Abstract
Acute stress is associated with beneficial as well as detrimental effects on cognition in different individuals. However, it is not yet known how stress can have such opposing effects. Stroop-like tasks typically show this dissociation: stress diminishes speed, but improves accuracy. We investigated accuracy and speed during a stroop-like task of 120 healthy male subjects after an experimental stress induction or control condition in a randomized, counter-balanced cross-over design; we assessed brain–behavior associations and determined the influence of individual brain connectivity patterns on these associations, which may moderate the effect and help identify stress resilience factors. In the mean, stress was associated to increase in accuracy, but decrease in speed. Accuracy was associated to brain activation in a distributed set of brain regions overlapping with the executive control network (ECN) and speed to temporo-parietal activation. In line with a stress-related large-scale network reconfiguration, individuals showing an upregulation of the salience and down-regulation of the executive-control network under stress displayed increased speed, but decreased performance. In contrast, individuals who upregulate their ECN under stress show improved performance. Our results indicate that the individual large-scale brain network balance under acute stress moderates cognitive consequences of threat.
Keywords: brain state, executive control, functional connectivity, salience, stress
Introduction
Acute stress is associated to both negative and positive influences on cognitive performance (Sandi, 2013). The negative consequences of stress on performance are especially critical in occupations in which small mistakes can have life-threatening consequences such as in pilots, surgeons, policemen or firefighters. Indeed, individual stress reactivity may be an important criterion for selection and health status for people with these occupations. It would thus be important to determine individual factors which relate to improved cognitive performance under stress.
In acute stress, a conceptual link between stress-related neurochemical processes, such as fast action of catecholamines and slower action of glucocorticoids, to large-scale brain network balance has been proposed (Hermans et al., 2014). The relative configuration of different large-scale brain networks may be a potential biomarker for vulnerability or resilience to stress. The model builds on the spatial selectivity and differential temporal effects of neuromodulators invoked by a stressor. Early and fast stress responses via catecholaminergic pathways may have spatially selective effects on a network of brain regions that have been labeled ‘salience network’ (SN, (Arnsten, 2009; Hermans et al., 2011; Sara and Bouret, 2012; Shirer et al., 2012)), which is often conceptualized as including the amygdala, the anterior middle cingulated (aMCC; dorsal anterior cingulate, dACC), anterior insula, thalamus, temporo-parietal cortices, striatum and the brainstem. Stress-related catecholamine increase leads to an upregulation of the salience network during acute stress. The model further proposes that the change in SN is mirrored by changes in the executive control network (ECN), which may show reciprocal effects. Thus, the ECN, which largely consists of the dorsolateral prefrontal cortex, dorso-medial prefrontal cortex and dorsal posterior parietal cortex (Smith et al., 2009; Hermans et al., 2014), is suppressed in acute stress and upregulated in the aftermath of stress. These large-scale brain network modulations reflect an adaptive response to threat. While the upregulation of the salience network at the cost of the ECN inherently explains the detrimental effect of stress on executive control tasks, it does not intuitively explain why certain executive control tasks may show an improvement under stress and what role inter-individual differences in large-scale network balance may play in the modulation of performance in such tasks (Easterbrook, 1959; Lupien et al., 2007; Sandi, 2013).
For example, stroop-like tasks have previously led to robust findings of improved cognitive performance (e.g. reaction times and accuracy), when subjects are under time pressure, under threat, subjected to aversive noise or preparing for an exam (Chajut and Algom, 2003; Kofman et al., 2006; Booth and Sharma, 2009; Hu et al., 2012). These results are explained by general shifts in cognition, optimizing cognitive capacity or motivational accounts (Chajut and Algom, 2003). In a series of experiments, strong support was found for a general attentional account, which is not modulated by motivational factors or cognitive capacity (Chajut and Algom, 2003).
A recent study found increased performance and reduced speed in a stroop-like task under stress and the authors proposed two separate underlying cognitive processes, as opposed to a simple speed–accuracy trade-off (Hu et al., 2012). They propose a general slowing of executive control processing demands, and a general reduction of processing of task-irrelevant information, which leads to performance facilitation in stroop like tasks under stress. Such differential effects on behavioral parameters under stress may be moderated by stress-related large-scale network dynamics and thus may provide a parsimonious opportunity to explain heterogeneity in behavioral patterns found in stroop-like tasks under stress.
In this study we investigated how speed and performance in a cognitive task may be modulated under acute stress and how this is influenced by stress-related large-scale network dynamics. We used the emotional conflict task (Etkin et al., 2006), which is a stroop-like task that requires categorical decisions on a target and in certain trials suppression of a predominant response. Responses in these incongruent trials were compared to responses on control trials (in which no response suppression is needed).
