Short abstract
A recent report has identified changes in embryonic gene expression that are associated with, and may halt, embryonic cell proliferation during diapause.
Abstract
In many mammalian species, embryonic cell proliferation can be reversibly arrested in an embryonic diapause at the time of embryo implantation. A recent report has identified changes in embryonic gene expression that are associated with, and may halt, embryonic cell proliferation.
We are all accustomed to the fact that most successful human pregnancies last approximately nine and a half months. But in some 100 mammalian species, the duration of pregnancy can vary among individuals within the same species, and even between pregnancies in a single individual. A variety of strategies have evolved for regulating the length of pregnancy, ranging from the storage and timed release of sperm for fertilization following mating to delaying development of the implanted embryo, as in some bat species. The most common method, however, is to allow the fertilized egg to develop to the blastocyst stage and then to arrest embryonic cell proliferation and metabolism. This arrest prevents the blastocyst from implanting into the uterus - an essential step for subsequent embryonic development. Implantational delay, or embryonic diapause, results in the blastocyst entering into a state of metabolic and proliferative quiescence. Once diapause is interrupted, the blastocycst regains an active metabolism, cell proliferation is initiated, the blastocyst implants in the uterus and development continues (Figure 1).
Figure 1.

The growth of diapausing blastocysts is reversibly arrested before implantation into the uterus. (a) A diapausing blastocyst (arrowhead) is shown in contact with the uterine luminal epithelium (LE). Note the loose fibroblastic morphology of the stroma (S) underlying the luminal epithelium. T, the trophoblast of the blastocyst. (b) Implantation after diapause starts with the luminal epithelium adjacent to the trophoblast of the blastocyst undergoing apoptosis as the trophoblast cells start to invade the uterus. After implantation, the stroma has undergone massive proliferation and differentiation to form the decidua (D).
The precise role of diapause in the reproductive strategies of mammals has not been fully established, but it appears to have evolved as a strategy to maximize the mammals' reproductive fitness and increase the probability that offspring will survive following birth. Diapause, therefore, usually results in the birth of offspring at a time of abundant food supply in a seasonal environment (reviewed in [1]). What is particularly fascinating about diapause is that it is an inducible, but reversible, mechanism that can halt the proliferation of early embryonic cells - cells that have an intrinsic ability to proliferate extremely rapidly. The mouse blastocyst can be considered as being quasi-malignant, because the surgical transfer of blastocysts to extrauterine sites, such as underneath the testis or kidney capsule, results in the embryo forming a rapidly growing teratocarcinoma, which within three to four weeks weighs several grams [2]. Recently, a study by Hamatani et al. [3] profiled the patterns of gene expression in diapausing mouse embryos and identified genes that may mediate this form of naturally induced growth arrest.
The principal factor regulating embryonic implantation and diapause is the hormonal state of the maternal uterine environment. High levels of progesterone, produced by the ovary, are required for both embryo implantation and sustaining post-implantation development. The other ovarian steroid hormone, estrogen, is first produced at ovulation and is required (in combination with progesterone) to initiate proliferative and differentiative changes in the uterus in preparation for the arrival of the blastocyst, thus ensuring a normal pregnancy. In rodents, a second increase in estrogen levels, the so-called nidatory rise, initiates blastocyst implantation [4]. Estrogen induction of embryo implantation is mediated by the expression of a cytokine, leukemia inhibitory factor (LIF), in the uterus (Figure 2). LIF is required so that the uterus is induced to become receptive to the blastocyst, allowing the embryo to implant: in the absence of LIF blastocysts do not implant [5]. Paradoxically, although LIF was first identified by the fact that it is required to maintain the undifferentiated proliferation of mouse embryonic stem (ES) cells in culture [6,7], LIF is not required in vivo by embryos for their normal development, even though ES cells are derived from the inner cell mass (ICM) of the blastocyst [5]. A single intraperitoneal injection of LIF into a LIF-deficient female carrying LIF-deficient blastocysts is sufficient to induce blastocyst implantation and subsequent development of the embryo to birth [8].
