Abstract
The cognitive regulation of emotion is impaired in major depressive disorder and has been linked to an imbalance of pre-frontal–subcortical brain activity. Despite suggestions that this relationship represents a neurodevelopmental marker of depression, few studies have examined the neural correlates of emotion regulation in depressed youth. We combined a ‘cognitive reappraisal’ paradigm with functional magnetic resonance imaging to study the neural correlates of emotional regulation in a large sample of non-medicated depressed adolescents and young adults (n = 53) and healthy controls (n = 64). As compared with healthy controls, young people with depression were less able to reduce negative affect during reappraisal, which corresponded to blunted modulation of amygdala activity. While in healthy individuals amygdala activation was modulated by age, no such relationship was observed in depressed individuals. Heightened activation of the ventromedial pre-frontal cortex (vmPFC) and reduced activation of the dorsal midline cortex was also found for the depressed group. Overall, these findings suggest that brain systems that support cognitive reappraisal are functionally altered in youth depression. We argue that excessive engagement of the vmPFC in particular, may be central to understanding how the process of putting a ‘positive spin’ on negative emotional material may be altered in depressed youth.
Keywords: major depressive disorder, adolescence, development, emotion regulation, reappraisal, fMRI
Introduction
Impaired emotion regulation is recognized as a characteristic feature of major depressive disorder (MDD; Rive et al., 2013; Berking et al., 2014; Joormann and Vanderlind, 2014; Lei et al., 2014; Millgram et al., 2015). Depressed individuals are less likely to use adaptive emotion regulation strategies like cognitive reappraisal: a form of regulation that involves re-interpreting the meaning of a negative stimulus or event in order to improve one’s mood (Garnefski et al., 2002; Ochsner and Gross, 2005; D’Avanzato et al., 2013; Gross, 2013). It has been hypothesized that neurodevelopmental processes that contribute to improved emotion regulation across adolescence and young adulthood (i.e. youth) may be particularly affected in depression (McRae et al., 2012; Ahmed et al., 2015; Ho et al., 2015)—which typically has its first onset during this life period (Dahl, 2004; Nelson et al., 2005; Giedd et al., 2008; Casey et al., 2010; Powers and Casey, 2015)—although this hypothesis requires more direct examination.
In adults with depression, neuroimaging studies have consistently implicated functional alterations of pre-frontal cortical regions that mediate ‘top-down’ emotional control—in particular the dorsolateral pre-frontal cortex—as well as alterations of ‘bottom up’ limbic-subcortical regions (including the amygdala) which contribute to the generation of negative emotional responses (Mayberg, 1997; Siegle et al., 2007; Phillips et al., 2008; Carter, 2009; Price and Drevets, 2010). When performing cognitive reappraisal tasks, unmedicated depressed adults have demonstrated heightened engagement of lateral pre-frontal and dorsal midline cortical regions (Beauregard et al., 2006; Johnstone et al., 2007; Sheline et al., 2010), which has been suggested to index greater difficulty in applying reappraisal strategies (Beauregard et al., 2006). In parallel, these studies have also reported weaker modulation of amygdala activity to negative stimuli during reappraisal (Beauregard et al., 2006; Erk et al., 2010; Greening et al., 2014), with amygdala down-regulation being a robust correlate of successful emotion regulation in non-depressed individuals (Schaefer et al., 2002; Ochsner et al., 2004; Phan et al., 2005; Buhle et al., 2014; Frank et al., 2014).
The period from adolescence to young adulthood has long been cited as a difficult period for the regulation of emotion, and likely related to this, it is when most first episodes of depression emerge (Lewinsohn et al., 1998; Lee et al., 2014; Giedd et al., 2008). Yet, despite an increased focus on the neurobiology of MDD in youth (Kerestes et al., 2014), few neuroimaging studies have examined reappraisal processes in these populations. Studying reappraisal processes in depressed youth is however important, because at this early stage symptoms are less likely to be entrenched (via the natural course of illness) and potential confounds from treatment interventions are likely to be minimized (Hulvershorn et al., 2011). Further, as many of the neural substrates relevant to emotion regulation undergo substantial development during this period (Giedd et al., 1999; Cunningham et al., 2002; Ahmed et al., 2015), examination of the pathophysiology of depression in youth may reveal a role for abnormal developmental processes in the emergence of depressive pathology.
To date, the vast majority of neuroimaging research examining reappraisal has been focused on adults with MDD. In one of the only reappraisal studies of adolescents to date, Perlman et al. (2012) examined depressed participants aged between 13 and 17 years. They reported a group difference between patients and controls in amygdala response in their task. However, this was driven by greater amygdala response to negative stimuli among patients during a ‘maintain’ (i.e. non-emotion regulation) condition, rather than impaired amygdala down-regulation during reappraisal, per se. The lack of obvious alteration in this group with respect to reappraisal ability and regulation of amygdala activity converges with results in child/pre-adolescent populations (Belden et al., 2015) and appears contrary to the idea that core deficits in emotion regulation underlie depressive vulnerability in youth (Garnefski et al., 2002; Silk et al., 2003; Waller et al., 2014). However, considering the modest sample size of this study (14 patients), further investigations are needed to examine the neural correlates of emotion regulation in depressed youth. In addition, more research is needed to determine the impact of depression on developmentally mediated improvements in reappraisal ability and top-down regulation of the amygdala previously reported to occur across this age period (Pitskel et al., 2011; Silvers et al., 2012, 2015; Vink et al., 2014; Martin and Ochsner, 2016; Stephanou et al., 2016).
