Abstract
Health communication via mass media is an important strategy when targeting risky drinking, but many questions remain about how health messages are processed and how they unfold their effects within receivers. Here we examine how the brains of young adults—a key target group for alcohol prevention—‘tune in’ to real-life health prevention messages about risky alcohol use. In a first study, a large sample of authentic public service announcements (PSAs) targeting the risks of alcohol was characterized using established measures of message effectiveness. In the main study, we used inter-subject correlation analysis of fMRI data to examine brain responses to more and less effective PSAs in a sample of young adults. We find that more effective messages command more similar responses within widespread brain regions, including the dorsomedial prefrontal cortex, insulae and precuneus. In previous research, these regions have been related to processing narratives, emotional stimuli, self-relevance and attention towards salient stimuli. The present study thus suggests that more effective health prevention messages have greater ‘neural reach’, i.e. they engage the brains of audience members’ more widely. This work outlines a promising strategy for assessing the effects of health communication at a neural level.
Keywords: fMRI, health communication, public service announcements, inter-subject correlation, self, alcohol
Introduction
Risky drinking constitutes a major public health problem in the population at large and young adults in particular. For instance, alcohol contributes to 5% of the global burden of disease and about a quarter of all deaths between ages 20 and 40 (World Health Organization, 2014). Mass media health messages, particularly public service announcements (PSAs), are frequently used to combat such alcohol- or other behavior-related health problems. While many factors contribute to the effectiveness of public media health messages, a core ingredient of effective campaigns seems to be the potential to address individuals in a self- and emotionally-relevant way (Burnkrant and Unnava, 1995; Dillard and Peck, 2000; Tannenbaum et al., 2015). Functional neuroimaging offers a unique opportunity to measure neural responses to health risk communication media, and this study was designed to shed light on how more and less effective health messages about alcohol are received and processed in the brains of a target audience.
Previous neuroimaging studies provide first insights into neural responses to health communication with varying message characteristics, such as message sensation value and argument strength (for a review, see Kaye et al., 2016). While the former refers to visual and auditory features, novelty and intensity of a message (e.g. Palmgreen et al., 2002; Kang et al., 2006), the latter refers to whether a message is logical, backed up by strong reasons, and persuasive overall (e.g. Kang et al., 2006; Zhao et al., 2011). Health messages varying on these constructs prompted differential blood oxygen level-dependent (BOLD) signal in the medial prefrontal cortex and the precuneus (Langleben et al. 2009; Wang et al., 2013; Weber et al., 2014). Responses in these regions also differed between tailored and non- or less-tailored health messages (Chua et al., 2009, 2011; Wang et al., 2016) and have recently been linked to message-driven behavioral outcomes (Chua et al., 2011; Falk et al., 2010a, 2011). Furthermore, emerging evidence points to the engagement of anterior cingulate and insular regions during the reception of naturalistic risk and health information (Schmälzle et al., 2013; Wang et al., 2013, 2016). These findings align well with previous research in cognitive, social and affective neuroscience linking medial prefrontal, anterior cingulate and insular cortices as well as the precuneus to the processing of self-relevant and emotionally salient stimuli (Amodio and Frith, 2006; Northoff et al., 2006; Seeley et al., 2007; Uddin, 2015). Finally, health message characteristics were also linked to activation in occipital visual (Langleben et al., 2009; Seelig et al., 2014) and inferior parietal brain regions (Wang et al., 2013), likely reflecting differences in attentional processing related to the format and content of the health message.
Most previous research on health message reception has focused on mean level BOLD signal changes. Whereas classical neuroimaging approaches prefer short, simplified stimuli, the inter-subject correlation (ISC) analysis technique can assess audience-wide brain responses towards complex and naturalistic stimuli (Hasson et al., 2004; Hasson et al., 2010). In particular, ISC analysis measures the similarity of fMRI time courses across individuals who are exposed to the same message and reveals where and to what extent message-evoked brain responses concur across receivers. For instance, Schmälzle et al. (2015) observed reliable differences in ISC for powerful political speeches in bilateral superior temporal regions and the medial prefrontal cortex, suggesting increased neural engagement across listeners. Furthermore, a recent ISC study in the health domain examined the reception of an H1N1 documentary that aired on national TV during the epidemic and provided evidence that ISC coupling varied according to preexisting H1N1 risk perceptions (Schmälzle et al., 2013). This suggests that the ISC approach offers a way to reveal how health messages engage the brains of their target audiences. Importantly, ISC might differentiate between more and less effective health messages according to their ability to motivate recipients to process the information, a core variable in current models of effective persuasion and attitude change (Burnkrant and Unnava, 1995; Dillard and Peck, 2000; Petty et al., 2009; Tannenbaum et al., 2015).
The main goal of the present study was to reveal differences of more compared to less effective PSAs in the ability to evoke commonly shared neural processing in neural regions related to motivated stimulus processing. To this end, a large sample of anti-alcohol PSAs from German-speaking public media campaigns was collected. In a first study, ten of the most and ten of the least effective PSAs of this sample were characterized using three established self-report measures of effectiveness: ad effectiveness (Falk et al., 2012), perceived argument strength (Zhao et al., 2011), and perceived message sensation value (Palmgreen et al., 2002). In a second study, these PSAs were shown to young adults while fMRI data was recorded. Young adults are a main target group of anti-alcohol campaigns as around 40% of young men and around 30% of young women in Germany show risky alcohol use (Robert Koch Institute, 2014). We hypothesized that both more and less effective health messages would prompt correlated neural processes across sensory regions. However, more effective health messages should also have greater impact on the audience and lead to enhanced inter-subject correlations of neural activity, particularly in regions associated with self-relevant information processing, emotional salience, and selective attention modulation.
Study 1
Methods
Participants
93 introductory psychology students (MAge = 22.02, SD = 7.36, 71 females, 80 reported drinking alcohol on regular base) participated for class requirements. Experiments were conducted in mixed-gender groups of 8–12 students and experimental procedures were approved by the ethical committee of the University of Konstanz.
