Introductory Paragraph
The international TEsticular CAncer Consortium (TECAC) combined five published genome-wide association studies of testicular germ cell tumors (TGCT; 3,558 cases and 13,970 controls) to identify novel susceptibility loci. We conducted a fixed effects meta-analysis, including the first analysis of the X chromosome. Eight new loci mapping to 2q14.2, 3q26.2, 4q35.2, 7q36.3, 10q26.13, 15q21.3, 15q22.31, and Xq28 achieved genome-wide significance (P < 5×10−8). Most loci harbor biologically plausible candidate genes. We refined previously reported associations at 9p24.3 and 19p12 by identifying one and three additional independent SNPs, respectively. In aggregate, the 39 independent markers identified to date explain 37% of father-to-son risk, 8% of which can be attributed to the 12 new signals reported here. Our findings substantially increase the number of known TGCT susceptibility alleles, move the field closer to a comprehensive understanding of the underlying genetic architecture of TGCT, and provide further clues into the etiology of TGCT.
In Europe and the United States, testicular germ cell tumors (TGCT) are the most common cancers in young men aged 20 to 39 years1. The incidence of TGCT is rising, and is highest in men of Northern European and lowest in men of African ancestry1–3. Risk factors for TGCT include cryptorchidism, adult height, prior diagnosis and familial history of TGCT4–8; its heritability ranges from 37% to 49%9,10. Despite the multiple lines of evidence demonstrating a considerable genetic component of TGCT risk, linkage and candidate gene approaches to find rare, highly-penetrant susceptibility genes involved in TGCT etiology were unsuccessful11.
In contrast, genome wide association studies (GWAS) of TGCT have had remarkable success in identifying susceptibility loci with strong effects. Of the 27 replicated loci, most were discovered using GWAS chip-based microarray platforms12–18, with 13 identified after replication on the iCOGs array19–21 and one identified as a candidate region22. The genes mapping at or near identified susceptibility loci have revealed several biological themes that are highly likely to be important to TGCT development, including male germ cell maturation and differentiation, KIT-MAPK signaling, DNA damage response, and chromosomal segregation.
We imputed each of five published TGCT GWAS scans12,18,20,23, and combined the association test statistics for a total of 8,960,654 autosomal and 249,696 chromosome X single nucleotide polymorphisms (SNPs), after excluding those with INFO score < 0.3 or minor allele frequency (MAF) < 0.01. We conducted a fixed-effects meta-analysis for 3,558 cases and 13,970 controls (Methods and Supplementary Table 1). The genomic control factor λ = 1.037 suggests little systematic inflation from population stratification (Supplementary Fig. 1). We identified eight new TGCT susceptibility loci surpassing genome-wide significance, and an additional four novel independent loci in two previously established regions (9p24.3 and 19p12) (Table 1). Two of these loci (rs6837349 and rs12912292) showed evidence of effect measure heterogeneity (I2 > 0.50) across the five sample sets. We also determined the Bayes false discovery probability (BFDP)24 for these 12 loci using a prior probability of 0.0001 and odds ratio of 1.2 (Supplementary Table 2). Two loci, rs61408740 and rs17336718, failed to surpass a BFDP < 0.05, likely because of their low minor allele frequencies (0.023 and 0.053, respectively).
Table 1.
TGCT meta-analysis association results for novel loci and new independent SNPs in established loci
| Cytoband | Gene Neighborhood | SNP | Position | Study | Info | Controls | Cases | Reference allele | Effect allele | Effect allele frequency | OR | CI | P | Phet | I2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Case | |||||||||||||||
| 2q14.2 | TFCP2L1 | rs2713206 | 122007941 | NCI | 0.92 | 1055 | 581 | C | T | 0.15 | 0.20 | 1.45 | (1.18–1.79) | 4.19E-04 | ||
| UK | 0.98 | 4945 | 985 | C | T | 0.15 | 0.17 | 1.15 | (1.00–1.31) | 4.43E-02 | ||||||
