Abstract
The recent call to move from focus on one brain’s functioning to two-brain communication initiated a search for mechanisms that enable two humans to coordinate brain response during social interactions. Here, we utilized the mother–child context as a developmentally salient setting to study two-brain coupling. Mothers and their 9-year-old children were videotaped at home in positive and conflictual interactions. Positive interactions were microcoded for social synchrony and conflicts for overall dialogical style. Following, mother and child underwent magnetoencephalography while observing the positive vignettes. Episodes of behavioral synchrony, compared to non-synchrony, increased gamma-band power in the superior temporal sulcus (STS), hub of social cognition, mirroring and mentalizing. This neural pattern was coupled between mother and child. Brain-to-brain coordination was anchored in behavioral synchrony; only during episodes of behavioral synchrony, but not during non-synchronous moments, mother’s and child's STS gamma power was coupled. Importantly, neural synchrony was not found during observation of unfamiliar mother-child interaction Maternal empathic/dialogical conflict style predicted mothers’ STS activations whereas child withdrawal predicted attenuated STS response in both partners. Results define a novel neural marker for brain-to-brain synchrony, highlight the role of rapid bottom-up oscillatory mechanisms for neural coupling and indicate that behavior-based processes may drive synchrony between two brains during social interactions.
Keywords: social synchrony, magnetoencephalography, MEG, gamma-band activity, superior temporal sulcus, mother–child interaction
Introduction
Social neuroscientists have recently called to move from focus on one-brain functioning to understanding how two brains dynamically coordinate during real-life social interactions (Stanley and Adolphs, 2013; Hasson et al., 2016). Hari et al. (2015) have further emphasized that the brain’s default modus operandi is not solipsistic but situated, evolved to constantly receive information from, respond to, update predictions and monitor actions in accordance with the online communicative signals of social partners. As social neuroscience is moving towards a two-person perspective, there is a growing need to describe mechanisms that underpin two-brain coordination. Yet, a major issue in studying this topic is ecological validity (Gilam and Hendler, 2016). Notwithstanding the variety of novel approaches employed in recent years, such as human–Avatar or human–robot interactions (Wykowska et al., 2016), two individuals lying in separate magnets (Bilek et al., 2015) or magnetoencephalography (MEG) machines (Baess et al., 2012; Hirata et al., 2014), and coordinated brain response to commercial movies (Hasson et al., 2004), it is important to study two-brain coupling in relation to natural social contexts, not under conditions where the entire social envelop is altered. Furthermore, it has been suggested that brain rhythms likely define a central mechanism underpinning the brain’s capacity to coordinate online with another brain and that research should capitalize on natural moments of peak emotional intensity between interacting partners (Hari et al., 2015). These aspects, however, have not yet been integrated into a single study.
‘Social synchrony’ is defined as the coordination of behavior among affiliative members during moments of social contact and has been proposed as a key mechanism supporting two-brain coordination via bottom-up, behavior-based processes (Feldman, 2016, 2017). One approach for assessing two-brain coordination without compromising ecological validity is by studying the perception of social synchrony among affiliative partners. Prior neuroimaging studies demonstrated overlapping neural circuits underpinning the perception and experience of social functions, including emotions (Bastiaansen et al., 2009), pain (Singer et al., 2004) and social action (Mukamel et al., 2010). These findings lend support to the hypothesis that observation of social synchrony may trigger overlapping neural circuits to those activated during participation in real-life social synchrony. Cumulative evidence indicates that the perception of ecologically valid vignettes marked by high degree of social synchrony activates critical nodes of the social brain (Atzil et al., 2011, 2014; Abraham et al., 2014, 2016; Levy et al., 2016). This was found to be especially the case where the observed vignettes involve ‘similar to me’ interactions. For instance, mothers of 4-month-old infants showed greater activations to vignettes depicting social synchrony between mothers and her 4-month-old infants compared to non-synchronous mother–infant interactions (Atzil et al., 2014), and soldiers trained for coordinated action showed greater response to a movie depicting a synchronous unit in combat (Levy et al., 2016). This suggests that familiarity with the perceived social context and the degree of synchrony vs non-synchrony may enhance or diminish brain-to-brain coordination. As real-time hyper-scanning of interacting individuals under ecologically valid conditions is currently methodologically challenging (Hari et al., 2015, 2016), the perception of social synchrony among closely affiliated partners in designs that measure brain response to episodes of social synchrony vs non-synchrony may afford a novel and useful vantage point on brain-to-brain coordination.
The mother–child context provides a prototypical, developmentally salient setting where synchrony is first experienced and encoded in the brain during early sensitive periods (Feldman, 2015a,b). As mammals, our brain develops in the context of the mother–infant ‘nursing dyad’ through processes of biobehavioral synchrony, the coupling of mother’s and child’s physiological and behavioral signals during moments of social contact (Feldman, 2012a, 2016). Animal studies demonstrate that the coordination of mother and offspring’s physiological systems emerges through bottom-up behavioral processes and is based on the expression of maternal behavior (Champagne et al., 2001; Shahrokh et al., 2010). Human research has similarly shown that moments of social synchrony, the coupling of mother’s and child’s behavior in the affect and vocal modalities, induce coupling of physiological processes, such as heart rhythms or hormonal release (Feldman et al., 2010, 2011). Patterns of synchrony are dyad-specific, remain individually stable from infancy to adolescence (Feldman, 2007, 2010) and provide the template for synchrony with non-kin partners in later childhood, such as close friends (Feldman et al., 2013a) and caregivers (Feldman and Klein, 2003). Thus, social synchrony charts a central mechanism by which mothers tune their child’s brain to the social world during the early maturation of the social brain via behavior-based processes (Abraham et al., 2016). Interestingly, throughout human history, social synchrony has been used as a powerful tool to enhance group cohesion via cultural activities, such as group marching, joint harvesting or communal singing (Levy et al., 2016), and is regarded as an evolutionary mechanism that enhances species survival and enables social life (Wilson, 2012). Thus, the central hypothesis guiding this study is that the perception of mother–child social synchrony implicates a behavior-based process, that it rides on social synchrony, and that its neural signature may be best studied by focusing on the stable aspect of the mother–child synchronous relationship, which provides a window into the context where synchrony was initially acquired.
Two additional hypotheses were formulated in relation to the neural processes that may underlie child–mother neural coordination. First, we assumed that the bottom-up nature of mother–child synchrony may implicate bottom-up neural processes. Neural oscillations are a pervasive feature of neuronal activity in the cerebral cortex, reflecting periodic fluctuations of excitability in neural populations generated by transmembrane currents (Donner and Siegel, 2011). Several electroencephalography (EEG) or MEG hyper-scanning studies showed that brain-to-brain coordination—during motor, emotional, speech or musical tasks—is straightforwardly reflected through the synchrony of low-frequency neural oscillations (Dumas et al., 2010; Babiloni et al., 2012; Kawasaki et al., 2013; Müller et al., 2013; Konvalinka et al., 2014; Zhdanov et al., 2015). It thus appears that inter-subject synchrony builds on low-frequency neural oscillations, yet the perception of bottom-up behavioral processes between mother and child (Champagne et al., 2001; Shahrokh et al., 2010) may rather rely on the fast-paced bottom-up gamma rhythm (Fries, 2015).