Materials
We screened online video resources and contacted health agencies to obtain a comprehensive sample of German-speaking PSAs against risky alcohol use. This resulted in an initial database of 68 videos that aired on national television, the social web, and/or cinemas. The PSAs displayed typical contents of anti-alcohol health communication (see Supplementary Material, Table S1). Exemplars with insufficient physical quality (e.g. resolution, sound) were removed during an initial screening by MI and RS in order to obtain a sample of 50 PSAs, which were then rated towards perceived message effectiveness by an independent sample (N = 9, MAge = 24.1, SD = 4.51, 3 females, 6 reported drinking alcohol). Based on these ratings, we selected the 10 most and 10 least effective PSAs that were targeted at young adults and matched in length. These 20 videos were then resized to a 1280 × 720 pixels resolution, amended with a 2-s fade-in and fade-out transition and cropped in length to match a multiple of the 2.5 s TR. The sound level was normalized. The mean length of the more and the less effective PSAs did not differ (MMore = 58.5 s, SD = 25.93; MLess = 49.5 s, SD = 25.00; t18 = 0.43, P = 0.8, n.s.).
Procedure
Participants viewed the video stimuli on individual computers equipped with headphones. The series of 20 PSAs was shown in randomized order using E-Prime software (Psychology Software Tools). Self-report ratings for each video were obtained directly after each PSA. The type of self-report varied between subjects, however, each participant evaluated all PSAs for her/his respective scale items. One group (N = 33) rated the PSAs on the 10-item self-report scale of ad effectiveness, including items such as whether a PSA is believable, powerful or grabbed attention (Falk et al., 2012). A second group (N = 32) evaluated the PSAs on perceived message sensation value using a 17-item scale, including items related to physical characteristics of a message as well as dramatic impact, novelty, and emotional arousal (Palmgreen et al., 2002). A third group (N = 28) extracted the core argument of each PSA and evaluated it according to the perceived argument strength scale adapted from Zhao et al. (2011), including plausibility, convincibility, importance, and agreement with the arguments inherent in a message. The first group also evaluated the PSAs using one-item measures for perceived message effectiveness, perceived argument strength, physical production quality and SAM scales for arousal and valence (Bradley and Lang, 1994).
Results and discussion
The findings suggested pronounced differences in effectiveness among the large sample of health messages we obtained from authentic anti-alcohol campaigns. Considering established self-report scales, more as opposed to less effective PSAs were rated consistently higher regarding ad effectiveness, perceived message sensation value, or perceived argument strength (see Table 1). Furthermore, the analysis of single item measures of perceived message effectiveness and perceived argument strength confirmed these findings, with significantly higher ratings for more compared to less effective PSAs (see Table 1). In addition, more effective messages were also seen as more arousing but did not differ in valence. Finally, more effective PSAs were perceived as being higher in production quality—albeit with smaller effect size. Inter-rater agreement was very high for all measures (ICCs: 0.87–0.97, two-way random, absolute), suggesting high consistency for the rater’s evaluations. Moreover, the same results were obtained when excluding all participants who did not report alcohol consumption on a regular base (N = 13). Overall, these findings supported the a priori-categorization into more and less effective PSAs.
Table 1.
Self-report results of PSA characterizations in studies 1 and 2: mean ratings, statistical comparisons, effect sizes and inter-rater reliability
| Mean (SD) |
t value | P value | Cohen’s d | ICCb | ||
|---|---|---|---|---|---|---|
| More effective | Less effective | |||||
| STUDY 1 | ||||||
| Scales | ||||||
| Self-reported ad effectivenessa (N = 33) | 6.10 (0.64) | 3.81 (0.33) | 10.02 | < 0.001 | 4.50 | 0.97 |
| Perceived message sensation value (N = 32) | 6.07 (0.98) | 3.72 (0.64) | 6.35 | < 0.001 | 2.84 | 0.97 |
| Perceived argument strength (N = 28) | 6.15 (0.48) | 4.58 (0.37) | 8.26 | < 0.001 | 3.66 | 0.94 |
| Single item measures (N = 33) | ||||||
| Perceived message effectiveness | 6.61 (0.88) | 2.97 (0.61) | 10.76 | < 0.001 | 4.81 | 0.97 |
| Perceived argument strength | 6.58 (0.85) | 2.98 (0.74) | 10.09 | < 0.001 | 4.52 | 0.97 |
| Arousal | 4.73 (0.60) | 3.14 (0.30) | 7.47 | < 0.001 | 3.35 | 0.89 |
| Valencea | 4.34 (0.91) | 4.97 (0.45) | −1.98 | 0.064 | n.s. | 0.87 |
| Production quality | 6.68 (1.28) | 4.44 (0.94) | 4.48 | < 0.001 | 2.00 | 0.96 |
| STUDY 2 | ||||||
| Single item measures (N = 32) | ||||||
| Perceived message effectiveness | 6.51 (1.13) | 2.78 (0.64) | 9.14 | < 0.001 | 4.06 | 0.98 |
| Perceived argument strength | 6.52 (1.21) | 2.63 (0.54) | 9.24 | < 0.001 | 4.15 | 0.98 |
| Arousal | 4.65 (0.98) | 2.53 (0.46) | 6.19 | < 0.001 | 2.77 | 0.95 |
| Valencea | 4.77 (0.95) | 5.35 (0.39) | −1.78 | 0.100 | n.s. | 0.87 |
| Production quality | 6.75 (1.55) | 4.76 (1.07) | 3.35 | 0.004 | 1.49 | 0.96 |
Equal variances not assumed. bIntra-class correlation, two way random, absolute.
P-values were derived from two-sided, independent samples t-tests.