| PENN | 0.90 | 918 | 480 | C | T | 0.16 | 0.20 | 1.32 | (1.06–1.64) | 1.25E-02 | ||||||
| Norway/Sweden | 0.93 | 6687 | 1326 | C | T | 0.14 | 0.17 | 1.25 | (1.09–1.44) | 1.74E-03 | ||||||
| Denmark | 0.85 | 363 | 183 | C | T | 0.17 | 0.21 | 1.41 | (0.98–2.03) | 6.39E-02 | ||||||
| Combined | 13968 | 3555 | 1.26 | (1.16–1.36) | 1.68E-08 | 0.39 | 3.6 | |||||||||
| 3q26.2 | GPR160 | rs3755605 | 169756119 | NCI | 0.97 | 1055 | 581 | C | T | 0.41 | 0.44 | 1.14 | (0.98–1.33) | 9.31E-02 | ||
| UK | 0.98 | 4946 | 985 | C | T | 0.39 | 0.43 | 1.21 | (1.09–1.33) | 2.25E-04 | ||||||
| PENN | 0.97 | 918 | 480 | C | T | 0.41 | 0.43 | 1.08 | (0.92–1.27) | 3.54E-01 | ||||||
| Norway/Sweden | 0.98 | 6687 | 1326 | C | T | 0.40 | 0.44 | 1.24 | (1.12–1.37) | 2.08E-05 | ||||||
| Denmark | 0.99 | 363 | 183 | C | T | 0.39 | 0.43 | 1.19 | (0.91–1.55) | 1.94E-01 | ||||||
| Combined | 13969 | 3555 | 1.19 | (1.12–1.26) | 3.87E-09 | 0.67 | 0.0 | |||||||||
| 4q35.2 | ZFP42 | rs6837349 | 188921355 | NCI | 0.99 | 1055 | 581 | G | T | 0.66 | 0.63 | 0.84 | (0.72–0.98) | 2.71E-02 | ||
| UK | 0.99 | 4945 | 985 | G | T | 0.66 | 0.62 | 0.83 | (0.75–0.92) | 3.98E-04 | ||||||
| PENN | 0.69 | 918 | 480 | G | T | 0.66 | 0.68 | 1.15 | (0.94–1.40) | 1.80E-01 | ||||||
| Norway/Sweden | 0.99 | 6687 | 1326 | G | T | 0.68 | 0.63 | 0.77 | (0.70–0.86) | 1.18E-06 | ||||||
| Denmark | 0.74 | 363 | 183 | G | T | 0.66 | 0.65 | 0.96 | (0.70–1.32) | 8.00E-01 | ||||||
| Combined | 13968 | 3555 | 0.84 | (0.79–0.89) | 3.13E-08 | 0.02 | 67.4 | |||||||||
| 7q36.3 | NCAPG2 | rs11769858 | 158501492 | NCI | 0.93 | 1055 | 581 | T | C | 0.67 | 0.65 | 0.89 | (0.76–1.06) | 1.86E-01 | ||
| UK | 0.95 | 4945 | 985 | T | C | 0.69 | 0.65 | 0.84 | (0.76–0.94) | 1.51E-03 | ||||||
| PENN | 0.88 | 918 | 480 | T | C | 0.64 | 0.60 | 0.81 | (0.68–0.96) | 1.69E-02 | ||||||
| Norway/Sweden | 0.91 | 6687 | 1326 | T | C | 0.70 | 0.66 | 0.82 | (0.73–0.91) | 3.89E-04 | ||||||
| Denmark | 0.90 | 363 | 183 | T | C | 0.68 | 0.64 | 0.82 | (0.62–1.08) | 1.59E-01 | ||||||
| Combined | 13968 | 3555 | 0.84 | (0.79–0.89) | 2.38E-08 | 0.92 | 0.0 | |||||||||
| 9p24.3* | DMRT1 | rs55873183 | 878563 | NCI | 0.73 | 1055 | 581 | A | G | 0.06 | 0.07 | 1.34 | (0.93–1.93) | 1.14E-01 | ||
| UK | 0.82 | 4945 | 985 | A | G | 0.06 | 0.09 | 1.90 | (1.52–2.38) | 1.54E-08 | ||||||
| PENN | 0.73 | 918 | 480 | A | G | 0.06 | 0.09 | 1.97 | (1.36–2.84) | 3.05E-04 | ||||||
| Norway/Sweden | 0.80 | 6687 | 1326 | A | G | 0.07 | 0.11 | 2.08 | (1.70–2.54) | 1.21E-12 | ||||||
| Denmark | 0.81 | 363 | 183 | A | G | 0.07 | 0.11 | 1.84 | (1.11–3.03) | 1.75E-02 | ||||||
| Combined | 13968 | 3555 | 1.89 | (1.67–2.14) | 2.18E-23 | 0.36 | 8.1 | |||||||||
| 10q26.13 | LHPP | rs61408740 | 126274612 | NCI | 0.99 | 1056 | 582 | C | G | 0.02 | 0.04 | 1.68 | (1.09–2.60) | 1.89E-02 | ||
| UK | 0.95 | 4945 | 985 | C | G | 0.03 | 0.04 | 1.64 | (1.22–2.20) | 1.05E-03 | ||||||
| PENN | 0.94 | 919 | 480 | C | G | 0.03 | 0.04 | 1.92 | (1.22–3.03) | 4.92E-03 | ||||||
| Norway/Sweden | 1.00 | 6687 | 1326 | C | G | 0.02 | 0.03 | 1.53 | (1.12–2.09) | 7.79E-03 | ||||||
| Denmark | 0.96 | 363 | 183 | C | G | 0.02 | 0.03 | 1.61 | (0.69–3.76) | 2.75E-01 | ||||||
| Combined | 13970 | 3556 | 1.65 | (1.38–1.96) | 1.75E-08 | 0.95 | 0.0 | |||||||||
| 15q21.3 | PRTG | rs12912292 | 56038707 | NCI | 0.95 | 1055 | 581 | G | A | 0.51 | 0.55 | 1.25 | (1.07–1.46) | 4.76E-03 | ||
| UK | 0.99 | 4946 | 985 | G | A | 0.53 | 0.55 | 1.09 | (0.99–1.20) | 9.18E-02 | ||||||
| PENN | 0.95 | 918 | 480 | G | A | 0.47 | 0.56 | 1.44 | (1.23–1.70) | 8.74E-06 | ||||||
| Norway/Sweden | 0.95 | 6687 | 1326 | G | A | 0.52 | 0.58 | 1.26 | (1.14–1.39) | 3.42E-06 | ||||||
| Denmark | 0.95 | 363 | 183 | G | A | 0.51 | 0.57 | 1.30 | (1.00–1.69) | 4.81E-02 | ||||||
| Combined | 13969 | 3555 | 1.22 | (1.15–1.29) | 1.38E-11 | 0.03 | 61.4 | |||||||||
| 15q22.31 | MAP2K1, TIPIN | rs60180747 | 66663261 | NCI | 0.99 | 1055 | 582 | A | C | 0.26 | 0.30 | 1.27 | (1.07–1.50) | 5.59E-03 | ||
| UK | 0.99 | 4946 | 986 | A | C | 0.26 | 0.30 | 1.23 | (1.10–1.37) | 2.34E-04 | ||||||
| PENN | 0.99 | 919 | 481 | A | C | 0.27 | 0.34 | 1.37 | (1.15–1.63) | 4.53E-04 | ||||||
