNA Extraction and Genotyping
The DNA was extracted using the DNA salt extraction method [15]. Subsequently, the DNA was quantified using a Nanodrop® 1000 Spectrophotometer (Wilmington, DE, USA).
The PCR-allele technique was used for genotyping in duplicates with 100% of concordance [16]. This assay facilitates the simultaneous amplification and detection of DNA using a common reverse primer in each reaction tube and two marked primers with two different fluorophores that recognize specific sequences corresponding to each allele. A fluorescence reader was used to capture the emitted fluorescence signal for clusterizing and identifying genotypes.
The following six polymorphisms in the genes involved in folate metabolism were analyzed: 677C>T (rs1801133) and 1298A>C (rs1801131) of methylenetetrahydrofolate reductase—MTHFR; 2756A>G (rs1805087) of methionine synthase—MTR; 66A>G (rs1801394) of methionine synthase reductase—MTRR; 80G>A (rs1051266) of reduced folate carrier 1-RFC-1; and a 19-bp deletion in dihydrofolate reductase—DHFR. In this study, the genotyping call rate was >97% for the polymorphisms.
2.8. Statistical Analysis
For each polymorphism in the population, the minimum allele frequency was calculated, and the Hardy-Weinberg equilibrium was verified. The frequencies of female and male, age group, and self-declared races, and median of folate and homocysteine concentrations were stratified according to genotypes for each of the gene encoding enzymes or carrier proteins. The chi-square test and Kruskal-Wallis test were performed to verify differences between frequencies and medians, respectively. The folate concentration was also considered to evaluate the differences in homocysteine concentrations in the presence of genetic variants. Therefore, the population was divided into tertiles of the folate plasma concentration with mean, respectively: first (14.9 nmol/L), second (27.5 nmol/L), and third (50.7 nmol/L). A linear regression model was used to evaluate differences in the homocysteine concentrations in accordance with genetic variants by total population and folate concentration tertiles. The model considered the log-transformed homocysteine concentration as a dependent variable and the genetic variants as independent variables, divided into three categories: (1) homozygous wild-type, (2) heterozygous mutation, and (3) homozygous mutation for enzyme or carrier genotypes. The models were adjusted according to race, sex, age, estimated consumption of total folate (mcg of DFE/day), body mass index, serum concentrations of vitamins, folate, B6, and B12. In addition, we used the interaction terms of genotypes and folate plasma concentration in these linear regression models.
All statistical analyses were done using the STATA® software (version 10.0, 2007; College Station, TX, USA). A significance level of 5% was considered in all analyses.
3. Results
The present study involved a total of 750 participants as a representative sample of the population of São Paulo, Brazil, of which 58.1% of the participants were women and 41.9% of the participants were men, averaging 46.7 years of age (95% CI: 45.0–48.4 years). In relation to the nutritional status of the population according to the BMI classification, 46.1% of the population was overweight (BMI > 25 kg/m2). Moreover, in terms of race, most of the participants were self-declared Whites (59.2%), followed by Mixed (white/black) (30.7%), Blacks (8.3%), and Asians/Indigenous (1.9%). Non-smokers represented 85.2% of the population. The median of homocysteine and folate concentrations in the studied population were 8.8 μmol/L and 27.4 nmol/L, respectively.
The primary information concerning the polymorphisms studied in this population, such as gene location, change of DNA molecule bases and consequent amino acid alterations, Hardy-Weinberg equilibrium, and minor allele frequencies, are listed in Table 1. The chi-square test (p > 0.05) revealed that the population was in Hardy-Weinberg equilibrium for all of the alleles studied. The genotype frequencies of the genetic variants in the studied population according to sex, age group, and self-declared race, and the respective homocysteine and folate concentrations according to genotypes are listed in Table 2. Individuals presenting genotypes CT and TT for the MTHFR 677C>T polymorphism presented higher homocysteine levels than individuals with genotype CC (p = 0.026), whereas individuals presenting genotypes GA and GG for the MTR 2756A>G polymorphism presented lower folate concentrations than individuals with genotype AA (p = 0.015). Race was observed to show statistically significant differences in the genotypes of MTHFR 677>T (p = 0.035), MTRR 66A>G (p = 0.000), and RFC-1 80G>A (p = 0.003).
Table 1.
Panel of genetic variants, minimum allele frequency, and Hardy-Weinberg equilibrium of the six polymorphisms involved in one-carbon metabolism.
| Polymorphisms | Location | Gene | Changes | p a | MAF | |
|---|---|---|---|---|---|---|
| DNA | Amino acids | |||||
| rs1801131 | 1p36.3 | MTHFR | A→C | Glu→Ala | 0.392 | 0.24 |
| rs1801133 | 1p36.3 | MTHFR | C→T | Ala→Val | 0.428 | 0.33 |
| rs1805087 | 1q43 | MTR | A→G | Asp→Gly | 0.333 | 0.19 |
| rs1801394 | 5p15.31 | MTRR | A→G | - | 0.154 | 0.42 |
| rs1051266 | 21q22.3 | RFC1 | G→A | His→Arg | 0.141 | 0.46 |
| 19-bp deletion | 5q11.2–q13.2 | DHFR | - | - | 0.807 | 0.47 |
a p-value for Hardy-Weinberg equilibrium. MAF, minor allele frequency; RFC-1, reduced folate carrier 1; DHFR, dihydrofolate reductase; MTHFR, 5,10-methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase.