Correlation analysis of self-report measures suggested that scale and single-item measures share a substantial part of their variance. Specifically, the three scale and two single-item measures assessing effectiveness-related message characteristics were highly correlated (r’s > 0.87, P’s < 0.001). Also, the single item measure of perceived message effectiveness was highly correlated with the established scales measuring ad effectiveness, perceived message sensation value and perceived argument strength (r’s > 0.89, P’s < 0.001), justifying its use in examining PSA effectiveness in the fMRI study (Study 2).
Study 2
Methods
Participants
Thirty-six volunteers from 18 to 30 years of age participated in the study. Four participants were discarded due to excessive head movement, anatomical anomalies, or technical failure, resulting in a final sample size of 32 participants (16 females, MAge = 23.41; SD = 2.96). All participants had normal hearing, corrected-to-normal vision, and no history of neurological diseases. Participants received course credit or monetary reimbursement. Consent was obtained according to the Declaration of Helsinki and all procedures were approved by the local ethics committee.
Materials
We used the same 10 more and 10 less effective anti-alcohol PSAs described and characterized in Study 1 as stimulus materials.
Procedure
In a first session (T1), we assessed fMRI eligibility and obtained self-report measures related to alcohol consumption, risk perception, and behavior. In the main experimental session, participants were asked to attentively view the 10 more effective and 10 less effective PSAs (ITI = 10 s, fixation on blank background). PSAs were presented in pseudo-randomized order, and scanning lasted approximately 45 minutes. Upon leaving the scanner, participants answered the same questionnaires on alcohol-related risk perceptions (T2). Lastly, all PSAs were again presented to the participants and evaluated using the single-item measures established in Study 1, i.e. perceived message effectiveness, perceived argument strength, arousal, valence, and production quality. One week after the fMRI scan (T3), participants were invited to answer an online questionnaire containing the same measures as the baseline assessment.
Self-reported alcohol consumption, risk perception, drinking intention, and drinking behavior
Alcohol consumption across the participants was assessed using the AUDIT alcohol screening questionnaire (range: 0–40; Babor et al., 2001). According to recommendations for the German population (Rumpf et al., 2002), three quarters of the participants showed a tendency towards risky drinking (MAudit = 6.41; SD = 3.55; range: 1–17). Thus, the sample of young adults was a valid target audience for the anti-alcohol PSAs.
Risk perception and behavior concerning alcohol use were assessed using items adapted from previous work on health risk perception, i.e. baseline levels of alcohol consumption, risk perceptions, intentions, worries and perceived pressure to change (Renner, 2004; Renner and Reuter, 2012; Schmälzle et al., 2013). There were mostly small, non-significant differences for most measures of risk perceptions when comparing baseline (T1) and follow up (T3) measurements. However, the pattern of results consistently suggested positive changes in risk behavior and perceptions concerning alcohol use and participants reported a small reduction in drinking amount per drinking event (t31 = 2.95, P < 0.01, two-sided, one sample t-test, Cohen’s d = 0.52). These results are consistent with what would be expected after a one-shot exposure to a mixed sequence of anti-alcohol PSAs.
MRI acquisition, preprocessing and analysis
MRI data was recorded using a Siemens Skyra 3T System equipped with a 20 channel head coil. Blood oxygenation level-dependent (BOLD) contrast was acquired during health message viewing using a T2*-weighted Fast Field Echo-Echo Planar Imaging sequence (80° flip angle, TR = 2500 ms, TE = 30 ms). Data was obtained in ascending-interleaved slice order (36 axial slices; no gap; FOV = 240 × 240 mm; 80 × 80 acquisition matrix, 3 × 3 × 3.5 mm voxel size). 560 functional volumes were acquired during the audiovisual stimulation. Field of View was adjusted to AC-PC plane and tilted about 16° (Mennes et al., 2014). Structural images were obtained using a standard T1-weighted scan with 1 × 1 × 1 mm voxel size (FOV = 256 × 256 mm, 192 sagittal slices). Presentation software (Neurobehavioral Systems, Inc.) was used to present the PSAs and to synchronize MRI acquisition. PSAs were projected onto a translucent screen located in the back of the scanner bore. Participants viewed the stimuli via a mirror mounted to the head coil and heard the sound via MR-compatible headphones (MRConfon GmbH) that function optimally within the scanner bore.
Data was preprocessed and analyzed using the BrainVoyager 20 software package (BrainInnovation) and in-house MATLAB code (The MathWorks, Inc.). Functional data was corrected for slice scanning time using sinc-interpolation and corrected for 3D Motion (trilinear/sinc-interpolation). Participants exceeding 3 mm of head movement were excluded. Functional data was spatially smoothed (FWHM = 6 mm) and temporally filtered to remove linear trends and low-frequency shifts using a high pass-GLM-Fourier filter (up to 6 cycles). Functional and anatomical volumes were normalized into the Talairach coordinate system (Talairach and Tournoux, 1988). To compensate for psychological onset transients and signal saturation, the first six TRs were discarded prior to preprocessing. We extracted the single PSA data from the full time course with a shift of two TRs and thus compensated for the lag in hemodynamic responding. Based on visual inspection of the data, two initial TRs were discarded for each PSA to compensate for onset transients.