In this study, we recruited a large sample of unmedicated adolescents and young adults (which we refer to as ‘youth’) with moderate-to-severe MDD, who completed a cognitive reappraisal fMRI task that focused on social-affective imagery. Most reappraisal tasks have used a mixture of social and non-social imagery, but we focused on social images given the saliency of such contexts for youth depression (Casey et al., 2008; Burnett et al., 2011; Marroquín, 2011; Crone and Dahl, 2012) and our broader interest in the maturation of social–emotional processes in young people (Davey et al., 2011; Whittle et al., 2016). Depressed participants were included from an age range that extended from the middle teenage years to early adulthood (15–25 years), which accords with a clinical focus on youth mental health (McGorry, 1998) and is consistent with our current understanding of the continuities in brain and social development through this period (Giedd et al., 1999; Davey et al., 2008). On the basis of our recent work with the reappraisal paradigm in healthy youth (Stephanou et al., 2016), we were interested to examine the impact of MDD on activation of the amygdala and pre-frontal cortex as they were found to be robustly implicated by our task and have been linked to developmentally mediated changes in reappraisal ability across this age period (McRae et al., 2012; Silvers et al., 2015). Consistent with studies of depressed adults, we anticipated that young people with depression would show impaired pre-frontal cortical modulation of amygdala activity during reappraisal, and that typical age-related improvements in the regulation of amygdala activity—previously demonstrated in healthy adolescents (Stephanou et al., 2016)—would be specifically adversely impacted by the illness.
Materials and methods
Participants
Sixty-two medication-free outpatients with a primary diagnosis of MDD were recruited from specialized youth mental health clinics in Melbourne, Australia. All patients were experiencing a major depressive episode, as determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (SCID-IV; First et al., 2002). Depression severity was moderate-to-severe (≥ 20) as confirmed with the Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979) (Table 1 contains further patient information). Patients with a current or past diagnosis of a psychotic disorder, bipolar disorder, substance dependence disorder, acute or unstable medical disorder or intellectual disability were excluded, as were those who had received treatment with psychoactive or antidepressant medication in the last 4 weeks. Of the 62 participants who completed the full neuroimaging protocol, seven were subsequently excluded due to excessive head movement during scanning (see below); one other due to an incidental MRI finding; and another due to a technical problem during scanning.
Table 1.
Clinical characteristics of depressed and healthy control participants
| MDDa (n = 53) | HCb (n = 64) | Statistics | P value two-tailed | |
|---|---|---|---|---|
| Age (years) (S.D.)c | 19.72 (2.68) | 19.03 (2.45) | t(115) = 1.43 | 0.16 |
| Female, % (n) | 58.5 (31) | 62.5 (40) | χ2(1) = 0.19 | 0.66 |
| Education, mean years (S.D.)c | 12.15 (1.74) | 12.79 (2.09) | t(115) = 1.79 | 0.11 |
| FSIQd, mean years (S.D.)c | 105.06 (8.63) | 107.65 (6.86) | t(115) = 1.81 | 0.13 |
| MADRSe score, mean (S.D.)c | 32.80 (4.80) | 2.14 (2.99) | t(115) = 41.92 | 0.00** |
MDD, Major Depressive Disorder.
HC, healthy controls.
S.D., standard deviation.
FSIQ, Full Scale Intelligence Quotient.
MADRS, Montgomery–Åsberg Depression Rating Scale.
P < 0.001.
Healthy control participants were recruited from the community and had no current or past diagnosis of a psychiatric or neurological disorder, and were not taking psychoactive medication. Control participants represent a subsample of a larger group included in our previous study of emotion regulation (Stephanou et al., 2016). Controls were selected to match patients on the variables of age, gender, years of education and estimated IQ. The final sample was composed of 53 depressed patients (Mage = 19.7, s.d. = 2.7, range = 15–25 years; 31 females) and 64 healthy controls (Mage = 19.0, s.d. = 2.4, range = 15–25 years; 40 females), all with normal or corrected-to-normal vision. All participants (and their parents if < 18 years of age) provided written informed consent to complete this study, which was approved by the Melbourne Health Human Research Ethics Committee.
Experimental task
Our block-design cognitive reappraisal task (Stephanou et al., 2016) consisted of three experimental conditions: one containing neutral imagery (‘look-neutral’) and two containing negative imagery (‘look-negative’ and ‘reappraise’)—with each condition presented in eight blocks. Each block consisted of four consecutive image presentations (each 6 s) and at the beginning of each block, a word appeared for 2 s instructing participants to either ‘look’ or ‘reappraise.’ If the instruction was to ‘look,’ participants were required to attend to images of neutral or negative content without trying to alter their emotions in any way. If the instruction was to ‘reappraise,’ participants were instructed to use reappraisal strategies (see below) to attenuate their emotional response to the negative images. A total of 64 negative and 32 neutral pictures were presented. Characteristics of our picture stimuli have been reported previously (Stephanou et al., 2016): briefly, images depicted complex social scenes and were taken from the International Affective Picture System (IAPS) database (Lang et al., 2008), the Empathy Picture System (EPS) database (Geday et al., 2003) and online sources. At the end of each block the question ‘How bad do you feel?’ appeared on screen to which participants responded by pressing 1–4 on a button box with their dominant hand (1 = not at all bad; 4 = very bad). This was followed by a rest period in which participants viewed a fixation cross (10 s). The task was presented with Paradigm software (http://www.paradigmexperiments.com) on an LCD screen visible via a reverse mirror mounted to the participants’ head coil.
Participants received intensive training in reappraisal strategies 30 min prior to their scan. During training, participants demonstrated their ability to reappraise by narrating aloud their re-interpretation of several example images (not appearing in the experiment). In accordance with previous research, three types of re-interpretations were emphasized: (i) it is not real (e.g. it is just a scene from a movie); (ii) things will improve with time (e.g. whatever is going wrong will resolve over time); (iii) things are not as bad as they appear to be (e.g. the situation looks worse than it is, it could be a lot worse) (McRae et al., 2012). Once the participant’s narration indicated that they were able use reappraisal strategies quickly and effectively (within 6 s), participants independently performed several practice blocks that mimicked the task in preparation for the scan.
Following the scan a brief questionnaire was administered to assess the perceived frequency with which participants used the three reappraisal strategies, as well as their use of avoidance strategies (i.e. looking away or closing their eyes), which participants rated on a scale from 1 to 5 (1 = Never, to 5 = Always). With this data, we sought to determine whether perceived use of the different reappraisal or avoidance strategies was different in patients (as compared with controls) and if any group differences were associated with activation effects observed during fMRI.