Second, it was hypothesized that the perception of dyadic synchrony would implicate rhythmic processes in the social brain. The most integrative node of the social brain is the superior temporal sulcus (STS), which combines functions related to social perception, action observation and theory of mind (Yang et al., 2015). Moreover, the STS is a key node of the social brain with overlapping mirror and mentalizing properties and is thus involved in both fast-paced bottom-up sensorimotor coupling, such as biological motion (Jastorff et al., 2012), and slower higher-order socio-cognitive predictions, such as theory-of-mind and intentionality (Dufour et al., 2013). We have previously found that mothers’ STS responds (Blood Oxygenation Level Dependent signal (BOLD) response) to the perception of mother–child synchrony (Atzil et al., 2014), that mothers’ and fathers’ STS activations synchronize (BOLD response) in response to their own child (Atzil et al., 2012), and that salient experiences within social groups enhance STS response (alpha rhythm) to vignettes depicting social synchrony (Levy et al., 2016). Furthermore, gamma oscillations in the STS have been implicated in non-verbal emotional communication (for a recent review, see Symons et al., 2016) and the coupling of STS/STG activity was found to play a key role between pairs of communicators (Stolk et al., 2014, 2016). We thus hypothesized that gamma power in the STS could underpin the perception of mother–child synchrony in both mother’s and child’s brains and that this response would be coupled between mother and child.
To test neural synchrony between mother and child, we videotaped mother–child interactions in the home and used this interactive vignette as MEG stimulus for both mother and child. We used interactions videotaped 2 years prior to scanning in order to tap the stable component of the mother–child relationship in their natural habitat. Mother–child behavioral synchrony has been shown to be individually stable from infancy to adolescence (Feldman, 2007, 2010), and thus, the longitudinal design enabled us to filter out momentary fluctuations in the relationship and highlight its stable aspects, yet, it exposed partners to stimuli that were easily identifiable and were not reminiscent of a distant past. We selected to videotape mothers and children at 8–9 years as by this age children have already developed theory-of-mind abilities (Baird and Baldwin, 2001; Sommerville, 2010) and have undergone the first maturation of mentalizing brain structures (Decety, 2010).
Another question of interest was whether STS activations in mother or child would be predicted by individual differences in the general parameters of the mother–child relationship, including the degree of reciprocity, empathy and social withdrawal during observed interactions. Mother–child reciprocity is a central feature of the parent–child relationship and predicts adolescent adjustment and social competencies (Feldman, 2010; Feldman et al., 2013a), whereas social withdrawal has been linked with increased child psychopathology and compromised sociality (Apter-Levi et al., 2016). We thus expected that these two central parameters of the mother–child relationship would be associated with the degree of neural response to mother–child synchrony.
Materials and methods
Participants
Results for the MEG study mainly report findings on a sample of sixty participants: 26 children; age M ± s.d., 11.67 ± 0.89 (range =9.34–13.41 years) and 34 mothers; age; M ± s.d., 41.58 ± 4.69 (range 35.09–54.72 years). Yet, for the analyses of mother–child neural coupling, nine mothers and one child were not included as their dyadic partner was not MEG-compatible (i.e. wearing metallic tooth bracelets which disturb the brain’s magnetic field), and the synchrony findings are thus reported for 25 mother–child biological pairs (N = 50; 25 mothers and their 25 children). The original sample included 84 participants who participated in the MEG experiment, of which 24 participants were not included in the MEG analysis as they had no sufficient (or none) moments of dyadic synchrony and therefore could not contribute to the present study (for detail, see below ‘Micro-coding of social synchrony’). Home visits were conducted approximately 2 years before the MEG scan when children were between 8 and 9 years old (M ± s.d., 8.87 ± 1.08).
All participants were physically healthy and free of psychopathology, and children showed typical development since birth. All children were reared in two-parent families in which mothers were the primary caregiver since birth. The study received approval from the local ethics committee, and participants gave written informed consent before the experiment in line with the University’s Institutional Review Board. Subjects received monetary compensation for participation.
Procedure
Home visit
Mother and child were videotaped in two well-validated one-on-one interaction paradigms: a ‘positive dialogue’ and a ‘conflict dialogue’ (Schneiderman et al., 2012, 2014; Feldman et al., 2013b, 2014). For the positive dialogue, mother and child were asked to plan a ‘fun day’ to spend together; for the conflict dialogue, they were asked to choose a typical conflict in their daily life and discuss it for 7 min.
Behavioral coding
Coding was conducted offline by coders trained to reliability who were blind to all other information and two types of coding were used—microlevel second-by-second coding and global rating scales.
Micro-coding of social synchrony
As social synchrony increases during positive interactions (Feldman, 2007), we chose to micro-code synchrony during the ‘positive dialogue’. Mother’s and child’s affect, which is a primary channel of non-verbal social communications, was coded using a set of mutually exclusive codes consistent with our prior brain and behavioral research (Feldman and Eidelman, 2007; Atzil et al., 2011). Coding for mother and child was conducted in separate passes using a computerized system (Noldus, Waggenigen, The Netherlands) while the system was set to 0.01 s accuracy. We coded for mother’s and child’s Affect in the following dimensions: positive (high positive arousal/energy indicated by laugh, giggle or positive excitement), neutral (facial expression pleasant, arousal low), negative-withdrawn (sad or flat facial expression, disengagement) and negative-angry (high negative arousal indicated by angry voice, scream, scold, etc.). Reliability was computed on 20 interactions and inter-rater reliability exceeded 90% on all codes (κ = 0.87, range = 0.81–95).
Episodes of ‘synchrony’ were computed as conditional probabilities (i.e. mother in behavior x while child in behavior y) and complied with the following guidelines: (i) described moments of simultaneous positive affect (both mother and child express positive emotions), (ii) episodes were at least 2000 ms long, (iii) segments were rounded at multiples of 500 ms (e.g. a segment of 2224 ms was defined as 2000 ms whereas a segment of 2226 ms was defined as 2500 ms) to result in trial epochs of equal length, (iv) consecutive episodes separated by an interval smaller than 1000 ms were collapsed into a single episode, (v) the first five seconds of the interaction were not coded.
Episodes of ‘non-synchrony’ were matched to the number of episodes of synchrony but included trial epochs during which neither mother nor child expressed positive affect. They complied with the additional following guidelines: (vi) episodes of non-synchrony were nearest in time and preceding the episodes of synchrony and (vii) if however no preceding episodes of non-synchrony were found (until the former episode of synchrony), we selected those that followed the synchrony episodes but only those that occurred after a time-laps of at least 3000 ms from the episode of synchrony (or episode of positive affect for either mother or child).
Global rating of conflict discussion
The Coding Interactive Behavior (CIB) system (Feldman, 1998) was used to code the conflict discussion. The CIB is a well-validated global rating system for coding social interactions that includes 45 codes aggregated into several composites. The CIB has shown construct and predictive validity, test–retest reliability and sensitivity to cultural contexts, interacting partner, and multiple psychopathological conditions (for a review see Feldman, 2012b). Consistent with prior research, we used three CIB constructs; Reciprocity, Empathy and Withdrawal (Feldman et al., 2013b, 2014; Schneiderman et al., 2014; Weisman et al., 2015; Apter-Levi et al., 2016), each computed by averaging several CIB codes. The Reciprocity composite included the following codes: recognition, elaboration, affect synchrony, containment, constancy, warmth and support. The Empathy construct included cognitive, emotional and behavioral empathy. The Withdrawal composite included the following codes: parent’s withdrawal from child and from the interaction, parent’s depressed mood and parent’s avoidance of conflict. Inter-rater intraclass reliability computed on 20 interactions averaged r = 0.93 (range: 0.88–0.99).