To probe individual differences in the stress response we investigated a large group of male subjects who were confronted with stressful and neutral videos during two fMRI sessions in a randomized, counter-balanced cross-over study (van Marle et al., 2009; Cousijn et al., 2010; Hermans et al., 2011; Everaerd et al., 2015). All participants performed an emotional version of the stroop task after a stress induction procedure or a neutral control procedure (Etkin et al., 2006). To dissociate separate cognitive processes (underlying reaction time and accuracy) under the influence of stress (Hu et al., 2012), individual differences in speed and in accuracy were associated to stress-related changes in brain activation. Modulation of these brain behavior associations by stress-related brain network connectivity dynamics is assessed in a final step. This probes if individual differences in stress-related ‘brain states’ (different brain network connectivity profiles) effectively modulate the translation of brain activation to performance and thereby may serve as a biological marker of individual stress reactivity.
Materials and methods
Participants
The study sample consisted of 120 healthy men (see Supplementary Material for details). We excluded participants who reported a history of somatic diseases with a possible influence on brain structure or function, neurologic or psychiatric disorders, substance abuse and dependence (recreational drugs, alcohol), who reported medication or recreational drug use in the preceding 6 months, and conventional MRI contraindication. All subjects were paid a 60 Euros compensation for full participation in the study and received a complete description of the study after which written informed consent was obtained. The study was approved by the local ethics committee (CMO Region Arnhem-Nijmegen, Netherlands) and was conducted in accordance with the Declaration of Helsinki in its 2008 revised form.
Procedure
All subjects were measured under two conditions (stressful and neutral, separated by >5 days). On the first session, assignment to either the stress or the neutral condition was randomized and counterbalanced. All participants adhered to a highly standardized study protocol. Details on this protocol can be found in Supplementary Material. During the stress condition, highly aversive movie clips (depicting physical violence) were shown in the MRI scanner and in the neutral condition, participants were shown a neutral movie matched to the stress movie. The subjects were instructed to watch the video as if they would be witnessing the events.
Participants were requested to perform the emotion conflict resolution task (Etkin et al., 2006), which has been validated and applied in previous neuroimaging studies (Etkin et al., 2006; Egner et al., 2008). The tasks consists of viewing faces displaying either happy or fearful emotional expressions, which are overlaid with either the Dutch word for happy or the Dutch word for fearful written in red font. The facial display of emotion could either be incongruent or congruent. Stimuli were presented for one second, presentation of the stimulus was followed by a fixation cross (ISI, 3–5 s). The paradigm took approximately 13 min in total (see Supplementary Figure S1).
MR data acquisition
MR data were acquired on a 1.5 T Avanto MR scanner (Siemens, Erlangen, Germany) at the Donders Institute in Nijmegen, the Netherlands. A series of around 242 T2*-weighted functional images were acquired using gradient echo-planar imaging (EPI) with the following parameters: 32 oblique transverse slices, voxel size = 3.5 × 3.3 × 3.3 mm, repetition time (TR) = 2.34 s, flip angle α = 90°, echo time (TE) = 35 ms. A 3D magnetization-prepared rapid gradient echo (MPRAGE) anatomical T1-weighted image was acquired for normalization purposes (176 slices, 1.0 mm isotropic, TR = 2730 ms, TE = 2.95 ms).
Behavioral analyses
All behavioral analyses (including moderation analyses) were conducted with SPSS 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Full factorial generalized estimating equations (GEEs) procedure was used for analyzing reaction time and accuracy of responses during the emotional conflict task. GEE is a generalization of generalized linear model approaches that considers within-subject correlations and also allows explicit specification of link functions. Both models (reaction time and accuracy) included the within subject factor condition (stress vs control) and the within subject factor congruency (incongruent vs congruent). For reaction time data an identify link function and for the response data a probit link function (to better capture the unbalanced binomial distribution of responses) was implemented and an unstructured working correlation matrix was used for both.
Data analysis
Standard fMRI preprocessing procedures plus ICA-based denoising for movement artifacts was performed. For details see Supplementary Material. This pipeline was applied for both univariate analyses and functional connectivity.
GLM brain data analysis
The emotion conflict resolution task was modeled on preprocessed and denoised 4D files of each participant and session. Two regressors for correct congruent and incongruent trials were set up, additionally a third nuisance regressor was entered which included all incorrect trials as well as any instructions. In a second step, a fixed effects analysis of stress versus control was conducted for the incongruent and congruent trials and the differential contrast (IC vs C) on the subject level. For group statistics, we analyzed these models using the mixed effects approaches FLAME 1 + 2. Furthermore, we included subject-specific difference scores of percent correct and reaction times in this model, allowing for the analyses of covarying difference in brain activity with differing behavioral scores. That is, we analyzed brain activation change in relation to performance change (brain–behavior association; Figure 1). All reported Z-statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of P = 0.05 (Worsley, 2001).