Table 2.
Genotype frequencies of the enzymes MTHFR, MTR, MTRR, and DHFR and the carrier protein RFC-1 according to sex, age group, and race; and the medians of homocysteine and folate concentrations according to genotypes.
| SNP | MTHFR 677C>T | p-Value | MTHFR 1298A>C | p-Value | MTR 2756A>G | p-Value | MTRR 66A>G | p-Value | RFC1 80G>A | p-Value | DHFR Deletion | p-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | C:C | C:T | T:T | A:A | A:C | C:C | A:A | A:G | G:G | A:A | A:G | G:G | G:G | G:A | A:A | WT: WT | WT:del | del:del | ||||||
| Total (%) | 46.0 | 42.7 | 11.3 | 58.6 | 34.2 | 6.2 | 66.0 | 29.8 | 4.2 | 35.6 | 44.8 | 19.6 | 30.5 | 47.0 | 22.5 | 28.4 | 50.2 | 21.4 | ||||||
| Sex (%) 1 | ||||||||||||||||||||||||
| male | 43.4 | 44.3 | 12.3 | 0.462 | 60.6 | 34.5 | 4.9 | 0.376 | 67.8 | 27.7 | 4.6 | 0.548 | 35.0 | 45.2 | 19.8 | 0.961 | 31.6 | 45.1 | 23.4 | 0.682 | 20.1 | 52.9 | 27.0 | 0.455 |
| female | 47.8 | 41.6 | 10.6 | 57.2 | 35.6 | 7.21 | 64.7 | 31.3 | 3.9 | 36.0 | 44.5 | 19.5 | 29.7 | 48.4 | 21.9 | 22.4 | 48.2 | 29.4 | ||||||
| Age group (%) 1 | ||||||||||||||||||||||||
| 12–19 years | 42.0 | 44.0 | 14.0 | 0.662 | 61.9 | 33.6 | 4.5 | 0.593 | 69.5 | 26.6 | 3.9 | 0.139 | 33.3 | 44.4 | 22.2 | 0.193 | 28.5 | 45.7 | 25.8 | 0.763 | 24.4 | 48.1 | 27.6 | 0.801 |
| 20–59 years | 48.2 | 41.2 | 10.6 | 56.0 | 36.3 | 7.7 | 69.5 | 26.2 | 4.4 | 36.9 | 47.8 | 15.4 | 32.1 | 45.7 | 22.2 | 19.4 | 52.0 | 28.6 | ||||||
| 60+ years | 45.8 | 43.7 | 10.6 | 59.6 | 34.8 | 5.7 | 60.5 | 35.3 | 4.2 | 35.5 | 41.8 | 22.7 | 29.9 | 48.9 | 21.1 | 21.9 | 49.5 | 28.6 | ||||||
| Race (%) 1 | ||||||||||||||||||||||||
| White | 42.4 | 44.4 | 13.2 | 0.035 * | 54.9 | 37.5 | 7.6 | 0.056 | 68.4 | 28.9 | 2.8 | 0.183 | 28.7 | 46.2 | 25.2 | 0.000 * | 32.4 | 47.3 | 20.3 | 0.003 * | 30.3 | 51.3 | 18.5 | 0.120 |
| Black | 59.7 | 38.7 | 1.6 | 69.4 | 27.4 | 3.2 | 65.6 | 29.5 | 4.9 | 54.8 | 38.7 | 6.5 | 29.5 | 57.4 | 13.1 | 16.1 | 51.6 | 32.3 | ||||||
| Mixed (white/black) | 49.3 | 41.0 | 9.7 | 63.7 | 32.3 | 4.0 | 61.2 | 31.7 | 7.1 | 43.1 | 43.5 | 13.5 | 28.4 | 44.4 | 27.1 | 28.1 | 47.8 | 24.1 | ||||||
| Asian/Indigenous | 42.9 | 35.7 | 21.4 | 41.7 | 41.7 | 16.7 | 71.4 | 28.6 | 0.0 | 42.9 | 50.0 | 7.1 | 7.7 | 30.8 | 61.5 | 28.6 | 50.0 | 21.4 | ||||||
| Hcy, µmol/L (median) 2 | 8.3 | 9.0 | 9.3 | 0.026 * | 8.7 | 8.9 | 7.5 | 0.180 | 8.6 | 8.9 | 8.7 | 0.569 | 9.0 | 8.2 | 9.4 | 0.097 | 8.9 | 8.8 | 8.5 | 0.401 | 8.4 | 8.9 | 8.8 | 0.355 |
| Folate, nmol/L (median) 2 | 27.9 | 27.9 | 24.1 | 0.088 | 26.5 | 28.5 | 22.8 | 0.565 | 28.0 | 25.1 | 21.0 | 0.015 * | 26.6 | 27.0 | 28.1 | 0.423 | 29.0 | 27.4 | 25.4 | 0.396 | 27.5 | 28.0 | 25.6 | 0.935 |
1 p-value for the chi-square test; 2 p-value for the Kruskal-Wallis test; * A p-value < 0.05 was considered statistically significant.