MEG recordings and data preprocessing
The MEG experiment included presentation of the ‘positive dialogue’ video which was normalized across participants to a 1.5 min positive dialogue. To control for inter-brain coupling effects that are not driven by synchrony between self and attachment partner (i.e. mother and her own child), we included a control condition of age-matched similar positive dialog video between an unfamiliar mother and her child. The video appeared as 300 × 225 pixels at the center of a gray background on a 20 inch monitor, subtending a visual angle of 20.96° × 15.37° at a viewing distance of 50 cm. Participants lay in supine position inside the MEG system while facing a screen projecting the video. Subjects were asked to remain relaxed and not move their limbs and the experimenter observed their compliance using an infrared camera. We programmed and operated the experiment using E-Prime® software (Psychology Software Tools Incorporated).
We recorded ongoing brain activity (sampling rate, 1017 Hz, online .1–400 Hz band-pass filter) using a whole-head 248-channel magnetometer array (4-D Neuroimaging, Magnes® 3600 WH) inside a magnetically shielded room. Reference coils located approximately 30 cm above the head, oriented by the x, y and z axes enabled removal of environmental noise. We attached five coils to the participant’s scalp to record head position relative to the sensor. We performed analyses using MATLAB 7 (MathWorks®, Natick, MA, USA) and the FieldTrip software toolbox (Oostenveld et al., 2011). We segmented the data into 1000 ms epochs with an overlap of 500 ms between consecutive epochs. Four steps aimed to clean artifacts and noise: (i) We removed external noise (e.g. power-line, mechanical vibrations) and heartbeat artifacts from the data using a predesigned algorithm for that purpose (Tal and Abeles, 2013); (ii) we rejected trials containing muscle artifacts using visual inspection; (iii) we removed eye-blinks, eye movements, or any other potential noisy artifacts using spatial component analysis; and (iv) a final visual inspection of every trial verified any other noise/artifact to be removed from further analysis. We filtered the data in the 1–200 Hz range with 10 s padding and resampled them to 400 Hz.
Spectral analysis and source localization
We applied tapers to each time window to compute spectral power for each trial and to calculate the fast Fourier transform for short sliding time windows. We analyzed data in alignment to stimulus onset and then averaged the power estimates across tapers. Five Slepian multitapers (Percival and Walden, 1993) were applied using a fixed window length of 0.2 s, resulting in a frequency smoothing of 15 Hz in the gamma-frequency band (30–120 Hz). To localize neural sources, we built a single shell brain model for each subject, based on an MNI adult (for mothers) and early-to-advanced-puberty (for children) template brain (Fonov et al., 2011), which we modified to fit each subject’s digitized head shape using SPM8 (Wellcome Department of Imaging Neuroscience, University College London, www.fil.ion.ucl.ac.uk). Head shape underwent manual digitization (Polhemus FASTRAK® digitizer). We then divided the subject’s brain volume into a regular grid, obtaining the grid positions by their linear transformation in a canonical 1 cm grid. This procedure facilitates group analysis, because it requires no spatial interpolation of the volumes on reconstructed activity. For each grid position, we reconstructed spatial filters in the aim of optimally passing activity from the location of interest, while suppressing activity that was not of interest.
We applied adaptive spatial filtering (Gross et al., 2001) relying on partial canonical correlations. We computed the cross-spectral density (CSD) matrix between all MEG sensor pairs from the Fourier transforms of the tapered data epochs. We constructed spatial filters for each grid location, based on the identified frequency bins (50–60 Hz for mothers and 35–45 Hz for children), and projected the Fourier transforms of the tapered data epochs through the spatial filters. We extracted t-values of the synchrony vs no-synchrony contrast for the activation peak (in the right STS) and proceeded with brain-to-brain Pearson r correlation.
To calculate intra-dyadic synchrony, we extracted time-series from the activation peak (right STS) by applying a linear constrained minimum variance beamformer. We then followed two approaches to analyze the data: first, we calculated the inter-brain weighted phase lag index (wPLI) that reflects the phase synchrony of inter-brain activities from two individuals within the dyad. wPLI is an improved index of phase-synchronization for electrophysiological data with the capacity of circumventing sources of noise (e.g. volume-conduction, noise and sample-size bias) that may artificially induce functional connectivity (Vinck et al., 2011). Specifically, wPLI measures the distribution of phase angle differences of two channels, that is, if two the functional coupling between channels is strong, the resulting connectivity index will be high in a given frequency. Phase-coupled activity is an important mechanism in the functional communication between brain regions (Fries, 2005). Similarly, inter-brain phase synchrony can be estimated by examining two virtual channels from two individuals within a dyad, such as STS of mother and STS of child. This would therefore imply a form of communication between the targeted brain areas of mother and child, reflecting intra-dyadic synchrony. Second, we calculated the inter-subject correlation index (Chang et al., 2015) by averaging the epoch-to-epoch gamma-band power and correlating it between mother and child. The resulting index was shown to reflect a shared psychological perspective between individuals (Lahnakoski et al., 2014), and we assumed that under the present experimental setting it could reflect episodes of synchrony between mother and child. Finally, we correlated (Pearson r) brain-to-behavior: mother and child STS activity and social behavior indices (proportion of synchrony episodes and CIB constructs.
Statistical analysis
We assessed statistical significance of the power values using a randomization procedure (Maris and Oostenveld, 2007). This nonparametric permutation approach takes the cross-subject variance into account, as this variance is the basis for the width of the randomization distribution. This approach is valuable because it does not make any assumptions about the underlying distribution and is unaffected by partial dependence between neighboring time–frequency pixels. Specifically, in the first step of the procedure we computed t-values per subject, channel, frequency and time, representing the contrast between the conditions. Subsequently, we defined the test statistic by pooling the t-values over all participants. Here, we searched time–frequency clusters with effects that were significant at the random-effect level after correcting for multiple comparisons along the time and frequency dimensions. Testing the probability of this pooled t-value against the standard normal distribution would correspond to a fixed effect statistic. However, to make statistical inferences corresponding to a random effect statistic, we tested the significance of this group-level statistic by means of a randomization procedure: We randomly multiplied each individual t-value by 1 or by −1 and summed it over participants. Multiplying the individual t-value with 1 or −1 corresponds to permuting the original conditions in that subject.
We reiterated this random procedure 1000 times to obtain the randomization distribution for the group-level statistic. For each randomization, we retained only the maximal and the minimal cluster-level test statistic across all clusters, placing them into two histograms that we addressed as maximum/minimum cluster-level test statistic histograms. We then determined, for each cluster from the observed data, the fraction of the maximum/minimum cluster-level test statistic histogram that was greater/smaller than the cluster-level test statistic from the observed cluster. We retained the smaller of the two fractions and divided it by 1000, giving the multiple comparisons corrected significance thresholds for a two-sided test. The proportion of values in the randomization distribution exceeding the test statistic defines the Monte Carlo significance probability, which is also called a P-value (Nichols and Holmes, 2002; Maris and Oostenveld, 2007). This cluster-based procedure allowed us to obtain a correction for multiple comparisons in all brain analyses.