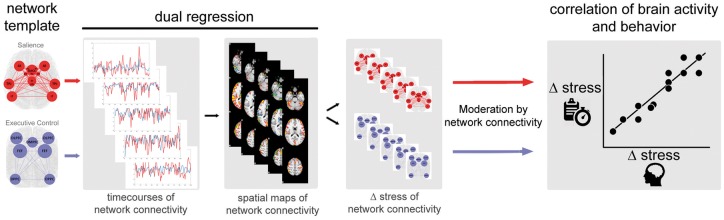
Fig. 1.
The processing pipeline and rationale for the moderated moderation is depicted in this figure. We investigated if network connectivity changes under stress influence the correlation between brain activity and behavioral performance. Network connectivity changes were determined by applying dual regression and calculating stress related changes of the salience and executive control networks. The influence of these stress-related network changes on the correlation of brain activity and behavior is analyzed in the moderated moderation.
Network connectivity strength
The preprocessed and denoised 4D files were also used for connectivity analyses applying dual regression. In our dual regression, unthresholded template maps of the salience and ECN (plus default mode network) were used to generate subject-wise network time courses. Regression of these time courses against the data resulted in individual spatial maps (Filippini et al., 2009). Templates of executive control and default mode network (DMN) maps were taken from Smith and colleagues (Smith et al., 2009) and a template for the salience network was acquired from the Greicius group (Shirer et al., 2012).
Network maps during stress and control were subtracted per subject. This resulted in one volume per subject and network that reflected change in connectivity between stress and control. By applying an inclusive mask of the template maps (thresholded at Z > 3) on the individual spatial difference maps, one mean connectivity change value per subject and network was extracted (Fig. 1; details in Supplementary Material). Prior to further analyses, network connectivity change scores were Z-standardized. The standardized mean of SN and ECN change were entered into the moderation analysis.
Moderation
The moderated moderation was conducted with the PROCESS macros (Hayes, 2015). In a moderated moderation, a simple regression (in our case the prediction of behavior change by brain activation change) is modulated by two moderator variables. We used the subject-wise extracted mean connectivity change values from salience and executive control as moderators. In this way, we formally tested the influence of change in salience network connectivity on the influence of change of ECN activity on the brain–behavior association (Figure 1).
For this purpose for each significant cluster of the analysis of brain–behavior association median, beta activation values were extracted. The different beta activation values per cluster were entered in moderation models as independent variable (one model per cluster). The outcome variable was task performance (accuracy and reaction time). This association of brain and performance is what has been analyzed in the above described GLM and called brain behavior association. The standardized mean connectivity change values were used as moderators. Significance was determined by determining significance of the three way interaction (Hayes, 2015). All models are tested using 1000 bootstrapping samples and a confidence interval of 95%.
Additional stress measures
During each session, saliva samples and different psychophysiological measures were obtained. Details on the acquisition and results of these measures are summarized in Supplementary Materials.
Results
Stress induction
Stress induction was successful, which replicates results of previous studies using a similar stress induction procedure (van Marle et al., 2009; Cousijn et al., 2010; Hermans et al., 2011). Details on the stress induction in this sample have been previously reported (Everaerd et al., 2015). Briefly, the stress condition led to higher salivary cortisol levels (stress mean: 101.4% of baseline, control mean: 90.9% of baseline, SD = 45.7%, t(112)=1.46, P = 0.016), higher systolic and diastolic blood pressure (systolic stress mean: 108.5 mm Hg, systolic control mean: 106.5 mm Hg, SD =6.7, t(116) = 3.24, P = 0.002; diastolic stress mean: 69.9 mm Hg, diastolic control mean: 68.4 mm Hg, SD = 4.7, t(117)=3.23, P = 0.002), higher heart rate (stress mean: 67.1 BPM, control mean: 63.9 BPM, SD = 7.6, t(110)=4.36, P = 0.001), decreased heart rate variability (stress mean: 62.2 ms, control mean: 68.6 ms, SD = 26.2, t(109) = 4.36, P = 0.012), and elevated self ratings of negative affect (stress mean: 17.1, control mean: 13.7, SD = 5.9, t(116) = 6.28, P = 0.001) compared to the neutral control condition.
Accuracy and speed analyses
Behavioral analyses of the emotional conflict task tested if speed or accuracy differed in trials in which the stimulus configuration (emotional face – emotional word) were congruent and trials in which this configuration was incongruent and as an additional factor on whether the subjects were stressed or not.
The GEE analysis for correct versus incorrect responses was significant for the factor congruency (Wald χ2 =5.205; P = 0.023), and the interaction between stress and congruency (Wald χ2 =4.419; P = 0.036), but no main effect of stress (P = 0.113). Subjects showed more correct responses in the congruent than in incongruent trials. The interaction was driven by better performance in incongruent trials in the stress condition compared to the neutral control condition (Figure 2A).