Figure 2.
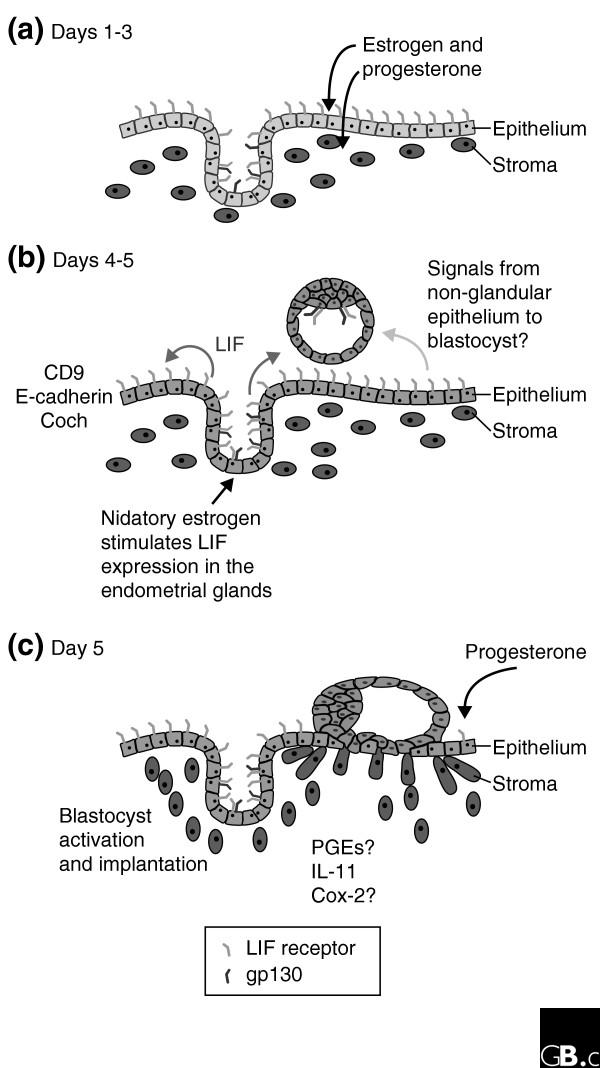
The time course of events leading to embryo implantation in the mouse. (a) On day 3 after fertilization the uterus is undergoing differentiation and proliferation under control of the ovarian steroid hormones estrogen and progesterone. (b) On day 4 the blastocyst is adjacent to the uterus and the production of leukemia inhibitory factor (LIF) is induced in the endometrial glands by nidatory estrogen. LIF is released into the uterine lumen, where it binds to LIF receptors on the luminal epithelium. LIF binding induces the expression of many genes, including the cell adhesion factors Coch and CD9, as the uterus becomes receptive to the embryo allowing the onset of implantation. Other adhesion molecules, such as E-cadherin, undergo redistribution in their expression. (c) On day 5 the blastocyst has started to invade the uterus and the stroma is undergoing decidualization, accompanied by the expression of prostaglandins (PGEs), which are regulated by Cox-2, and the cytokine interleukin-11 (IL-11), which is essential for decidualization.
In the absence of LIF (as occurs in LIF knockout mice), unimplanted blastocysts that are recovered from the uterus between three and four days after implantation should have occurred look remarkably like embryos undergoing diapause [5]. It appears that in the absence of LIF mouse blastocysts may enter, by default, into a state of diapause. Blastocysts also express the heterodimeric LIF receptor [9], but LIF activation of these embryonic receptors is not necessary for implantation, as blastocysts lacking functional LIF receptors are able to implant and undergo post-implantation development [10-12]. Blastocysts lacking gp130, one component of the LIF receptor, do not survive when undergoing prolonged diapause, however, because of the gradual loss of ICM cells [13]. LIF may therefore have multiple roles in regulating blastocyst implantation, diapause and blastocyst viability in mice. LIF is required by the uterus for the blastocyst to implant, and during a normal reproductive cycle embryos do not require LIF for their own development. If blastocysts undergo diapause, however, LIF (or another related factor that binds to gp130) may be required to sustain the ICM cells in conjunction with the arrest of ICM proliferation. This suggests that ES cells derived from the ICM depend on LIF as a survival factor. What drives ES cell proliferation is, however, unclear [14].