Results
Behavioral analysis
We analyzed the ‘positive dialog’ video (1.5 min length) corresponding to each participant, spotting discrete episodes of mother–child synchrony (Figure 1B) throughout the video (for more detail, see section ‘Micro-coding of social synchrony’). Twenty-four participants had either no episodes of synchrony or only a single epoch (i.e. 1 s) of synchrony and therefore were not included in the MEG analyses. The 60 participants who exhibited two or more episodes of mother–child synchrony had an average number of M ± s.d., 13.15 ± 10.25 ‘synchrony’ episodes. These ‘synchrony’ trial epochs were matched by an equal number of neighboring ‘non-synchrony’ episodes.
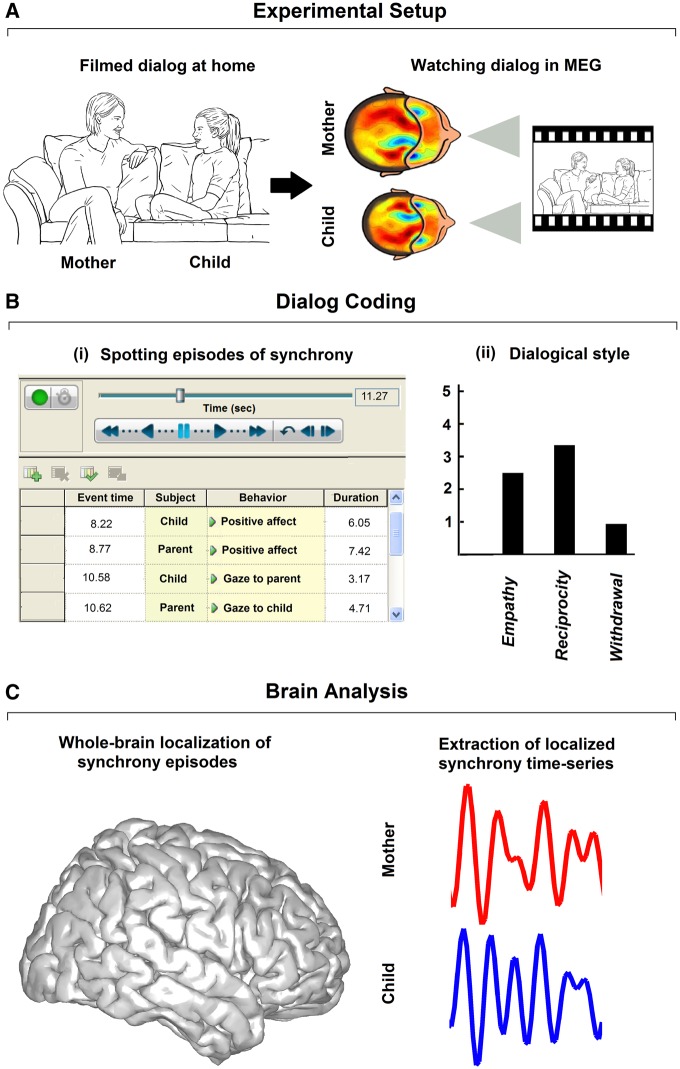
Fig. 1.
Experimental and analytical procedures. (A) Mother–child dyads were videotaped during social interaction (left panel). Two years later mother and child were invited to participate in MEG experiment where they each observed the videotaped interaction (right panel). (B) Micro-coding in second-to-second level (left panel) to define episodes of social synchrony and non-synchrony. Global assessment of dialogical style (right panel). (C) Brain signals were extracted from the localized brain region signaling heightened activity during moments of social synchrony (compared to non-synchrony).
In addition to analyzing the neural data during the perception of one’s ‘own’ dyadic interaction, we also analyzed the neural data during the perception of interaction of an unfamiliar mother–child dyad. This control dyadic interaction probed an identical ‘positive dialog’ video (1.5 min length), but with an exemplar dyad who was unfamiliar to all participants. This was to control for inter-brain synchrony effects that are not driven by own mother–child synchrony. This control interaction had seven synchrony episodes (and seven non-synchrony episodes).
Means (and s.d.) for the three global behavioral constructs during the conflict dialogue, each coded from 1 (low) to 5 (high) were as follows: Empathy: M ± s.d., 3.02 ± 1.00, Reciprocity: M ± s.d., 3.86 ± 0.93, and Withdrawal: M ± s.d., 0.83 ± 0.13 (see illustration on Figure 1A).
Brain-to-brain synchrony during episodes of synchrony vs non-synchrony
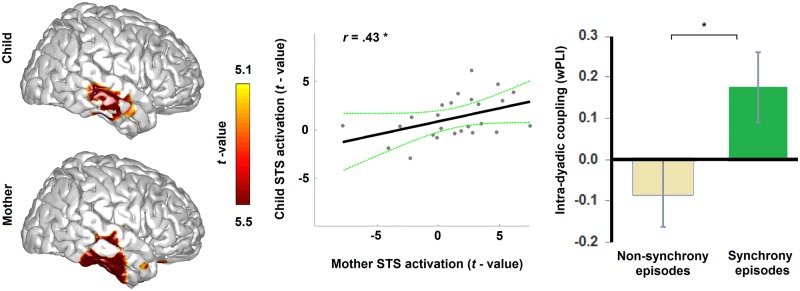
To localize the neural substrates characterizing brain-to-brain synchrony during episodes of synchrony vs non-synchrony, whole MEG sensor-array analysis detected that the neural response to social synchrony was expressed by enhanced gamma-band power, peaking between 50 and 60 Hz for mothers (Pcluster-cor = 0.003) and between 35 and 45 Hz for children (Pcluster-cor = 0.02). This effect in the gamma band was absent when probing synchrony vs non-synchrony while mothers (Pcluster-cor = 0.13) and children (Pcluster-cor = 0.59) were watching unfamiliar mother–child interaction. We then localized this effect and found it to peak in the right STS (Figure 2 left panel) in both mothers (Pcluster-cor = 0.04) and children (Pcluster-cor = 0.05), yet not significantly while watching stranger mother–child dyad in interaction (Pcluster-cor = 0.11 and Pcluster-cor = 0.89, respectively). Importantly, the extent of STS activation was significantly coupled (r = 0.43, P = 0.02) between mothers and their own children (Figure 2 left panel). This suggests that the described neural response—gamma power in the right STS to episodes of social synchrony—is a dyadic phenomenon at the neural level located to the STS.
Fig. 2.
Brain response of mother and child to vignettes probing social synchrony. The brain response of child (left upper panel) and mother (left lower panel) to episodes of own social synchrony was expressed in the gamma-band in the right STS. The overall extent of the STS response was coupled between child and mother (middle panel). The second-by-second STS phase showed significantly greater coupling between child and mother when observing episodes of synchrony compared to episodes of non-synchrony (right panel). Color-bar illustrates masked statistical significance on the overlaid cortical surface (Pcluster-cor ≤ 0.05). *P < 0.05, **P < 0.01.