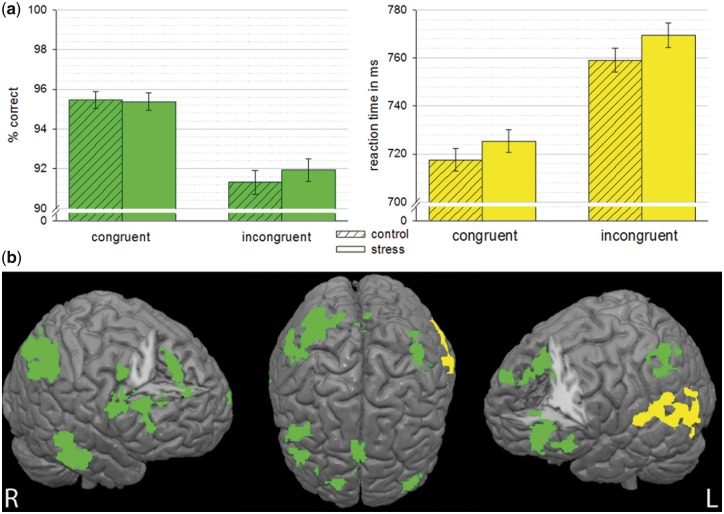
Fig. 2.
(a) Behavioral data for congruent and incongruent trials under stress (plain) and control (striped). Response accuracy given in percent correct responses (green). Mean reaction times (yellow) are displayed for correct response trials only. Error bars indicate 95% CI. (b) Association of increase in accuracy (green) and decrease in speed (yellow) in the task with increase in brain activation in stress compared to control (for incogruent trials only, controlled for main effect of stress). Results are cluster level corrected threshold (Z > 2.3, P < 0.05) and overlaid on an MNI template brain.
For reaction time data, the GEE analysis was significant for the factors congruency (Wald χ2 =278.3; P < 0.001), stress (Wald χ2 =272.3; P < 0.001) and the stress x congruency interaction (Wald χ2 =333.4; P = 0.001), with slower reactions in the stress condition compared to the neutral control condition, slower reaction times in the incongruent condition and slowest reaction times in the incongruent condition under stress (Figure 2A).
As we observed dissociation in behavioral results, with better accuracy but slower reactions under stress, we correlated these performance measures. We observed moderate inter-correlations between reaction time and accuracy (r between −0.4 to −0.31, P < 0.001) in stress and control separately, but the difference score did not show a significant correlation (r = −0.09, P = 0.3). Thus, a general speed–accuracy trade-off seems to be present, but there is no indication for a pronunciation of such an effect under stress. The decrease of speed and increase in accuracy under stress does not reflect a stress-related speed–accuracy trade off, rather some subjects get slower, while others become better.
Brain–behavior association
Speed and accuracy measures were entered as covariates in a single model at the group level, thereby only uniquely explained variance contributes to significant findings. Individual differences in responses accuracy (stress minus control) were positively associated with increases in brain activity from control to stress (in incongruent trials) in bilateral middle frontal gyrus, pregenual ACC extending into dorsal ACC and pre-SMA, right superior frontal gyrus, bilateral inferior frontal gyrus (pars triangularis), left inferior and middle temporal gyrus, bilateral supramarginal gyrus and angular gyrus extending into precuneus (Fig. 2b; Supplementary Table S3). For reaction time differences we observed a negative association with differential reaction times and differential brain activation in a cluster extending from the left temporo-parietal junction over middle and superior temporal gyrus into the planum temporale, with faster responses associated to higher activation under stress (see Figure 2b and Supplementary Table S3). See Supplementary Material for results on the main effect of stress and the covariance analyses for congruent trials.
Modulation of brain–behavior association by inter-individual differences in network connectivity
We did not find any significant mean differences over the whole group in ECN, SN and DMN using a non-parametric test of mean differences between stress and control (dual-regression of the three networks templates, all P > 0.112). However, when investigating whether brain–behavior associations were modulated by individual profiles of network connectivity change under stress in SN and ECN, we found a clear dissociation (by using a ‘moderated moderation’ (Hayes, 2015), details on the procedure in Supplementary Material). We tested if brain network connectivity profiles (e.g. change in both SN and ECN connectivity taken together) modulate the translation of brain activation to performance (for speed and accuracy, respectively). The influence of mean connectivity change in ECN and SN under stress on the brain–behavior association can be described by looking at prototypical brain state profiles: The prototypic profiles are: (1) Increase in SN and decrease in ECN under stress, (2) increase in ECN and decrease in SN under stress, (3) Increase in connectivity in stress in both ECN and SN under stress, (4) decrease in ECN and SN under stress. A significant moderation is marked by a significant difference between profiles regarding the correlation between brain and behavior. That is, how ECN and SN change under stress modulated the correlation between brain and behavior.
