Abstract
Experiences such as mind-wandering illustrate that cognition is not always tethered to events in the here-and-now. Although converging evidence emphasises the default mode network (DMN) in mind-wandering, its precise contribution remains unclear. The DMN comprises cortical regions that are maximally distant from primary sensory and motor cortex, a topological location that may support the stimulus-independence of mind-wandering. The DMN is functionally heterogeneous, comprising regions engaged by memory, social cognition and planning; processes relevant to mind-wandering content. Our study examined the relationships between: (i) individual differences in resting-state DMN connectivity, (ii) performance on memory, social and planning tasks and (iii) variability in spontaneous thought, to investigate whether the DMN is critical to mind-wandering because it supports stimulus-independent cognition, memory retrieval, or both. Individual variation in task performance modulated the functional organization of the DMN: poor external engagement was linked to stronger coupling between medial and dorsal subsystems, while decoupling of the core from the cerebellum predicted reports of detailed memory retrieval. Both patterns predicted off-task future thoughts. Consistent with predictions from component process accounts of mind-wandering, our study suggests a 2-fold involvement of the DMN: (i) it supports experiences that are unrelated to the environment through strong coupling between its sub-systems; (ii) it allows memory representations to form the basis of conscious experience.
Keywords: default mode network, resting state functional connectivity, perceptual decoupling, component process account, mind-wandering
Introduction
Thoughts and feelings unrelated to the here-and-now occupy up to half of waking thought (Klinger and Cox, 1987; Killingsworth and Gilbert, 2010; Poerio et al., 2013). Despite their ubiquity, we currently lack a clear understanding of how these complex everyday experiences are produced. Our capacity for self-generated thought allows us to think about people and places that are not in the immediate environment, a process hypothesized to depend on retrieving internally stored representations that capture memories of past episodes and conceptual knowledge (Smallwood et al., 2016). These unconstrained experiences can have beneficial effects, including creativity (Baird et al., 2012; Smeekens and Kane, 2016), the alleviation of loneliness (Poerio et al., 2015, 2016a), psycho-social adaptation (Poerio et al., 2016b), and the refinement of goals (Medea et al., 2016). Despite these benefits of mind-wandering, the experience can also derail performance on concurrent tasks (McVay and Kane, 2009).
Contemporary accounts of mind-wandering suggest that it is best understood as the combination of different component processes (Smallwood, 2013a,b; Smallwood and Schooler, 2015). One component is the decoupling of attention from perceptual input that explains why mind-wandering is often linked with poor performance on external tasks. This component process is hypothesized to provide the mechanism that allows cognition to become independent of events taking place in the external environment. Support for the decoupling hypothesis comes from empirical evidence showing that evoked responses to external input are reduced during mind-wandering (e.g. Baird et al., 2014). Another key component process is the retrieval of episodic and semantic knowledge which is thought to provide the mnemonic representations upon which internal perceptually decoupled thought is based. In support of this, studies have shown that the hippocampus, a region important in episodic memory, is: (i) active early during mind-wandering (Ellamil et al., 2016) and (ii) shows a pattern of enhanced connectivity with the medial pre-frontal cortex for participants who engage in mental time travel during mind-wandering (Karapanagiotidis et al., 2017). In combination, the processes of perceptual decoupling and episodic memory retrieval are considered necessary conditions for the self-generation of a train of thought unrelated to the external environment (Smallwood and Schooler, 2015; Mittner et al., 2016).