To further probe whether this neural coupling is dynamic at a moment-by-moment level and responds online to episodes of behavioral synchrony, we calculated intra-dyadic activity coupling by tapping into both power (Pearson’s r) and phase (wPLI) components of STS activity in mother and child with epoch-by-epoch steps. Results reveal that correlations between mother’s and child’s STS gamma power were significant during episodes of ‘synchrony’, r = 0.22, P = 0.008, but not during episodes of ‘non-synchrony’, r = 0.14, P = 0.12; but there was no significant (P = 0.60) difference between the extent of correlation values in the two episodes. However, findings indicate that phase coupling between mother’s and child’s STS gamma activity was significantly (P = 0.05) positive during episodes of ‘synchrony’ (wPLI = 0.17), and was significantly higher (P = 0.02) than during episodes of ‘non-synchrony’ (wPLI = −.08) (Figure 2). Hence, although intra-dyadic coupling of both activity component (power and phase) was significant during episodes of synchrony, only phase coupling robustly differentiated episodes of synchrony from non-synchrony. This may imply that intra-dyadic behavioral synchrony may reflect intra-dyadic communication, which is sustained by phase coupling (Fries, 2005) rather than shared psychological perspectives, which is sustained by power coupling (Lahnakoski et al., 2014).
Social behavior as longitudinal predictors of STS activations in mother and child
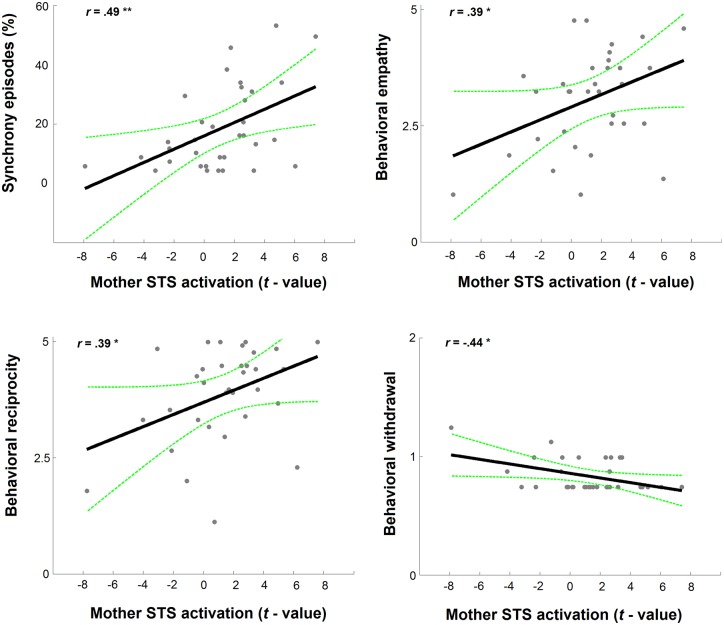
Finally, we examined associations between micro-level synchrony during positive interaction and global reciprocity, empathy, and withdrawal during conflict discussion and mother’s and child’s STS activations 3 years later. Results revealed that higher proportions of ‘synchrony’ episodes (M ± s.d., 18.52% ±13.95) during the positive dialogue predicted higher maternal STS activations, r = 0.49, PFDR-cor = 0.003, df = 31 (see Figure 3 upper left panel); yet, child’s STS activations were not significantly associated with the prevalence of synchrony, r = 0.31, P = 0.13, df = 24. Although this raises the possibility that mother’s STS activity may have exerted an influence on the extent of intra-dyadic coupling during synchrony episodes, the fact that the two variables were not significantly correlated r = 0.09, P = 0.65, df = 24, does not lend support to this possibility.
Fig. 3.
Associations between social behavior during dyadic interaction and mother’s and child’s STS activation. Behavioral empathy (left) and withdrawal (middle) correlated with mothers’ STS activation and with child’s STS activation (right). *P < 0.05.
Similar findings emerged for the global constructs, which were found to predict STS activations mainly in the mother. Mother’s STS activations were associated with higher Empathy (r = 0.39, PFDR-cor = 0.02, df=31; Figure 3 upper right panel), higher Reciprocity (r=0.39, PFDR-cor = 0.02, df = 31; Figure 3 lower left panel) and with lower Withdrawal (r = 0.44, PFDR-cor = 0.01, df = 31; Figure 3 lower right panel) during conflict discussion. (Figure 3). Child’s STS activations did not correlate significantly with Empathy, r = 0.10, P = 0.64, df = 24, or Reciprocity, r = 0.08, P = 0.69, df = 24. However, low levels of Withdrawal predicted higher child’s STS activation, r = −0.41, PFDR-cor = 0.03, df = 24. It thus appears that the longitudinal associations between the putative neural marker for dyadic synchrony, that is, STS activation in the gamma band, and social behavior show closer behavior–brain links for mothers compared children and mother’s STS activation during episodes of synchrony were impacted by both the proportion of synchronous episodes during positive interactions and maternal overall empathic style during the management of conflict.
Discussion
Recent models in social neuroscience have advocated the need to find novel paradigms to elucidate mechanisms that enable the human brain to coordinate response with that of another human’s. To our knowledge this is the first study to use the mother–child context to assess neural coordination by utilizing individually tailored stimuli collected in the natural habitat, focus on brain rhythms and anchor neural coupling in moments of behavioral synchrony. Our findings indicate that brain-to-brain synchrony emerges during the perception of synchronous patterns that are specific to the attachment context, and that neural synchrony was not observed in response to unfamiliar mother–child interaction. Such self-own-child neural synchrony implicates bottom-up processes at both the neural and behavioral levels and was localized to a key integrative node of the social brain, the STS. We further found that neural coupling in both partners’ brain rode on fast, bottom-up gamma-band rhythms and was anchored in second-by-second coordination of non-verbal social signals. In addition to moment-by-moment coordination of gamma power in the STS, mother’s and child’s STS activity was associated with global aspects of their relationship, including reciprocity and social withdrawal. Specifically, mother–child reciprocity, a dyadic-systemic feature of the relationship, predicted greater maternal gamma-band STS activations, while child social withdrawal correlated with attenuated STS response in both mother and child. Overall, our findings accord with the ‘neurobiology of human attachments’ model (Feldman, 2017), which suggests that early relationships build the brain’s mechanisms for social participation through the coordination of social behavior.
Research has shown that neural rhythms play an important role in dyadic behavioral synchrony, for instance when two individuals match each other’s movements, speech or musical production (Dumas et al., 2010; Babiloni et al., 2012; Kawasaki et al., 2013; Müller et al., 2013; Konvalinka et al., 2014; Zhdanov et al., 2015), but also during the observation of vignettes marked by high behavioral synchrony (Levy et al., 2016). In fact, authors have suggested that the synchrony of neural oscillations in the gamma-band is a mechanism underpinning neural communication (Fries, 2005). Hari et al. (2015), in their recent call to move to research of engaged and interacting participants, provide evidence that neural synchrony within one brain could also underpin the human brain’s ability to synchronize with other brains. The mother–child context provides an especially powerful setting to test the role of oscillations in two-brain-coordination; beginning in fetal life the human brain is primed by the mother’s cerebral functioning (Keverne, 2015) and it is likely that such neural imprinting includes cerebral rhythms, which may continue in postnatal life. Such coupling within the ‘nursing dyad’ may enable the child’s brain to gradually adapt to those of other conspecifics during social moments. The current findings provide the first evidence for these propositions and show that perception of social synchrony during mother–child interactions, compared to episodes of non-synchrony as well as with those of unfamiliar mother and child, not only induces gamma-band power enhancement within the social brain but that this oscillatory pattern is synchronized between mother and child.