Image acquisition and pre-processing
A 3T General Electric Signa Excite system equipped with an eight-channel phased-array head coil was used in combination with ASSET parallel imaging (Sunshine Hospital, Western Health, Melbourne). The functional sequence consisted of a single shot gradient recalled EPI sequence in the steady state (repetition time, 2000 ms; echo time, 35 ms; and pulse angle, 90°) in a 23 cm field of view, with a 64 × 64-pixel matrix and a slice thickness of 3.5 mm (no gap). Thirty-six interleaved slices were acquired parallel to the anterior–posterior commissure line with a 20° anterior tilt to achieve more optimal coverage of ventral pre-frontal cortical brain regions. The total sequence time was 16 min, corresponding to 485 whole-brain echoplanar imaging volumes and was acquired in a single run. The first four volumes from each run were discarded to allow for T1 equilibration effects. Additionally, a T1-weighted high-resolution anatomical image was also acquired for each participant to assist with functional time-series co-registration using the following 3D BRAVO sequence: 140 contiguous slices; repetition time, 7900 ms; echo time, 3000 ms; flip angle, 13°; in a 25.6 cm field of view, with a 256 × 256 pixel matrix and a slice thickness of 1 mm (no gap). To assist with noise reduction, all participants used foam insert earplugs. To assist with head immobility, foam-padding inserts were placed around the participants’ head inside the coil.
Imaging data were transferred and processed on a Linux platform running MATLAB version 8.2 (The MathWorks Inc., Natick, MA, USA). Pre-processing was performed with Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, UK). Motion correction was performed by aligning each participant’s time series to the first image using least squares minimization and a six-parameter (rigid body) spatial transformation. Participants were excluded from the analysis if their gradual or frame to frame movement in the translational or rotational planes exceeded 2 mm or 2°, respectively. Realigned functional images were then co-registered to each participant’s respective T1 anatomical scan. Using the unified segmentation method, anatomical scans were segmented and spatially normalized to the International Consortium for Brain Mapping template, and the normalization parameters were applied to the co-registered functional images, which were then resliced to 2 mm isotropic resolution. Functional images were smoothed with a 6 mm (full-width, half maximum) Gaussian filter.
Behavioral analysis
Mean in-scanner negative affect ratings were derived for each participant corresponding to the ‘look-negative,’ ‘look-neutral’ and ‘reappraise’ conditions. Emotional reactivity and reappraisal success scores were estimated for each participant by computing simple differences between mean condition ratings (i.e. look-negative > look-neutral for emotional reactivity, and look-negative > reappraise for reappraisal success). We assessed for group differences between patients and controls on behavioral indices of emotional reactivity and reappraisal success in Statistical Package for the Social Science version 20 (SPSS; Chicago, IL, USA). For our patient group, we assessed if symptom severity (i.e. MADRS score) correlated with these behavioral indices. In addition, we assessed for age effects (linear or quadratic) on reported emotional reactivity and reappraisal success within participants and tested for age × group interactions using multiple regression in SPSS. Finally, with respect to our post-scan measure, we utilized t-tests in SPSS to examine group differences in the perceived frequency with which participants used the various reappraisal or avoidance strategies, with results corrected for multiple comparisons using the Benjamini–Hochberg procedure (FDR, P < 0.05).
Imaging analysis
First-level (single-subject) SPM contrast images were estimated for the following primary effects of interest: (i) look-negative > look-neutral to identify brain regions associated with emotional reactivity to aversive social-affective images; (ii) reappraise > look-negative to identify brain regions activated during reappraisal of aversive images and (iii) look-negative > reappraise to identify brain regions that showed reduced activation (or were down-regulated) by reappraisal. For these analyses, the BOLD response at each voxel was convolved with a canonical hemodynamic response function and its temporal derivative (using a 128 s high-pass filter). In second-level analyses, for the main task effects, within-group activation maps were estimated separately for patients and controls thresholded at PFDR < 0.05 (false-discovery rate corrected for the whole-brain volume) with a minimum cluster size extent (KE) of 10 contiguous voxels. To identify brain regions that were significantly down-regulated by reappraisal, within-group results corresponding to the look-negative > reappraise contrast were inclusively masked to only include voxels that were significantly activated by negative imagery (i.e. look-negative > look-neutral), PFDR < 0.05, whole-brain corrected.
Between-group differences between patients and controls for the three primary contrasts of interest were assessed with two-sample t-tests. To accurately interpret the direction of between-group differences across the different contrasts, we have used global conjunction masking to constrain these tests. That is, for a given contrast (e.g. reappraise > look-negative), we masked the between-group test by regions that were activated to this contrast at the within-group level in one group and/or the other (PFDR < 0.05, whole-brain corrected). Although within-group activations for patients and controls were highly overlapping, masking group differences using this global conjunction method ensured that all regions activated by the task in either group were assessed for potential group differences. This masking method also ensured that group differences identified for the reappraise > look-negative contrast were only in regions that were activated by reappraisal in either patients or controls (i.e. we did not detect deactivation differences), and that group differences for the look-negative > reappraise contrast were only in regions that were down-regulated during reappraisal (Contreras-Rodríguez et al., 2014; Kerestes et al., 2015). Within this activation mask, significant group differences were identified using a whole-brain uncorrected voxel threshold of P < 0.001 with at least 10 contiguous voxels per cluster, which allowed us to compare current results to studies from others that have used comparable significance thresholds (Erk et al., 2010; Fitzgerald et al., 2017; Rive et al., 2013) and which has been recommended as producing a desirable balance between Type I and Type II error rates (Lieberman and Cunningham, 2009). As our a priori region of interest (ROI) was the amygdala, activation in the amygdala was small-volume corrected for multiple comparisons (PFWE-SVC < 0.05) using a bilateral anatomically defined mask derived from the SPM Anatomy toolbox (Version 2.0, Eickhoff et al., 2005) that consisted of amygdala maximum probability maps recommended by Eickhoff et al. (2006).