ISC analysis
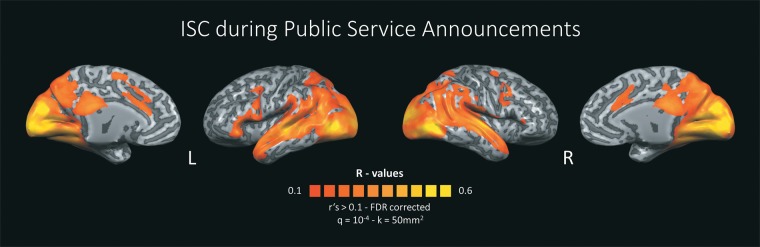
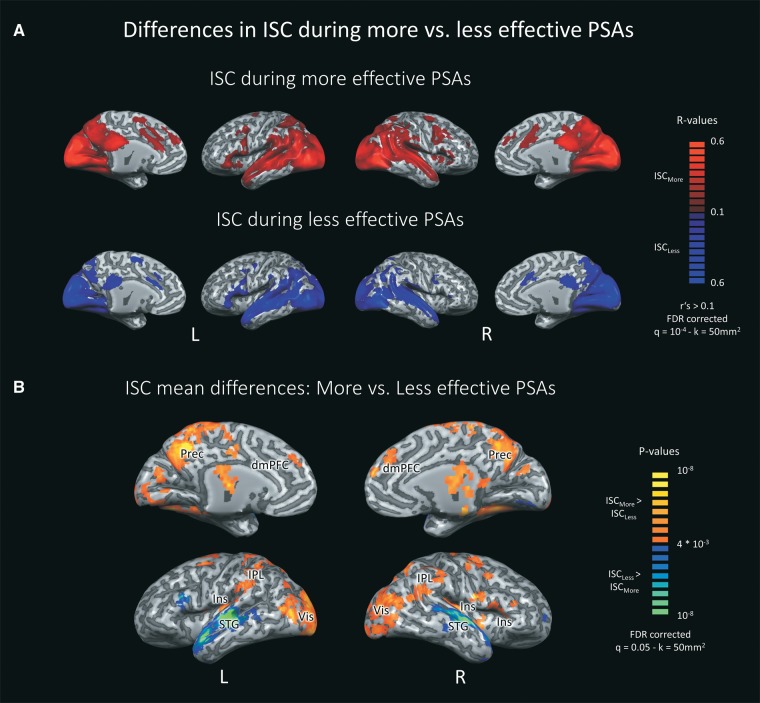
Functional data was analyzed using the inter-subject correlation (ISC) approach (Hasson et al., 2004, 2010), which assesses the voxel-by-voxel correlations between fMRI time courses from different individuals. Because all viewers are exposed to the identical material, ISC result maps provide a measure of the intersubjective similarity of continuous neural processing at the level of individual brain regions. A two-step analysis scheme was adopted in which we first assessed reliably correlated responses within each PSA. Towards this end, voxel-by-voxel ISC values were computed for the time courses of each single PSA using the response time courses of each subject and the average time course of the rest of the group. After applying the Fisher Z-transformation to the individual coefficients, we calculated the average groupwise correlation coefficient r for each voxel. Statistical significance for the correlation coefficients was computed via a bootstrapping procedure: Using phase randomization, we generated 1000 phase-shifted bootstrapping time series for every empirical time course in every voxel. The empirical r values were then compared against the obtained null distribution. This procedure was performed separately for all PSAs. This step yielded twenty correlation maps indexing reliable neural processing across participants within each PSA. Then, average correlation maps were calculated for all PSAs (Figure 1) as well as separately for more and less effective PSAs (Figure 2A). To correct for multiple comparisons, we used FDR correction with q = 10−4 (Benjamini and Hochberg, 1995) and a cluster threshold of 50 mm2.
Fig. 1.
Average inter-subject correlation (ISC) during all anti-alcohol PSAs. Raw ISC values > 0.1 were displayed on an inflated, anatomical rendering of the Colin27 Average Brain (Holmes et al., 1998). Statistical values were FDR corrected with q = 10−4, smoothed and a voxel contiguity threshold of 50 mm2 was applied. L = Left hemisphere, R = Right hemisphere.
Fig. 2.
Differences in inter-subject correlation (ISC) during more compared to less effective PSAs. (A) Differential reach of ISC during more and less effective PSAs throughout the brain. Average raw ISC values > 0.1 were displayed for more (red) and less (blue) effective PSAs. FDR corrected with q = 10−4. (B) Illustration of significantly higher ISC during more (red/yellow) vs less (blue/green) effective PSAs. P-values were derived from two-sided, paired t-tests and FDR corrected with q = 0.05. All statistical values were overlaid onto a Talairach normalized, anatomical rendering of the Colin27 Average Brain (Holmes et al., 1998), and a voxel contiguity threshold of 50 mm2 was applied. L = Left hemisphere, R = Right hemisphere, dmPFC = Dorsomedial prefrontal cortex, Ins = Insula, IPL = Inferior parietal lobe, Prec = Precuneus, STG = Superior temporal gyrus, Vis = Visual system.
In a second analysis step, we extracted the individual correlation values for each PSA and for each participant separately. Then, mean correlation values were calculated for the two PSA categories for each participant. For each voxel, this procedure resulted in two vectors containing the mean correlation values between one message recipient and the rest of the group for each of the PSA categories, respectively. These values were Fisher Z-transformed and submitted to a two-sided, dependent sample t-test on a voxel-by-voxel basis. This procedure reveals voxels exhibiting significant differences in ISC between the two PSA categories. As in previous research (e.g. Schmälzle et al., 2013; Cantlon and Li, 2013), ISC mean difference effects were corrected for multiple comparisons using FDR correction (q = 0.05, 50 mm2 cluster threshold). Only voxels revealing reliable ISC for either the more or the less effective PSAs (one sample t-test with P < 10−5, uncorr.) were included in this analysis. The obtained clusters (Figure 2B) were labeled using a Talairach-transformed (Lancaster et al., 2007) maximum probability tissue atlas from the OASISproject (as provided in SPM12 by Neuromorphometrics Inc., under academic subscription). Figures were created using a Talairach-transformed anatomical rendering of the Colin27 Average Brain (Holmes et al., 1998).
Results
Perceived message effectiveness
As shown in Table 1, self-report measures confirmed the findings from Study 1. Most importantly, more compared to less effective PSAs were perceived as higher in perceived message effectiveness and perceived argument strength. Moreover, the self-report measures again showed a very high agreement between the raters (ICCs: 0.87–0.98).