Brain-to-brain coupling, while riding on the brain’s oscillatory patterns, is suggested here to include both mirror mechanisms, expressed in fast sensorimotor coupling, and mentalizing mechanisms, involved in slower sociocognitive processes. Thus, neural coupling requires the integration of bottom-up and top-down processing in ways that may be unique to the person, context and relationship history of the interacting partners. Moreover, researchers have called to capitalize on discrete, well-demarcated moments within real-life social interactions as a first step to understanding mechanisms that support two-brain coordination (Hari et al., 2015). Accordingly, our study is the first to probe the brain’s oscillatory response to specific moments of heightened social significance within attachment relationships, moments of nonverbal synchrony that not only echo the first parent–infant interactions experienced in infancy but also integrate the infant’s early biological rhythms, such as heart rhythms and sleep-wake cycles, with the rhythms of the first social dialogue (Feldman, 2006). These moments of salient emotional meaning for mother and child are shown here to serve as one mechanism that triggers brain-to-brain synchrony, highlighting the behavior-based dyadic-signal relevance of behavioral synchrony for neural coupling.
We found that social synchrony was translated into neural synchrony in the STS, a key node of the social brain that integrates both mirror and mentalizing properties. The STS has shown to be critically involved in both biological motion, which requires fast bottom-up sensorimotor coupling (Jastorff et al., 2012) as well as socio-cognitive, regulatory top-down theory-of-mind abilities (Dufour et al., 2013), which are reported to operate slowly (Liu et al., 2004; Vistoli et al., 2011; Ferguson et al., 2015). This study thus strengthens prior evidence regarding the reliance of behavioral matching on neural synchrony at different neural substrates and rhythms (Dumas et al., 2010; Babiloni et al., 2012; Kawasaki et al., 2013; Müller et al., 2013; Konvalinka et al., 2014; Zhdanov et al., 2015; Levy et al., 2016), and suggests that gamma synchrony in the STS selectively underlies social synchrony.
Behavioral synchrony is a bottom-up mechanism, utilizing the human-specific repertoire of maternal non-verbal behavior that emerges immediately after birth and integrates the infant’s heart rhythms and sleep cycles into the first social dialogue (Champagne et al., 2001; Feldman, 2006; Shahrokh et al., 2010). The present findings of STS gamma coupling reflecting bottom-up mechanism are well-explained by the nature of the neural signature detected here. Indeed, gamma oscillations are bottom-up-directed (Fries, 2015), and STS is a key node in bottom-up processing of social perception (Allison et al., 2000) and mirror neurons network activity (Iacoboni and Dapretto, 2006). At the same time, the STS equally integrates top-down processing (Dufour et al., 2013; Yang et al., 2015), and gamma oscillations are assumed to be top-down-controlled by lower-frequency oscillations (Fries, 2015). In the same vein, early split-second events of parent–infant synchrony is integrated into higher-order stretches of behavioral matching in later childhood that also involve symbolic and verbal dialogue, perspective taking, and the ability to negotiate conflict with empathy and involvement (Feldman, 2015a, 2016). Thus, across oscillatory rhythms, brain structures, and concrete social behavior, we find that brain-to-brain synchrony integrates bottom-up with top-down processes to create online bio-behavioral coordination between two interacting humans.
Mothers’ STS activations were predicted by individual differences in global social behavior during conflict interaction, including greater amount of synchronous episodes, higher empathy and increased reciprocity. Child’s STS activations, on the other hand, were unrelated to these interactive parameters and were only predicted by lower social withdrawal. The finding that maternal STS activations were more sensitive to behavior than the child’s STS activations requires much further research, but it is possible that mother’s brain–behavior coupling is the mechanism that drives the child’s social brain activations, thereby tuning the child’s brain to the social world. This finding is in line with a recent study showing that parental STS activation in infancy is shaped by parent–infant behavioral synchrony (Abraham et al., 2014) and predicts the development of children’s social competencies during the preschool years, including theory-of-mind, socialization and complex emotion regulation skills (Abraham et al., 2016). Children’s STS, on the other hand, was unrelated to the degree of interactive reciprocity. Possibly, children’s STS response may be more reactive to the global aspects of the relationship, such as whether interactions were involved or withdrawn, rather than fine-tuned aspects related to the amount of reciprocity or empathy. Yet, this study is the first to show that not only does parental STS tune to dyadic interaction (Atzil et al., 2011, 2014; Abraham et al., 2014) but also that the child’s STS shows similar tuning. Most importantly, our findings demonstrate for the first time that the STS is the template upon which brain-to-brain coupling between mother and child is registered.
The central limitation of this study is that mother’s and child’s brains were not measured concurrently during social interactions, but each responded to real-life social interaction in their natural habitat—the home environment. Another limitation is that the longitudinal aspect of our study was only from behavior at T1 to brain at T2 and no longitudinal brain data were available. Such repeated observations of mother’s and child’s brain response to synchronous interactions across development mark an important area for future research. It is important to note that all prior studies addressing brain-to-brain coupling and using technologies that enable good spatial localizations, such as fMRI or MEG, did not use real-life social interactions and future technological advances are required for such research. For instance, Bilek et al. (2015) tested unfamiliar individual pairs who were situated within two scanners and responded to a computer presentation of shapes, yet partners did not see each other’s faces or engaged in ‘real’ social interactions. The brain findings were then correlated with the complexity of the individuals’ social network size, but not with actual social behavior. The methodological approach used here is based on the assumption of overlapping neural circuits underpinning the perception and experience of social functions, including emotions (Bastiaansen et al., 2009), pain (Singer et al., 2004) and social action (Mukamel et al., 2010). The current findings are consistent with previous research showing that the perception of ecologically valid vignettes marked by high social synchrony activates critical nodes of the social brain (Atzil et al., 2011, 2012, 2014; Abraham et al., 2014, 2016; Levy et al., 2016). Yet, much future research is required to test online coordination of two brains during naturalistic person-to-person social interactions.
Since the early days of attachment theory authors have suggested that the mother–child relationship, the first and most significant social relationship throughout life, shapes the brain network that underpins participation in social life. Such socially related network has been termed ‘internal working model’ in attachment theory and was thought to re-activate each time the primary relationship is probed (Bowlby, 1969). Research in the attachment tradition following children from infancy to adulthood demonstrates that early mother–child relational patterns of dyadic reciprocity are individually stable and guide future interactions with both intimate partners and members of the larger social world (Sroufe, 2005). Consistent with this model, our study that focuses on careful observations and longitudinal assessment of mother’s and child’s brain using MEG, an imaging venue integrating excellent temporal resolution with adequate spatial localization which has rarely been utilized in social neuroscience, may contribute to mechanism detection, hypothesis generation and future research. Building on the findings from this study, we found, using hyper-scanning two-person EEG, that during real-life social interactions between romantic partners brain-to-brain coupling was similarly detected in gamma-band oscillations, but not in any other oscillatory band, and that this rhythm was localized to the temporal cortex (Kinreich et al., Submitted). Consistent with the notion that the parent–infant bond provides the neurobiological template for pair bonding in both humans and animals (Numan and Young, 2016), our findings indicate that the neural signature detected in the context of the mother–child bond translates into other meaningful relationships throughout life. The current findings can thus contribute to theory and research on the brain basis of human social life, inform two-person neuroscience perspectives and guide future studies on the social brain in action, a direction that may enrich human social neuroscience research.