The relationship between brain activation and variables of interest—age, reappraisal success and symptom severity (i.e. MADRS score for the patient group)—was assessed at the whole-brain level, by repeating the above group level analyses with these variables specified as covariates of interest. We examined the effect of covariates only within regions identified in the former analyses as significantly activated or down-regulated during reappraisal. For example, to identify the influence of age and age × group effects on brain activation associated with the reappraise > look-negative contrast, we inclusively masked age-effects by the estimated reappraise > look-negative main effect (as determined above). For reappraisal success, we sought to assess if our behavioral measure of regulation success would account for any group differences in reappraisal-related brain activation observed. Lastly, we sought to assess if depression symptom severity (i.e. MADRS score) influenced reappraisal-related brain activation within our patient group. These covariates were entered as regressors in the corresponding second-level analysis, voxel thresholded at P < 0.001 uncorrected (KE ≥ 10). As in the between-group analysis, associations with amygdala activation were corrected for multiple comparisons (PFWE-SVC < 0.05) and where significant effects were found, mean beta values from the clusters were extracted (using the first eigenvariate) to generate scatterplots for visual inspection in SPSS, in order to clarify effects within our sample (i.e. patients and controls).
Results
Behavioral analysis
Within groups, both patients and controls reported significantly greater negative affect to negative vs neutral stimuli; (i.e. patients, MDIFF = 1.60 (s.d. = 0.80), t52 = 14.44, P < 0.001; controls, MDIFF = 1.82 (s.d. = 0.66), t63 = 22.02, P < 0.001). Reduced negative affect during reappraisal (vs look-negative trials)—an index of reappraisal success—was also significant for both patients (MDIFF = 0.43 (s.d. = 0.44), t52 = 6.89, P < 0.001), and controls (MDIFF = 0.82 (s.d. = 0.59), t63 = 11.06, P < 0.001). There was no significant difference in the magnitude of emotional reactivity to aversive stimuli between patients and controls (F1, 115 = 2.59, P = 0.110). There was, however, a significant difference between the groups in terms of reappraisal success: reappraisal was associated with greater reductions in negative affect in controls relative to patients (F1, 115 = 15.20, P < 0.001; Supplementary Figure S1). Further, within the patient group symptom severity (i.e. MADRS score) was inversely associated with reappraisal success (F1,51 = 6.93, P = 0.011, R2 = 0.13), but was not associated with emotional reactivity. There were no significant age effects (linear or quadratic) on reported emotional reactivity or reappraisal success within participants, nor were there any age × group interactions. Finally, there were no significant differences between the groups in reported use of reappraisal and avoidance strategies as assessed by our post-scan measure.
Imaging results
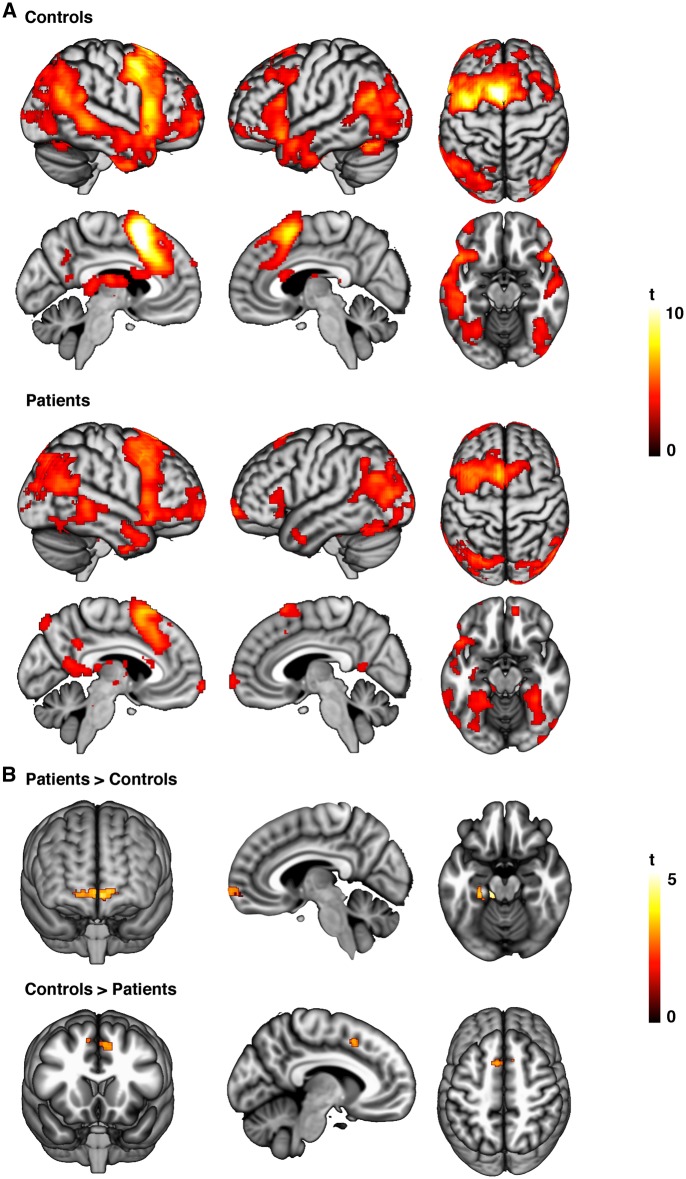
In response to negative imagery (look-negative > look-neutral), both groups showed significant activation bilaterally across a large expanse of visual association cortex, including the fusiform gyrus and lateral occipital cortex; the intraparietal sulcus and primary somatosensory cortex; the hippocampus–amygdala complex, dorsal midbrain, medial thalamus, caudate (head) and ventral anterior insula; and the ventrolateral pre-frontal cortex, lateral pre-motor cortex, pre-supplementary motor cortex and dorsomedial pre-frontal cortex (at PFDR < 0.05, whole-brain corrected; see Supplementary Table S1 and Supplementary Figure S2). Between-group analysis identified significantly reduced activation in patients, as compared with controls, bilaterally in the anterior temporal cortex, extending to the right-sided anterior insula, putamen and pallidum; the right-sided ventrolateral and dorsolateral pre-frontal cortex; and the bilateral orbitofrontal cortex (P < 0.001, uncorrected, KE ≥ 10; see Supplementary Table S2 and Supplementary Figure S2).