The association between genetic variants involved in one-carbon metabolism and homocysteine concentration was assessed (Table 3). Only MTHFR 677C>T was significantly associated with Hcy concentration in the total population (p = 0.000). The increasing risk allele (T) was related to the increase in Hcy concentration. In the presence of genetic variants stratified by folate level tertiles, the same effect was observed in MTHFR 677C>T. The individuals carrying the risk allele had higher Hcy concentrations than those of non-carriers in the first tertile (p = 0.006) and third tertile (p = 0.038) of folate concentration. However, no interaction was observed between genotypes and folate concentration on homocysteine concentration (p > 0.05).
Table 3.
Associations between the genotypes involved in one-carbon metabolism and homocysteine concentration by total population and folate concentration tertiles.
| Homocysteine 1 | Total | Folate Concentration | p-Interaction 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First Tertile | Second Tertile | Third Tertile | |||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| MTHFR 677C>T | |||||||||
| C:C | 9.3 | 0.2 | 9.7 | 0.4 | 9.6 | 0.4 | 8.9 | 0.3 | 0.208 |
| C:T | 10.3 | 0.4 | 10.5 | 0.8 | 10.0 | 0.4 | 10.5 | 1.0 | |
| T:T | 12.6 | 1.3 | 12.6 | 1.3 | 10.2 | 1.7 | 13.9 | 3.9 | |
| p-value 2 | 0.000 * | 0.006 * | 0.162 | 0.038 * | |||||
| MTHFR 1298A>C | |||||||||
| A:A | 10.5 | 0.4 | 11.0 | 0.6 | 9.5 | 0.4 | 10.9 | 1.0 | 0.327 |
| A:C | 9.7 | 0.2 | 10.3 | 0.5 | 10.0 | 0.5 | 9.0 | 0.3 | |
| C:C | 9.2 | 0.7 | 7.1 | 0.6 | 12.1 | 1.6 | 8.4 | 1.3 | |
| p-value 2 | 0.304 | 0.121 | 0.190 | 0.187 | |||||
| MTR 2756A>G | |||||||||
| A:A | 10.0 | 0.3 | 10.1 | 0.4 | 9.5 | 0.3 | 10.4 | 0.8 | 0.397 |
| A:G | 10.4 | 0.5 | 11.5 | 1.2 | 10.5 | 0.7 | 9.2 | 0.4 | |
| G:G | 9.1 | 0.5 | 9.6 | 0.7 | 8.5 | 1.0 | 9.8 | 1.5 | |
| p-value 2 | 0.439 | 0.583 | 0.859 | 0.374 | |||||
| MTRR 66A>G | |||||||||
| A:A | 10.2 | 0.4 | 10.4 | 0.5 | 10.0 | 0.6 | 10.6 | 1.1 | 0.825 |
| A:G | 10.0 | 0.4 | 10.6 | 0.8 | 9.5 | 0.5 | 9.6 | 0.9 | |
| G:G | 10.1 | 0.4 | 9.8 | 0.9 | 10.1 | 0.6 | 10.4 | 0.7 | |
| p-value 2 | 0.576 | 0.59 | 0.612 | 0.388 | |||||
| RFC1 80G>A | |||||||||
| G:G | 10.7 | 0.5 | 11.7 | 1.2 | 11.0 | 0.8 | 9.2 | 0.5 | 0.214 |
| G:A | 10.1 | 0.4 | 9.6 | 0.4 | 9.7 | 0.4 | 11.2 | 1.2 | |
| A:A | 9.4 | 0.3 | 10.5 | 0.7 | 8.5 | 0.5 | 9.2 | 0.5 | |
| p-value 2 | 0.281 | 0.920 | 0.001 * | 0.396 | |||||
| deletion DHFR | |||||||||
| WT:WT | 10.4 | 0.6 | 10.5 | 1.3 | 9.7 | 0.8 | 10.8 | 1.3 | 0.535 |
| WT:del | 10.1 | 0.3 | 10.2 | 0.4 | 9.8 | 0.4 | 10.1 | 0.8 | |
| del:del | 10.1 | 0.4 | 11.2 | 0.8 | 10.3 | 0.6 | 8.9 | 0.5 | |
| p-value 2 | 0.312 | 0.071 | 0.667 | 0.836 | |||||
1 Hcy concentration data are mean and SEM. Hcy concentration values were Log transformed before analysis. 2 Linear regression model adjusted by race, sex, age, estimated consumption of total folate (mcg of DFE/day), body mass index, serum concentrations of vitamins folate, B6, and B12. 3 p-value of genotypes-folate concentration interaction term at the linear regression model. * p-values were considered significant (p < 0.05).