Acknowledgements
We thank Galit Schneider, Shahar Aberbach and Tirza Ben-Ari for their assistance in collecting and processing data.
Conflict of interest. None declared.
Funding
This work was supported by the Simms-Mann Foundation, the Irving B. Harris Foundation and by the ICORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant No. 51/11).
References
- Abraham E., Hendler T., Shapira-Lichter I., Kanat-Maymon Y., Zagoory-Sharon O., Feldman R. (2014). Father’s brain is sensitive to childcare experiences. Proceedings of the National Academy of Sciences of the United States of America, 111, 9792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E., Hendler T., Zagoory-Sharon O., Feldman R. (2016). Network integrity of the parental brain in infancy supports the development of children’s social competencies. Social Cognitive and Affective Neuroscience, 11, 1707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T., Puce A., McCarthy G. (2000). Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences, 7, 267–78. [DOI] [PubMed] [Google Scholar]
- Apter-Levi Y., Pratt M., Vakart A., Feldman M., Zagoory-Sharon O., Feldman R. (2016). Maternal depression across the first years of life compromises child psychosocial adjustment; relations to child HPA-axis functioning. Psychoneuroendocrinology, 64, 47–56. [DOI] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. (2011). Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology, 36, 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. (2014). The brain basis of social synchrony. Social Cognitive and Affective Neuroscience, 9, 1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Zagoory-Sharon O., Winetraub Y., Feldman R. (2012). Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 798–811. [DOI] [PubMed] [Google Scholar]
- Babiloni C., Buffo P., Vecchio F., et al. (2012). Brains “in concert”: frontal oscillatory alpha rhythms and empathy in professional musicians. NeuroImage, 60, 105–16. [DOI] [PubMed] [Google Scholar]
- Baess P., Zhdanov A., Mandel A., et al. (2012). MEG dual scanning: a procedure to study real-time auditory interaction between two persons. Frontiers in Human Neuroscience, 6, 83.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J.A., Baldwin D. (2001). Making sense of human behavior: action parsing and intentional inference In: Malle, B.F., Moses, L.J., Baldwin, D.A., editors.Intentions and Intentionality Foundations of Social Cognition, 193–206, Cambridge, MA, US: The MIT Press. [Google Scholar]
- Bastiaansen J.A., Thioux M., Keysers C. (2009). Evidence for mirror systems in emotions. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 364, 2391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilek E., Ruf M., Schäfer A., et al. (2015). Information flow between interacting human brains: identification, validation, and relationship to social expertise. Proceedings of the National Academy of Sciences of the United States of America, 112, 5207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. (1969). Attachment and loss: Volume I: Attachment. The International Psycho-Analytical Library, 79, 1–401. [Google Scholar]
- Champagne F., Diorio J., Sharma S., Meaney M.J. (2001). Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America, 98, 12736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.T., Jääskeläinen I.P., Belliveau J.W., et al. (2015). Combined MEG and EEG show reliable patterns of electromagnetic brain activity during natural viewing. NeuroImage, 114, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. (2010). The neurodevelopment of empathy in humans. Developmental Neuroscience, 32, 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner T.H., Siegel M. (2011). A framework for local cortical oscillation patterns. Trends in Cognitive Sciences, 15, 191–9. [DOI] [PubMed] [Google Scholar]
- Dufour N., Redcay E., Young L., et al. (2013). Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS One, 8, e75468.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G., Nadel J., Soussignan R., Martinerie J., Garnero L. (2010). Inter-brain synchronization during social interaction. PLoS One, 5, e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. (2006). From biological rhythms to social rhythms: physiological precursors of mother-infant synchrony. Developmental Psychology, 42, 175–88. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2007). Mother-infant synchrony and the development of moral orientation in childhood and adolescence: direct and indirect mechanisms of developmental continuity. American Journal of Orthopsychiatry, 77, 582–97. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2010). The relational basis of adolescent adjustment: trajectories of mother-child interactive behaviors from infancy to adolescence shape adolescents’ adaptation. Attachment & Human Development, 12, 173–92. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2012a). Parent-infant synchrony: a biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77, 42–51. [Google Scholar]
- Feldman R. (2012b). Parenting behavior as the environment where children grow In: Mayes L. C., Lewis M., editors. The Cambridge Handbook of Environment in Human Development, 533–67, New York; Cambridge University Press. [Google Scholar]
- Feldman R. (2015a). Sensitive periods in human social development: new insights from research on oxytocin, synchrony, and high-risk parenting. Development and Psychopathology, 27, 369–95. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2015b). The adaptive human parental brain: implications for children’s social development. Trends in Neurosciences, 38, 387–99. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2016). The neurobiology of mammalian parenting and the biosocial context of human caregiving. Hormones and Behavior, 77, 3–17. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2017). The neurobiology of human attachments. Trends in Cognitive Sciences, 21, 80–99. [DOI] [PubMed] [Google Scholar]
- Feldman R., Bamberger E., Kanat-Maymon Y. (2013a). Parent-specific reciprocity from infancy to adolescence shapes children’s social competence and dialogical skills. Attachment and Human Development, 15, 407–23. [DOI] [PubMed] [Google Scholar]
- Feldman R., Eidelman A.I. (2007). Maternal postpartum behavior and the emergence of infant–mother and infant–father synchrony in preterm and full-term infants: the role of neonatal vagal tone. Developmental Psychobiology, 49, 290–302. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Influs M., Gutbir T., Ebstein R.P. (2013b). Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38, 1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. (2010). The cross-generation transmission of oxytocin in humans. Hormones and Behavior, 58, 669–76. [DOI] [PubMed] [Google Scholar]
- Feldman R., Klein P.S. (2003). Toddlers’ self-regulated compliance to mothers, caregivers, and fathers: implications for theories of socialization. Developmental Psychology, 39, 680–92. [DOI] [PubMed] [Google Scholar]
- Feldman R., Magori-Cohen R., Galili G., Singer M., Louzoun Y. (2011). Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development, 34, 569–77. [DOI] [PubMed] [Google Scholar]
- Feldman R., Rosenthal Z., Eidelman A.I. (2014). Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first ten years of life. Biological Psychiatry, 75, 56–64. [DOI] [PubMed] [Google Scholar]
- Ferguson H.J., Cane J.E., Douchkov M., Wright D. (2015). Empathy predicts false belief reasoning ability: evidence from the N400. Social Cognitive and Affective Neuroscience, 10, 848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L.; Brain Development Cooperative Group (2011). Unbiased average age-appropriate atlases for pediatric studies. NeuroImage, 54, 313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences, 9, 474–80. [DOI] [PubMed] [Google Scholar]