| Norway/Sweden | 1.00 | 6687 | 1326 | A | C | 0.26 | 0.28 | 1.18 | (1.05–1.31) | 3.89E-03 | ||||||
| Denmark | 1.00 | 363 | 183 | A | C | 0.27 | 0.31 | 1.22 | (0.93–1.60) | 1.59E-01 | ||||||
| Combined | 13970 | 3558 | 1.23 | (1.16–1.32) | 1.10E-10 | 0.70 | 0.0 | |||||||||
| 19p12* | ZNF728 | rs58521262 | 23205184 | NCI | 0.99 | 1056 | 581 | G | A | 0.151 | 0.106 | 0.69 | (0.55–0.85) | 6.64E-04 | ||
| UK | 1.00 | 4946 | 985 | G | A | 0.140 | 0.106 | 0.74 | (0.64–0.86) | 4.83E-05 | ||||||
| PENN | 0.98 | 919 | 481 | G | A | 0.154 | 0.123 | 0.78 | (0.63–0.98) | 2.95E-02 | ||||||
| Norway/Sweden | 0.99 | 6687 | 1326 | G | A | 0.173 | 0.136 | 0.74 | (0.65–0.84) | 4.90E-06 | ||||||
| Denmark | 0.99 | 363 | 183 | G | A | 0.183 | 0.137 | 0.71 | (0.51–1.00) | 5.10E-02 | ||||||
| Combined | 13971 | 3556 | 0.74 | (0.68–0.80) | 4.87E-14 | 0.95 | 0.0 | |||||||||
| 19p12* | ZNF726 | rs34601376 | 24050828 | NCI | 0.84 | 1055 | 581 | A | T | 0.216 | 0.220 | 1.04 | (0.85–1.28) | 6.77E-01 | ||
| UK | 0.92 | 4945 | 985 | A | T | 0.202 | 0.242 | 1.29 | (1.14–1.46) | 5.08E-05 | ||||||
| PENN | 0.83 | 918 | 480 | A | T | 0.191 | 0.229 | 1.32 | (1.07–1.64) | 1.02E-02 | ||||||
| Norway/Sweden | 0.88 | 6687 | 1326 | A | T | 0.195 | 0.238 | 1.39 | (1.22–1.58) | 4.19E-07 | ||||||
| Denmark | 0.69 | 363 | 183 | A | T | 0.146 | 0.168 | 1.32 | (0.85–2.05) | 2.23E-01 | ||||||
| Combined | 13968 | 3555 | 1.29 | (1.20–1.39) | 2.40E-11 | 0.23 | 29.2 | |||||||||
| 19p12* | ZNF257 | rs73019876 | 22267849 | NCI | 0.93 | 1055 | 581 | T | G | 0.45 | 0.42 | 0.89 | (0.76–1.04) | 1.35E-01 | ||
| UK | 0.96 | 4946 | 985 | T | G | 0.45 | 0.40 | 0.83 | (0.75–0.91) | 1.51E-04 | ||||||
| PENN | 0.95 | 918 | 480 | T | G | 0.51 | 0.49 | 0.91 | (0.78–1.07) | 2.59E-01 | ||||||
| Norway/Sweden | 0.95 | 6687 | 1326 | T | G | 0.43 | 0.41 | 0.85 | (0.77–0.94) | 1.22E-03 | ||||||
| Denmark | 0.94 | 363 | 183 | T | G | 0.44 | 0.36 | 0.72 | (0.55–0.93) | 1.25E-02 | ||||||
| Combined | 13969 | 3555 | 0.85 | (0.80–0.90) | 2.04E-08 | 0.54 | 0.0 | |||||||||
| Xq28 | TKTL1 | rs17336718 | 153536119 | NCI | 0.97 | 1056 | 582 | C | T | 0.05 | 0.09 | 1.33 | (1.07–1.64) | 8.85E-03 | ||
| UK | 0.63 | 4945 | 986 | C | T | 0.05 | 0.07 | 1.46 | (1.19–1.80) | 3.71E-04 | ||||||
| PENN | 0.78 | 918 | 480 | C | T | 0.05 | 0.09 | 1.59 | (1.23–2.06) | 4.06E-04 | ||||||
| Denmark | 0.84 | 363 | 183 | C | T | 0.06 | 0.08 | 1.15 | (0.78–1.69) | 4.71E-01 | ||||||
| Combined | 7282 | 2231 | 1.41 | (1.25–1.59) | 3.84E-08 | 0.51 | 0.0 | |||||||||
New independent SNPs in established loci
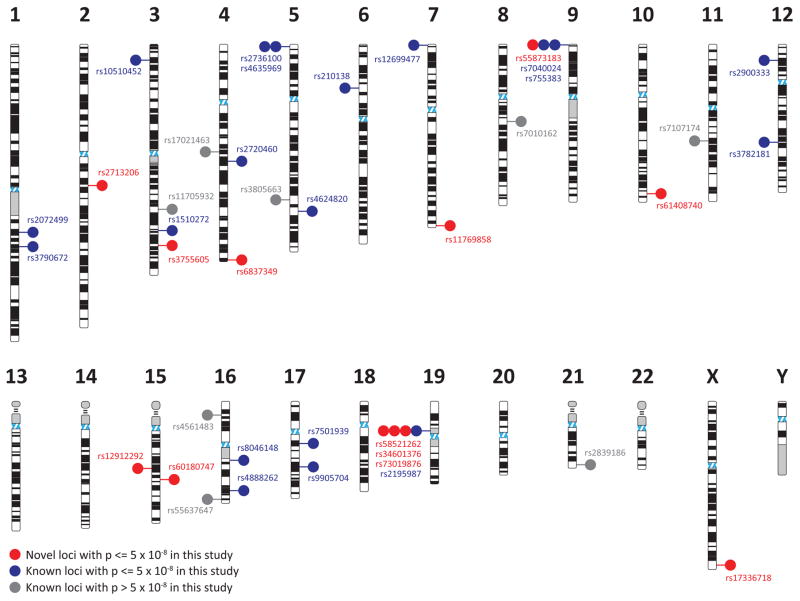
Prior reports identified 27 independent SNPs, at 25 distinct regions, and the gr/gr deletion associated with TGCT susceptibility (Fig. 1). In our current study, 19 of the SNPs (at 17 loci) reached a level of genome-wide significance (Table 2). Eight previously reported susceptibility markers were not identified in our meta-analysis likely related to limited study power, staged replication of GWAS chip-based array results in published studies, and possible residual population substructure. Considering these limitations and to further place context around our findings, we calculated the BFDP for these 27 loci using a prior probability of 0.10, which assumes 10% of the previously established loci are true positives (Supplementary Table 2). This threshold is more liberal than one that would be used to identify novel loci, but still may be too conservative for the re-identification of previously identified susceptibility markers. Only one locus of all 27, rs11705932, failed to surpass a BFDP < 0.05.
Figure 1. All identified SNP markers associated with TGCT susceptibility to date.
In the ideogram, red dots and red rs number annotation indicate SNPs identified and described in the current study (P ≤ 1 × 10−8); blue dots and blue rs number annotation represent previously identified SNP markers achieving genome wide significance (P ≤ 1 × 10−8) in the current study; and gray dots and gray rs number annotation are previously identified SNPs that fail to achieve genome wide significance in this study (P > 1 × 10−8).
Table 2.