We clearly know a reasonable amount about how the mother regulates diapause in the mouse, but what happens in the blastocyst? Previous studies have shown that the diapausing blastocysts enter into a state of proliferative and metabolic quiescence. Cell proliferation ceases, as does amino-acid uptake and overall metabolism [15,16]. A recent report [3] has now taken the characterization of the diapausing blastocyst a step further by using microarray technology to identify genes that are differentially expressed between delayed blastocysts and blastocysts that have been activated following diapause. In this study, mRNA was isolated from approximately 100 diapausing and activated embryos, amplified and screened against the National Institute on Aging (NIA) mouse microarrays, which are enriched for genes expressed during different stages of mouse development [17]. The report by Hamatani et al. [3] identifies 229 genes (out of a total of around 22,000, so roughly 1% of the genes on the array) that are differentially expressed between activated and delayed blastocysts. Delayed blastocysts had 80 genes that showed increased expression levels compared to 149 genes that were relatively upregulated in the activated blastocysts.
The genes showing altered levels of expression between the arrested and activated blastocysts were clustered into six functional groups: specifically, genes involved in cell-cycle or cell-proliferation control; energy pathways and carbohydrate metabolism; signaling; nuclear transport; chromatin remodeling; and adhesion. Consistent with the physiological state of the embryos, delayed blastocysts showed decreased levels of expression of genes involved in cell-cycle progression, together with an increase in the levels of expression of genes that arrest cell proliferation at G0 or G1 stages in the cell cycle - for example, Btg1 and the cell-cycle inhibitor p21Cip1, which is regulated by p53 [18]. But p53, which can cause cell-cycle arrest when upregulated, did not show any difference in mRNA levels between the arrested and activated blastocysts. Intriguingly, one of the genes upregulated in the diapausing embryos is the maternally expressed imprinted gene encoding the Igf2 receptor (Igf2r), which retards cell proliferation when overexpressed [19]. Activated blastocysts also showed relative upregulation in the expression of genes that function in the glucose and pyruvate energy pathways. Another gene that was identified as upregulated in delayed embryos was Irs1, encoding insulin receptor 1 substrate, a docking protein that is involved in the binding and activation of signal transduction molecules after being phosphorylated by the insulin receptor kinase. Mutations in the Drosophila homolog of Irs1, Chico, result in a significant increase in the lifespan of the mutant flies [20], and Irs1 may therefore be involved in sustaining blastocyst viability and longevity during developmental delay. Surprisingly, no alterations in levels of expression of the LIF receptor or gp130 (which together comprise the heterodimeric LIF receptor) were detected in the delayed blastocysts.
The last category of genes investigated by Hamatani et al. [3] was that involved in mediating cell adhesion and/or migration. In the mouse, implantation is marked by the active invasion of the apoptosing luminal epithelium by the embryonic trophoblast. Six genes whose products are associated with tight-junction integrity, cell migration, cell-to-cell adhesion and focal adhesions were all upregulated in the activated blastocysts. In addition, the heparin-binding epidermal growth factor (HB-EGF), which shows a remarkable localization in expression at the site of blastocyst attachment [21], was also upregulated in the activated blastocysts. This finding was particularly intriguing as HB-EGF interacts with EGF receptors and loss of a functional EGF receptor in some strains of mouse causes peri-implantation lethality [22]. Although previous results have suggested a critical role for HB-EGF in mediating blastocyst implantation, recent reports indicated that HB-EGF is not localized to the cell membrane until after blastocyst attachment [23], and mice lacking HB-EGF are fertile [24], indicating that HB-EGF may not be essential for blastocyst activation or implantation.