Converging evidence suggest that mind-wandering is associated with a large-scale neural network known as the default mode network (DMN) (Raichle et al., 2001; Weissman et al., 2006; Mason et al., 2007; Christoff et al., 2009; Stawarczyk et al., 2011; Allen et al., 2013). The DMN consists of cortical regions with the greatest geodesic distance from the input/output systems of the brain in visual and motor cortex (Margulies et al., 2016). This topological location may facilitate the expression of stimulus-independent aspects of cognition that characterize mind-wandering because these regions are thought to be less tethered to the input/output systems of the cortex (Buckner and Krienen, 2013). The DMN is also implicated in specific domains of cognition that are critical during mind-wandering, including social cognition, semantic and episodic memory, and future planning (for meta-analyses see Spreng et al., 2009; Andrews-Hanna et al., 2014). As such, the DMN may support the contents of experience when the mind wanders. In line with this perspective, the DMN contains subsystems that relate to the two amodal long-term memory systems in the brain (Andrews-Hanna et al., 2010a,b, 2014). First, the dorsal-medial subsystem of the DMN encompasses regions of medial prefrontal and anterior temporal cortex, regions implicated in the representation and retrieval of conceptual knowledge of the world (e.g. Jackson et al., 2016; Lambon Ralph et al., 2017). Second, the medial-temporal subsystem involves regions of medial temporal lobe, including the hippocampus, that are important in episodic memory (Moscovitch et al., 2016). Both of these subsystems, as well as the DMN core, integrate information from multiple cortical regions that contain modality-specific aspects of experience such as regions that represent faces, places, words, actions, smells and sounds (Horner et al., 2015; Patterson et al., 2007). Neural substrates supporting episodic memory may bind such information into a single representation, while substrates supporting semantic memory may extract commonalities across experiences giving rise to general knowledge. These representational codes may be subsequently integrated into the core of the DMN based on its capacity to echo neural responses from across the cortex (Leech et al., 2011, 2012). Given that the DMN has been linked to both the capacity to generate experiences that do not reflect the state of the external world, and memory representation and retrieval, its contribution to mind-wandering might reflect the decoupling of attention from input/output systems during mind-wandering, the availability of memory representations that reflect the contents of our thoughts, or both.
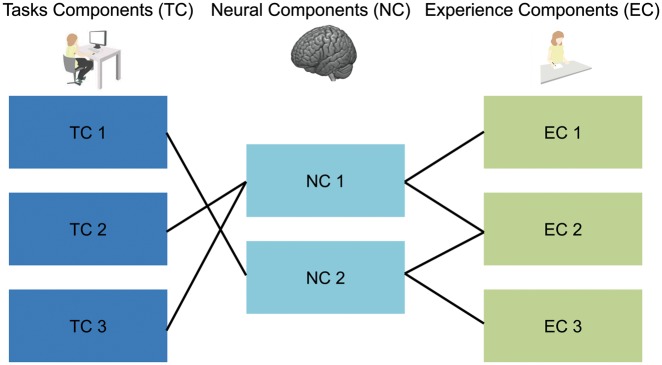
The current study used an individual difference analysis to delineate potential roles for the DMN in mind-wandering. Based on evidence of spatial overlap between patterns of neural activity observed in both task based conditions (e.g. Smith et al., 2009) and from studies providing evidence that individual differences in brain organization at rest are associated with the ability to perform a task mimicking those seen online during task performance (e.g. Krieger-Redwood et al., 2016), we used resting-state functional magnetic resonance imaging to describe trait differences in neural organization and linked them to trait differences in mind-wandering. We recruited 157 participants and measured their brains at rest on the first day of our study. On three subsequent days we assessed the content and form of their naturally occurring thoughts in the laboratory in order to provide a relatively stable measure of trait-like mind-wandering. Next, in a subset of 80 participants we measured how they performed on a battery of tasks selected to measure core cognitive processes linked to the DMN (including episodic and semantic memory, planning and social cognition). We generated descriptions of the higher-order components underlying (i) task performance (what we call ‘task components’—TC) and (ii) self-reports produced using experience sampling of thoughts (what we call ‘experience components’—EC). Next, we identified ‘neuro-cognitive components’ (NCs) by examining the relationship between different features of task performance and patterns of functional connectivity exhibited by the DMN. Finally, we used these NCs to identify the brain–behavior relationships predictive of the patterns of experiences that emerge during unconstrained thought. In particular, we were interested in whether brain–behavior–experience patterns highlighted a relationship to the potential contents of thought (e.g. patterns of memory retrieval, social cognition or planning). Alternatively, we might predict that brain–behavior–experience patterns would relate more to the features that allow mind-wandering to occur such as being internally focused or performing poorly on tasks requiring external engagement (e.g. McVay and Kane, 2009), which would speak to the role of the DMN in stimulus-independent features of mind-wandering. The rationale for this experiment is summarized in Figure 1.