- Fries P. (2015). Rhythms for cognition: communication through coherence. Neuron, 88, 220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilam G., Hendler T. (2016). With love, from me to you: embedding social interactions in affective neuroscience. Neuroscience & Biobehavioral Reviews, 68, 590–601. [DOI] [PubMed] [Google Scholar]
- Gross J., Kujala J., Hamalainen M., Timmermann L., Schnitzler A., Salmelin R. (2001). Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 98, 694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R., Henriksson L., Malinen S., Parkkonen L. (2015). Centrality of social interaction in human brain function. Neuron, 88, 181–93. [DOI] [PubMed] [Google Scholar]
- Hari R., Sams M., Nummenmaa L. (2016). Attending and neglecting people : bridging neuroscience, psychology and sociology. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 371, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Frith C.D., Frith C.D. (2016). Mirroring and beyond: coupled dynamics as a generalized framework for modelling social interactions. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 371, pii: 20150366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. (2004). Intersubject synchronization of cortical activity during natural vision. Science, 303, 1634–40. [DOI] [PubMed] [Google Scholar]
- Hirata M., Ikeda T., Kikuchi M., et al. (2014). Hyperscanning MEG for understanding mother-child cerebral interactions. Frontiers in Human Neuroscience, 8, 118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Dapretto M. (2006). The mirror neuron system and the consequences of its dysfunction. Nature Reviews. Neuroscience, 7, 942–51. [DOI] [PubMed] [Google Scholar]
- Jastorff J., Popivanov I.D., Vogels R., Vanduffel W., Orban G.A. (2012). Integration of shape and motion cues in biological motion processing in the monkey STS. NeuroImage, 60, 911–21. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Yamada Y., Ushiku Y., Miyauchi E., Yamaguchi Y. (2013). Inter-brain synchronization during coordination of speech rhythm in human-to-human social interaction. Scientific Reports, 3, 1692.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne E.B. (2015). Genomic imprinting, action, and interaction of maternal and fetal genomes. Proceedings of the National Academy of Sciences of the United States of America, 112, 6834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinka I., Bauer M., Stahlhut C., Hansen L.K., Roepstorff A., Frith C.D. (2014). Frontal alpha oscillations distinguish leaders from followers: multivariate decoding of mutually interacting brains. NeuroImage, 94, 79–88. [DOI] [PubMed] [Google Scholar]
- Lahnakoski J.M., Glerean E., Jääskeläinen I.P., et al. (2014). Synchronous brain activity across individuals underlies shared psychological perspectives. NeuroImage, 100, 316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Goldstein A., Zagoory-Sharon O., et al. (2016). Oxytocin selectively modulates brain response to stimuli probing social synchrony. NeuroImage, 124, 923–30. [DOI] [PubMed] [Google Scholar]
- Liu D., Sabbagh M.A., Gehring W.J., Wellman H.M. (2004). Decoupling beliefs from reality in the brain: an ERP study of theory of mind. Neuroreport, 15, 991–5. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. (2007). Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods, 164, 177–90. [DOI] [PubMed] [Google Scholar]
- Mukamel R., Ekstrom A.D., Kaplan J., Iacoboni M., Fried I. (2010). Single-neuron responses in humans during execution and observation of actions. Current Biology, 20, 750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V., Sänger J., Lindenberger U. (2013). Intra- and Inter-brain synchronization during musical improvisation on the guitar. PLoS One, 8, e73852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M., Young L.J. (2016). Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Hormones and Behavior, 77, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. (2011). FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival D.B., Walden A.T. (1993). Spectral Analysis for Physical Applications: Multitaper and Conventional Univariate Techniques. Cambridge: Cambridge University Press. [Google Scholar]
- Schneiderman I., Kanat-Maymon Y., Zagoory-Sharon O., Feldman R. (2014). Mutual influences between partners’ hormones shape conflict dialogue and relationship duration at the initiation of romantic love. Social Neuroscience, 9, 337–51. [DOI] [PubMed] [Google Scholar]
- Schneiderman I., Zagoory-Sharon O., Leckman J.F., Feldman R. (2012). Oxytocin at the first stages of romantic attachment: relations to couples’ interactive reciprocity. Psychoneuroendocrinology, 37, 1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh D.K., Zhang T.Y., Diorio J., Gratton A., Meaney M.J. (2010). Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology, 151, 2276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Seymour B., Doherty J.O., Kaube H., Dolan R.J., Frith C.D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303, 1157–63. [DOI] [PubMed] [Google Scholar]
- Sommerville J.A. (2010). Detecting causal structure: the role of interventions in infants’ understanding of psychological and physical causal relations In: Causal Learning: Psychology, Philosophy, and Computation, Oxford University Press. [Google Scholar]
- Sroufe L.A. (2005). Attachment and development: a prospective, longitudinal study from birth to adulthood. Attachment & Human Development, 7, 349–67. [DOI] [PubMed] [Google Scholar]
- Stanley D.A., Adolphs R. (2013). Toward a neural basis for social behavior. Neuron, 80, 816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk A., Noordzij M.L., Verhagen L., et al. (2014). Cerebral coherence between communicators marks the emergence of meaning. Proceedings of the National Academy of Sciences of the United States of America, 111, 18183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk A., Verhagen L., Toni I. (2016). Conceptual alignment: how brains achieve mutual understanding. Trends in Cognitive Sciences, 20, 180–91. [DOI] [PubMed] [Google Scholar]
- Symons A.E., El-Deredy W., Schwartze M., Kotz S.A. (2016). The functional role of neural oscillations in non-verbal emotional communication. Frontiers in Human Neuroscience, 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal I., Abeles M. (2013). Cleaning MEG artifacts using external cues. Journal of Neuroscience Methods, 217, 31–8. [DOI] [PubMed] [Google Scholar]
- Vinck M., Oostenveld R., Van Wingerden M., Battaglia F., Pennartz C.M.A. (2011). An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. NeuroImage, 55, 1548–65. [DOI] [PubMed] [Google Scholar]
- Vistoli D., Brunet-Gouet E., Baup-Bobin E., Hardy-Bayle M.C., Passerieux C. (2011). Anatomical and temporal architecture of theory of mind: a MEG insight into the early stages. NeuroImage, 54, 1406–14. [DOI] [PubMed] [Google Scholar]
- Weisman O., Feldman R., Burg-Malki M., et al. (2015). Mother-child interaction as a window to a unique social phenotype in 22q11.2 Deletion syndrome and in Williams syndrome. Journal of Autism and Developmental Disorders, 45, 2567–77. [DOI] [PubMed] [Google Scholar]
- Wilson E. (2012). The Social Conquest of Earth. New York: Basic. [Google Scholar]
- Wykowska A., Chaminade T., Cheng G. (2016). Embodied artificial agents for understanding human social cognition. Royal Society of London, 371, 20150375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.Y.-J., Rosenblau G., Keifer C., Pelphrey K.A. (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience and Biobehavioral Reviews, 51, 263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanov A., Nurminen J., Baess P., et al. (2015). An internet-based real-time audiovisual link for dual MEG recordings. PLoS One, 10, e0128485.. [DOI] [PMC free article] [PubMed] [Google Scholar]