4. Discussion
Herein, we conducted a population-based study of individuals in the city of São Paulo, Brazil, to evaluate the frequencies of the primary polymorphisms associated with one-carbon metabolism, the associations between these genotypes and sex, age, and self-declared race, and the differences in metabolic responses considering genetic variants. The results showed that (1) the allele frequencies in the population were in equilibrium and similar to those in other populations; (2) the genotype frequencies of MTHFR 677C>T, MTRR 66A>G, and RFC-1 80G>A were significantly different according to race; and (3) statistically significant differences between the mean homocysteine concentrations according to the genotypes of MTHFR 677C>T were observed independently of folate level.
Among the described polymorphisms of the enzyme MTHFR, the variants 677C>T and 1298A>C are the most well studied. Americans of Hispanic origin presented a higher prevalence of homozygotes TT for MTHFR 677C>T, which was found in 25% of the population. The prevalence of this genotype among white Americans was between 10% and 15%, and a lower prevalence of this genotype was observed for African and African-Americans, with 0% and 1%, respectively [4,17,18]. The results from case-control studies showed that the homozygote prevalence (TT) for this variant (677C>T) was 9.5% in control Brazilian individuals who were considered to be healthy [19], while the homozygote prevalence was 1.9% in individuals of African origin and 11.8% in individuals of Caucasian origin [20]. Herein, we observed that 11.3% of the population presented as being homozygous (TT) for the variant 677C>T, considering a homozygote prevalence of 13.2% and 1.6% among White and Black races, respectively. In a study concerning the polymorphism 1298A>C, 40% heterozygotes (AC) and 6% mutant homozygotes CC were observed in a healthy Brazilian population [21], consistent with the results of the present study (34.2% AC and 6.2% CC). In the United Kingdom, 44.8% heterozygotes AC and 9.3% mutant homozygotes CC were observed in an elderly population (n = 1041) [22].
The prevalence of 3.7% homozygotes GG and 19.7% heterozygotes AG for the MTR variant 2756A>G was observed in the control individuals from a Brazilian population [23], whereas in the present study, we observed a prevalence of 4.2% homozygotes and 29.8% heterozygotes. The homozygotes GG were detected at a frequency of 2–3% in Japanese, Chinese, Korean, and European individuals and at approximately 1–5% in Americans [24]. However, the MTRR polymorphism is extremely common. The prevalence of this genotype among the individuals examined in the present study was 44.8% heterozygotes AG and 19.6% homozygotes GG. However, in a previous study, the prevalence of the MTRR polymorphism among Brazilians was 54.3% and 17.6%, respectively [23].
The RFC-1 genetic variant is a mutation with high prevalence among the studied populations. Homozygotes AA showed a prevalence of 22.3% and heterozygotes AG showed a prevalence of 48.4% among a Brazilian population that attended a public health care center [25]. In contrast, in the present study, we detected a 22.5% prevalence of homozygotes AA and 47% prevalence of homozygotes AG, whereas in a British elderly population, the observed prevalence was 18.6% for AA and 50% for AG [22]. A 19-base pair deletion in the DHFR gene was also prevalent among these populations. The homozygote and heterozygote variants for this deletion accounted for 17.2% and 51.3%, respectively, of the population in the United States [26]. However, in the Brazilian population, the DHFR polymorphism has only been reported in studies of individuals with DS, presenting a frequency of genotype Del:Del in 20.9% of these individuals [27]. In the present study, the observed genotype frequency of Del:Del was 21.4%.
In relation to the homocysteine concentration, several studies have shown that an increase in folate consumption, particularly folic acid via fortification or supplementation, consequently increases folate intake and serum vitamin concentrations, and decreases homocysteine concentrations [28]. Additional studies have shown no alterations in the homocysteine concentration when folate is adequate. However, with low folate concentrations, there is a significant increase in the homocysteine concentration in individuals presenting genetic variants as methylenetetrahydrofolate reductase C677T mutation [29]. In contrast, the results of the present study showed that the mean homocysteine concentration was significantly lower (p < 0.05) in individuals with wild-type compared to the increasing risk allele (T) for the MTHFR 677C>T polymorphism regardless of folate concentrations tertiles. Similar results were found in other studies of folic acid supplementation. The mutation MTHFR TT was associated with lower folate concentrations and higher homocysteine concentration, and the trend of TT compared to CC was maintained even at different folic acid doses [30]. Indeed, for the remethylation of Hcy into methionine, the enzyme MTHFR is responsible for converting 5.10-methyltetrahydrofolate into 5-methyltetrahydrofolate, the circulating and physiologically active form of folate and primary methyl group donator for remethylation [3]. The results of a previous study showed that homozygotes (TT) for the MTHFR 677C>T mutation presented one-third of the expected activity for this enzyme [31]. It has been suggested that folic acid from fortified food primarily increases the intake and serum concentration of folate [32]. The synthetic form of folate, folic acid, needs to be converted into 5-methyltetrahydrofolate (5MeTHF) through the enzyme MTHFR. However, the presence of the MTHFR 677C>T polymorphism would restrict this conversion, thereby decreasing homocysteine remethylation and consequently increasing homocysteine concentrations in the blood [3,30]. On the other hand, the effect of natural sources of folate on plasma homocysteine has not been assessed in studies. It is known that common folate form in food without fortification, i.e., naturally occurring folate, is 5MeTHF after absorption, and this form does not require conversion by enzyme MTHFR in the metabolism [33]. Thus, we emphasized the need for further research studies to elucidate this gap.