Neural processing of Anti-Alcohol PSAs
Figure 1 illustrates significant inter-subject correlation of spatiotemporal brain activity patterns for both more and less effective PSAs in medial cortical structures extending from anterior and posterior cingulate to the precuneus, and extended parietal cortices as well as to widespread posterior and temporal brain regions. These findings align well with previous research using naturalistic, audiovisual stimuli (Hasson et al., 2004, 2010; Jääskeläinen et al., 2008; Schmälzle et al., 2013) and indicate that health messages collectively engage large-scale brain systems involved in visual, auditory, higher order semantic and attentional functions.
More vs less effective anti-alcohol PSAs
We next compared the level of ISC during more vs less effective PSAs to identify differences in health message reception according to PSA effectiveness. Figure 2A shows ISC during more and less effective PSAs separately, and Figure 2B reveals brain regions exhibiting significant ISC differences. As can be seen in Figure 2A, both categories engaged auditory and visual brain regions. However, marked differences in the relative spatial distribution of ISC are apparent: More effective PSAs elicited heightened ISC in dorsomedial prefrontal (dmPFC), precuneus, and posterior cingulate cortices. Moreover, significant differences were identified in bilateral insula extending to both inferior frontal and posterior insular cortices as well as the right anterior insula. Finally, enhanced ISC emerged for more effective PSAs in supramarginal and superior parietal regions as well as bilateral occipital regions encompassing primary and extrastriate visual cortices.
The reverse pattern, i.e. larger ISC for less effective PSAs, was observed in temporal regions involved in auditory and language processing. This effect might be due to differences in the amount of spoken language in the PSAs. To test this hypothesis, we performed a control analysis that excluded all PSAs with more than two spoken sentences (2 more/4 less effective PSAs). Limiting the analysis in this way largely diminished the effect of heightened ISC in temporal regions for the less effective messages (see Supplementary Material, Figure S1). Thus, larger ISC for less effective PSAs in these regions seems to depend mainly on the amount of spoken language. Furthermore, when controlling for the amount of speech, the finding of increased ISC for more compared to less effective PSAs was accentuated.
To address potential influences of familiarity with PSAs, we re-ran all analyses excluding PSAs that were recognized, as assessed during the post-scanning questionnaire. Three more effective PSAs were recognized by one to four participants each. Control analysis excluding these PSAs confirmed the main results (Supplementary Material, Figure S2).
General discussion
A core ingredient of effective health media messages is the ability to evoke recipients’ attention and engage them in a self- and emotionally relevant fashion. Furthermore, to be able to evoke effects in large audiences, mass media-based health messages need to have mass appeal, i.e. they have to cater to the common denominator within a recipient group (Freimuth and Quinn, 2004). Here we employed ISC-fMRI to capture the degree of shared brain responses towards fully realistic health messages. The main finding is that effective PSAs about risky alcohol use prompt enhanced cross-recipient brain coupling in cortical midline regions (i.e. dmPFC and precuneus) as well as the insular, parietal and occipito-temporal regions known to be involved in the processing of self-relevant, emotional and salient stimuli. These results suggest functional neuroimaging as a suitable measure for tracking the neural effects of health messages.
The present study shows that effective anti-alcohol messages prompted more similar neural responses across receivers’ brains. This effect was particularly strong in regions of the cortical midline, encompassing the dmPFC and precuneus, two regions often implicated in the processing of narrative and social content (Ferstl et al., 2008; Mar, 2011). Other lines of research linked these regions to a general role in processing self-related stimuli (Kelley et al., 2002; Amodio and Frith, 2006; Northoff et al., 2006) and self-referential introspection (Gusnard et al., 2001) as well as imagining personally relevant past and future episodes (Addis et al., 2007; D’Argembeau et al., 2010; Spreng et al., 2009). Given this broad set of self-related functions, the heightened ISC in these regions for more effective PSAs may indicate stronger personal involvement, which may—according to the elaboration likelihood model of persuasion—prompt deeper engagement and elaborate message processing (Petty et al., 2009). The suggested role of personal or self-relevance for effective health messaging is also supported by research showing stronger activity in the mPFC and precuneus for self-tailored compared to less or untailored anti-smoking and safer sex messages (Chua et al., 2009, 2011; Wang et al., 2016). Our findings also coincide with work that suggests the dmPFC as part of a neural network related to persuasion (Falk et al., 2010b). Overall, we suggest that effective health messages lead to deeper personal engagement across multiple viewers, as reflected by shared brain responses in cortical midline regions, which lastly might lead to persuasion.
Accompanying the effects in cortical midline regions, more compared to less effective PSAs also evoked stronger ISC in bilateral insulae. Recent work on the perception of health risk messages also found effects in the anterior insula (Schmälzle et al., 2013; Wang et al., 2013, 2016). For instance, enhanced ISC in the anterior insulae during an H1N1 documentary was linked to risk perception (Schmälzle et al., 2013). Moreover, activations in the anterior insula also varied with HIV risk perceptions towards unacquainted persons (Häcker et al., 2015), and were modulated by anti-smoking health messages (Wang et al., 2013). Similarly, the insula, and especially the anterior insula, is consistently observed in studies on emotionally salient stimulus processing, and has been suggested as a hub regulating attention and working memory processes (Menon and Uddin, 2010). According to these findings, effective PSAs appear to evoke similar signals across viewers in regions involved in saliency, attention and working memory. While our findings align with the suggested role of the insula in switching between large-scale networks, future research using e.g. dynamic connectivity (Bassett et al., 2015; Fornito et al., 2015) and inter-subject functional correlation methods (Simony et al., 2016) is needed to determine the interplay of systems involved in saliency and self-relevance processing with sensory-perceptual systems.