TGCT meta-analysis association results for previously published susceptibility loci
| Cytoband | Gene Neighborhood | SNP | Position | OR | CI | P | Phet | I2 |
|---|---|---|---|---|---|---|---|---|
| 1q22 |
KIAA0446 SLC25A44 |
rs2072499 | 156169610 | 1.20 | (1.13–1.27) | 1.63E-09 | 0.78 | 0.0 |
| 1q24.1 | UCK2 | rs3790672 | 165873392 | 1.27 | (1.20–1.35) | 2.15E-14 | 0.84 | 0.0 |
| 3p24.3 | DAZL | rs10510452 | 16625048 | 0.82 | (0.77–0.87) | 3.36E-10 | 0.76 | 0.0 |
| 3q23* |
TFDP2 DKFZp434G222 |
rs11705932 | 141818850 | 0.88 | (0.82–0.94) | 3.51E-04 | 0.90 | 0.0 |
| 3q25.31 | rs1510272 | 156300724 | 0.83 | (0.78–0.88) | 7.15E-09 | 0.45 | 0.0 | |
| 4q22.3* |
SMARCAD1 HPGDS |
rs17021463 | 95224812 | 0.87 | (0.82–0.92) | 1.36E-06 | 0.06 | 56.5 |
| 4q24 |
CENPE CENPE variant protein |
rs2720460 | 104054686 | 0.78 | (0.74–0.83) | 9.88E-17 | 0.92 | 0.0 |
| 5p15.33 |
TERT hTERT |
rs2736100 | 1286516 | 1.29 | (1.22–1.37) | 7.69E-20 | 0.41 | 0.0 |
| 5p15.33 | CLPTM1L | rs4635969 | 1308552 | 1.46 | (1.37–1.57) | 2.83E-27 | 0.15 | 41.0 |
| 5q31.1* |
CATSPER3 PITX1 AK026965 |
rs3805663 | 134366200 | 0.88 | (0.83–0.93) | 9.16E-06 | 0.36 | 8.2 |
| 5q31.3 | SPRY4 | rs4624820 | 141681788 | 1.51 | (1.42–1.59) | 2.59E-46 | 0.51 | 0.0 |
| 6p21.31 |
BAK1 AY383626 C6orf227 |
rs210138 | 33542538 | 1.55 | (1.44–1.66) | 2.51E-34 | 0.66 | 0.0 |
| 7p22.3 | MAD1L1 | rs12699477 | 1968953 | 1.21 | (1.14–1.28) | 2.24E-10 | 0.13 | 43.7 |
| 8q13.3* | PRDM14 | rs7010162 | 70976505 | 0.86 | (0.81–0.91) | 1.42E-07 | 0.33 | 13.5 |
| 9p24.3 | DMRT1 | rs7040024** | 845516 | 0.67 | (0.62–0.71) | 1.21E-32 | 0.04 | 59.3 |
| 9p24.3 | DMRT1 | rs755383** | 863635 | 1.49 | (1.41–1.58) | 6.52E-41 | 0.52 | 0.0 |
| 11q14.1* | GAB2 | rs7107174 | 77997936 | 1.19 | (1.10–1.29) | 6.35E-06 | 0.36 | 8.7 |
| 12p13.1 |
ATF7IP PLBD1 |
rs2900333 | 14653867 | 0.85 | (0.80–0.90) | 2.71E-08 | 0.20 | 33.6 |
| 12q21.32 | KITLG | rs3782181 | 88953561 | 2.02 | (1.88–2.18) | 1.32E-76 | 0.90 | 0.0 |
| 16p13.13* |
BCAR4 CATX-11 RSL1D1 |
rs4561483 | 11920037 | 0.86 | (0.81–0.91) | 4.19E-07 | 0.45 | 0.0 |
| 16q12.1 |
HEATR3 AF086132 |
rs8046148 | 50142944 | 1.24 | (1.15–1.33) | 3.15E-09 | 0.21 | 32.2 |
| 16q23.1 | RFWD3 | rs4888262 | 74670458 | 0.83 | (0.78–0.88) | 5.65E-11 | 0.08 | 52.7 |
| 16q24.2* | ZFPM1 | rs55637647 | 88549264 | 1.18 | (1.11–1.26) | 1.33E-07 | 0.40 | 1.4 |
| 17q12 | HNF1B | rs7501939 | 36101156 | 1.26 | (1.19–1.34) | 1.27E-14 | 0.42 | 0.0 |
| 17q22 | TEX14 | rs9905704 | 56632543 | 1.27 | (1.19–1.35) | 1.99E-14 | 0.68 | 0.0 |
| 19p12 | AK125686 | rs2195987 | 24149545 | 1.23 | (1.15–1.32) | 1.21E-09 | 0.89 | 0.0 |
| 21q22.3* |
MCM3APAS MCM3AP |
rs2839186 | 47690068 | 1.13 | (1.07–1.20) | 2.00E-05 | 0.02 | 67.1 |
Indicates sub genome-wide statistical significance.
Pairwise r2=0.38.
rs12912292 is the most statistically significant novel SNP marker in our study, (OR=1.22; P = 1.38 ×10−11), marking a 131 kb haploblock on 15q21.3 (Table 1 and Supplementary Fig. 2a). This region contains only a single gene, PRTG, a member of the immunoglobulin superfamily implicated in neurogenesis25. PRTG is highly expressed in thyroid, testes and uterus (Supplementary Fig. 3a). No variant in this region is an eQTL in either normal testes or TGCT.
The SNP marker rs60180747 (OR=1.23; P = 1.10 ×10−10) marks a 261 kb haploblock on 15q22.31 that contains several genes, including TIPIN (TIMELESS-interacting protein) MAP2K1 (mitogen-activated protein kinase kinase 1), DIS3L (DIS3 like exosome 3′–5′ exoribonuclease), SNAPC5 (small nuclear RNA activating complex, polypeptide 5), and LCTL (lactose-like) (Table 1 and Supplementary Fig. 2b). Several of these proteins, particularly ZWILCH and TIPIN, have high and somewhat specific expression in testes (Supplementary Fig. 3b–g). TIPIN coordinates the DNA replication checkpoint by interacting with Replication protein A26; ZWILCH is a kinetochore protein important for proper chromatid alignment during cell division27. A missense mutation in ZWILCH, p.Ser230Gly, lies within the LD block (Supplementary Table 3). The LD block also contains a single eQTL to RP11-653J6.1, which is a long non-coding (lnc) RNA highly specific to testes (Supplemental Fig. 3h). RP11-653J6.1 levels and eQTLs were not measured in TGCT Cancer Genome Atlas (TCGA). Further dissection of this signal is needed to pinpoint the candidate gene.
The SNP marker rs3755605 (OR=1.19; P = 3.87 ×10−9) identifies a 213 kb haploblock containing three genes, GPR160 (G protein-coupled receptor 160), PHC3 (polyhomeotic homolog 3), and PRKCI (protein kinase C, iota form) on 3q26.2 (Table 1 and Supplementary Fig. 2c). Several SNPs across this block are eQTLs for GPR160 (Supplementary Table 3, Supplementary Fig. 4a); the risk allele is associated with increased expression in both normal testes and TGCT (Supplementary Fig. 5a and Supplementary Fig. 6a). Several SNPs in the haploblock also are eQTLs in normal testes with RP11-469J4.3, a lncRNA of unknown function. RP11-469J4.3 is highly expressed in normal testes, but lies outside the haploblock (Supplementary Fig. 3l, Supplementary Fig. 4b, and Supplementary Fig. 5b). RP11-469J4.3 expression was not measured in the TCGA.
The SNP marker rs2713206 (OR = 1.26; P = 1.68 ×10−8) lies within a smaller LD region of only 48 kb on 2q14.2. The gene TFCP2L1 (transcription factor CP2-like 1) overlies the entirety of the haploblock (Table 1 and Supplementary Fig. 2d); SNPs in the region are eQTLs (Supplementary Table 3) with the risk allele associated with decreased expression of TFCP2L1 in TGCT (Supplementary Fig. 6b). TFCP2L1 is not expressed in normal adult testes (Supplementary Fig. 3m) but it is highly expressed in fetal gonocytes and in germ cell neoplasia in situ, the precursor of TGCT28,29. TFCP2L1 is upregulated in human primordial germ cells during embryogenesis at the time of epigenetic reprogramming30, but downregulated during transition from fetal gonocytes into spermatogonia29. The SNP marker rs6837349 (OR = 0.84; P = 3.13 ×10−8) localizes to an intron of ZFP42 (zinc finger protein 42) on 4q35.2, and marks a small 11 kb haploblock containing no other genes (Table 1 and Supplementary Fig. 2e). The region has no eQTLs in either normal testes or TGCT, although ZFP42 is expressed exclusively in normal testes (Supplementary Fig. 3n), specifically in human spermatogonia and TGCT29,31. Additionally, both ZFP42 and TFCP2L1 are involved in embryonal stem cell pluripotency30,32.