Fig. 1.
Schematic description of the hypothesized relationship between latent neuro-cognitive components and both task performance and descriptions of experience. TC, task component; NC, neuro-cognitive component; EC, experiential component.
We were particularly interested in testing two features of the component process account of mind-wandering. First, that perceptual decoupling is an enabling condition that allows memory processes to contribute to thought content during the mind-wandering state (Smallwood 2013a,b). We therefore examined whether individual differences in memory performance were related to mind-wandering and, if so, whether poor performance on tasks requiring external attention would moderate this relationship. Second, that key component processes involved in the mind-wandering state (perceptual decoupling, memory retrieval—revealed by our TCs) are anchored by neural processes in the DMN. We expected that patterns of functional connectivity of one or more subsystems of this large scale network would be related to individual differences in mind-wandering, memory retrieval, poor external engagement and/or a combination of all three.
Materials and methods
Participants and procedure
One hundred and fifty-seven participants (60% female, Mage = 20.43 s.d. = 2.63; range = 18–31) took part in an initial study where their resting-state brain activity and naturally occurring thoughts in the laboratory were recorded. A sub-sample of 80 then completed the subsequent task battery (64% female; Mage = 20.29; s.d. = 2.26, range = 18–29). All participants had a resting state scan prior to any laboratory testing and underwent three days of testing during which we acquired descriptions of their naturally occurring thoughts while performing a simple non-demanding cognitive task. These measurements took place at the beginning of each session after which they completed a number of measures not relevant to our current research questions. These participants were compensated £80 or a commensurate amount of course credits. The subset of 80 then took part in a laboratory session lasting ∼1.5 h during which they completed the tasks described below in a random order (with the exception of the non-social semantic relatedness task, which was part of a previous battery of tasks). Participants also completed several questionnaires online prior to the laboratory session, which are not relevant to the specific research question addressed here. Participants were compensated with £20 for their time or a commensurate amount of course credits. Ethical approval was obtained from the University of York Psychology Department and the York Neuroimaging Centre and the research was conducted in accordance with the Helsinki Declaration.
Resting state MRI acquisition
Structural and functional data were acquired using a 3T GE HDx Excite MRI scanner utilizing an eight-channel phased array head coil (GE) tuned to 127.4 MHz, at the York Neuroimaging Centre, University of York.
Structural MRI acquisition
Structural scans in all participants were based on a T1-weighted 3D fast spoiled gradient echo sequence (TR = 7.8 s, TE = minimum full, flip angle= 20°, matrix size = 256 × 256, 176 slices, voxel size = 1.13 × 1.13 × 1 mm3).
Functional MRI acquisition
Resting-state functional Magnetic Resonance Imaging activity was recorded from the whole brain using single-shot 2D gradient-echo-planar imaging (TR = 3s, TE = minimum full, flip angle = 90°, matrix size = 64 × 64, 60 slices, voxel size = 3 × 3 × 3 mm3, 180 volumes). Participants viewed a fixation cross with eyes open for the durations of the functional MRI resting state scan (9 min). A T1 weighted FLAIR scan with the same orientation as the functional scans was collected to improve co-registration between subject-specific structural and functional scans (TR = 2560 ms, TE = min full, matrix size = 64 × 64, voxel size = 3 × 3 × 3 mm3).
Resting state pre-processing and first level analysis
Functional and structural data were pre-processed and analyzed using FMRIB’s Software Library (FSL version 4.1, www.fmrib.ox.ac.uk/fsl). Individual FLAIR and T1-weighted structural brain images were extracted using Brain Extraction Tool. Structural images were linearly registered to the MNI-152 template using FMRIB's Linear Image Registration Tool (FLIRT). The resting state functional data were pre-processed and analyzed using the FMRI Expert Analysis Tool (FEAT). The individual subject analysis involved: motion correction using MCFLIRT; slice-timing correction using Fourier space time-series phase-shifting; spatial smoothing using a Gaussian kernel of FWHM 6 mm; high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 100 s); Gaussian low-pass temporal filtering, with sigma = 2.8 s; six motion parameters (as estimated by MCFLIRT) were regressed out; cerebrospinal fluid and white matter signal were regressed out (top five PCA components, CompCor method).