During cognitive reappraisal (reappraise > look-negative), both groups exhibited significant activation in pre-dominately left-lateralized regions including the pre-supplementary motor area (which in the control group extended to the dorsal anterior cingulate cortex and dorsomedial frontal cortex); the dorsolateral pre-frontal cortex, and ventrolateral pre-frontal cortex, extending to the ventral anterior insula and caudate body; and bilaterally in the angular gyrus, posterior superior temporal gyrus and fusiform gyrus (PFDR < 0.05, whole-brain corrected; see Supplementary Table S3 and Figure 1). Compared with controls, patients showed significantly greater activation in the bilateral medial and lateral regions of the anterior pre-frontal cortex extending to the right ventromedial pre-frontal cortex (vmPFC); the left fusiform gyrus extending to the parahippocampal gyrus; and in left cerebellar-pontine regions (P < 0.001, uncorrected, KE ≥ 10). In addition, patients showed significantly weaker activation in the left pre-supplementary motor area (P < 0.001, uncorrected, KE ≥ 10; Table 2 and Figure 1).
Fig. 1.
(A) Patterns of significant within-group activation associated with reappraisal (reappraise > look-negative) in healthy control and depressed participants. (B) Between-group activation differences associated with reappraisal.
Table 2.
Group differences in regions activated during reappraisal (reappraise > look-negative) and down-regulated during reappraisal (look-negative > reappraise)
| Anatomy |
Stats |
||||
|---|---|---|---|---|---|
| X c | Y c | Z c | KE | Z d | |
| Reap > Look-Neg (Patient > Control)a | |||||
| Left cerebellum | −14 | −32 | −30 | 149 | 3.98 |
| Left anterior medial pre-frontal cortex | −4 | 70 | −8 | 204 | 3.78 |
| Right anterior medial pre-frontal cortex | 16 | 66 | −6 | 3.16 | |
| Right ventral medial pre-frontal cortex | 8 | 60 | −8 | 3.14 | |
| Left fusiform gyrus | −32 | −6 | −34 | 38 | 3.48 |
| Left parahippocampal gyrus | −32 | −10 | −28 | 3.14 | |
| Reap > Look-Neg (Control > Patient)a | |||||
| Left pre-supplementary motor area | −8 | 14 | 46 | 53 | 3.61 |
| Look-Neg > Reap (Control > Patient)b | |||||
| Right amygdala | 22 | −2 | −14 | 11 | 3.37 |
P < 0.001 uncorrected, KE ≥ 10 voxels.
PFWE < 0.05 small-volume corrected, KE ≥ 10 voxels.
Anatomical co-ordinates (x, y, z) are given in MNI space (mm).
Z values represent peak activation for cluster.
Examination of the look-negative > reappraise contrast revealed that reappraisal led to significant reductions in activation (i.e. down-regulation) of brain regions previously identified to be responsive to the negative imagery. This included the left primary somatosensory cortex in both controls and patients. In controls, additional down-regulation was observed in the left primary somatosensory cortex (area 1, 2 and 3b) and bilateral supramarginal gyrus; the right putamen, extending to the right dorsal amygdala, ventral pallidum and posterior insula; and the right ventrolateral pre-frontal cortex (PFDR < 0.05, whole-brain corrected; see Supplementary Table S4). Analysis of group differences indicated that down-regulation of the right amygdala was significantly reduced in patients, as compared with controls (PFWE-SVC < 0.05; Table 2 and Figure 2).
Fig. 2.
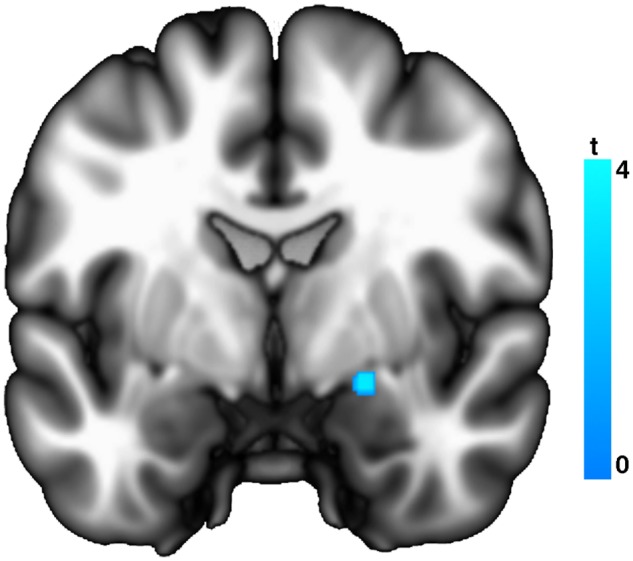
Significantly greater down-regulation of amygdala responses during reappraisal (look-negative > reappraise) in healthy control vs depressed participants.
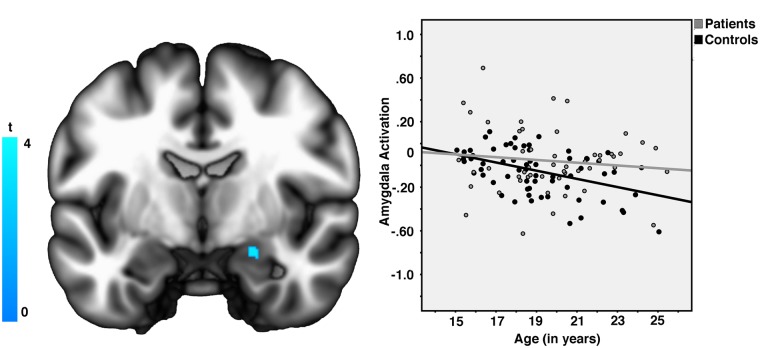
No significant age effects were observed for the reappraise > look-negative contrast in a whole-brain analysis. For the look-negative > reappraise contrast, we observed a significant positive effect of age on down-regulation of the right amygdala ROI (PFWE-SVC < 0.05, peak Z = 3.56, [x, y, z] = [18, 0, −20]; KE = 19 voxels; see Figure 3). When examining the groups separately, we observed that this association between age and amygdala activation was significant in controls (PFWE-SVC < 0.05, peak Z = 4.11; KE = 32 voxels), but not patients (PFWE-SVC > 0.05). However, the age × group interaction for the amygdala failed to reach significance. There was no significant effect of reappraisal success on observed group differences in reappraisal-related activation, nor was there any association between depression symptom severity and reappraisal-related activation within our patient group.