Another important finding is related to differences in genetic variations according to race. In the present study, race was differently presented (p < 0.05) among MTHFR 677>T, MTRR 66A>G, and RFC-1 80G>A genotypes. Some researchers criticize the use and limitations of self-declared race and consider the use of individual genetic ancestry to be the best measure of racial differences in genetic variations [34]. Nevertheless, significant differences in the prevalence of polymorphisms among self-declared races were observed, suggesting the importance of population miscegenation [35]. Therefore, the race variable must be considered in future population studies evaluating potential differences in serum concentrations of folate, homocysteine, B vitamins, and other molecules, consistent with previous studies [36,37].
Accordingly, the knowledge of genetic variability in epidemiological studies remains scarce in Brazil, primarily in representative samples of healthy populations. However, several studies have attempted to associate disease outcomes with genetic variability among individual populations. In the last two decades, several studies have reported that MTHFR polymorphisms, particularly 677C>T and 1298A>C, are associated with an increased risk for neural tube defects [38], cardiovascular diseases [39], schizophrenia [40], neoplasia [41,42,43], and hyperhomocysteinemia [44]. In contrast with other diseases related to folate metabolism, the MTR variant has not been associated with hyperhomocysteinemia, an increased risk of neural tube defects, or vascular diseases [4,45]. Several studies have investigated the association of the MTR variant with the development of neoplasia; however, the results are conflicting. The results of a meta-analysis of the MTRR 66A>G polymorphism showed that the genotype GG is associated with an increase in risk of carcinomas [46]. With respect to RFC-1, several studies have related the genetic variants for RFC-1 with an increased risk for neural tube defects [47]. The 19-base pair deletion in the DHFR gene has only been reported in studies concerning individuals with Down syndrome [27,48].
Despite these findings, a potential limitation of the present study was the use of self-declared race, as this variable reflects the influence of social-economic and cultural status, although, until recently, genetic ancestry analysis was not performed in population-based studies in the city of São Paulo. Accordingly, even self-declared race is not an adequate genetic indicator, but rather this variable could represent an exposition indicator of social factors [49]. Therefore, epidemiological studies with representative samples are important to promote these results for further discussions concerning this issue.
5. Conclusions
In conclusion, adequate folate levels are an important point in this discussion, as Brazil is a country that has a public health policy of mandatory folic acid fortification. Folic acid fortification considerably reduces the inadequacy of folate prevalence within levels considered to be safe in the inhabitant population of São Paulo [32]; however, the profile of genetic variability among this population remains unknown. Indeed, the presence of these polymorphisms modifies the metabolism and alters the concentrations of these metabolites, as differences in homocysteine concentrations were observed in individuals with genetic variants of MTHFR 677C>T. These effects in health and disease are inconclusive; therefore, the evaluation of such variants must be considered in future epidemiological studies. Consequently, studies concerning the frequencies and factors associated with genetic variants in the enzymes involved in metabolic processes are becoming increasingly important.
Acknowledgments
This work was supported by Municipal Health Secretariat of São Paulo; National Counsel of Technological-CNPq; and Scientific Development and São Paulo Research Foundation–FAPESP (Grant Numbers: 2010/19899-5, 2011/19788-1, and 2012/05505-0).