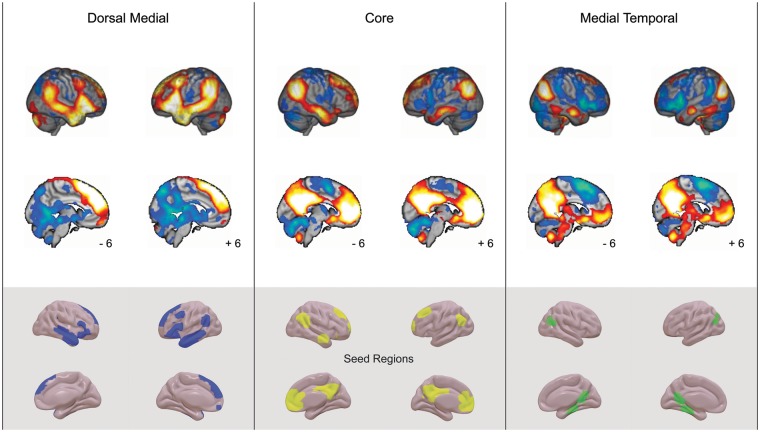
Studies have highlighted three DMN subsystems (Andrews-Hanna et al., 2010b, 2014; Yeo et al., 2011): (i) the core of the network is focused on medial regions in posterior cingulate cortex and medial prefrontal cortex (mPFC), and the angular gyrus, (ii) the dorsal-medial subsystem encompasses regions of dorsal mPFC, anterior and lateral temporal cortex and ventral, anterior prefrontal cortex, (iii) the medial-temporal subsystem engages retrosplenial cortex, ventro-medial prefrontal cortex and parahippocampus. After preprocessing, we used the DMN maps described by Yeo et al. (2011) to drive functional connectivity analyses. We selected networks 15 (medial-temporal subsystem), 16 (core subsystem) and 17 (dorsal-medial subsystem) from the 17-network solution. The parcellations were obtained in FreeSurfer surface space from http://www.freesurfer.net/fswiki/CorticalParcellation_Yeo2011. After calculating the time series for each of these networks, we performed a functional connectivity analysis separately for each subject. The resulting maps were compared at the group level using FMRIB’s Local Analysis of Mixed Effects. These maps were thresholded at a Z = 2.3 and whole brain cluster corrected P < 0.05 FWE. The resulting positive and negative maps are presented in Figure 2 and the individual maps were used as the dependent variables in a series of multiple regressions (see Results). To facilitate the transparency of our analyses we uploaded all maps produced in our study to a publicly available collection at Neurovault (http://neurovault.org/collections/2115/).
Fig. 2.
Identifying the functional connectivity of different subsystems of the default mode network. Results of a functional connectivity analysis in which the three default mode network subsystems as defined by Yeo et al. (2011) were used as seed regions. These maps are thresholded at Z = 2.3 and are corrected for multiple comparisons at P <0.05 FWE. The maps in the gray sub panel reflect the networks that were used as the seed regions (dorsal medial = blue; core = yellow; medial temporal = green).
Measures of experience
The contents of experience were measured using multi-dimensional experience sampling (MDES) (Ruby et al., 2013a,b; Engert et al., 2014; Medea et al., 2016; Smallwood et al., 2016). This technique uses experience sampling to periodically assess the content and form of a participant’s naturally occurring thoughts and experiences. In this case, participants performed a simple task that alternated in working memory load between a 0-back condition and a 1-back condition (see Konishi et al., 2015). Participants completed this task, which lasted ∼25 min, on three separate occasions. We sampled experience on 3 days to minimize potential state-related influences on this measure.