Fig. 3.
(Left) Across participants, age predicted greater down-regulation of right amygdala responses during reappraisal (look-negative > reappraise). (Right) The association between age and right amygdala activity was significant in healthy control but not depressed participants.
Discussion
The results of this study suggest that the use of cognitive reappraisal to reduce negative affect is impaired in young people with depression, reflected by reduced down-regulation of amygdala responses to aversive images. The absence of age-related changes in amygdala modulation in depressed youth also suggests a likely impact of the disorder on the maturation of subcortical systems involved in affect generation. When reappraising aversive imagery, depressed participants instead demonstrated greater engagement of the vmPFC. Activation in this region, which has previously been implicated in elaboration of context and self-relevance during reappraisal, may help explain how the process of putting a ‘positive spin’ on negative affective stimuli may be altered in depressed youth as compared with controls.
It has been hypothesized that in depression over-activation of subcortical systems linked to affect generation may both precede, and over time give rise to, the dysregulation of dorsal pre-frontal systems that facilitate the cognitive regulation of emotion (Ressler and Mayberg, 2007; Drevets et al., 2008). Specifically, disturbed function of the amygdala—which is broadly implicated in the processing of affective salience—is thought to be centrally involved (Anderson and Phelps, 2001; Siegle et al., 2002; Drevets, 2003; Barrett et al., 2007; Davey et al., 2011; Mingtian et al., 2012). Several studies in adult populations have reported a failure of depressed participants to down-regulate amygdala responses during cognitive reappraisal (Beauregard et al., 2006; Erk et al., 2010; Greening et al., 2014). Ours is the first study to confirm this pattern of findings in younger depressed participants, and our findings also suggest that depression may interfere with the normative maturation of subcortical affective systems (Guyer et al., 2008; Dahl and Gunnar, 2009).
Patients’ difficulties in regulating negative affect during reappraisal were accompanied by a heightened activation of the anterior vmPFC. Dysfunction of this region has been broadly emphasized in contemporary neural systems models of mood disorders, including major depression (Price and Drevets, 2010; Farb et al., 2011; Lemogne et al., 2012; Myers-Schulz and Koenigs, 2012). One recent perspective, consistent with these models, is that the vmPFC may specifically contribute to the higher-level computation of ‘affective meaning’: specifying it as the integrative hub of a large scale pre-frontal–subcortical system mediating adaptive emotional responses (Urry et al., 2006; Roy et al., 2012; Motzkin et al., 2015). In this capacity, vmPFC function may be specialized for integrating conceptual information—regarding the self, others and contextual knowledge—with affective sensory cues in order to facilitate adaptive regulation of emotion (Price, 2007; Ochsner et al., 2012; D'Argembeau, 2013; Viviani, 2014).
Applying this model to our current findings, one possibility is that depressed participants become differentially engaged in processes of reconceptualizing the meaning of aversive stimuli in order to more positively reframe their immediate reactions and feelings towards them. In other words, depressed participants may become engaged in the internalized process of meaning generation—including evaluation of the context or self-relevance of a stimulus—at the expense of stimulus-focused processing needed for effective reappraisal (Mathews et al., 1996; Sheline et al., 2010; Diekhof et al., 2011; Roy et al., 2012). Because the vmPFC has been specifically linked to positively vs negatively valanced affective processing (Lindquist et al., 2016), one interpretation is that hyper-activation observed in depressed participants represents abnormal processing associated with generating more positive—or alternatively stated, less negative—appraisals of negative imagery in particular. Depressed participants also demonstrated reduced activation of dorsal midline cortex, which in the context of other reappraisal studies, has been shown to mediate the influence of lateral pre-frontal cortex on reappraisal success (Wager et al., 2008), and thus seems to play an intermediary role in engaging broader cognitive control systems (Lau et al., 2004; Wager et al., 2004; Sumner et al., 2007; Pizzagalli, 2011). Taken together, depression in young people therefore seems to be characterized by disturbances in both ventral pre-frontal–subcortical ‘generative’ as well as dorsal ‘regulatory’ systems that contribute to adaptive emotional processing. Thus, the results of this study suggest that dysregulation of pre-frontal–subcortical systems previously observed in reappraisal studies of depressed adults may also be evident in the early stages of the disorder (Kerestes et al., 2014; Miller et al., 2015), although further studies will be needed to confirm this observation.
This study has several limitations. With the exception of our amygdala ROI, the reported group differences would not survive formal statistical correction for multiple comparisons. As such, these findings should be considered exploratory and need to be confirmed in subsequent studies, particularly those utilizing voxel-wise correction and permutation methods (Eklund et al., 2016). Further, while the reappraisal strategies incorporated in our task were influenced by cognitive theory and psychotherapy for depression (Teasdale and Barnard, 1993; Gotlib and Joormann, 2010; Berking et al., 2013; Beck and Haigh, 2014), they are nonetheless only an approximation of real-life regulatory processes, which limits the generalization of these results.
Notwithstanding these limitations, it is important to acknowledge that the uncorrected P value used within our study exceeded a threshold (P < 0.001) that previously has been found to optimize the trade-off between type I and type II error (Lieberman and Cunningham, 2009), and group differences in our amygdala ROI are consistent with those previously reported in prior adult MRI studies (Greening et al., 2014). Further, as the largest study in a young unmedicated help-seeking sample to date, our findings regarding the vmPFC and amygdala, if corroborated, may help to refine therapeutic strategies aimed at improving emotion regulation in youth with MDD. For example, targeted therapy approaches that reduce focus on internally generated affective meaning and encourage greater external awareness and reasoned reflection—such as mindfulness-based cognitive therapy—may be particularly beneficial for young people with depression (Teasdale et al., 2000; Modinos et al., 2010; Troy et al., 2013). Whether cause or consequence of depression, we propose that impaired ability to adaptively regulate emotions and ‘put a more positive spin’ on negative events during adolescence and young adulthood is likely to have adverse effects on long-term developmental trajectories and should be an important target for future research.