Author Contributions
J.S., R.M.F. and D.M.M. conceived and designed the research. G.J.F.G. performed the experiments. J.S., A.M.C., A.A.F.C. and A.M. analyzed the data. J.S., A.M.C., A.A.F.C., A.M. and D.M.M. drafted the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ulrich C.M. Nutrigenetics in cancer research—Folate metabolism and colorectal cancer. J. Nutr. 2005;135:2698–2702. doi: 10.1093/jn/135.11.2698. [DOI] [PubMed] [Google Scholar]
- 2.Stover P.J. Polymorphisms in 1-carbon metabolism, epigenetics and folate-related pathologies. J. Nutr. Nutr. 2011;4:293–305. doi: 10.1159/000334586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams K.T., Schalinske K.L. New insights into the regulation of methyl group and homocysteine metabolism. J. Nutr. 2007;137:311–314. doi: 10.1093/jn/137.2.311. [DOI] [PubMed] [Google Scholar]
- 4.Eldibany M.M., Caprini J.A. Hyperhomocysteinemia and thrombosis: An overview. Arch. Pathol. Lab. Med. 2007;131:872–884. doi: 10.5858/2007-131-872-HATAO. [DOI] [PubMed] [Google Scholar]
- 5.Clarke R., Halsey J., Lewington S., Lonn E., Armitage J., Manson J.E., Bønaa K.H., Spence J.D., Nygård O., Jamison R., et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch. Intern. Med. 2010;170:1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 6.DeVos L., Chanson A., Liu Z., Ciappio E.D., Parnell L.D., Mason J.B., Tucker K.L., Crott J.W. Associations between single nucleotide polymorphisms in folate uptake and metabolizing genes with blood folate, homocysteine, and DNA uracil concentrations. Am. J. Clin. Nutr. 2008;88:1149–1158. doi: 10.1093/ajcn/88.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacques P.F., Selhub J., Bostom A.G., Wilson P.W.F., Rosenberg I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 8.Ganji V., Kafai M.R. Trends in serum folate, RBC folate, and circulating total homocysteine concentrations in the United States: Analysis of data from National Health and Nutrition Examination Surveys, 1988–1994, 1999–2000, and 2001–2002. J. Nutr. 2006;136:153–158. doi: 10.1093/jn/136.1.153. [DOI] [PubMed] [Google Scholar]
- 9.Marchioni D.M.L., Fisberg R.M. Manual de Avaliação do Consumo Alimentar em Estudos Populacionais: A Experiência do Inquérito de Saúde em São Paulo (ISA) Faculdade de Saúde Pública da USP; São Paulo, Brazil: 2012. [Google Scholar]
- 10.Verly E., Jr., Steluti J., Fisberg R.M., Marchioni D.M. A quantile regression approach can reveal the effect of fruit and vegetable consumption on plasma homocysteine levels. PLoS ONE. 2014;9:e111619. doi: 10.1371/journal.pone.0111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haubrock J., Noethlings U., Volatier J.L., Dekkers A., Ocke M., Harttig U., Illner A.K., Knüppel S., Andersen L.F., Boeing H., et al. Estimating Usual Food Intake Distributions by Using the Multiple Source Method in the EPIC-Potsdam Calibration Study. J. Nutr. 2011;141:914–920. doi: 10.3945/jn.109.120394. [DOI] [PubMed] [Google Scholar]
- 12.WHO—World Health Organization . Obesity: Preventing and Managing the Global Epidemic Report of a World Health Organization Consultation. World Health Organization; Geneva, Switzerland: 2000. p. 256. [PubMed] [Google Scholar]
- 13.Bagley P.J., Selhub J. Analysis of folate from distribution by affinity followed by reversed-phase chromatography with electrical detection. Clin. Chem. 2000;46:404–411. [PubMed] [Google Scholar]
- 14.Rybak M.E., Jain R.B., Pfeiffer C.M. Clinical vitamin B6 analysis: An interlaboratory comparison of pyridoxal 5′-phosphate measurements in serum. Clin. Chem. 2005;51:1223–1231. doi: 10.1373/clinchem.2005.050278. [DOI] [PubMed] [Google Scholar]
- 15.Miller S., Dykes D., Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myakishev M.V., Khripin Y., Hu S., Hamer D.H. High-throughput SNP genotyping by allele-specific PCR with universal energy-transfer-labeled primers. Genome Res. 2001;11:163–169. doi: 10.1101/gr.157901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider J.A., Rees D.C., Liu Y.T., Clegg J.B. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am. J. Hum. Genet. 1998;62:1258–1260. doi: 10.1086/301836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botto L.D., Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HuGE review. Am. J. Epidemiol. 2000;151:862–877. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- 19.Shinjo S.K., Oba-Shinjo S.M., da Silva R., Barbosa K.C., Yamamoto F., Scaff M. Methylenetetrahydrofolate reductase gene polymorphism is not related to the risk of ischemic cerebrovascular disease in a Brazilian population. Clinics. 2007;63:295–300. doi: 10.1590/S1807-59322007000300014. [DOI] [PubMed] [Google Scholar]
