Short abstract
Microtubule-associated proteins (MAPs) of the MAP2/Tau family include the vertebrate proteins MAP2, MAP4, and Tau and homologs in other animals. They are best known for their microtubule-stabilizing activity roles regulating microtubule networks but, accumulating evidence suggests a much broader range of functions. regulation of microtubule-mediated transport. Tau is also implicated in Alzheimer’s disease and other dementias.
Abstract
Microtubule-associated proteins (MAPs) of the MAP2/Tau family include the vertebrate proteins MAP2, MAP4, and Tau and homologs in other animals. All three vertebrate members of the family have alternative splice forms; all isoforms share a conserved carboxy-terminal domain containing microtubule-binding repeats, and an amino-terminal projection domain of varying size. MAP2 and Tau are found in neurons, whereas MAP4 is present in many other tissues but is generally absent from neurons. Members of the family are best known for their microtubule-stabilizing activity and for proposed roles regulating microtubule networks in the axons and dendrites of neurons. Contrary to this simple, traditional view, accumulating evidence suggests a much broader range of functions, such as binding to filamentous (F) actin, recruitment of signaling proteins, and regulation of microtubule-mediated transport. Tau is also implicated in Alzheimer's disease and other dementias. The ability of MAP2 to interact with both microtubules and F-actin might be critical for neuromorphogenic processes, such as neurite initiation, during which networks of microtubules and F-actin are reorganized in a coordinated manner. Various upstream kinases and interacting proteins have been identified that regulate the microtubule-stabilizing activity of MAP2/Tau family proteins.
Gene organization and evolutionary history
Several types of microtubule-associated protein (MAP) have evolved in eukaryotes, including microtubule motors, microtubule plus-end-binding proteins, centrosome-associated proteins, enzymatically active MAPs, and structural MAPs. We focus here on the MAP2/Tau family of structural MAPs, which along with the MAP1A/1B family form one of the 'classical', well-characterized families of MAPs. In mammals, the family consists of the neuronal proteins MAP2 and Tau and the non-neuronal protein MAP4 (Table 1).
Table 1.
Properties of human MAP2/Tau family genes
| Gene | Locus | Predicted exons | Splice form | Number of microtubule-binding repeats | Alternatively spliced exons |
| MAP2 | 2q34-q35 | 18 | Isoform 1 (MAP2b) | 3 | +9, +10, +11, -16 |
| Isoform 2 (MAP2c) | 3 | -9, -10, -11, -16 | |||
| Isoform 3 | 4 | +9, +10, +11, +16 | |||
| Isoform 4 (MAP2d) | 4 | -9, -10, -11, +16 | |||
| MAP2a | Unknown | +8, +9, +10, +11, (16?) | |||
| Tau | 17q21.1 | 17 | Isoform 1 (HMW-tau) | 4 | +2, +3, +4A, +6, +10 |
| Isoform 2 (tau 4R/2N) | 4 | +2, +3, -4A, -6, +10 | |||
| Isoform 3 (tau 4R/0N) | 4 | -2, -3, -4A, -6, +10 | |||
| Isoform 4 (tau 3R/0N) | 3 | -2, -3, -4A, -6, -10 | |||
| MAP4 | 3p21 | 23 | Various isoforms | 3-5 | Various |
Chromosomal localization and sequence information about reviewed alternative splice forms were obtained from LocusLink [75]. Commonly used designations for splice forms are indicated in brackets.
It has been proposed that the Escherichia coli protein ZipA, which interacts with the bacterial tubulin homolog FtsZ [1], might be an ancient prototype of MAP2/Tau family members [2]. ZipA contains a region with limited homology to MAP2/Tau proteins, but this region is neither sufficient nor necessary for FtsZ binding [3]. A single, unambiguous functional ortholog of MAP2/Tau proteins is found in Caenorhabditis elegans (alternative splice forms PTL-1A and PTL-1B [4,5]) and in Drosophila melanogaster (CG31O57 [6]; see Figure 1). Both contain microtubule-binding domains related to those in mammalian MAP2/Tau proteins. In contrast, the genome of the frog Xenopus laevis has an ortholog of each member of the family. At least three distinct MAP2/Tau related genes have been identified in the Tetraodon (pufferfish) genome: CAF98218 and CAGO9246 appear similar to MAP2, whereas CAGO3O2O appears similar to Tau [7]. Additional MAP2/Tau-related genes appear to be present in Tetraodon, but the limited sequence information and lack of mapping data make it difficult to evaluate their significance. No homologs have been found in eukaryotes outside animals. Mammalian MAP2/Tau genes span multiple exons, which are spliced to produce several alternative isoforms [8,9] (Table 1 and see below).
Figure 1.
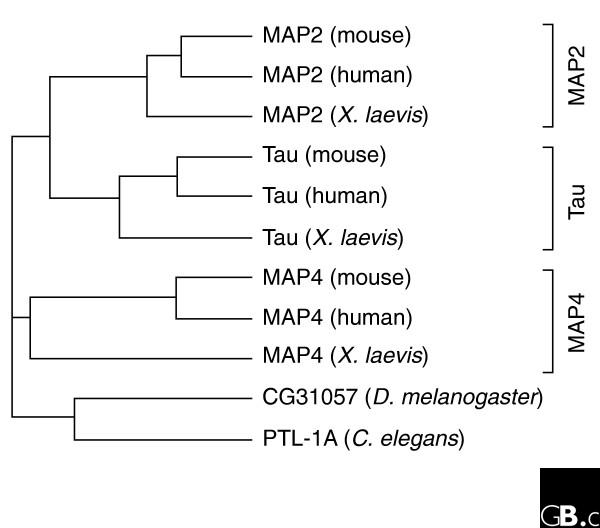
Phylogenetic analysis of MAP2/Tau family proteins. Homologous protein sequences of the microtubule-binding repeats of MAP2 (using splice forms (with three microtubule-binding repeats), Tau (four-repeat isoforms), MAP4 (five-repeat isoforms) and the invertebrate MAPs CG31057 and PTL-1A (five-repeat isoforms) were analyzed using the program Phylip 76; gaps were ignored. The available Tetraodon sequences are incomplete and were therefore not included in the analysis.