It is important to note that in the moderation the above described profiles and the visualization in the figure are not simply subsets of individuals, but ‘profiles’ of prototypical individuals showing an increase or decrease in brain networks: These prototypical individuals are based on the regression equation of the ‘moderated moderation’. For interpretation and visualization, coefficients are fixed at an increase or decrease of one standard deviation in connectivity strength in ECN and SN. The different profiles thus represent the variance in network connectivity observed in our sample and ultimately we tested if this variance influences brain–behavior associations.
For accuracy–brain associations, all clusters showed significant brain network moderation effects. The moderation analysis for speed–brain associations was not significant (in the interaction term), but the strength of speed–brain association differs in significance in different brain connectivity profiles (Figure 3, see Supplementary Material for details on statistical parameters and complete results tables for different clusters).
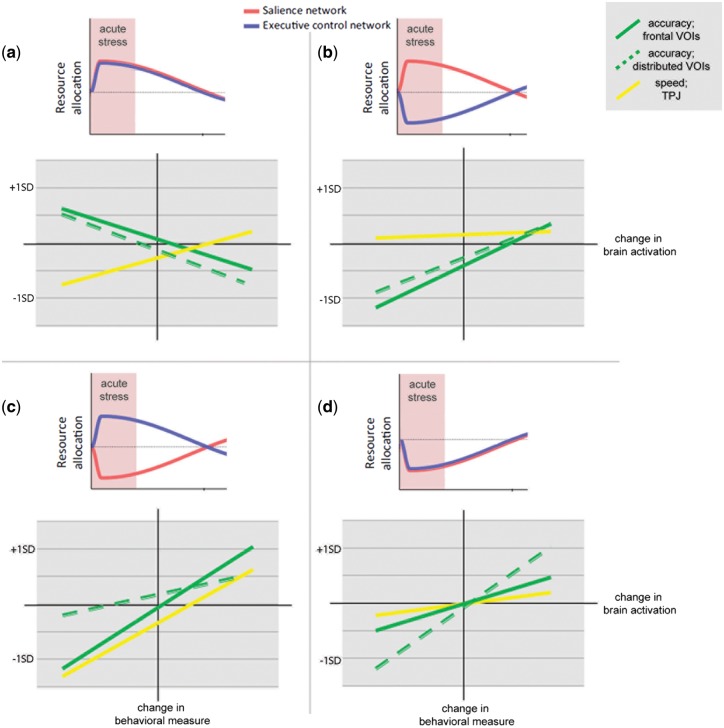
Fig. 3.
The moderation analysis can be illustrated at four prototypic ‘brain network connectivity profiles’. These profiles were characterized by a distinct pattern in change of connectivity in the two networks in stress. (a) profile which showed increase in connectivity in stress in SN and ECN (b) profile which showed increase in connectivity only in SN and decrease in ECN (c) profile which showed increase in ECN and decrease in SN and (d) profile which showed decrease in connectivity in both. For each brain state, the correlation between brain and behavior is displayed, the plots depict the correlation from the VOIs (frontal VOIs: DLPFC, IFG, pgACC, distributed VOIs: DMPFC, AG, MTG). Change in reaction time or percent correct is plotted on the y-axis and change in brain activation on the x-axis. Positive values represent an increase of activity in stress and negative a decrease in stress. Slopes and offset were generated by the moderated moderation based on + 1 and −1 SD in all scales, that is the different profiles represent the variance in network connectivity observed in our sample and how this variance influenced brain–behavior associations.
The moderation effects for accuracy in bilateral angular gyri, the MTG and the DMPFC were very similar, as well as DLPFC and IFG + pgACC, therefore these were grouped and referred to as distributed volumes of interest (VOIs) and frontal VOIs, respectively. These accuracy–brain associations which showed a spatial overlap to the ECN, were based on their moderation pattern separated into a set of frontal regions (frontal VOIs) and a set of more posterior, but distributed regions (distributed VOIs). This was solely done for display purposes.
Each profile is discussed in terms of strength of correlation and in the offset or overall performance/speed level.
The first profile (SN↑ + ECN↑) displayed a reversed brain-accuracy correlation in both VOIs. In the brain–speed association the correlation was moderate, but the mean speed was reduced (Figure 3a).
In the second profile (SN↑ + EC↓), brain–accuracy association was in all VOIs moderate. Compared to other brain states, more brain activation increase was necessary to reach a similar accuracy level, thus mean accuracy was lowered. Conversely, the brain–speed association was virtually non-existent with a moderate mean increase in speed at a given level of brain activation (Figure 3b). Overall, this profile demonstrates the negative influence of up-regulation of SN and down-regulation of ECN on accuracy, while at the same time speed of responses in increased in this profile.