- 20.Voetsch B., Damasceno B.P., Camargo E.C., Massaro A., Bacheschi L.A., Scaff M., Annichino-Bizzacchi J.M., Arruda V.R. Inherited thrombophilia as a risk factor for the development of ischaemic stroke in young adults. Thromb. Haemost. 2000;83:229–233. [PubMed] [Google Scholar]
- 21.Oliveira K.C., Bianco B., Verreschi I.T.N., Guedes A.D., Galera B.B., Galera M.F., Barbosa C.P., Lipay M.V. Prevalence of the Polymorphism MTHFR A1298C and not MTHFR C677T Is Related to Chromosomal Aneuploidy in Brazilian Turner Syndrome Patients. Arq. Bras. Endocrinol. Metabol. 2008;52:1374–1381. doi: 10.1590/S0004-27302008000800028. [DOI] [PubMed] [Google Scholar]
- 22.Devlin A.M., Clarke R., Birks J., Evans J.G., Halsted C.H. Interactions among polymorphisms in folate-metabolizing genes and serum total homocysteine concentrations in a healthy elderly population. Am. J. Clin. Nutr. 2006;3:708–713. doi: 10.1093/ajcn.83.3.708. [DOI] [PubMed] [Google Scholar]
- 23.Lima C.S., Ortega M.M., Ozelo M.C., Araujo R.C., De Souza C.A., Lorand-Metze I., Annichino-Bizzacchi J.M., Costa F.F. Polymorphisms of methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR), methionine synthase reductase (MTRR), and thymidylate synthase (TYMS) in multiple myeloma risk. Leuk. Res. 2008;32:401–405. doi: 10.1016/j.leukres.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Sharp L., Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: A HuGE review. Am. J. Epidemiol. 2004;159:423–443. doi: 10.1093/aje/kwh066. [DOI] [PubMed] [Google Scholar]
- 25.Barnabé A., Aléssio A.C., Bittar L.F., de MoraesMazetto B., Bicudo A.M., de Paula E.V., Höehr N.F., Annichino-Bizzacchi J.M. Folate, vitamin B12 and Homocysteine status in the post-folic acid fortification era in different subgroups of the Brazilian population attended to at a public health care center. Nutr. J. 2015;14:19. doi: 10.1186/s12937-015-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalmbach R.D., Choumenkovitch S.F., Troen A.P., Jacques P.F., D’Agostino R., Selhub J. A 19-base pair deletion polymorphism in dihydrofolate reductase is associated with increased unmetabolized folic acid in plasma and decreased red blood cell folate. J. Nutr. 2008;138:2323–2327. doi: 10.3945/jn.108.096404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendes C.C., Raimundo A.M., Oliveira L.D., Zampieri B.L., Marucci G.H., Biselli J.M., Goloni-Bertollo E.M., Eberlin M.N., Haddad R., Riccio M.F., et al. DHFR 19-bp Deletion and SHMT C1420T Polymorphisms and Metabolite Concentrations of the Folate Pathway in Individuals with Down Syndrome. Genet. Test. Mol. Biomark. 2013;17:274–277. doi: 10.1089/gtmb.2012.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoey L., McNulty H., Askin N., Dunne A., Ward M., Pentieva K., Strain J., Molloy A.M., Flynn C.A., Scott J.M. Effect of a voluntary food fortification policy on folate, related B vitamin status, and homocysteine in healthy adults. Am. J. Clin. Nutr. 2007;86:1405–1413. doi: 10.1093/ajcn/86.5.1405. [DOI] [PubMed] [Google Scholar]
- 29.Girelli D., Friso S., Trabetti E., Olivieri O., Russo C., Pessotto R., Faccini G., Pignatti P.F., Mazzucco A., Corrocher R. Methylenetetrahydrofolate reductase C677T mutation plasma homocysteine, and folate in subjects from northern Italy with or without angiographically documented severe coronary atherosclerotic disease: Evidence for an important genetic-environmental interaction. Blood. 1998;9:4158–4163. [PubMed] [Google Scholar]
- 30.Crider K.S., Zhu J.H., Hao L., Yang Q.H., Yang T.P., Gindler J., Maneval D.R., Quinlivan E.P., Li Z., Bailey L.B., et al. MTHFR 677C→T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am. J. Clin. Nutr. 2011;93:1365–1372. doi: 10.3945/ajcn.110.004671. [DOI] [PubMed] [Google Scholar]
- 31.Rozen R. Genetic predisposition to hyperhomocysteinemia: Deficiency of methylenetetrahydrofolate reductase (MTHFR) Thromb. Haemost. 1997;78:523–526. [PubMed] [Google Scholar]
- 32.Marchioni D.M.L., Verly E., Jr., Steluti J., Cesar C.L.G., Fisberg R.M. Ingestão de folato nos períodos pré e pós-fortificação mandatória: Estudo de base populacional em São Paulo, Brasil Folic acid intake before and after mandatory fortification: A population-based study in Sao Paulo, Brazil. Cad. Saúde Pública. 2013;29:2083–2092. doi: 10.1590/0102-311X00084712. [DOI] [PubMed] [Google Scholar]
- 33.FAO/WHO—Food and Agriculture Organization/World Health Organization . FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements. FAO; Rome, Italy: 2001. Folate and Folic Acid; pp. 53–63. [Google Scholar]