Characteristic structural features
All MAP2/Tau family proteins have microtubule-binding repeats near the carboxyl terminus [10], each containing a conserved KXGS motif that can be phosphorylated (Figure 2) [11,12]. In addition, each family member contains an amino-terminal projection domain of varying size. In MAP2 and Tau, this domain has a net negative charge and exerts a long-range repulsive force as shown by atomic force microscopy [13]. Each protein has several isoforms, with variation in the length of the projection domain and the number of microtubule-binding repeats [8,9]. The main forms of MAP2 are MAP2C, which is relatively short, and MAP2a and MAP2b, which have longer projection domains.
Figure 2.
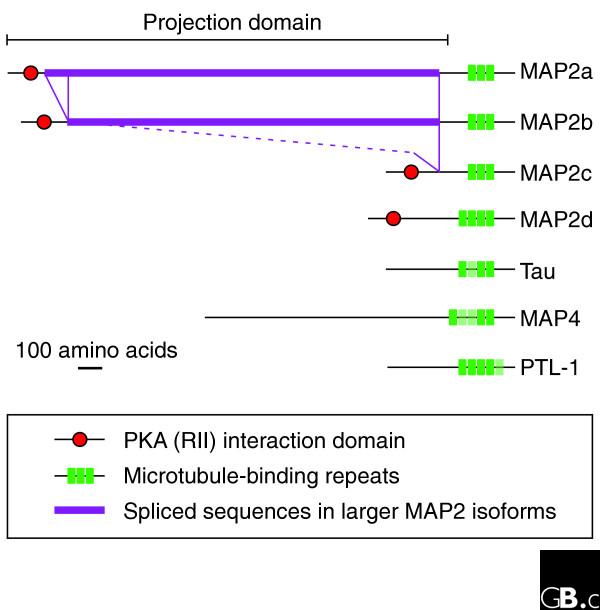
The domain organization of MAP2/Tau family proteins. Selected isoforms of the human members of the family are shown, as well as the nematode homolog PTL-1. All family members have alternative splice forms with varying numbers of carboxy-terminal microtubule-binding repeats and amino-terminal projection domains of varying lengths. PKA (RII) indicates a domain interacting with the RII subunit of protein kinase A. Repeats that are not present in all major isoforms are shown lighter.
MAP2/Tau family members are natively unfolded molecules and, like other proteins in this class, are thought to adopt specific conformations upon binding to their targets (microtubules, F-actin and potentially other molecules) [14]. Most regions of MAP2/Tau proteins seem to be devoid of secondary structure. The only region of MAP2 that appears to form a secondary structure is an amino-terminal domain (residues 86-5103), which is found in all isoforms and interacts with the regulatory subunit of protein kinase A (PKA). Like the related domain in the A-kinase anchoring protein AKAP79/150, this region is predicted to form an amphipathic helix [15].
MAP2 also can interact directly with F-actin [16]; interestingly, the F-actin-binding site is located within the domain containing the microtubule-binding repeats. Although the MAP2 repeat region is highly similar to that of Tau, neither wild-type Tau nor MAP2 chimeras containing the Tau microtubule-binding repeats can bind to F-actin directly. However, F-actin binding is conferred on Tau if its microtubule-binding domain is exchanged for the corresponding region of MAP2 [16].
Localization and function
Developmental and regional expression
Mammalian MAP2 is expressed mainly in neurons, but MAP2 immunoreactivity is also detected in some non-neuronal cells such as oligodendrocytes. Its expression is very weak in neuronal precursors and then becomes strong about 1 day after expression of neuron-specific tubulin isoform βIII [17]. MAP2c is the juvenile isoform and is downregulated after the early stages of neuronal development [18], whereas MAP2b is expressed both during development and adulthood. MAP2a becomes expressed when MAP2c levels are falling and is not detected uniformly in all mature neurons [19]. In the brain, smaller splice forms of Tau (of 50-565 kDa) are differentially expressed during early development. Specifically, Tau isoforms with three microtubule-binding repeats are predominantly expressed during early development, whereas isoforms with four repeats are expressed during adulthood [20,21]. High-molecular-weight variants of Tau (110-5120 kDa) are expressed in peripheral neurons and also at a much lower level in the brain [22]. MAP4 is expressed in various organs, including brain, adrenal gland, lung and liver [23], but it is not ubiquitously expressed: in the brain, for example, MAP4 is expressed only in non-neuronal cells and is absent from neurons [24].
Shortly after axonogenesis in developing cortical and hippocampal neuronal cultures, Tau gradually segregates into axons, while MAP2 segregates into the nascent dendrites (at this stage dendrite precursors are called 'minor neurites') [25]. It is believed that a combination of protein stability [26], differential protein sorting [27], and dendrite-specific transport of MAP2 mRNA [28] are responsible for this spatial segregation of the two MAPs. Thus, in mature neurons Tau is present mainly in axons whereas MAP2 is restricted to cell bodies and dendrites (Figure 3).
Figure 3.

A neuron from a culture of rat brain hippocampus, showing the distinct subdomains of MAP2 and Tau enrichment in mature neurons. MAP2 is found specifically in dendrites (arrow), whereas Tau is mainly axonal (arrowhead). Note the fine meshwork of axons from neighboring cells outside the field of view that make numerous synaptic connections among the neurons in the culture.
Functions of MAP2 and Tau in neurons
MAP2/Tau family proteins were originally discovered for and characterized by their ability to bind and stabilize microtubules. Ultrastructural analyses revealed the presence of these MAPs along the sides of microtubules [29-31]. MAP2 and Tau also increase microtubule rigidity [32] and induce microtubule bundles in heterologous cell systems [33-35]. Microtubule bundle formation induced by MAP2 was suggested to be an indirect effect of its stabilization of microtubules within the confinement of cell borders [36], but more recent results suggest that MAP2-induced bundles can form even within the interior of the cell [37], indicating the existence of crosslinks. Evidence for direct crosslinking of microtubules by MAP2/Tau family proteins is lacking, leaving open the possibility that additional proteins are necessary.