Overall, the expression of many of the genes that differ between the diapausing and activated blastocysts is consistent with the cellular and physiological events that are expected to change with activation. No one factor has yet been identified as being the 'key' to regulating diapause, unless it is among the approximately 30% of the 229 differentially expressed genes to which no function has yet been assigned. Nevertheless, the list of genes does reveal some possible candidates that may be essential for mediating growth arrest in the implanting blastocyst. With the current availability of knockout lines of mice carrying mutations in many of the genes identified in this screen it will be possible to systematically determine which of the genes may be required for diapause, as has already been shown for the gp130 receptor. In turn this may provide both profound and fascinating insights into the molecular mechanisms regulating cell proliferation and growth control in the mammalian embryo.
References
- Renfree MB, Shaw G. Diapause. Annu Rev Physiol. 2000;62:353–375. doi: 10.1146/annurev.physiol.62.1.353. [DOI] [PubMed] [Google Scholar]
- Stevens LC. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970;21:364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci USA. 2004;101:10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos A. Endocrine control of egg implantation. In: Greep RO, Astwood EB, editor. In Handbook of Physiology. Vol. 2. Baltimore: Williams and Wilkins; 1973. pp. 187–215. [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141:4365–4372. doi: 10.1210/en.141.12.4365. [DOI] [PubMed] [Google Scholar]
- Nichols J, Davidson D, Taga T, Yoshida K, Chambers I, Smith A. Complementary tissue-specific expression of LIF and LIF-receptor mRNAs in early mouse embryogenesis. Mech Dev. 1996;57:123–131. doi: 10.1016/0925-4773(96)00531-X. [DOI] [PubMed] [Google Scholar]
- Ware CB, Horowitz MC, Renshaw BR, Hunt JS, Liggitt D, Koblar SA, Gliniak BC, McKenna HJ, Papayannopoulou T, Thoma B, et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121:1283–1299. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C, Chambers I, Johnstone S, Robertson M, Ebrahimi B, Saito M, Taga T, Li M, Burdon T, Nichols J, et al. Paracrine induction of stem cell renewal by LIF-deficient cells: a new ES cell regulatory pathway. Dev Biol. 1998;203:149–162. doi: 10.1006/dbio.1998.9026. [DOI] [PubMed] [Google Scholar]
- Nichols J, Chambers I, Taga T, Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–2339. doi: 10.1242/dev.128.12.2333. [DOI] [PubMed] [Google Scholar]
- Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/S0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Dickson AD. Temporal and spatial aspects of oestrogen-induced RNA, protein and DNA synthesis in delayed-implantation mouse blastocysts. J Anat. 1975;119:453–459. [PMC free article] [PubMed] [Google Scholar]
- Spindler RE, Renfree MB, Gardner DK. Carbohydrate uptake by quiescent and reactivated mouse blastocysts. J Exp Zool. 1996;276:132–137. doi: 10.1002/(SICI)1097-010X(19961001)276:2<132::AID-JEZ6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Carter MG, Piao Y, Dudekula DB, Qian Y, VanBuren V, Sharov AA, Tanaka TS, Martin PR, Bassey UC, Stagg CA, et al. The NIA cDNA project in mouse stem cells and early embryos. C R Biol. 2003;326:931–940. doi: 10.1016/j.crvi.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Kuo ML, Duncavage EJ, Mathew R, den Besten W, Pei D, Naeve D, Yamamoto T, Cheng C, Sherr CJ, Roussel MF. Arf induces p53-dependent and -independent antiproliferative genes. Cancer Res. 2003;63:1046–1053. [PubMed] [Google Scholar]
- Hernandez L, Kozlov S, Piras G, Stewart CL. Paternal and maternal genomes confer opposite effects on proliferation, cell-cycle length, senescence, and tumor formation. Proc Natl Acad Sci USA. 2003;100:13344–13349. doi: 10.1073/pnas.2234026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Isaacs J, Murphy CR. Heparin-binding EGF-like growth factor is seen on the extracellular surface of uterine epithelial cells only after the initial stages of blastocyst attachment. Histochem J. 2002;34:339–343. doi: 10.1023/A:1023334727288. [DOI] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]