The third profile (SN↓ + ECN↑), was characterized by a relatively higher mean level of accuracy and for the frontal VOIs a stronger correlation between accuracy and brain activation. For brain–speed associations, the correlation was in relation to other groups increased and the mean speed was decreased (Figure 3c). Overall, this profile demonstrates the positive influence of ECN up-regulation (and parallel SN down-regulation) on accuracy.
The fourth profile (SN↓ + ECN↓) showed a moderate correlation in brain accuracy for the frontal VOIs and a moderate to low correlation in the brain–speed association, while the brain-accuracy correlation in the distributed VOIs was relatively higher (Figure 3d).
In summary, ECN upregulation compared to down-regulation seemed to have a positive influence on quality of executive processing (accuracy), while for speed–brain association ECN up-regulation lead to an increase in correlation between brain activity (TPJ) and speed, which may reflect a higher cognitive flexibility. Up-regulation of SN and down-regulation of ECN had a detrimental effect on accuracy, while at the same time speed of responses was increased.
Discussion
Acute stress can give rise to cognitive facilitation, but also to cognitive disturbance, based on circumstances and individual differences in stress reactivity (Hermans et al., 2011; Sandi, 2013). Applying a task in which previous research has demonstrated two separate stress-related effects on cognition (Hu et al., 2012), we aimed to investigate if we can elucidate underlying neuronal patterns of such a dissociative effect on cognition during stress and if this effect is moderated by inter-individual differences in stress-related large-scale network dynamics or ‘brain connectivity profiles’ in a large group of male subjects. In other words we aimed to investigate why some people become mainly faster under stress, while others become better in the same task. Our results indicate that the state of brain connectivity change in the ECN and the salience network (SN) of an individual explains this dissociation at the behavioral level.
We were able to show that our stress induction is associated with generally reduced stroop interference (accuracy), but also with slower reaction times (speed) at the group level. This replicates the finding of two dissociable effects of stress on cognitive processes (Hu et al., 2012). Similarly to our results, reaction time slowing under stress was in this study apparent over all conditions, while reduction of the error rate was only present in the incongruent condition. These results are explained as reflecting two (opposing) effects of stress on cognitive processing, with a general slowing of executive functions, which is accompanied by a reduction of processing of task-irrelevant information leading to reduced interference. The notion of two separate (cognitive) processes receives additional support by our finding that behavioral change scores were not correlated with each other. Thus, there is no evidence for a modulation of speed–accuracy trade-off under stress. Nevertheless, effect sizes are small, which reflects the heterogeneous nature of stress reactivity. As intended when designing the task, this inter-individual variance was subsequently targeted in the analysis of brain–behavior associations and most importantly in the analysis of the influence of brain connectivity profiles on translation of brain activation to behavior.
Increased accuracy in stress was significantly associated to increasing activity in a set of brain regions that strongly overlap with the ECN (Smith et al., 2009; Hermans et al., 2014). This network showing increasing activity with increasing accuracy encompassed bilateral IFG and DLPFC, the pregenual ACC, pre-SMA, extending into the aMCC, bilateral angular gyrus and precuneus (Fig.1b). In general, the association of these regions to executive control is very well established (Smith et al., 2009; Cieslik et al., 2013; Niendam et al., 2012; Rottschy et al., 2012a). Such an integration of individual differences in cognitive neuroscience studies is increasingly popular (Braver et al., 2010). Some studies have found an association between activity increase in the parietal cortex and working memory load (Vogel and Machizawa, 2004; Todd and Marois, 2005) or increase in fronto-parietal attention network with increasing demand (Bavelier et al., 2012). Nevertheless, the stronger recruitment of a widespread network of brain areas associated to performance increase in an executive control task is to our knowledge a novel finding. In line with the reasoning of Hu and colleagues (Hu et al., 2012), this effect may be mediated by enhanced selective attention to relevant stimuli under stress which might engage the ECN to a larger extent. As the ECN has in a meta-analysis been shown to have strong overlap with brain networks proposed to underlie selective attention (Rottschy et al., 2012b), these finding would lend support to the notion that stress improves accuracy via a stronger engagement of selective attention brain networks.