As described above, MAP2 can bind both microtubules and F-actin, and both activities have been mapped to its microtubule-binding-repeat domain. It is not yet known whether a single molecule can crosslink an actin filament to a microtubule. MAP2 can bundle actin filaments in vitro [16]. MAP2c by itself can induce neurites in Neuro-2a neuroblastoma cells; its microtubule-stabilizing activity is necessary for this effect but is not sufficient, and F-actin dynamics also need to be altered [38]. MAP2's ability to interact with F-actin appears to be key to this specific biological function. Unlike MAP2C, neither Tau nor chimeric MAP2C containing the Tau microtubule-binding domain can trigger neurite initiation, an observation that correlates with their lack of F-actin binding in vitro [16]. This suggests that MAP2c's ability to interact with both microtubules and F-actin is essential for its neurite-initiation activity.
Knockout experiments in mice suggest that neither MAP2 nor Tau is essential by itself, but each single knockout leads to detectable morphological phenotypes. Tau expression was undetectable after targeted deletion of the first Tau exon, which includes the protein start codon [39]. Homozygous animals showed no major defects in brain morphology, but the microtubule density in small-caliber axons was reduced. Similarly, MAP2 expression was undetectable after deletion of one exon encoding a portion of the MAP2 microtubule-binding domain [40]. Again, homozygous animals showed no major defects in brain morphology, but microtubule density in dendrites was reduced. In addition, dendrite length in cultured neurons was reduced, suggesting a role for MAP2 in supporting dendrite elongation.
The phenotypes of single knockouts suggest specific but nonessential roles for Tau and MAP2 in the morphogenesis of the nervous system. However, these proteins probably have multiple roles in other pathways and can be compensated for by other proteins with redundant functions. Interestingly, the structurally unrelated microtubule-associated protein MAP1B appears to have some redundant roles with both Tau [41,42] and MAP2 [43]. Simultaneous inhibition of either MAP1B and Tau or MAP1B and MAP2 resulted in more severe phenotypes than those seen in single knockouts. Taken together, these experiments suggest a role for Tau, MAP2 and MAP1B in both neuronal migration and outgrowth of neurites. Redundancy among MAP2, Tau and MAP4 has not been adequately tested in mammalian systems. It is also possible that other classes of MAP such as stable tubule only protein (STOP), adenomatous polyposis coli (APC), doublecortin, or spectraplakins might provide additional redundancy with MAP functions.
MAP2/Tau family proteins have been shown to interact with numerous proteins; Table 2 provides an overview of identified interaction partners and briefly describes the proposed function of each interaction. Binding of MAP2 to the RII regulatory subunit of PKA is one of the best-characterized examples of a classical MAP functioning as an adaptor protein. The interaction site was mapped to the amino terminus of MAP2 and is present in all common MAP2 splice forms in mammals [44] but absent in Tau. Knockout mice show that MAP2 is essential for linking PKA to microtubules in various brain regions [40]. Interestingly, the absence of MAP2 affects the phosphorylation of cAMP-responsive element binding protein (CREB), suggesting a role for the MAP2-PKA interaction in CREB-mediated signal transduction [40]. Deletion of the PKA-binding site in MAP2c reduces its ability to induce neurites in neuroblastoma cells [38].
Table 2.
Selected interaction partners of MAP2/Tau family proteins
| Family member | Interacting protein | Proposed function of the interaction | Reference |
| MAP2 | Microtubules | Stabilization of microtubules; inhibition of depolymerization (catastrophes); increase in microtubule rigidity, neurite initiation | [77] |
| F-actin | Modulation of neurite initiation | [16] | |
| Regulatory subunit RII of PKA | Localization of PKA to hippocampal dendrites; facilitation of cAMP-responsive element binding protein (CREB) phosphorylation; modulation of neurite initiation | [44] | |
| Tyrosine kinase Src | Signal transduction and integration | [78] | |
| Adapter protein Grb2 | Signal transduction and integration | [78] | |
| Tyrosine kinase Fyn | Signal transduction and integration | [79] | |
| Neurofilaments | Crossbridges between microtubules and neurofilaments | [80] | |
| Class C L-type calcium channels | Linking PKA to channels | [81] | |
| MAP2-RNA transacting proteins MARTA1 and MARTA2 | Interaction with MAP2 mRNA: targets MAP2 mRNA to dendrites | [82] | |
| Tau | Microtubules | Stabilization of microtubules; inhibition of depolymerization (catastrophes); increase in microtubule rigidity | [83] |
| Fyn | Modulation of microtubule organization; pathogenesis of Alzheimer's disease | [84] | |
| Src | Unknown | [84] | |
| Presenilin 1 | Links Tau to glycogen synthase kinase 3β; pathogenesis of Alzheimer's disease | [85] | |
| Apolipoprotein E | Regulation of Tau metabolism; pathogenesis of Alzheimer's disease | [86] | |
| Calmodulin | Regulation of microtubule assembly | [87] | |
| Calmodulin-related protein S100b | Regulation of Tau phosphorylation by protein kinase C | [87] | |
| MAP4 | Microtubules | Stabilization of microtubules; inhibition of depolymerization (catastrophes) | [49] |
| Cyclin B | Links p34cdc2 kinase to microtubules; regulation of M-phase microtubule dynamics | [51] |
Tau has been studied extensively for its involvement in neurofibrillary tangle formation in Alzheimer's Disease and in frontotemporal dementias associated with chromosome 17 (FTDP-17); see several excellent discussions of Tau pathology [45-48].