In both the 0-back and 1-back tasks, participants viewed different pair of shapes (non-targets) that appeared on screen separated by a vertical line. There were six possible shape pairings: circle and square, circle and triangle, and square and triangle (each with opposite left-right pairings). After a series of non-target blocks, participants were presented with a target trial requiring them to make a manual response (left or right arrow button press). The target was a small shape in the middle of the vertical line. In the 0-back condition, the target (e.g. a small square in the middle of a vertical line) was flanked by one of two shapes (e.g. a square on the left; a circle on the right) and participants had to indicate via button press which shape matched the target shape (e.g. left). In the 1-back condition, the target was flanked by two question marks and participants had to indicate which shape on the previous trial (i.e. 1-back) matched the target. There were eight blocks—each consisting of two to four mini-blocks containing either the 0- or 1-back condition. Each mini-block contained one target trial; the number of preceding non-target trials varied between one and six. Participants were informed of the change in condition (from 0 to 1-back and vice versa) by the presentation of the word ‘SWITCH’ that remained on-screen for 5 s. Stimuli presentation rates were jittered: fixation cross presentations ranged from 1.3 to 1.7 s, non-target presentations ranged from 0.8 to 1.2 s and target presentations ranged from 2.1 to 2.5 s.
Participants’ thoughts were sampled on a number of dimensions using quasi-random thought probes that occurred during the 0- and 1-back tasks. Participants received an average of 14.07 probes (s.d. = 3.30, Range: 6–25) during each session of the task. Probes asked participants to report on the contents of their conscious experience in the moment immediately preceding the interruption. Participants always rated their level of task focus first (My thoughts were focused on the task I was performing) from 0 (completely off-task) to 1 (completely on-task). Participants then rated their thought at the moment before the probe on a further 12 dimensions (described in Table 1) that captured core features of experience. All ratings were made on a sliding scale from 0 to 1 and were answered in a random order. The analyses we report in this paper focused on the experiences regardless of the two tasks participants performed. We also looked for task differences but found no evidence that the patterns reported in this paper varied according to the demands of the task.
Table 1.
Experience sampling questions used
| Dimensions | Questions | 0 | 1 |
|---|---|---|---|
| Focus | My thoughts were focused on the task I was performing. | Not at all | Completely |
| Future | My thoughts involved future events. | Not at all | Completely |
| Past | My thoughts involved past events. | Not at all | Completely |
| Self | My thoughts involved myself. | Not at all | Completely |
| Other | My thoughts involved other people. | Not at all | Completely |
| Emotion | The content of my thoughts was: | Negative | Positive |
| Images | My thoughts were in the form of images. | Not at all | Completely |
| Words | My thoughts were in the form of words. | Not at all | Completely |
| Vivid | My thoughts were vivid as if I was there. | Not at all | Completely |
| Vague | My thoughts were detailed and specific. | Not at all | Completely |
| Habit | This thought has recurrent themes similar to those I have had before. | Not at all | Completely |
| Evolving | My thoughts tended to evolve in a series of steps. | Not at all | Completely |
| Spontaneous | My thoughts were: | Spontaneous | Deliberate |
Measures of memory and cognition
Tasks were selected to provide a broad coverage of aspects of cognition and memory with an emphasis on elements of cognition that have been previously associated with mind-wandering and the DMN: (i) autobiographical planning (e.g. Spreng et al., 2010; Gerlach et al., 2014), (ii) social cognition (Amodio and Frith, 2006; Schilbach et al., 2008) and (iii) episodic and semantic memory retrieval (Binder et al., 2009; Spreng and Grady, 2010).