The SNP marker rs61408740 (OR= 1.65; P = 1.75 ×10−8) localizes to an intron of LHPP (phospholysine phosphohistidine inorganic pyrophosphate phosphatase) on 10q26.13 (Table 1 and Supplementary Fig. 2f). Only two SNPs were identified with pair-wise r2>0.4, one in the region of the second gene, FAM175B (Supplementary Table 3); neither are eQTLs. LHPP encodes an inorganic diphosphatase that functions in oxidative phosphorylation33.
The SNP marker rs11769858 (OR = 0.84; P = 2.38 ×10−8) identifies an 82 kb LD block on 7q36.3 that contains a large portion of NCAPG2 (non-SMC condensin II complex subunit G2) (Table 1 and Supplementary Fig. 2g). NCAPG2 encodes a regulatory subunit of the condensin II complex, which is highly expressed in testes (Supplementary Fig. 3q), and plays a role in chromosome assembly and segregation during mitosis34.
We also identified a locus marked by SNP rs17336718 (OR = 1.41; P = 3.84 ×10−8) on chromosome Xq28 (Table 1 and Supplementary Fig. 2h) in an intron of TKTL1 (transketolase-like 1), which is highly expressed in normal testes. The SNP is an eQTL for TKTL1 in the TGCT TCGA data (Supplementary Figure 6c). TKTL1 converts sedoheptulose to ribose and glyceraldehyde to xylulose, linking the pentose phosphate pathway to the glycolytic pathway. Interestingly, although overexpression of TKTL1 is associated with the Warburg effect and poor prognosis in several cancer types35–38, the risk allele is associated with lower expression.
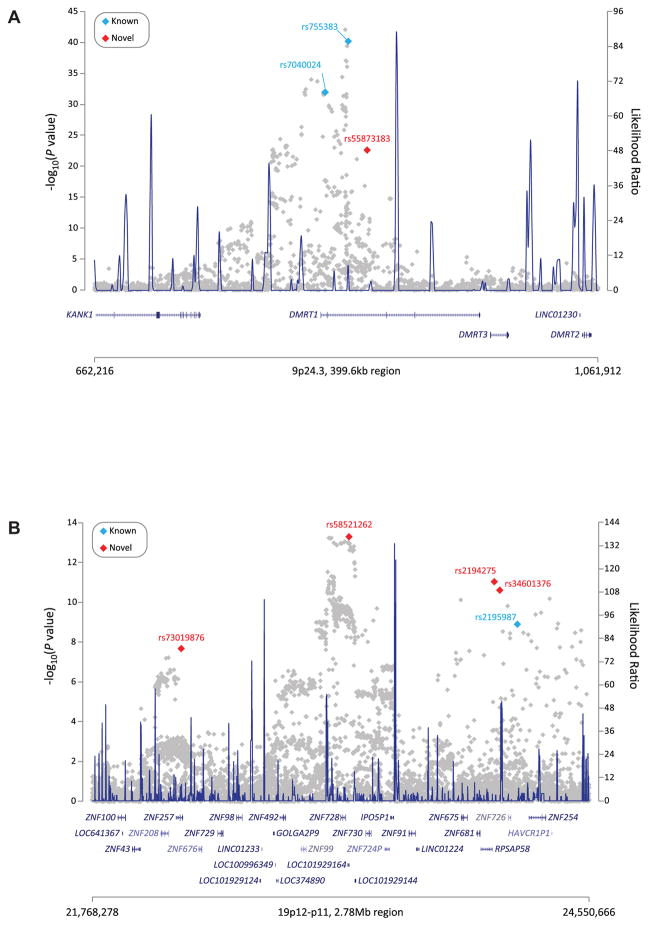
At the previously-reported TGCT susceptibility locus DMRT1 on chromosome 9, we identified a third independent signal, rs55873183 (OR = 1.89; P = 2.18 ×10−23) (Table 1, Fig. 2a, and Supplementary Table 4a). This intronic SNP marker has an r2 of 0.03 and 0.06 with the two previously published SNP markers, rs7040024 and rs75538314,17, respectively; it retained genome-wide significance in conditional analysis (Table 1, Supplementary Table 4b, Supplementary Fig. 2i). We also identified three additional independent signals at 19p12: rs58521262, rs34601376 and rs73019876 (Table 1, Fig. 2b, Supplementary Tables 5a and 5b, and Supplementary Figs. 2j, 2k, 2l). We identified a SNP, rs2194275 (P = 9.23 ×10−12; OR = 0.76), in moderate LD (r2 = 0.7) with the previously published rs2195987 (P = 1.21 ×10−9; OR = 0.81), which was more statistically significant in this meta-analysis (Supplementary Table 5b). 19p12 contains a cluster of Krüppel-associated box zinc finger genes (KRAB-ZFPs)39. KRAB-ZFPs are highly and differentially expressed in germ cells, and important for the epigenetic reprogramming requisite for normal germ cell development30. A number of different GWA studies, including one for telomere length40, have identified significant SNPs in this region. The 19p12 LD blocks are large: rs58521262 marks a 219 kb block, and rs73019876 marks a 184 kb block, each containing over 200 relevant SNPs and several genes. The rs73019876 haploblock is extremely eQTL rich, with ZNF729 and ZNF676 both eQTLs in normal testes (Supplemental Figs. 4c and 4d). rs58521262 is an eQTL with ZNF728 and CTD-2291D10.2, which like ZNF729 (Supplemental Figs. 3t), are only expressed in normal testes (Supplemental Fig. 3v). Associated SNPs in the LD block also include two putative missense mutations in ZNF728 (Supplemental Table 3) not predicted to be deleterious. Given the multiple independent signals in this region, further study will be required to determine which, if any, are causally involved in TGCT.
Figure 2. Genetic association between SNP markers and TGCT risk for regions with multiple independent signals.
The strength of the association signals (−log10 P-values) for individual SNPs at (a) 9p24.3 and (b) 19p12-11 are plotted on the Y-axis relative to their genomic locations (GRCh37) along the X-axis. Red diamonds are the newly identified independent SNPs, blue diamonds are previously reported SNP markers, and all other SNPs are colored gray. The line graph shows likelihood ratio statistics (right Y-axis) for recombination hotspots calculated with SequenceLDhot software using 1000 Genomes Project CEU population data. Gene annotation along the X-axis is based on NCBI RefSeq genes from the UCSC Genome Browser.