In line with the notion that effective messages engage the brains of their recipients more consistently, we also found differences between more and less effective PSAs in regions involved in attention regulation and expression. Specifically, stronger ISC effects for more effective PSAs were seen in extended regions of the inferior and superior parietal cortices, overlapping the dorsal and the ventral attention networks (Corbetta and Shulman, 2002; Fox et al., 2006). Furthermore, more effective PSAs also evoked stronger ISC in occipital visual and adjacent parietal regions, presumably reflecting increased visual attention. This interpretation is supported by research showing increased activations in visual-associative regions towards emotional images, e.g. mutilation and injury (Junghöfer et al., 2005; Sabatinelli et al., 2005; Lang and Bradley, 2010). Overall, our results suggest that the PSAs that were more effective in drawing visual attention were also evaluated as being more effective by both independent raters as well as those whose brains we scanned.
The current findings are compatible with models of persuasion, such as the elaboration likelihood model (ELM, Petty and Cacioppo, 1986), the extended ELM (Slater, 2002), and fear appeal messaging more broadly (e.g. Witte and Allen, 2000; Tannenbaum et al., 2015). For instance, the increased ISC in dorsomedial prefrontal cortex for more effective PSAs might relate to higher personal relevance, a key variable for elaborate stimulus processing in the ELM (Petty et al., 2009). Similarly, increased ISC in regions of the saliency network and in visual-associative brain regions suggests attentional tuning and deeper message processing. Typical of the genre, the anti-alcohol PSAs employed in this study mostly presented their message via short stories (see Supplementary Material, Table S1) that vividly exemplify the risks of alcohol consumption (Zillmann, 2002). Furthermore, research on narrative transportation has shown that attentional tuning is accentuated when negative outcomes (e.g. harm, injury) are emphasized (Bezdek and Gerrig, 2016), which is also compatible with the finding of enhanced ISC across viewers during effective PSAs. The anti-alcohol messages in the present study revealed large differences not only in terms of perceived message effectiveness, but also for other message characteristics. Thus, more work is needed to disentangle effects of individual message characteristics on message effectiveness as well as on measures of collective neural engagement. Furthermore, the more effective messages often contained several features at once (e.g. dramatic images, narratives or highlighting negative consequences). This makes it difficult to identify which features make a health message more effective, and features may likely interact and have gestalt-like, emergent effects. In addition, while our sample of college students is an important target audience for anti-alcohol PSAs, this homogeneity naturally also limits generalizability. The study of larger samples could not only increase confidence, but could also reveal group differences based on attitudes, intentions and behaviors. Specifically, future work might follow up on existing research (Schmälzle et al., 2013; Weber et al., 2014) and examine whether participants who do or do not engage in a risk behavior also respond more similarly to messages, or specific elements therein. In sum, more work is needed to examine causes, mechanisms, and consequences of health messages, and emerging work in the area of health prevention neuroimaging has begun to address these issues (e.g. Langleben et al., 2009; Schmälzle et al., 2013; Weber et al., 2014; Cooper et al., 2015).
We propose that the brain responses to effective messages are likely precursors of shifts in risk perception. Theoretical accounts of health risk perception make an important distinction between the general awareness of a risk and personal risk perception (Renner et al., 2015). While the former represents probabilistic knowledge or factual information that is not necessarily action-relevant or remains detached from experience, the latter seems to be what drives preventive efforts by fostering feelings of being personally at risk (Weinstein, 1989; Slovic and Peters, 2006; Renner and Schupp, 2011; Schmälzle et al., 2011). Such an authentic sense of vulnerability could be achieved by effective messages that are attended, deeply processed and accepted by the receiver. We suggest that larger ISC during highly effective anti-alcohol PSAs in neural regions associated to self-relevance, and risk perception (i.e. dmPFC, precuneus and insula) might index such a proximal form of message success. Once this is achieved, it should also be more likely for the message to have distal effects, i.e. changes in risk perception and behavior. Thus, neural effects within midline regions and the insula might indicate whether a message ‘got under the skin’ and successfully engaged processes related to risk perception or affect more broadly. Related to this issue, recent research showed that mPFC-activity in response to health messages can predict behavior change such as smoking or sunscreen use (Chua et al., 2009, 2011; Cooper et al., 2015; Falk et al., 2010a, 2011). Furthermore, a growing body of evidence suggests that large scale population effects can be predicted by neural activity of small-scale target audiences in regions similar to those found in the current study (Falk et al., 2012, 2016). As is often the case, no metrics related to population-level effectiveness were available for the present set of public health campaigns (e.g. Noar, 2006). Thus, future research needs to explore whether effective messages that achieve greater ‘neural reach’ within a sample audience also have greater ‘mass reach’ in the public. Given the advantage of ISC to capture neural responses to content-rich audiovisual PSAs, typically used in health prevention, expanding on the present work may lead to the development of fMRI-based signatures of message effectiveness (cf. Wager et al., 2013). Specifically, to the extent that we can differentiate signatures of more from less effective messages, we might also invert this model and attempt to forecast whether new messages will ‘catch on’.
To conclude, we demonstrate collective-level brain effects of effective health messages about the risks of alcohol on a group of young adults. The main finding is that more compared to less effective health messages evoke more consistent neural responses in brain regions implicated in self-relevant and emotional processing as well as the modulation of attention. Increased ability to capture the dynamic engagement of audiences in health-related PSAs will promote better understanding of the mediating mechanisms between message exposure and subsequent effects. As such, assessing the ‘neural reach’ of mass media campaigns within recipients contributes to the emerging field of communication neuroscience (Falk, 2010) at the intersection of communication, health, and neuroscience.
Supplementary Material
Acknowledgements
We thank Emily Falk, Tobias Flaisch, Martina Gamp, Ursula Kirmse, Christoph Becker and Frank Häcker for invaluable help, enriching discussions and expertise. We also thank Delia Decroupet and Ursa Grau for help in data acquisition.
Supplementary data
Supplementary data are available at SCAN online.
Funding
This work was supported in part by the German Research Foundation [DFG, FOR 2374 and RE 3430] and the Lienert Foundation for Training in Biopsychological Research Methods [stipend to MI].