Supplementary Material
Acknowledgements
We thank staff from the Medical Imaging Department, Western Health, Sunshine Hospital (St. Albans, Melbourne) for their support and contributions to this work.
Funding
This study was funded by National Health and Medical Research Council of Australia (NHMRC) Project Grants 1064643 (Principal Investigator BJH) and 1024570 (Principal Investigator CGD). BJH is supported by a NHMRC Clinical Career Development Award (I.D. 1124472).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Ahmed S.P., Bittencourt-Hewitt A., Sebastian C.L. (2015). Neurocognitive bases of emotion regulation development in adolescence. Developmental Cognitive Neuroscience, 15, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.K., Phelps E.A. (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411(6835), 305–9. [DOI] [PubMed] [Google Scholar]
- Barrett L.F., Bliss-Moreau E., Duncan S.L., Rauch S.L., Wright C.I. (2007). The amygdala and the experience of affect. Social Cognitive Affective Neuroscience, 2(2), 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M., Paquette V., Levesque J. (2006). Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport, 17(8), 843–6. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Haigh E.A. (2014). Advances in cognitive theory and therapy: the generic cognitive model. Annual Review of Clinical Psychology, 10, 1–24. [DOI] [PubMed] [Google Scholar]
- Belden A.C., Pagliaccio D., Murphy E.R., Luby J.L., Barch D.M. (2015). Neural activation during cognitive emotion regulation in previously depressed compared to healthy children: evidence of specific alterations. Journal of the American Academy of Child and Adolescent Psychiatry, 54(9), 771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M., Ebert D., Cuijpers P., Hofmann S.G. (2013). Emotion regulation skills training enhances the efficacy of inpatient cognitive behavioral therapy for major depressive disorder: a randomized controlled trial. Psychotherapy and Psychosomatics, 82(4), 234–45. [DOI] [PubMed] [Google Scholar]
- Berking M., Wirtz C.M., Svaldi J., Hofmann S.G. (2014). Emotion regulation predicts symptoms of depression over five years. Behaviour Research and Therapy, 57, 13–20. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S., Sebastian C., Cohen Kadosh K., Blakemore S.J. (2011). The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience & Biobehavioral Reviews, 35(8), 1654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C.S. (2009). The ups and downs of emotion regulation. Biological Psychiatry, 65(5), 359–60. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Levita L., et al. (2010). The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology, 52(3), 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodríguez O., Pujol J., Batalla I., et al. (2014). Disrupted neural processing of emotional faces in psychopathy. Social Cognitive Affective Neuroscience, 9, 505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–50. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. (2002). Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. The Journal of Comparative Neurology, 453(2), 116–30. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. (2013). On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Frontiers in Human Neuroscience, 7, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avanzato C., Joormann J., Siemer M., Gotlib I. (2013). Emotion regulation in depression and anxiety: examining diagnostic specificity and stability of strategy use. Cognitive Therapy and Research, 37(5), 968–80. [Google Scholar]
- Dahl R.E. (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences, 1021, 1–22. [DOI] [PubMed] [Google Scholar]
- Dahl R.E., Gunnar M.R. (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Development and Psychopathology, 21(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Allen N.B., Harrison B.J., Yucel M. (2011). Increased amygdala response to positive social feedback in young people with major depressive disorder. Biological Psychiatry, 69(8), 734–41. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Yucel M., Allen N.B. (2008). The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews, 32(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. (2011). Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage, 58(1), 275–85. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. (2003). Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Sciences, 985, 420–44. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function, 213(1–2), 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–35. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Heim S., Zilles K., Amunts K. (2006). Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage, 32(2), 570–82. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113, 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S., Mikschl A., Stier S., et al. (2010). Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience, 30(47), 15726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A., Anderson A.K., Bloch R.T., Segal Z.V. (2011). Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry, 70(4), 366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P), New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fitzgerald J.M., MacNamara A., Kennedy A.E., et al. (2017). Individual differences in cognitive reappraisal use and emotion regulatory brain function in combat-exposed veterans with and without PTSD. Depression & Anxiety, 34, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., et al. (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews, 45, 202–11. [DOI] [PubMed] [Google Scholar]
- Garnefski N., Legerstee J., Kraaij V.V., Van Den Kommer T., Teerds J. (2002). Cognitive coping strategies and symptoms of depression and anxiety: a comparison between adolescents and adults. Journal of Adolescence, 25(6), 603–11. [DOI] [PubMed] [Google Scholar]
- Geday J., Gjedde A., Boldsen A.S., Kupers R. (2003). Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. NeuroImage, 18(3), 675–84. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2(10), 861–63. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Keshavan M., Paus T. (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9(12), 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. (2010). Cognition and depression: current status and future directions. Annual Review of Clinical Psychology, 27(6), 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening S.G., Osuch E.A., Williamson P.C., Mitchell D.G. (2014). The neural correlates of regulating positive and negative emotions in medication-free major depression. Social Cognitive Affective Neuroscience, 9(5), 628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (2013). Emotion regulation: taking stock and moving forward. Emotion, 13(3), 359–65. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., et al. (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Connolly C.G., Henje Blom E., et al. (2015). Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biological Psychiatry, 78(9), 635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn L., Cullen K., Anand A. (2011). Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior, 5(4), 307–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal–subcortical circuitry in major depression. The Journal of Neuroscience, 27(33), 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Vanderlind W.M. (2014). Emotion regulation in depression: the role of biased cognition and reduced cognitive control. Clinical Psychological Science, 2(4), 402–21. [Google Scholar]
- Kerestes R., Davey C.G., Stephanou K., Whittle S., Harrison B.J. (2014). Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clinical, 4, 209–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R., Harrison B.J., Dandash O., et al. (2015). Specific functional connectivity alterations of the dorsal striatum in young people with depression. Neuroimage Clinical, 7, 266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P., Bradley M., Cuthbert B. (2008) International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-8, Gainesville, FL: Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lau, H.C., Rogers, R.D., Haggard, P., Passingham, R.E. (2004). Attention to Intention. Science, 303,1208–10. [DOI] [PubMed] [Google Scholar]