- 34.Parra F.C., Amado R.C., Lambertucci J.R., Rocha J., Antunes C.M., Pena S.D. Color and genomic ancestry in Brazilians. Proc. Natl. Acad. Sci. USA. 2003;100:177–182. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez-Kurtz G. Pharmacogenomics and the genetic diversity of the Brazilian population. Cad. Saude Publica. 2009;25:1650–1651. doi: 10.1590/S0102-311X2009000800001. [DOI] [PubMed] [Google Scholar]
- 36.Rady P.L., Szucs S., Grady J., Hudnall S.D., Kellner L.H., Nitowsky H., Tyring S.K., Matalon R.K. Genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) in ethnic populations in Texas; a report of a novel MTHFR polymorphic site, G1793A. Am. J. Med. Genet. 2002;107:162–168. doi: 10.1002/ajmg.10122. [DOI] [PubMed] [Google Scholar]
- 37.Yang Q.-H., Botto L.D., Gallagher M., Friedman J., Sanders C.L., Koontz D., Nikolova S., Erickson J.D., Steinberg K. Prevalence and effects of gene-gene and gene-nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: Findings from the third National Health and Nutrition Examination Survey DNA Bank. Am. J. Clin. Nutr. 2008;88:232–246. doi: 10.1093/ajcn/88.1.232. [DOI] [PubMed] [Google Scholar]
- 38.Pangilinan F., Molloy A.M., Mills J.L., Troendle J.F., Parle-McDermott A., Signore C., O’Leary V.B., Chines P., Seay J.M., Geiler-Samerotte K., et al. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med. Genet. 2012;13:62. doi: 10.1186/1471-2350-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehlig K., Leander K., De Faire U., Nyberg F., Berg C., Rosengren A., Björck L., Zetterberg H., Blennow K., Tognon G., et al. The association between plasma homocysteine and coronary heart disease is modified by the MTHFR 677C>T polymorphism. Heart. 2013;99:1761–1765. doi: 10.1136/heartjnl-2013-304460. [DOI] [PubMed] [Google Scholar]
- 40.Muntjewerff J.W., Kahn R.S., Blom H.J., Heijer M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: A meta-analysis. Mol. Psychiatry. 2006;11:143–149. doi: 10.1038/sj.mp.4001746. [DOI] [PubMed] [Google Scholar]
- 41.Safarinejad M.R., Shafiei N., Safarinejad N. Relationship between three polymorphisms of Methylenetetrahydrofolate reductase (MTHFR C677T, A1298C, and G1793A) gene and risk of Prostate cancer: A case control study. Prostate. 2010;70:1645–1657. doi: 10.1002/pros.21200. [DOI] [PubMed] [Google Scholar]
- 42.Arslan S., Karadayi S., Yildirim M.E., Ozdemir O., Akkurt I. The association between methylene-tetrahydrofolate reductase gene polymorphism and lung cancer risk. Mol. Biol. Rep. 2011;38:991–996. doi: 10.1007/s11033-010-0194-z. [DOI] [PubMed] [Google Scholar]
- 43.Yu L., Chen J. Association of MHTFR Ala222Val (rs1801133) polymorphism and breast cancer susceptibility: An update meta-analysis based on 51 research studies. Diagn. Pathol. 2012;7:171. doi: 10.1186/1746-1596-7-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husemoen L.L.N., Skaaby T., Jørgensen T., Thuesen B.H., Fenger M., Grarup N., Sandholt C.H., Hansen T., Pedersen O., Linneberg A. MTHFR C677T genotype and cardiovascular risk in a general population without mandatory folic acid fortification. Eur. J. Nutr. 2014;53:1549–1559. doi: 10.1007/s00394-014-0659-2. [DOI] [PubMed] [Google Scholar]
- 45.Wilson A., Platt R., Wu Q., Leclerc D., Christensen B., Yang H., Gravel R.A., Rozen R. A Common Variant in Methionine Synthase Reductase Combined with Low Cobalamin (Vitamin B12) Increases Risk for Spina Bifida. Mol. Genet. Metab. 1999;67:317–323. doi: 10.1006/mgme.1999.2879. [DOI] [PubMed] [Google Scholar]
- 46.Hu J., Zhou G.W., Wang N., Wang Y.J. MTRR A66G polymorphism and breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2010;124:779–784. doi: 10.1007/s10549-010-0892-1. [DOI] [PubMed] [Google Scholar]
- 47.Wang H.G., Wang J.L., Zhang J., Zhao L.X., Zhai G.X., Xiang Y.Z., Chang P. Reduced folate carrier A80G polymorphism and susceptibility to neural tube defects: A meta-analysis. Gene. 2012;510:180–184. doi: 10.1016/j.gene.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Biselli J.M., Zampieri B.L., Goloni-Bertollo E.M., Haddad. R., Fonseca M.F., Eberlin M.N., Vannucchi H., Carvalho V.M., Pavarino E.C. Genetic polymorphisms modulate the folate metabolism of Brazilian individuals with Down syndrome. Mol. Biol. Rep. 2012;39:9277–9284. doi: 10.1007/s11033-012-1629-5. [DOI] [PubMed] [Google Scholar]
- 49.Laguardia J. O uso da variável “raça” na pesquisa em saúde. Physis. 2004;4:197–234. doi: 10.1590/S0103-73312004000200003. [DOI] [Google Scholar]