Functions of MAP4 and non-neuronal functions of MAP2 and Tau
The widely expressed non-neuronal member of the MAP2/Tau family, MAP4, shares many features with other members of the family, including the presence of microtubule-binding repeats [49] and microtubule-stabilizing activity [50]. MAP4 has been proposed to play a role in regulating mitotic microtubule dynamics during metaphase [51]. However, using function-blocking antibodies that interfere with the MAP4-microtubule interaction, a more recent study [52] failed to detect an obvious phenotype in mitosis or during interphase, suggesting that MAP4 might be a component of a functionally redundant system. Muscle-specific MAP4 isoforms have been shown to be required for myogenesis [53], but the exact role of MAP4 is not known in this process.
Although MAP2 is primarily neuronal, some isoforms are also present in certain astrocytes [54], oligodendrocytes [55], as well as in the testis [56]. The testicular isoform of MAP2 contains a functional nuclear localization sequence [56] and is enriched in nuclei of germ cells. Like MAP2, the primarily neuronal Tau is also expressed in oligodendrocytes [57]. Interestingly, alternative splicing of MAP2 [55] and Tau [58] is similar during the maturation of oligodendrocytes and neurons. In oligodendrocytes, Tau and its regulation by the Fyn tyrosine kinase are proposed to be involved in process outgrowth [59].
Mechanism and regulation
Microtubules exhibit dynamic instability, an intrinsic behavior characterized by alternating phases of growth, shortening, and pausing. The switch from growth to shortening and the switch from shortening to growth are called catastrophes and rescues, respectively. MAP2/Tau proteins bind along the length of microtubules and stabilize microtubules by altering this dynamic behavior [31,60,61]. The small isoform MAP2c stabilizes microtubules primarily by reducing the frequency and duration of catastrophes [60]. Under conditions where its concentration is non-saturating, MAP2 can also form clusters on microtubules, and microtubule catastrophes stop at such clusters [62]. Interestingly, isoforms of Tau containing three or four microtubule-binding repeats have distinct effects on microtubule dynamics, with four-repeat isoforms protecting microtubules from depolymerization much more robustly than three-repeat isoforms [61]. In cells, microtubules still exhibit dynamic behavior even when stabilizing MAPs are highly expressed [63], perhaps because their binding is regulated by phosphorylation and other factors.
A detailed cryo-electron microscopy (cryo-EM) analysis has suggested a possible mechanism by which MAP2/Tau might reduce catastrophes and thus stabilize microtubules. This study revealed that the microtubule-binding repeats interact in an elongated fashion on the outer microtubule lattice, spanning two tubulin dimers along a single protofilament rather than bridging adjacent protofilaments [31]. Tau appeared to show a similar pattern. Several other experiments confirm that MAP2 binds to the outside of microtubules in vivo. First, the projection domain of MAP2 can regulate microtubule spacing [64]. In addition, an EM study that compared wild-type to knockout animals suggested that electron-dense structures on the outer surface of microtubules contain MAP2 [40]. Another cryo-EM analysis suggested that Tau binds to the inner surface of microtubules [65], but the role of this binding is not yet clear. Tau might be able to bind to multiple sites, both inside and outside the microtubule lattice. This idea is consistent with the observation that Tau has different kinetic properties when bound to pre-polymerized microtubules than when co-polymerized with microtubules [66].
MAP2/Tau family proteins can inhibit kinesin- and dynein-dependent transport along microtubules [67-71]. Observations in vitro suggest that this inhibition of microtubule motor activity occurs by direct competition of MAP2/Tau proteins with dynein and kinesin for microtubule binding and also suggest a major role for the projection domain of the MAP2/Tau proteins in this competition [69,71]. In cells, overexpression of Tau interferes with kinesin-based transport and alters the balance of plus-end- versus minus-end-directed transport [67,68]. In vivo, the MAP2 and Tau projection domains appear to be involved in regulating microtubule spacing [64]. Such control over microtubule spacing might facilitate efficient organelle transport.
Binding of MAP2/Tau family proteins to microtubules can be regulated by phosphorylation of the KXGS motif within each microtubule-binding repeat. For both MAP2 and Tau, these motifs are phosphorylated by multiple protein kinases, including PKA [11] and the microtubule affinity regulating kinase (MARK) [12], and phosphorylation leads to decreased affinity for microtubules. Recent evidence also links the Jun kinase (Jnk) pathway to phosphorylation of MAP2 [72]. Many other protein kinases can phosphorylate MAP2/Tau proteins in vitro, but for most the identity of the targeted residues in vivo and the functional consequences of phosphorylation remain to be determined. For example, in the olfactory bulb, a site in the amino-terminal domain of MAP2 is phosphorylated in vivo in a manner that is regulated by sensory-driven neural activity; the function of this phosphorylation is not yet known, however [73]. The regulation of MAPs, including the MAP2/Tau family, has been summarized in a comprehensive review [74].
Frontiers
Since their original identification over 20 years ago, classical structural MAPs of the MAP2/Tau family have been extensively characterized in vitro and in vivo. A major challenge for further illuminating their function is the vast number of interaction partners and protein kinases predicted and confirmed to phosphorylate MAP2/Tau proteins. Although some key pathways controlling their activity have been elucidated, a broader and more precise analysis of phosphorylation and other post-translational modifications is needed to fully understand MAP2/Tau protein function in signaling networks controlling the morphogenesis of neurons. Recent progress in understanding the molecular mechanisms underlying MAP-microtubule and MAP-actin interactions in vitro is promising, but biological functions remain elusive. Future studies will need to correlate the effects of MAP2/Tau proteins in vivo with molecular knowledge gained from in vitro analyses. The apparent functional redundancies and cross-talk with other MAPs and cytoskeletal regulators are challenges that will require creative experimental strategies if we are to elucidate the specific functions of MAP2/Tau family proteins in cytoskeletal organization and morphological change.
Acknowledgments
Acknowledgements
We thank Julia Braga for preparation of the neuronal cultures shown in Figure 3. This work was supported by grants from the National Institutes of Health.