- Lee F.S., Heimer H., Giedd J.N., et al. (2014). Adolescent mental health—opportunity and obligation. Science, 346, 547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., Zhang X., Cai L., Wang Y., Bai M., Zhu X. (2014). Cognitive emotion regulation strategies in outpatients with major depressive disorder. Psychiatry Research, 218(1–2), 87–92. [DOI] [PubMed] [Google Scholar]
- Lemogne C., Delaveau P., Freton M., Guionnet S., Fossati P. (2012). Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders, 136(1–2), 1–11. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P.M., Rohde P., Seeley J.R. (1998). Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clinical Psychology Review, 18, 765–94. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience, 4, 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. (2016). The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26(5), 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquín B. (2011). Interpersonal emotion regulation as a mechanism of social support in depression. Clinical Psychology Review, 31(8), 1276–90. [DOI] [PubMed] [Google Scholar]
- Martin R.E., Ochsner K.N. (2016). The neuroscience of emotion regulation development: implications for education. Current Opinion in Behavioral Sciences, 10, 142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A., Ridgeway V., Williamson D.A. (1996). Evidence for attention to threatening stimuli in depression. Behaviour Research and Therapy, 34(9), 695–705. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S. (1997). Limbic-cortical dysregulation: a proposed model of depression. The Journal of Neuropsychiatry & Clinical Neurosciences, 9(3), 471–81. [DOI] [PubMed] [Google Scholar]
- McGorry P. (1998). Beyond adolescent psychiatry: the logic of a youth mental health model. The Australian and New Zealand Journal of Psychiatry, 32(1), 138–40. [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., et al. (2012). The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive Affective Neuroscience, 7(1), 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.H., Hamilton J.P., Sacchet M.D., Gotlib I.H. (2015). Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiatry, 72(10), 1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millgram Y., Joormann J., Huppert J.D., Tamir M. (2015). Sad as a matter of choice? Emotion-regulation goals in depression. Psychological Science, 26(8), 1216–28. [DOI] [PubMed] [Google Scholar]
- Mingtian Z., Shuqiao Y., Xiongzhao Z., et al. (2012). Elevated amygdala activity to negative faces in young adults with early onset major depressive disorder. Psychiatry Research, 201(2), 107–12. [DOI] [PubMed] [Google Scholar]
- Modinos G., Ormel J., Aleman A. (2010). Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Social Cognitive Affective Neuroscience, 5(4), 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. (1979). A new depression scale designed to be sensitive to change. British Journal of Psychiatry, 134, 382–9. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77(3), 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B., Koenigs M. (2012). Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molecular Psychiatry, 17(2), 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(2), 163–74. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23(2), 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman, G., Simmons, A.N., Wu, J., et al. (2012). Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. Journal of Affective Disorders, 139(1), 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–9. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. (2011). How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Developmental Cognitive Neuroscience, 1(3), 324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A. (2011). Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology, 36(1), 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A., Casey B.J. (2015). The adolescent brain and the emergence and peak of psychopathology. Journal of Infant, Child and Adolescent Psychotherapy, 14(1), 3–15. [Google Scholar]
- Price J.L. (2007). Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences, 1121, 54–71. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35(1), 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler K.J., Mayberg H.S. (2007). Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neuroscience, 10(9), 1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhe H.G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(10), 2529–53. [DOI] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. (2012). Ventromedial prefrontal–subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer S.M., Jackson D.C., Davidson R.J., Aguirre G.K., Kimberg D.Y., Thompson-Schill S.L. (2002). Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience, 14(6), 913–21. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. (2002). Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51(9), 693–707. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Thompson W., Carter C.S., Steinhauer S.R., Thase M.E. (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry, 61(2), 198–209. [DOI] [PubMed] [Google Scholar]
- Silk J.S., Steinberg L., Morris A.S. (2003). Adolescents' emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Development, 74(6), 1869–80. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., McRae K., Gabrieli J.D., Gross J.J., Remy K.A., Ochsner K.N. (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12(6), 1235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Shu J., Hubbard A.D., Weber J., Ochsner K.N. (2015). Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Developmental Science, 18(5), 771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou K., Davey C.G., Kerestes R., et al. (2016). Brain functional correlates of emotion regulation across adolescence and young adulthood. Human Brain Mapping, 37(1), 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P., Nachev P., Morris P., et al. (2007). Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron, 54(5), 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale J.D., Barnard P.J. (1993) Affect, Cognition and Change: Re-Modelling Depressive Thought, Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Teasdale J.D., Segal Z.V., Williams J.M., Ridgeway V.A., Soulsby J.M., Lau M.A. (2000). Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology, 68(4), 615–23. [DOI] [PubMed] [Google Scholar]
- Troy A.S., Shallcross A.J., Davis T.S., Mauss I.B. (2013). History of mindfulness-based cognitive therapy is associated with increased cognitive reappraisal ability. Mindfulness, 4(3), 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H.L., Van Reekum C.M., Johnstone T., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience, 26, 4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Derks J.M., Hoogendam J.M., Hillegers M., Kahn R.S. (2014). Functional differences in emotion processing during adolescence and early adulthood. NeuroImage, 91, 70–6. [DOI] [PubMed] [Google Scholar]
- Viviani R. (2014). Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Frontiers in Psychiatry, 5, 123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Jonides J., Reading S. (2004). Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage, 22(4), 1679–93. [DOI] [PubMed] [Google Scholar]
- Waller J.M., Silk J.S., Stone L.B., Dahl R.E. (2014). Co-rumination and co-problem solving in the daily lives of adolescents with major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 53(8), 869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Liu K., Bastin C., Harrison B.J., Davey C.G. (2016). Neurodevelopmental correlates of proneness to guilt and shame in adolescence and early adulthood. Developmental Cognitive Neuroscience, 19, 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.