Similar to prior reports, several of the loci identified in the current study contain biologically plausible genes implicating pathways involved in male germ cell development and pluripotency (TFCP2L1, ZFP42), kinetochore function (ZWILCH), DNA damage response (TIPIN), and metabolic mitochondrial function (TKTL1 and LHPP). We identified eight such loci in novel genomic regions and four in previously identified regions. These additional loci bring the cumulative total of independent susceptibility alleles for TGCT to 40. Interestingly, racial differences in risk allele frequencies that parallel population-specific TGCT risk also continue to be apparent. Of the 40 identified susceptibility loci, the allele frequencies of all but one differ significantly across continents based on analysis of data from the AFR, AMR, ASN and EUR populations available in the 1000 Genomes project41, with most comparisons having a P-value surpassing strict Bonferroni correction (P < 0.00125) (Supplementary Table 6). The 12 newly identified susceptibility alleles account for 5.3% of the genetic risk to the brothers and 8.0% of risk to the sons of TGCT patients, increasing estimates of heritability to 25% and 37% of the risk to brothers and sons, respectively. The newly identified TGCT susceptibility markers continue to demonstrate moderate effects with ORs that range from 1.17 to 1.89. In comparison with other cancer types, we have accounted for a high proportion of site-specific heritability with fewer loci42–44.
ONLINE METHODS
Studies
Detailed characteristics and genotype quality control metrics of the study populations (Denmark, NCI [STEED, FTCS], Norway/Sweden, Penn, UK) have been previously described12,15,18,19,23. Subjects used in the current study are all of European descent, and data from each study were collected and analyzed in accordance with local ethical permissions and informed consent.
Genotype imputation
Genotype imputation was conducted by each center following a similar protocol. SNPs with a call rate < 95% or Hardy-Weinberg proportion test P-value < 0.000001 or MAF < 1% were removed prior to imputation. Imputation was conducted using IMPUTE2 software version 2.2.2 and version 3 of the 1,000 Genomes Project Phase 1 data as the reference set. First, the genomic coordinates were lifted over from NCBI human genome build 36 to build 37 using the UCSC lift over tool. Second, the strand of the inference data was aligned with the 1,000 Genomes data informed by allele state comparison or allele frequency matching for A/T and G/C SNPs. A pre-phasing strategy with SHAPEIT software version 1 was adopted to improve the imputation performance. The phased haplotypes from SHAPEIT were imported directly into the IMPUTE2 program. We applied sliding windows of 4Mb with 250kb as an overlapping buffer and generated 744 segments for imputing autosomes. For Chromosome X, the pseudoautosomal region 1 (PAR1), PAR2, and the remaining region, which was split into 37 sections, were imputed separately. We excluded imputed loci with INFO score < 0.3 or MAF < 0.01 from further association analysis. Further, we acknowledge the limitations of imputation, including that the accuracy of imputation depends on the linkage disequilibrium between markers in the reference panel and markers to be imputed, and that the quality of imputation across scans differs because of imperfect population matching of data sets to the reference panel.
Statistical analysis
Within each data set, a test for trend was performed for each SNP using SNPTEST software version 2.2 or 2.5. Fixed-effects meta-analysis was used to combine individual within-study association estimates from five imputed GWAS scans. Genetic effect heterogeneity across studies was assessed by using I2 and P-value calculated from the Cochran’s Q statistic. To refine the association signals of each risk region, we first performed LD pruning using pairwise R2 > 0.3 and then conducted the conditional association analyses to estimate the independent effect of each SNP by simultaneously including all specified SNPs from the same region and with their unconditional P-values < 5 × 10−8 into the same logistic regression model.
Heritability analysis
To evaluate the familial risk explained by the new loci identified in our study, we estimated the contribution of each SNP based on the formula h2SNP = β2 × 2f (1–f), where β is the log per allele odds ratio and f is the risk allele frequency45. We calculated the proportion of familial risk explained by dividing the summed contribution of all h2SNP by the total heritability, which was derived from the log relative risk (RR), where RR = 4 for affected father and RR = 8 for affected brothers46.
In silico bioinformatics analysis
We used HaploReg v4.1 and RegulomeDB v1.1 to explore potential non-coding functional annotation within the ENCODE database in the genomic region surrounding our SNPs of interest, with particular attention to annotations in induced pluripotent stem cells (IPSC) and embryonic stem cells (ESC), as we considered these tissue types as best proxies for TGCT. Specifically, we interrogated the linkage disequilibrium (LD) block of neighboring SNPs in a haploblock defined as pair-wise r2 > 0.4 with the index SNP (Supplementary Table 4). We also searched the GTEx v6 database to determine whether the haploblock SNPs were implicated as eQTLs in their sample of 157 normal adult testis tissues with available genotype. Of note, the normal testis contains an abundance of stromal cells (i.e., Sertoli and Leydig cells), so may not be an exact surrogate for germ cells, and in particular the primordial germ cells from which TGCT is believed to develop. Finally, we assessed our 12 novel susceptibility loci for eQTLs among the 128 cases of TGCT with linked genotype data available in The Cancer Genome Atlas.
For the correlation between genotype and expression data from the TGCT TCGA data set, the genotype data was downloaded from the NCI’s Genomic Data Commons (https://gdc.nci.nih.gov/). Data was converted to PLINK v1.0747 format. Subjects were screened for discordant sex, insufficient genotype call rate (>0.05), and excessive heterozygosity (> +/− 3 SD from the mean). SNPs were screened for MAF (>0.01), Hardy-Weinberg equilibrium violations (p<0.0001), and missingness (>0.01). All quality control steps were performed in PLINK. A total of five subjects were removed (all for heterozygosity violations), leaving 145 valid for analysis. 1000 Genomes Phase 341 was used as the reference set. Alignment to the reference set and haplotype estimation was performed using Shapeit v248, and additional SNPs were imputed using Impute249. Imputed SNPs with an info score <0.4 were discarded. For the 12 SNPs of interest, the risk allele was calculated as the allele with increased odds of TGCT (OR>1). For each subject, the zygosity with respect to the risk allele was calculated, and genotypes were tabulated.
All available TCGA TGCT data were retreived from the TCGA Data Coordinating Center and processed through the TCGA pipeline at the TCGA Genome Data Analysis Center at the Institute for Systems Biology. Gene expression matrices were generated for 133 primary tumor samples using available (TCGA Level 3) gene expression values from RNA sequencing, expressed as RSEM values50. Imputed genotypes for all novel SNPs reported in this paper were related to gene expression, for the 128 cases with both genotype and gene expression levels available. Assocations were tested using a linear regression model (using the lm function in R).
Technical validation of imputed SNPs
To technically validate our imputation findings, we optimized TaqMan assays (Applied Biosystems) for 12 loci based on the standard pipeline at the Cancer Genomics Research Laboratory at National Cancer Institute (Supplementary Table 7). For six loci that failed initial TaqMan assay design, LD surrogate SNPs were used. We randomly selected about 1000 samples previously scanned in one of three GWAS (~300 each from NCI, Penn and Norway/Sweden) for TaqMan genotyping. For the imputed probabilistic genotypes, a threshold of 0.80 was applied to derive the discrete genotypes. The average concordance rates are 0.98, 0.97 and 0.93 for NCI, Penn and Norway/Sweden respectively (Supplementary Table 7).