References
- Hale CA, de Boer PA. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/S0092-8674(00)81838-3. The interaction between the bacterial tubulin homolog FtsZ and an ancestral MAP, ZipA, is described. [DOI] [PubMed] [Google Scholar]
- RayChaudhuri D. ZipA is a MAP-Tau homolog and is essential for structural integrity of the cytokinetic FtsZ ring during bacterial cell division. EMBO J. 1999;18:2372–2383. doi: 10.1093/emboj/18.9.2372. The functional significance of the ancestral MAP ZipA in bacterial cell division is described and its relation to MAP2/Tau is proposed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, Rhee AC, de Boer PA. ZipA-induced bundling of FtsZ polymers mediated by an interaction between C-terminal domains. J Bacteriol. 2000;182:5153–5166. doi: 10.1128/JB.182.18.5153-5166.2000. The FtsZ interaction domain on ZipA is mapped to its carboxyl terminus, a region unrelated to MAP2/Tau, suggesting that ZipA is not a functional homolog of MAP2/Tau proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Baur CP, Ahringer J, Jakes R, Hasegawa M, Spillantini MG, Smith MJ, Hill F. PTL-1, a microtubule-associated protein with tau-like repeats from the nematode Caenorhabditis elegans. J Cell Sci. 1996;109:2661–2672. doi: 10.1242/jcs.109.11.2661. Describes the cloning of a MAP2/Tau homolog from C. elegans, expression analyses, microtubule binding and stabilization experiments, and overexpression studies. [DOI] [PubMed] [Google Scholar]
- McDermott JB, Aamodt S, Aamodt E. ptl-1, a Caenorhabditis elegans gene whose products are homologous to the tau microtubule-associated proteins. Biochemistry. 1996;35:9415–9423. doi: 10.1021/bi952646n. The first characterization and cloning of the C. elegans MAP2/Tau homolog. [DOI] [PubMed] [Google Scholar]
- Heidary G, Fortini ME. Identification and characterization of the Drosophila tau homolog. Mech Dev. 2001;108:171–178. doi: 10.1016/S0925-4773(01)00487-7. Cloning, expression and subcellular localization studies of the fly MAP2/Tau homolog are described. [DOI] [PubMed] [Google Scholar]
- Tetraodon Genome Browser http://www.genoscope.cns.fr/externe/tetranew/
- Kalcheva N, Albala J, O'Guin K, Rubino H, Garner C, Shafit-Zagardo B. Genomic structure of human microtubule-associated protein 2 (MAP-2) and characterization of additional MAP-2 isoforms. Proc Natl Acad Sci USA. 1995;92:10894–10898. doi: 10.1073/pnas.92.24.10894. The human MAP2 gene is sequenced and analyzed and additional splice forms are characterized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A. Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol. 1989;9:1389–1396. doi: 10.1128/mcb.9.4.1389. The bovine Tau gene is sequenced and analyzed and additional splice forms are described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Wang D, Cowan NJ. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242:936–939. doi: 10.1126/science.3142041. This paper defined the relationship between MAP2 and Tau and thereby defined the MAP2/Tau family by showing that the two proteins contain related microtubule-binding domains. [DOI] [PubMed] [Google Scholar]
- Ozer RS, Halpain S. Phosphorylation-dependent localization of microtubule-associated protein MAP2c to the actin cytoskeleton. Mol Biol Cell. 2000;11:3573–3587. doi: 10.1091/mbc.11.10.3573. The phosphorylation of MAP2c by PKA and its relevance for MAP2-microtubule and MAP2-F-actin interaction is reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow EM, Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. Purification of a novel kinase and characterization of its role in regulating the microtubule-Tau interaction. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Hoh JH. AFM force measurements on microtubule-associated proteins: the projection domain exerts a long-range repulsive force. FEBS Lett. 2001;505:374–378. doi: 10.1016/S0014-5793(01)02844-7. The authors measured a repulsive, entropic force generated by arrays of bovine brain MAPs (of which 70% was made up of the MAP2a and MAP2b isoforms). [DOI] [PubMed] [Google Scholar]
- Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269:2–12. doi: 10.1046/j.0014-2956.2001.02649.x. A review of the basic biochemical characteristics of natively unfolded proteins, such as the MAP2/Tau proteins. [DOI] [PubMed] [Google Scholar]
- Malmendal A, Halpain S, Chazin WJ. Nascent structure in the kinase anchoring domain of microtubule-associated protein 2. Biochem Biophys Res Commun. 2003;301:136–142. doi: 10.1016/S0006-291X(02)02989-3. Characterization of the structural properties of the PKA-RII-binding domain of MAP2 using limited proteolysis, nuclear magnetic resonance spectroscopy and circular dichroism spectroscopy. [DOI] [PubMed] [Google Scholar]
- Roger B, Al Bassam J, Dehmelt L, Milligan RA, Halpain S. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol. 2004;14:363–371. doi: 10.1016/j.cub.2004.01.058. This key paper demonstrates that binding of MAP2/Tau proteins to F-actin correlates with their ability to induce neurites. It is also the first paper to measure this binding quantitatively. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. The temporal sequence of expression of neuronal markers β-III-tubulin and MAP2 is analyzed with respect to the behavior of migrating neurons and dividing neuronal precursors in the developing brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CC, Brugg B, Matus A. A 70-kilodalton microtubule-associated protein (MAP2c), related to MAP2. J Neurochem. 1988;50:609–615. doi: 10.1111/j.1471-4159.1988.tb02954.x. The cloning and characterization of the small MAP2 isoform MAP2c is reported. [DOI] [PubMed] [Google Scholar]
- Chung WJ, Kindler S, Seidenbecher C, Garner CC. MAP2a, An alternatively spliced variant of microtubule associated protein 2. J Neurochem. 1996;66:1273–1281. doi: 10.1046/j.1471-4159.1996.66031273.x. The cloning and characterization of the alternatively spliced adult MAP2 isoform MAP2a is described. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–26. doi: 10.1016/0896-6273(89)90210-9. Characterization using RNAse protection assays of developmentally regulated isoforms of Tau that differ in the number of microtubule-binding repeats. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron. 1989;2:1389–1397. doi: 10.1016/0896-6273(89)90077-9. Identification of isoforms of Tau and their developmental expression, using northern blots. [DOI] [PubMed] [Google Scholar]