Autobiographical planning
Participants completed a version of the Means End Problem Solving Test (Platt and Spivack, 1975). Participants were presented with six different social scenarios in a random order. For each scenario they were provided with an initial situation where a problem has to be solved (e.g. a person’s friends are ignoring them) and a desired end point (e.g. the person’s friends like him/her again). Participants were required to complete the middle potion of each social scenario providing means whereby the initial solution becomes the desired end point. For each scenario participants were provided with 4 min to write about the steps that they would take to solve the problem in each scenario. Responses were coded according to (i) the number of relevant means (i.e. the problem solving steps); (ii) the solution effectiveness (defined as one that maximizes positive and minimizes negative short- and long-term consequences) (D'Zurilla and Goldfried, 1971) which was rated from 1 (not at all effective) to 7 (extremely effective) and (iii) the solution specificity (i.e. a detailed and specific problem solution) which was rated from 1 (not at all specific) to 7 (extremely specific). Each problem solution was individual coded and scores for each domain (relevant means, effectiveness, specificity) were averaged across each of the six problems. A random 25% of the problem solutions were second coded by an independent rater; inter-rater reliability was calculated with intra-class correlation coefficient (two-way random). The reliability coefficient was 0.73 for relevant means, 0.46 for effectiveness and 0.52 for specificity indicating fair to good interrater reliability (Hallgren, 2012).1
The MEPs provides information on how individuals plan the steps between from a starting social situation to desired end goal. To provide a non-social control for the process of planning, participants completed a computerized version of the Tower of London task (see Spreng et al., 2010). For each problem, participants were presented with pictures depicting three rods of different heights and three colored discs. The tallest rod can hold three discs, the middle rod can hold two discs and the shortest rod can hold only one disc. In each problem, participants were presented with two pictures: the bottom picture showed the ‘goal’ state (i.e. how the discs should be positioned on the rods); the top picture showed the ‘initial’ state (i.e. how the discs are currently positioned). Participants’ task was to mentally plan the steps needed to reach the goal state from the initial state and indicate the minimum number of moves that it would take, with the conditions that discs can only be moved one at a time and that only the top disc on a rod can be moved. Participants viewed the goal state for 5 s and then the start configuration for up to 15 s (or until they provided a response). Accuracy and reaction time were used to create an efficiency score where higher scores reflected fast and accurate responses.
Social cognition
We acquired behavioral measures of two aspects of social cognition: mentalizing and theory of mind. To assess mentalizing, participants completed a measure of perspective taking (Stiller and Dunbar, 2007) in which they read five short stories of social interactions involving a number of characters. After reading each story twice participants were presented with 20 true/false questions. Half the questions concerned facts about the story; half required participants to correctly infer the mental states of the story characters which differed in their levels of intentionality (the most complex metalizing questions for example involved tracking the mental states of all characters in the story). Fact and metalizing questions were ordered randomly, as were the order of the stories. Participants’ answers to each question were weighted according to their level of complexity (such that harder questions were given more weight) and then averaged separately for fact and metalizing questions across the five stories. Participants also completed the Reading the mind in the eyes test, a theory of mind measure comprising mental state attribution and complex emotion recognition (Baron-Cohen et al., 2015). Participants were presented with 36 photographs of people’s eye areas, and, for each photograph, they were instructed to select one metal state word (from four) that best described what the person in the photograph is feeling or thinking. Photographs and word choices were presented in a random order and were displayed on screen until participants responded or 20 s had elapsed (recorded as an incorrect response). Accuracy and reaction time were used to create an efficiency score where higher scores reflected fast and accurate responses.
Memory
We assessed aspects of both semantic and episodic memory since both of these have been implicated in the DMN (Binder et al., 2009; Spreng and Grady, 2010). To index autobiographical memory, participants completed an adapted version of the Autobiographical Memory Interview (Madore et al., 2014). Participants saw a random selection of six pictures (from a larger set of 18) which were used as cues to recall an autobiographical event from the past few years. Participants were instructed to describe a specific event in detail through their own eyes; they were given 3 min to write down as much detail about each event. Responses were scored using the adapted Autobiographical Interview scoring manual (Addis et al., 2008). This scoring system provided an individual score for each event regarding internal event details (i.e. episodic details regarding the event including place, time, sensory and mental state details related to the event) and external event details (i.e. non-episodic details such as semantic statements and repetitive or off-topic details); these were averaged for each participant across each of the six descriptions. Descriptions were also given a rating of episodic richness (Levine et al., 2002) to index the extent to which a feeling of experiencing of the event was conveyed; this was rated on a six-point scale. A random 25% of the event descriptions were second coded by an independent rater; inter-rater reliability was calculated with intra-class correlation coefficient (two-way random). The reliability coefficient was 0.89 for internal details, 0.86 for external details and 0.87 for episodic richness, indicating excellent interrater reliability (Hallgren, 2012).