The opposing cognitive effect of slower speed was significantly correlated with decreasing activity in the parietal operculum, the superior temporal gyrus (STG) and the inferior parietal lobule (IPL; Figure 1b). The IPL and STG have (as areas forming the functional label TPJ) been thought to play an important role in stimulus-driven, bottom-up attention processes, especially when having to detect rare or deviant events (Desimone and Duncan, 1995). TPJ activity has been shown to increase with task relevance in a stimulus detection paradigm (Downar et al., 2001). Thus, we tentatively assume that activation decrease in this area is associated to slower reactions under stress that may reflect a focus away from processing of the target stimulus (eg the face). While bottom-up modulated attention is thought to be mainly right lateralized, there is evidence for bilateral and also mainly left lateralized bottom-up attentional processing (DiQuattro and Geng, 2011; Vossel et al., 2014), which is thought to depend on the context. Thus, this patterns shows overlap with the ventral attention network supporting bottom-up stimulus driven processing (Corbetta and Shulman, 2002) or exogenous attention (Hermans et al., 2014)
Furthermore, these bottom-up stimulus driven detection effects can be seen as conceptually different from the more dorsal, cognitive attentional processes (Corbetta and Shulman, 2002) or endogenous attention (Hermans et al., 2014), which is associated to executive control. These two opposing patterns could be seen as indicative support for two separate modes of cognitive processing affected by stress in this task; e.g. an influence on more bottom-up, contextual stimulus driven versus an influence on more top-down facilitation of selective attention.
That is, our mild stressor may in certain individuals induce a shift towards enhanced selective attention or executive control, which in a top-down modulatory manner is associated to higher accuracy. In other subjects this stressor may engage bottom-up, stimulus driven responses, which modulate reaction times and is associated to increasing TPJ activity with faster responses. In order to further elucidate these individualized reactivity patterns, we computed the moderation analysis.
The moderation analyses displayed how the stress-related connectivity profile of two large-scale brain networks influences the translation of brain activation to behavior in our task. In other words, we investigated if increase or decrease in large scale brain networks under stress change the correlation of brain and behavior during the task. As stress-related changes in speed and accuracy are thought to represent two distinct cognitive mechanisms, we aimed to investigate if speed and accuracy are also differentially modulated by the change in ECN and SN and their interaction. We found moderation effects of brain states on brain–accuracy associations in all clusters at issue, but there was no significant moderation for brain–speed associations. Nevertheless, as there was a change of significance in the correlation between different levels of change in ECN and SN (different ‘brain connectivity profiles’) and for the purpose of comparison to the brain-accuracy moderation, we also discuss and display these results.
The most marked changes to the brain-accuracy correlation are apparent in the profile characterized by an increase in both ECN and SN in stress. Here the brain–behavior association is reversed compared to the group average: increasing brain activity in all clusters is associated to decreasing accuracy. This reversed correlation could indicate that increase in ECN and SN under stress may reflect an optimal brain connectivity state for coping with stress in which further increase in executive control related brain activation is not beneficial to accuracy, but indeed impairs performance. The second profile marked by an increase of ECN and a decrease of SN in stress was characterized by elevated mean accuracy accompanied by positive brain–accuracy associations.
Taken together, an increase in ECN network connectivity (regardless of the state of SN) under stress seemed to be beneficial for coping. ECN increase under stress is associated to a stable high level of performance. Individuals showing an increase in both ECN and SN connectivity would maybe have to employ different strategies to increase accuracy when necessary compared to individuals who only show an increase in ECN connectivity under stress, in which increasing effort and focus on executive control may be beneficial. Nevertheless, one putative biomarker of high-stress resilient individuals seems be the ability to elevate ECN connectivity in response to a stressor. An interesting venue for future research could be, whether training to increase ECN connectivity may positively modulate performance when stressed.
Our results are in line with the recent proposal that the combined reaction of ECN and SN after acute stress characterize the brain stress response and that this is related to the detrimental effect of stress on cognition (Hermans et al., 2014). This detrimental effect of stress on cognition indeed is also apparent in our data the profile marked by an increase in SN and a decrease in ECN.
The moderation analyses also gave some insight on the assumption of two separate processes underlying these opposing effects on accuracy and speed under stress. Here we propose an influence of executive-control/selective attention on accuracy opposed to an influence on bottom-up stimulus driven processes. A first indication of separate processes may be seen in the fact that brain profiles moderate brain–accuracy associations only, but not brain–speed associations, which should not be the case if these effects have common underlying cognitive correlates. Furthermore, one could argue that executive-control/selective attention should be more reliant on changes in ECN, while stimulus driven processes would be more reliant on changes in SN connectivity. Indeed, we find ECN connectivity increase to be beneficial to accuracy, while on the other hand increased mean speed is observable in the group that shows a selective increase in SN. The latter is in line with the stronger focus on salient stimuli when individuals are stressed (Arnsten, 2009; Hermans et al., 2014) and findings of elevated amygdala responsivity, which is a core hub of SN, to emotional facial stimuli under stress (van Marle et al., 2009). In summary, we propose two separate individual response patterns related to executive control and salience processing based on differential network connectivity.