- Mavilia C, Couchie D, Nunez J. Diversity of high-molecular-weight tau proteins in different regions of the nervous system. J Neurochem. 1994;63:2300–2306. doi: 10.1046/j.1471-4159.1994.63062300.x. An analysis of the regional expression of specific high-molecular-weight Tau splice forms. [DOI] [PubMed] [Google Scholar]
- Kotani S, Murofushi H, Maekawa S, Aizawa H, Sakai H. Isolation of rat liver microtubule-associated proteins. Evidence for a family of microtubule-associated proteins with molecular mass of around 200,000 which distribute widely among mammalian cells. J Biol Chem. 1988;263:5385–5389. Cloning of MAP4 and analysis of its tissue expression. [PubMed] [Google Scholar]
- Parysek LM, del Cerro M, Olmsted JB. Microtubule-associated protein 4 antibody: a new marker for astroglia and oligodendroglia. Neuroscience. 1985;15:869–875. doi: 10.1016/0306-4522(85)90084-3. The expression of MAP4 in the murine brain is analyzed. [DOI] [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins and the determination of neuronal form. J Physiol (Paris) 1990;84:134–137. A review of the subcellular localization and expression patterns of MAP2/Tau family proteins. [PubMed] [Google Scholar]
- Hirokawa N, Funakoshi T, Sato-Harada R, Kanai Y. Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J Cell Biol. 1996;132:667–679. doi: 10.1083/jcb.132.4.667. The stability of Tau and MAP2c in axons and dendrites was measured by injection of biotinylated exogenous proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Hirokawa N. Sorting mechanisms of tau and MAP2 in neurons: suppressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron. 1995;14:421–432. doi: 10.1016/0896-6273(95)90298-8. This study uses mutational analysis to examine the differential sorting of MAP2 and Tau into axons or dendrites. [DOI] [PubMed] [Google Scholar]
- Garner CC, Tucker RP, Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988;336:674–677. doi: 10.1038/336674a0. The localization of MAP2 mRNA to dendrites is reported. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Shiomura Y, Okabe S. Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol. 1988;107:1449–1459. doi: 10.1083/jcb.107.4.1449. An ultrastructural analysis of Tau binding to microtubules is reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Hisanaga S-I, Shiomura Y. MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies. J Neurosci. 1988;8:2769–2779. doi: 10.1523/JNEUROSCI.08-08-02769.1988. The structure of MAP2 in microtubule arrays is characterized using electron microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol. 2002;157:1187–1196. doi: 10.1083/jcb.200201048. This paper is the first direct visualization of the structure of MAP2 and Tau on microtubules at 20 Å resolution using cryo-electron microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner H, Frank R, Biernat J, Mandelkow EM, Mandelkow E, Ludin B, Matus A, Schliwa M. Domains of neuronal microtubule-associated proteins and flexural rigidity of microtubules. J Cell Biol. 1997;138:1067–1075. doi: 10.1083/jcb.138.5.1067. An analysis of the molecular basis of MAP2/Tau-induced flexural rigidity of microtubules using optical tweezers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B, Doll T, Matus A. Reorganisation of the microtubular cytoskeleton by embryonic microtubule-associated protein 2 (MAP2c). Development. 1992;116:1151–1161. doi: 10.1242/dev.116.4.1151. The organization of microtubules in non-neuronal cells exogenously expressing MAP2c. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Ivanov IE, Lee GH, Cowan NJ. Organization of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989;342:498–505. doi: 10.1038/342498a0. This paper describes microtubule bundle formation in transfected cells induced by MAP2 and Tau, and a potential mechanism is proposed. See also [35]. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Cowan N. Microtubule bundling. Nature. 1990;345:674. doi: 10.1038/345293a0. A letter providing additional data leading to a reinterpretation of the proposal in [34]. [DOI] [PubMed] [Google Scholar]
- Burgin KE, Ludin B, Ferralli J, Matus A. Bundling of microtubules in transfected cells does not involve an autonomous dimerization site on the MAP2 molecule. Mol Biol Cell. 1994;5:511–517. doi: 10.1091/mbc.5.5.511. Given the lack of a high-affinity dimerization site on MAP2c, this article proposes that microtubule stabilization by itself, through the physical restraint of the cell borders, is responsible for microtubule bundling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R, Okabe S, Umeyama T, Hirokawa N. Polarity orientation and assembly process of microtubule bundles in nocodazole-treated, MAP2c-transfected COS cells. Mol Biol Cell. 1995;6:981–996. doi: 10.1091/mbc.6.8.981. MAP2c-induced microtubule bundle assembly is analyzed by live-cell microscopy and the polarity of the resulting bundles is determined by electron microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23:9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003. Cytoskeletal rearrangements during spontaneous and MAP2c-induced neurite initiation are characterized using live-cell microscopy and MAP2 deletion analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. Generation and characterization of a Tau knockout mouse, which has defects in axon ultrastructure. [DOI] [PubMed] [Google Scholar]
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. MAP2 knockout mice show defects in dendrite outgrowth and targeting of the RII subunit of PKA to dendrites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTella MC, Feiguin F, Carri N, Kosik KS, Caceres A. MAP-1B/TAU functional redundancy during laminin-enhanced axonal growth. J Cell Sci. 1996;109:467–477. doi: 10.1242/jcs.109.2.467. The results of inhibition of MAP1B and Tau expression by antisense oligonucleotides suggests functional redundancy of the two proteins. [DOI] [PubMed] [Google Scholar]
- Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. This paper reports the crossing of MAP1B and Tau knockout animals; anatomical analysis shows defects in axon outgrowth and neuronal migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J, Takei Y, Harada A, Nakata T, Chen J, Hirokawa N. Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. J Cell Biol. 2001;155:65–76. doi: 10.1083/jcb.200106025. The first MAP2 knockout mouse is described. Crossing of MAP1B and MAP2 knockout animals leads to defects in dendrite outgrowth and neuronal migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar RA, Dingus J, Bayley H, Vallee RB. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron. 1989;3:639–645. doi: 10.1016/0896-6273(89)90274-2. Mapping of the PKA-RII-binding domain on MAP2 is reported. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. This review gives a general overview of tauopathies, diseases thought to be linked to alterations in Tau behavior. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Berry RW, Binder LI. Modeling tau polymerization in vitro: a review and synthesis. Biochemistry. 2003;42:15009–15017. doi: 10.1021/bi035722s. A review of biochemical analyses of Tau polymerization and its relevance for tauopathies. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Tau phosphorylation, tangles, and neurodegeneration: the chicken or the egg? Neuron. 2003;40:457–460. doi: 10.1016/S0896-6273(03)00681-0. A review of the role of Tau phosphorylation in neurodegenerative diseases. [DOI] [PubMed] [Google Scholar]
- Goedert M, Ghetti B, Spillantini MG. Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Their relevance for understanding the neurogenerative process. Ann NY Acad Sci. 2000;920:74–83. doi: 10.1111/j.1749-6632.2000.tb06907.x. The role of Tau mutations in the specific tauopathy FTDP-17 is reviewed. [DOI] [PubMed] [Google Scholar]
- Chapin SJ, Bulinski JC. Non-neuronal 210 × 10(3) Mr microtubule-associated protein (MAP4) contains a domain homologous to the microtubule-binding domains of neuronal MAP2 and tau. J Cell Sci. 1991;98:27–36. doi: 10.1242/jcs.98.1.27. This paper reports the cloning of MAP4 and comparison of its sequence with MAP2 and Tau. [DOI] [PubMed] [Google Scholar]
- Nguyen HL, Chari S, Gruber D, Lue CM, Chapin SJ, Bulinski JC. Overexpression of full- or partial-length MAP4 stabilizes microtubules and alters cell growth. J Cell Sci. 1997;110:281–294. doi: 10.1242/jcs.110.2.281. Stabilization of cellular microtubules by MAP4 is reported. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, Hotani H, Okumura E, Tachibana K, Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. This study reports an interaction of MAP4 with cyclin B and discusses its potential functional relevance for regulation of microtubules during mitosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Peloquin JG, Zhai Y, Bulinski JC, Borisy GG. Removal of MAP4 from microtubules in vivo produces no observable phenotype at the cellular level. J Cell Biol. 1996;132:345–357. doi: 10.1083/jcb.132.3.345. In cultured cells, MAP4 was blocked using a function-blocking antibody. No phenotype was detected, suggesting that MAP4 is a component of a functionally redundant system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan ME, Olmsted JB. A muscle-specific variant of microtubule-associated protein 4 (MAP4) is required in myogene-sis. Development. 1996;122:771–781. doi: 10.1242/dev.122.3.771. Defects in myogenesis in a muscle cell line lacking the muscle-specific MAP4 isoform were found. [DOI] [PubMed] [Google Scholar]
- Papasozomenos SC, Binder LI. Microtubule-associated protein 2 (MAP2) is present in astrocytes of the optic nerve but absent from astrocytes of the optic tract. J Neurosci. 1986;6:1748–1756. doi: 10.1523/JNEUROSCI.06-06-01748.1986. A report of the expression of MAP2 in specific astrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouyiouklis DA, Brophy PJ. Microtubule-associated proteins in developing oligodendrocytes: transient expression of a MAP2c isoform in oligodendrocyte precursors. J Neurosci Res. 1995;42:803–817. doi: 10.1002/jnr.490420609. The expression of the early neuronal MAP2 isoform MAP2c is analyzed during oligodendrocyte differentiation. [DOI] [PubMed] [Google Scholar]
- Loveland KL, Herszfeld D, Chu B, Rames E, Christy E, Briggs LJ, Shakri R, de Kretser DM, Jans DA. Novel low molecular weight microtubule-associated protein-2 isoforms contain a functional nuclear localization sequence. J Biol Chem. 1999;274:19261–19268. doi: 10.1074/jbc.274.27.19261. The discovery of nuclear MAP2 isoforms containing an alternatively spliced nuclear localization sequence. [DOI] [PubMed] [Google Scholar]
- LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci USA. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. Expression of Tau in oligodendrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R, Heinrich M, Heck S, Blohm D, Richter-Landsberg C. Expression of microtubule-associated proteins MAP2 and tau in cultured rat brain oligodendrocytes. Cell Tissue Res. 1997;288:239–249. doi: 10.1007/s004410050809. Expression of both Tau and MAP2 was analyzed in oligodendrocytes and compared to neurons. [DOI] [PubMed] [Google Scholar]
- Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J. Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. J Neurosci. 2002;22:698–707. doi: 10.1523/JNEUROSCI.22-03-00698.2002. The role of an interaction between Fyn and Tau is analyzed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Nachmanoff K, Halpain S, Williams RCJ. Recombinant microtubule-associated protein 2c reduces the dynamic instability of individual microtubules. Biochemistry. 1996;35:12576–12586. doi: 10.1021/bi961135d. A study of the effect of purified, recombinant MAP2c on microtubule dynamics in vitro. [DOI] [PubMed] [Google Scholar]
- Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc Natl Acad Sci USA. 2003;100:9548–9553. doi: 10.1073/pnas.1633508100. The effects of different Tau isoforms on microtubule dynamics are reported and the relevance for neurodegenerative diseases is discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K, Kitazawa H, Iguchi Y, Hotani H, Itoh TJ. Visualization of the stop of microtubule depolymerization that occurs at the high-density region of microtubule-associated protein 2 (MAP2). J Mol Biol. 2001;312:107–118. doi: 10.1006/jmbi.2001.4934. An analysis of the clustering of MAP2 on microtubules and its relevance for microtubule dynamics. [DOI] [PubMed] [Google Scholar]