Supplementary Material
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does the mention of trade names, commercial products or organization indicate endorsement by the U.S. Government. The authors thank Ms. Benita Weathers for her coordination of TECAC, Mr. John Pluta for biostatistical assistance, and Mr. Kurt D’Andrea for expert assistance with SNP genotyping. We thank Drs. Douglas R. Stewart and Jennifer T. Loud for critical support of the NCI Clinical Genetics Branch Familial Testicular Cancer Project (NCI 02-C-0178; NCT-00039598). The authors also thank all previous contributors to the GWAS analyzed in this study, including Drs. Nils Weinhold, Daniel Edsgärd, Henrik Leffers, and Profs. Anders Juul, Niels E. Skakkebæk, and Søren Brunak from the Danish study.
The Testicular Cancer Consortium is supported by the National Institutes of Health grant U01CA164947 to KLN, PAK and SMS. A portion of this work was supported by the Intramural Research Program of the National Cancer Institute and by a support services contract HHSN26120130003C with IMS, Inc. The Penn GWAS (Penn) was supported by the Abramson Cancer Center at the University of Pennsylvania and National Institute of Health grant CA114478 to KLN and PAK. The UK testicular cancer study was supported by the Institute of Cancer Research, Cancer Research UK and made use of control data generated by the Wellcome Trust Case Control Consortium (WTCCC) 2. CT is supported by the Movember foundation. KL is supported by a PhD fellowship from Cancer Research UK. LCP is supported by T32-GM008638. The contribution from the University of Leeds was funded by Cancer Research UK. Norwegian/Swedish study was supported by the Norwegian Cancer Society (grants number 418975 – 71081 – PR-2006-0387 and PK01-2007-0375); the Nordic Cancer Union (grant number S-12/07) and the Swedish Cancer Society (grant numbers 2008/708, 2010/808, 2011/484, and CAN2012/823). The Danish GWAS was supported by Villum Kann Rasmussen Foundation, a NABIIT grant from the Danish Strategic Research Council, the Novo Nordisk Foundation, the Danish Cancer Society, and the Danish Childhood Cancer Foundation.
Testicular Cancer Consortium
-
Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland, USA
Katherine McGlynn, Mark Greene, Stephen Chanock
-
Department of Growth and Reproduction, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark
Ewa Rajpert-De Meyts
-
Section of Epidemiology and Biostatistics, Leeds Institute of Cancer and Pathology, University of Leeds, Leeds, UK
D. Timothy Bishop, Jeremie Nsengimana
-
Center of Biological Sequence Analysis, Department of Systems Biology, Technical University of Denmark, Kongens Lyngby, Denmark
Ramneek Gupta
-
Cancer Registry of Norway, Oslo, Norway
Tom Grotmol
-
Faculty of Health Sciences, Oslo and Akershus University College of Applied Sciences, Oslo, Norway
Trine B. Haugen
-
Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
Fredrik Wiklund, Robert Karlsson
-
Division of Genetics and Epidemiology, Institute of Cancer Research, London, UK
Clare Turnbull
-
Department of Medicine, Division of Translational Medicine and Human Genetics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA
Katherine L. Nathanson
-
Fred Hutchinson Cancer Research Center, Seattle, Washington, USA
Stephen M. Schwartz
-
Genomics England, London, UK
Clare Turnbull
-
Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA
Peter A. Kanetsky
-
Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA
Katherine L. Nathanson
-
Department of Medicine, Unit of Andrology and Reproductive Medicine, University of Padova, Padova, Italy
Alberto Ferlin
-
Medical Oncology, University Medical Center Groningen, Groningen, The Netherlands
Jourik A. Gietema
-
Department of Clinical Preventive Medicine, Krek School of Medicine at University of Southern California, Los Angeles, CA
Victoria Cortessis
-
Department of Environmental Health, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA
Russ Hauser
-
Department of Epidemiology, Division of OVP, Cancer Prevention and Population Sciences, The University of Texas MD Anderson Cancer Center, Houston, TX
Michelle Hildebrandt
-
Radboud University Medical Centre, Radhoud Institute for Health Sciences, Nijmegen, Nijmegen, The Netherlands
Lambertus A. Kiemeney
-
Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Davor Lessel, Christian Kubisch
-
deCode Genetics, Reykjavik, Iceland
Thorunn Rafnar
-
Department of Biostatistics and Epidemiology, University of Turin, Turin, Italy
Lorenzo Richiardi
-
Department of Molecular Oncology, Institute for Cancer Research, Oslo University Hospital, Oslo, Norway
Rolf Skotheim
-
Department of Epidemiology, Brown University, Providence, RI
Tongzhang Zheng
Footnotes
Conflict of Interest
There are no conflicts of interest.
Author Contributions
K.L.N. and P.A.K. supervised the overall study. K.A.M., E.R-D.M, D.T.B., M.D.D., M.H.G., R.G., T.G., T.B.H., K.L., K.N., S.M.S., F.W., C.T., P.A.K, and K.L.N. contributed to recruitment, study and data management. Z.W., K.A.M., E.R-D.M, D.T.B., M.D.D., M.H.G., R.G., T.G., T.B.H., R.K., K.L., N.M., K.N., S.V., F.W., C.T., S.J.C., P.A.K, and K.L.N. contributed to genotyping or association analysis of individual studies. Z.W., C.C.C., L.C.P., V.T., S.J.C, P.A.K. and K.L.N. carried out the meta-analysis and the additional bioinformatics analyses, including using GTeX and TCGA TGCT data. Z.W., P.A.K. and K.L.N. drafted the initial manuscript, and all authors reviewed and contributed to the manuscript.
Data Availability
Individual level data from the UK GWAS data has been deposited into European Genome-phenome Archive (EGA) accession number EGAS00001001302. Individual level data has been deposited into dbGAP from University of Pennsylvania (phs001307.v1.p1) and NCI (phs001303.v1.p1). The summary data from the TECAC meta-analysis also has been deposited into dbGAP (phs001349.v1.p1).