Conflict of interest. None declared.
References
- Addis D.R., Wong A.T., Schacter D.L. (2007). Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45(7), 1363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–77. [DOI] [PubMed] [Google Scholar]
- Babor T.F., Higgins-Biddle J.C., Saunders J.B., Monteiro M.G. (2001). AUDIT: The Alcohol Use Disorders Identification Test.Guidelines for Use in Primary Care. Geneva, Switzerland. [Google Scholar]
- Bassett D.S., Yang M., Wymbs N.F., Grafton S.T. (2015). Learning-induced autonomy of sensorimotor systems. Nature Neuroscience, 18(5), 744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bezdek M.A., Gerrig R.J. (2016). When narrative transportation narrows attention: changes in attentional focus during suspenseful film viewing. Media Psychology, 1–30. [Google Scholar]
- Bradley M.M., Lang P.J. (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Burnkrant R.E., Unnava H.R. (1995). Effects of self-referencing on persuasion. Journal of Consumer Research, 22(1), 17–26. [Google Scholar]
- Cantlon J.F., Li R. (2013). Neural activity during natural viewing of Sesame Street statistically predicts test scores in early childhood. PLoS Biology, 11(1), e1001462.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua H.F., Ho S.S., Jasinska A.J., et al. (2011). Self-related neural response to tailored smoking-cessation messages predicts quitting. Nature Neuroscience, 14(4), 426–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua H.F., Liberzon I., Welsh R.C., Strecher V.J. (2009). Neural correlates of message tailoring and self-relatedness in smoking cessation programming. Biological Psychiatry, 65(2), 165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–15. [DOI] [PubMed] [Google Scholar]
- Cooper N., Tompson S., Brook O’Donnell M., Emily B.F. (2015). Brain activity in self- and value-related regions in response to online antismoking messages predicts behavior change. Journal of Media Psychology, 27(3), 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A., Stawarczyk D., Majerus S., et al. (2010). The neural basis of personal goal processing when envisioning future events. Journal of Cognitive Neuroscience, 22(8), 1701–13. [DOI] [PubMed] [Google Scholar]
- Dillard J.P., Peck E. (2000). Affect and persuasion emotional responses to public service announcements. Communication Research, 27(4), 461–95. [Google Scholar]
- Falk E.B. (2010). Communication neuroscience as a tool for health psychologists. Health Psychology, 29(4), 355–7. [DOI] [PubMed] [Google Scholar]
- Falk E.B., Berkman E.T., Lieberman M.D. (2012). From neural responses to population behavior: neural focus group predicts population-level media effects. Psychological Science, 23(5), 439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Berkman E.T., Mann T., Harrison B., Lieberman M.D. (2010a). Predicting persuasion-induced behavior change from the brain. Journal of Neuroscience, 30(25), 8421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Berkman E.T., Whalen D., Lieberman M.D. (2011). Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology, 30(2), 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., O'Donnell M.B., Tompson S., et al. (2016). Functional brain imaging predicts public health campaign success. Social Cognitive and Affective Neuroscience, 11(2), 204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Rameson L., Berkman E.T., et al. (2010b). The neural correlates of persuasion: a common network across cultures and media. Journal of Cognitive Neuroscience, 22(11), 2447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl E.C., Neumann J., Bogler C., Cramon D. Y v. (2008). The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Human Brain Mapping 29(5), 581–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Bullmore E.T. (2016). Fundamentals of Brain Network Analysis. San Diego: Academic Press. [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimuth V.S., Quinn S.C. (2004). The contributions of health communication to eliminating health disparities. American Journal of Public Health, 94(12), 2053–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(7), 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker F.E.K., Schmälzle R., Renner B., Schupp H.T. (2015). Neural correlates of HIV risk feelings. Social Cognitive and Affective Neuroscience, 10(4), 612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Malach R., Heeger D.J. (2010). Reliability of cortical activity during natural stimulation. Trends in Cognitive Sciences, 14(1), 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. (2004). Intersubject synchronization of cortical activity during natural vision. Science, 303(5664), 1634–40. [DOI] [PubMed] [Google Scholar]
- Holmes C.J., Hoge R., Collins L., Woods R., Toga A.W., Evans A.C. (1998). Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography, 22(2), 324–33. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen I.P., Koskentalo K., Balk M.H., et al. (2008). Inter-subject synchronization of prefrontal cortex hemodynamic activity during natural viewing. The Open Neuroimaging Journal, 2, 14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M., Schupp H.T., Stark R., Vaitl D. (2005). Neuroimaging of emotion: empirical effects of proportional global signal scaling in fMRI data analysis. NeuroImage, 25(2), 520–6. [DOI] [PubMed] [Google Scholar]
- Kang Y., Cappella J., Fishbein M. (2006). The attentional mechanism of message sensation value: interaction between message sensation value and argument quality on message effectiveness. Communication Monographs, 73(4), 351–78. [Google Scholar]
- Kaye S.-A., White M.J., Lewis I. (2016). The use of neurocognitive methods in assessing health communication messages: a systematic review. Journal of Health Psychology, doi: 10.1177/1359105316630138. [DOI] [PubMed] [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14(5), 785–94. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28(11), 1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M. (2010). Emotion and the motivational brain. Biological Psychology, 84(3), 437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben D.D., Loughead J.W., Ruparel K., et al. (2009). Reduced prefrontal and temporal processing and recall of high "sensation value" ads. NeuroImage, 46(1), 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R.A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–34. [DOI] [PubMed] [Google Scholar]