Participants also completed a semantic relatedness task to provide an indication of semantic memory performance. Since the DMN is also implicated in social cognition (see above) we used a task in which both semantic and social relatedness judgments were made. Participants were asked to determine the semantic relatedness between a probe word and three alternative choices only one of which was related in meaning to the probe. Each trial started with 500 ms blank screen and the three choices were then presented on the bottom of the screen for 900 ms. The probe was presented in the top middle section of the screen. Probe and choices remained visible until participants responded or for a maximum of 3 s. In the non-social version of this task that consisted of 60 probe words for objects that were selected from a large database used in previous experiments (e.g. Davey et al., 2015; Krieger-Redwood et al., 2015). The social version of this task consisted of 30 positive and 30 negative words describing abstract social concepts (e.g. impolite-tactless) adapted from Zahn et al. (2007). Accuracy and reaction time were used to create an efficiency score where higher scores reflected fast and accurate responses.
Results
Analytic aims
Our study aimed to understand the potential roles for the DMN in mind-wandering. To this end, we first identified latent components describing task performance and patterns of experience. Next, we conducted a series of resting-state functional connectivity analyses to identify the neural patterns associated with the DMN that described variation in task performance. Having characterized latent components in brain, behavior and experience, we examined if: (i) the experience components (EC) could be explained by individual differences in task components (TC) and (ii) if any of the patterns linking experience to behavior were also related to the neural components (NCs) that reflect patterns of neural activity that the DMN exhibited at rest that could be explained by measures of task performance.
Identifying components of task performance and experience
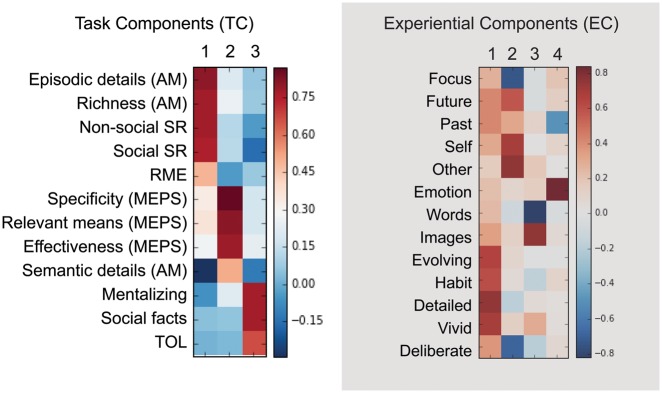
We decomposed the task performance measures and measures of experience using principal components analysis (PCA) to reveal latent variables that described these measurements. For the task measures (Table 2 for descriptive statistics), the PCA was conducted using the 12 measures resulting from the battery of tasks administered. This revealed three task components (TCs) with eigenvalues greater than one and with a clear elbow after the third component observed in the scree plot. The three orthogonal components accounted for 61% of the total variance and varimax rotation produced component loading patterns shown in Figure 3 and described below. We computed standardized component scores for each of our three components for each participant and used these as independent variables in the subsequent analyses.
Table 2.
Means and standard deviations of key task variables
| Construct and variables | M | s.d. |
|---|---|---|
| Autobiographical planning | ||
| MEPS—number of relevant means | 7.60 | 1.83 |
| MEPS—effectiveness (rated 1–7) | 5.01 | 0.85 |
| MEPS—specificity (rated 1–7) | 4.63 | 0.92 |
| TOL (efficiency score—expressed as reaction time) | −44.96 | 74.58 |
| Social cognition | ||
| Mentalizing (expressed as a proportion) | 0.76 | 0.06 |
| Social facts (expressed as a proportion) | 0.85 | 0.07 |
| RME (efficiency score—expressed as reaction time) | −6.57 | 2.29 |
| Memory | ||
| AM—episodic details | 20.95 | 5.36 |
| AM—semantic details | 2.37 | 1.67 |
| AM—richness (rated 0–6) | 3.81 | 0.82 |
| Non-social SR (efficiency score—expressed as reaction time) | −1.76 | 0.32 |
| Social SR (efficiency score—expressed as reaction time) | −2.76 | 0.62 |
Fig. 3.