- Kaech S, Ludin B, Matus A. Cytoskeletal plasticity in cells expressing neuronal microtubule-associated proteins. Neuron. 1996;17:1189–1199. doi: 10.1016/S0896-6273(00)80249-4. The short- and long-term dynamics of microtubules in the presence of MAP2 or Tau are characterized. [DOI] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. Characterization of the role of MAP2 and Tau projection domains in microtubule spacing in axons and dendrites. [DOI] [PubMed] [Google Scholar]
- Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003;22:70–77. doi: 10.1093/emboj/cdg001. A cryo-EM study that reports the binding of Tau to the inner surface of microtubules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides V, Massie MR, Feinstein SC, Lew J. Evidence for two distinct binding sites for tau on microtubules. Proc Natl Acad Sci USA. 2004;101:6746–6751. doi: 10.1073/pnas.0400992101. Tau binding to preassembled microtubules is compared to Tau binding after co-assembly with microtubules. The results suggest that Tau can bind microtubules in two distinct ways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci. 1999;112:2355–2367. doi: 10.1242/jcs.112.14.2355. The effect of Tau on dynein- and kinesin-dependent cellular transport processes is reported. [DOI] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. The effect of Tau overexpression on kinesin-dependent transport processes is reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. Competition between motor molecules (kinesin and cytoplasmic dynein) and fibrous microtubule-associated proteins in binding to microtubules. J Biol Chem. 1994;269:3581–3589. A biochemical analysis of competition between MAPs and microtubule motors. [PubMed] [Google Scholar]
- Seitz A, Kojima H, Oiwa K, Mandelkow EM, Song YH, Mandelkow E. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 2002;21:4896–4905. doi: 10.1093/emboj/cdf503. Single-molecule analysis of kinesin movements on microtubules and the influence of Tau on movement parameters are measured. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LA, Sheetz MP. Steric inhibition of cytoplasmic dynein and kinesin motility by MAP2. Cell Motil Cytoskeleton. 1993;24:1–16. doi: 10.1002/cm.970240102. The effect of MAP2 and Tau on dynein and kinesin activity is measured using microtubule sliding assays. [DOI] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4:521–533. doi: 10.1016/S1534-5807(03)00094-7. This report shows a reduced association of MAP2 with microtubules in Jnk1 knockout mice. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Lim JH, Halpain S, Brunjes PC. Experience-dependent modifications in MAP2 phosphorylation in rat olfactory bulb. J Neurosci. 1997;17:9596–9604. doi: 10.1523/JNEUROSCI.17-24-09596.1997. A report of activity-dependent phosphorylation of a specific site on MAP2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L, Spittle C. Regulation of microtubule-associated proteins. Int Rev Cytol. 2001;210:163–226. doi: 10.1016/s0074-7696(01)10006-9. This substantial review summarizes the activity and regulation of animal cell MAPs, including Tau and MAP2. [DOI] [PubMed] [Google Scholar]
- LocusLink http://www.ncbi.nlm.nih.gov/
- Felsenstein J. PHYLIP: Phylogenetic Inference Package. 3.6a. Seattle: Department of Genetics, University of Washington; 2002. [Google Scholar]
- Kim H, Binder LI, Rosenbaum JL. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979;80:266–276. doi: 10.1083/jcb.80.2.266. A highly enriched MAP2 fraction was prepared from calf neurotubules and a MAP2-microtubule interaction and microtubule stabilization were found. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RWL, Halpain S. Regulated association of microtubule-associated protein 2 (MAP2) with Src and Grb2: evidence for MAP2 as a scaffolding protein. J Biol Chem. 2000;275:20578–20587. doi: 10.1074/jbc.M001887200. A report of the interaction of MAP2 with Src and Grb2 and regulation of this interaction by Erk2. [DOI] [PubMed] [Google Scholar]
- Zamora-Leon SP, Lee G, Davies P, Shafit-Zagardo B. Binding of Fyn to MAP-2c through an SH3 binding domain. Regulation of the interaction by ERK2. J Biol Chem. 2001;276:39950–39958. doi: 10.1074/jbc.M107807200. A report of the interaction of Fyn with MAP2c and the regulation of this interaction by Erk2. [DOI] [PubMed] [Google Scholar]
- Leterrier JF, Liem RK, Shelanski ML. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982;95:982–986. doi: 10.1083/jcb.95.3.982. An interaction of MAP2 with neurofilaments is reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Dong F, Rubin CS, Hell JW. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J Biol Chem. 1999;274:30280–30287. doi: 10.1074/jbc.274.42.30280. This paper describes a role for MAP2 as an AKAP (A-kinase anchoring protein) for class C L-type calcium channels. [DOI] [PubMed] [Google Scholar]
- Rehbein M, Kindler S, Horke S, Richter D. Two trans-acting rat-brain proteins, MARTA1 and MARTA2, interact specifically with the dendritic targeting element in MAP2 mRNAs. Brain Res Mol Brain Res. 2000;79:192–201. doi: 10.1016/S0169-328X(00)00114-5. Two proteins were cloned that interact specifically with MAP2 mRNA elements responsible for dendritic targeting. [DOI] [PubMed] [Google Scholar]
- Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;115:717–730. doi: 10.1083/jcb.115.3.717. Mapping of the microtubule binding site of Tau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Newman T, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111:3167–3177. doi: 10.1242/jcs.111.21.3167. The interaction between Fyn and Tau is reported. [DOI] [PubMed] [Google Scholar]
- Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S, Wolozin B. Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proc Natl Acad Sci USA. 1998;95:9637–9641. doi: 10.1073/pnas.95.16.9637. A report of the interaction of Presenilin 1 with GSK3-beta and Tau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:11183–11186. doi: 10.1073/pnas.91.23.11183. A report of the interaction between ApoE and Tau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudier J, Mochly-Rosen D, Newton A, Lee SH, Koshland DE, Jr, Cole RD. Comparison of S100b protein with calmodulin: interactions with melittin and microtubule-associated tau proteins and inhibition of phosphorylation of tau proteins by protein kinase C. Biochemistry. 1987;26:2886–2893. doi: 10.1021/bi00384a033. The interaction between S100b and Tau is reported. [DOI] [PubMed] [Google Scholar]