URLs
GLU module, http://code.google.com/p/glu-genetics/;
GTEx portal, http://www.gtexportal.org/home/;
HaploReg, http://www.broadinstitute.org/mammals/haploreg/haploreg.php;
IMPUTE2, http://mathgen.stats.ox.ac.uk/impute/impute_v2.html;
RegulomeDB, http://regulome.stanford.edu/;
SHAPEIT, http://www.shapeit.fr/;
SNPTEST, https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html;
The Human Protein Atlas, http://www.proteinatlas.org/;
UCSC lift over tool, http://hgdownload.cse.ucsc.edu/downloads.html
References
- 1.Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology. 2015;3:4–12. doi: 10.1111/andr.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; 2015. [Google Scholar]
- 3.Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International variations and trends in testicular cancer incidence and mortality. Eur Urol. 2014;65:1095–106. doi: 10.1016/j.eururo.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Bromen K, et al. Testicular, other genital, and breast cancers in first-degree relatives of testicular cancer patients and controls. Cancer Epidemiol Biomarkers Prev. 2004;13:1316–24. [PubMed] [Google Scholar]
- 5.Chia VM, et al. Risk of cancer in first- and second-degree relatives of testicular germ cell tumor cases and controls. Int J Cancer. 2009;124:952–7. doi: 10.1002/ijc.23971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimdal K, et al. Risk of cancer in relatives of testicular cancer patients. Br J Cancer. 1996;73:970–3. doi: 10.1038/bjc.1996.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonneveld DJ, et al. Familial testicular cancer in a single-centre population. Eur J Cancer. 1999;35:1368–73. doi: 10.1016/s0959-8049(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 8.McGlynn KA, Trabert B. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol. 2012;9:339–49. doi: 10.1038/nrurol.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litchfield K, et al. Quantifying the heritability of testicular germ cell tumour using both population-based and genomic approaches. Sci Rep. 2015;5:13889. doi: 10.1038/srep13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mucci LA, et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA. 2016;315:68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crockford GP, et al. Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Hum Mol Genet. 2006;15:443–51. doi: 10.1093/hmg/ddi459. [DOI] [PubMed] [Google Scholar]
- 12.Chung CC, et al. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat Genet. 2013;45:680–5. doi: 10.1038/ng.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanetsky PA, et al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat Genet. 2009;41:811–5. doi: 10.1038/ng.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanetsky PA, et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet. 2011;20:3109–17. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher FR, et al. Testicular germ cell tumor susceptibility associated with the UCK2 locus on chromosome 1q23. Hum Mol Genet. 2013;22:2748–53. doi: 10.1093/hmg/ddt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapley EA, et al. A genome-wide association study of testicular germ cell tumor. Nat Genet. 2009;41:807–10. doi: 10.1038/ng.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbull C, et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet. 2010;42:604–7. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristiansen W, et al. Two new loci and gene sets related to sex determination and cancer progression are associated with susceptibility to testicular germ cell tumor. Hum Mol Genet. 2015;24:4138–46. doi: 10.1093/hmg/ddv129. [DOI] [PubMed] [Google Scholar]
- 19.Litchfield K, et al. Identification of four new susceptibility loci for testicular germ cell tumour. Nat Commun. 2015;6:8690. doi: 10.1038/ncomms9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruark E, et al. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat Genet. 2013;45:686–9. doi: 10.1038/ng.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litchfield K, et al. Multi-stage genome-wide association study identifies new susceptibility locus for testicular germ cell tumour on chromosome 3q25. Hum Mol Genet. 2015;24:1169–76. doi: 10.1093/hmg/ddu511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathanson KL, et al. The Y deletion gr/gr and susceptibility to testicular germ cell tumor. Am J Hum Genet. 2005;77:1034–43. doi: 10.1086/498455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalgaard MD, et al. A genome-wide association study of men with symptoms of testicular dysgenesis syndrome and its network biology interpretation. J Med Genet. 2012;49:58–65. doi: 10.1136/jmedgenet-2011-100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakefield J. A Bayesian measure of the probability of false discovery in genetic epidemiology studies. Am J Hum Genet. 2007;81:208–27. doi: 10.1086/519024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong YH, et al. Protogenin defines a transition stage during embryonic neurogenesis and prevents precocious neuronal differentiation. J Neurosci. 2010;30:4428–39. doi: 10.1523/JNEUROSCI.0473-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unsal-Kacmaz K, et al. The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol. 2007;27:3131–42. doi: 10.1128/MCB.02190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams BC, et al. Zwilch, a new component of the ZW10/ROD complex required for kinetochore functions. Mol Biol Cell. 2003;14:1379–91. doi: 10.1091/mbc.E02-09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almstrup K, et al. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 2004;64:4736–43. doi: 10.1158/0008-5472.CAN-04-0679. [DOI] [PubMed] [Google Scholar]
- 29.Sonne SB, et al. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res. 2009;69:5241–50. doi: 10.1158/0008-5472.CAN-08-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang WW, et al. A Unique Gene Regulatory Network Resets the Human Germline Epigenome for Development. Cell. 2015;161:1453–67. doi: 10.1016/j.cell.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristensen DM, et al. Presumed pluripotency markers UTF-1 and REX-1 are expressed in human adult testes and germ cell neoplasms. Hum Reprod. 2008;23:775–82. doi: 10.1093/humrep/den010. [DOI] [PubMed] [Google Scholar]
- 32.Scotland KB, Chen S, Sylvester R, Gudas LJ. Analysis of Rex1 (zfp42) function in embryonic stem cell differentiation. Dev Dyn. 2009;238:1863–77. doi: 10.1002/dvdy.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoi F, Hiraishi H, Izuhara K. Molecular cloning of a cDNA for the human phospholysine phosphohistidine inorganic pyrophosphate phosphatase. J Biochem. 2003;133:607–14. doi: 10.1093/jb/mvg078. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, et al. The condensin component NCAPG2 regulates microtubule-kinetochore attachment through recruitment of Polo-like kinase 1 to kinetochores. Nat Commun. 2014;5:4588. doi: 10.1038/ncomms5588. [DOI] [PubMed] [Google Scholar]
- 35.Schwaab J, et al. Expression of Transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. BMC Cancer. 2011;11:363. doi: 10.1186/1471-2407-11-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahopelto K, Bockelman C, Hagstrom J, Koskensalo S, Haglund C. Transketolase-like protein 1 expression predicts poor prognosis in colorectal cancer. Cancer Biol Ther. 2015:1–6. doi: 10.1080/15384047.2015.1121347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayachandran A, et al. Transketolase-like 1 ectopic expression is associated with DNA hypomethylation and induces the Warburg effect in melanoma cells. BMC Cancer. 2016;16:134. doi: 10.1186/s12885-016-2185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kayser G, et al. Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology. 2011;43:719–24. doi: 10.1097/PAT.0b013e32834c352b. [DOI] [PubMed] [Google Scholar]
- 39.Eichler EE, et al. Complex beta-satellite repeat structures and the expansion of the zinc finger gene cluster in 19p12. Genome Res. 1998;8:791–808. doi: 10.1101/gr.8.8.791. [DOI] [PubMed] [Google Scholar]
- 40.Mangino M, et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet. 2012;21:5385–94. doi: 10.1093/hmg/dds382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auton A, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiao S, et al. Estimating the heritability of colorectal cancer. Hum Mol Genet. 2014;23:3898–905. doi: 10.1093/hmg/ddu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancuso N, et al. The contribution of rare variation to prostate cancer heritability. Nat Genet. 2016;48:30–5. doi: 10.1038/ng.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michailidou K, et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47:373–80. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JH, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42:570–5. doi: 10.1038/ng.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hemminki K, Li X. Familial risk in testicular cancer as a clue to a heritable and environmental etiology. Br J Cancer. 2004;90:1765–1770. doi: 10.1038/sj.bjc.6601714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013;10:5–6. doi: 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- 49.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.