- Mennes M., Jenkinson M., Valabregue R., Buitelaar J.K., Beckmann C., Smith S. (2014). Optimizing full-brain coverage in human brain MRI through population distributions of brain size. NeuroImage, 98, 513–20. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function, 214(5-6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar S.M. (2006). A 10-year retrospective of research in health mass media campaigns: where do we go from here?. Journal of Health Communication, 11(1), 21–42. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., Greck M. d., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain–A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–57. [DOI] [PubMed] [Google Scholar]
- Palmgreen P., Stephenson M.T., Everett M.W., Baseheart J.R., Francies R. (2002). Perceived message sensation value (PMSV) and the dimensions and validation of a PMSV scale. Health Communication, 14(4), 403–28. [DOI] [PubMed] [Google Scholar]
- Petty R.E., Cacioppo J.T. (1986). The elaboration likelihood model of persuasion In Communication and Persuasion (pp. 1–24). New York: Springer. [Google Scholar]
- Petty R.E., Brinol P., Priester J.R. (2009). Mass media attitude change: Implications of the Elaboration Likelihood Model of Persuasion In Bryant J., Oliver M. D. (Eds.), Media Effects: Advances in Theory and Research (pp. 125–164). New York: Routledge. [Google Scholar]
- Renner B. (2004). Biased reasoning: adaptive responses to health risk feedback. Personality & Social Psychology Bulletin, 30(3), 384–96. [DOI] [PubMed] [Google Scholar]
- Renner B., Reuter T. (2012). Predicting vaccination using numerical and affective risk perceptions: the case of A/H1N1 influenza. Vaccine, 30(49), 7019–26. [DOI] [PubMed] [Google Scholar]
- Renner B., Schupp H.T. (2011). The perception of health risks In Friedman H. S. (Ed.), Oxford Handbook of Health Psychology (pp. 637–665). New York: Oxford University Press. [Google Scholar]
- Renner B., Gamp M., Schmälzle R., Schupp H.T. (2015). Health Risk Perception In Wright J. D., editors. International Encyclopedia of the Social and Behavioral Sciences (pp. 702–709). Amsterdam: Elsevier. [Google Scholar]
- Robert Koch-Institute. (2014). Daten und Fakten: Ergebnisse der Studie »Gesundheit in Deutschland aktuell 2012«. Beiträge zur Gesundheitsberichterstattung des Bundes. Berlin, Germany. Retrieved from http://www.rki.de/DE/Content/Gesundheitsmonitoring/Gesundheitsberichterstattung/GBEDownloadsB/GEDA12.pdf?__blob=publicationFile (accessed 22 August 2016).
- Rumpf H.-J., Hapke U., Meyer C., John U. (2002). Screening for alcohol use disorders and at-risk drinking in the general population: psychometric performance of three questionnaires. Alcohol and Alcoholism, 37(3), 261–8. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Bradley M.M., Fitzsimmons J.R., Lang P.J. (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. NeuroImage, 24(4), 1265–70. [DOI] [PubMed] [Google Scholar]
- Schmälzle R., Häcker F.E.K., Honey C.J., Hasson U. (2015). Engaged listeners: Shared neural processing of powerful political speeches. Social Cognitive and Affective Neuroscience, 10(8), 1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmälzle R., Häcker F.E.K., Renner B., Honey C.J., Schupp H.T. (2013). Neural correlates of risk perception during real-life risk communication. Journal of Neuroscience, 33(25), 10340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmälzle R., Schupp H.T., Barth A., Renner B. (2011). Implicit and explicit processes in risk perception: neural antecedents of perceived HIV risk. Frontiers in Human Neuroscience, 5, 43.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig D., Wang A.-L., Jagannathan K., et al. (2014). Low message sensation health promotion videos are better remembered and activate areas of the brain associated with memory encoding. PloS One, 9(11), e113256.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simony E., Honey C.J., Chen J., et al. (2016). Dynamic reconfiguration of the default mode network during narrative comprehension. Nature Communications, 7, 12141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M.D. (2002). Involvement as goal-directed strategic processing: extending the elaboration likelihood model In Dillard J. P., Pfau M., editors. The Persuasion Handbook: Developments in Theory and Practice. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Slovic P., Peters E. (2006). Risk Perception and Affect. Current Directions in Psychological Science, 15(6), 322–5. [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: G. Thieme; Thieme Medical Publishers. [Google Scholar]
- Tannenbaum M.B., Hepler J., Zimmerman R.S., et al. (2015). Appealing to fear: A meta-analysis of fear appeal effectiveness and theories. Psychological Bulletin, 141(6), 1178–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.-W., Kross E. (2013). An fMRI-based neurologic signature of physical pain. The New England Journal of Medicine, 368(15), 1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.-L., Lowen S.B., Shi Z., Bissey B., Metzger D.S., Langleben D.D. (2016). Targeting modulates audiences' brain and behavioral responses to safe sex video ads. Social Cognitive and Affective Neuroscience, 11(10), 1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.-L., Ruparel K., Loughead J.W., et al. (2013). Content matters: neuroimaging investigation of brain and behavioral impact of televised anti-tobacco public service announcements. Journal of Neuroscience, 33(17), 7420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R., Huskey R., Mangus J.M., Westcott Baker A., Turner B.O. (2014). Neural predictors of message effectiveness during counterarguing in antidrug campaigns. Communication Monographs, 82(1), 4–30. [Google Scholar]
- Weinstein N.D. (1989). Effects of personal experience on self-protective behavior. Psychological Bulletin, 105(1), 31–50. [DOI] [PubMed] [Google Scholar]
- Witte K., Allen M. (2000). A meta-analysis of fear appeals: implications for effective public health campaigns. Health Education & Behavior, 27(5), 591–615. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2014). Global status report on alcohol and health Geneva, Switzerland. Available: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ [August 22, 2016].
- Zhao X., Strasser A., Cappella J.N., Lerman C., Fishbein M. (2011). A measure of perceived argument strength: reliability and validity. Communication Methods and Measures, 5(1), 48–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillmann D. (2002). Exemplification theory of media influence In Bryant J., Zillmann D., editors. Media Effects: Advances in Theory and Research. Media Effects: Advances in Theory and Research (2nd ed., pp. 19–41). Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.