Conclusion
We were able to demonstrate general facilitation of performance and slowing of responses under stress in a stroop like task in a large group of male subjects. Our results support the assumption of two parallel processes mediating performance change in this task (Hu et al., 2012). These two processes are related to distinct patterns of brain activity and also individual differences in brain connectivity profiles, which may reflect a bottom-up, stimulus driven response pattern and a top-down selective attention/executive-control response pattern. Furthermore, inter-individual differences in stress-induced changes of ECN and SN connectivity enabled us to detect optimal brain profiles in relation to accuracy in this stroop-like task and also lent further support to the hypothesis that acute stress and the detrimental influence of stress on cognition is related to elevated SN and decrease ECN connectivity. Taken together, we demonstrate that large-scale brain network profiles may serve as a biomarker for stress vulnerability and resilience, as they moderate translation of brain activation to behavior.
Supplementary Material
Acknowledgements
We would like to thank M.D. Greicius and C.M. Price for providing us with the maps of the Salience network and Linda de Voogd for help in preparation of data-analysis.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Arnsten A.F.T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D., Achtman R.L., Mani M., Foecker J. (2012). Neural bases of selective attention in action video game players. Vision Research, 61, 132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R., Sharma D. (2009). Stress reduces attention to irrelevant information: Evidence from the Stroop task. Motivation and Emotion, 33, 412–8. [Google Scholar]
- Braver T.S., Cole M.W., Yarkoni T. (2010). Vive les differences! Individual variation in neural mechanisms of executive control. Current Opinion in Neurobiology, 20, 242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajut E., Algom D. (2003). Selective attention improves under stress: implications for theories of social cognition. Journal of Personality and Social Psychology, 85, 231–48. [DOI] [PubMed] [Google Scholar]
- Cieslik E.C., Zilles K., Caspers S., et al. (2013). Is there “One” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cerebral Cortex, 23(11): 2677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–15. [DOI] [PubMed] [Google Scholar]
- Cousijn H., Rijpkema M., Qin S., et al. (2010). Acute stress modulates genotype effects on amygdala processing in humans. Proceedings of the National Academy of Sciences USA, 107, 9867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Duncan J.S. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- DiQuattro N.E., Geng J.J. (2011). Contextual knowledge configures attentional control networks. Journal of Neuroscience, 31, 18026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. (2001). The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage, 14, 1256–67. [DOI] [PubMed] [Google Scholar]
- Easterbrook J.A. (1959). The effect of emotion on cue utilization and the arganization of behavior. Psychological Review, 66, 183–201. [DOI] [PubMed] [Google Scholar]
- Egner T., Etkin A., Gale S., Hirsch J. (2008). Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex, 18, 1475–84. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–82. [DOI] [PubMed] [Google Scholar]
- Everaerd D., Klumpers F., van Wingen G., Tendolkar I., Fernández G. (2015). Association between neuroticism and amygdala responsivity emerges under stressful conditions. Neuroimage, 112, 218–24. [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences USA, 106, 7209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. (2015). An index and test of linear moderated mediation. Multivariate Behavioral Research, 50, 1–22. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., Henckens M.J.A.G., Joëls M., Fernández G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neuroscience, 37, 304–14. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., van Marle H.J.F., Ossewaarde L., et al. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science, 334, 1151–3. [DOI] [PubMed] [Google Scholar]
- Hu K., Bauer A., Padmala S., Pessoa L. (2012). Threat of bodily harm has opposing effects on cognition. Emotion, 12, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman O., Meiran N., Greenberg E., Balas M., Cohen H. (2006). Enhanced performance on executive functions associated with examination stress: evidence from task-switching and Stroop paradigms. Cognition & Emotion, 20, 577–95. [Google Scholar]
- Lupien S.J., Maheu F., Tu M., Fiocco A., Schramek T.E. (2007). The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain and Cognition, 65, 209–37. [DOI] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective & Behavioral Neuroscience, 12, 241–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C., Caspers S., Roski C., et al. (2012a). Differentiated parietal connectivity of frontal regions for “what” and “where” memory. Brain Structure & Function, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C., Langner R., Dogan I., et al. (2012b). Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage, 60, 830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C. (2013). Stress and cognition. Wiley Interdisciplinary Reviews, 4, 245–61. [DOI] [PubMed] [Google Scholar]
- Sara S.J., Bouret S. (2012). Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron, 76, 130–41. [DOI] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22, 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T.M., Miller K.L., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106, 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J.J., Marois R. (2005). Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cognitive, Affective, & Behavioral Neuroscience, 5, 144–55. [DOI] [PubMed] [Google Scholar]
- van Marle H.J.F., Hermans E.J., Qin S., Fernández G. (2009). From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry, 66, 649–55. [DOI] [PubMed] [Google Scholar]
- Vogel E.K., Machizawa M.G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–51. [DOI] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. (2014). Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist, 20, 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K.J. (2001). Statistical analysis of activation images. In: Functional MRI: An Introduction to Methods, 251–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.