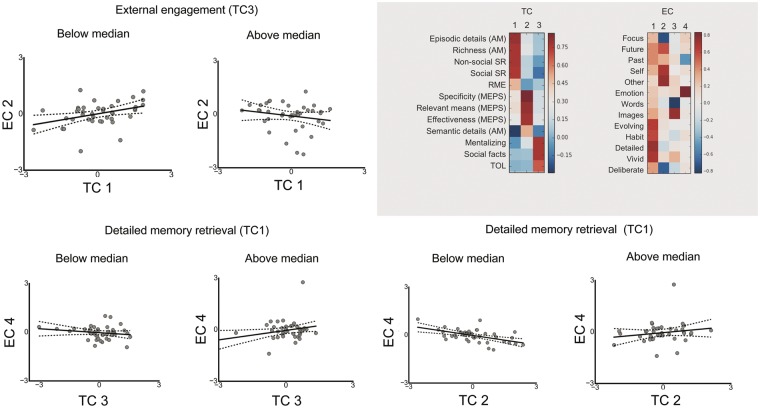
Identifying the components underlying task performance and experience. The results of decomposition of the multi-dimensional experience sampling data (MDES) and the task battery to produce components of experience (EC) and components of task performance (TC). In both cases, we employed exploratory factor analysis and used varimax rotation. The number of solutions was selected based on the elbow from the scree plot. TC1 = detailed memory retrieval; TC2 = social problem solving; TC3 = external engagement; EC1 = immersive thoughts; EC2 = spontaneous off-task future thoughts; EC3 = modality of thoughts; EC4 = positive thoughts. EC, experiential components; TC, task components; AM, autobiographical memory; SR, semantic retrieval; MEPS, means ends problem solving; TOL, tower of London; RME, reading the mind in the eyes; TOM, theory of mind.
TC1—Detailed memory retrieval—accounted for 35% of the overall variance and individuals with a high weighting on this component produced more episodic details and richer descriptions from autobiographical memory; they also performed efficiently on tasks involving the retrieval of social and non-social semantic associations. These individuals tended not to produce generic semantic information in autobiographical memory retrieval. While they were stronger on average at recalling autobiographical, social and semantic information, these participants were not stronger on tasks that involved encoding and planning (such as Tower of London) or encoding, understanding and recalling stories (weaker fact and metalizing scores), suggesting that this component did not correspond to the capacity to encode ongoing events, or to memory ability in general, but rather to the capacity to retrieve detailed and specific information.
TC2—Social problem solving—accounted for 17% of the overall variance and individuals with a high weighting on this component performed well on all indices of social problem solving. This capacity for problem-solving did not extend to a non-social domain (Tower of London).
TC3—External engagement—accounted for 10% of the overall variance and individuals with a high weighting on this component performed well on the Tower of London task, and well on both fact-based and metalizing questions about information that was described in the social stories. Notably, these tasks differ from others in the battery because they rely to a much greater extent on encoding information during the task (rather than for example being able to rely on pre-existing knowledge or experience). This component did not predict good performance on semantic or episodic retrieval tasks.
We decomposed the experience sampling data at the trial level (for prior demonstrations of this approach see Ruby et al., 2013a,b; Engert et al., 2014; Medea et al., 2016; Smallwood et al., 2016). This revealed four experiential components (ECs) with a clear elbow after the fourth component observed in the scree plot.
EC1—Immersive thoughts—accounted for 26% of the overall variance and described thoughts that were detailed, evolving, vivid and habitual.
EC2—Spontaneous off-task future thoughts—accounted for 19% of the overall variance and described spontaneous off-task thoughts involving the self, others, and future events.
EC3—Modality of thoughts—accounted for 10% of the overall variance and distinguished visual from verbal thoughts.
EC4—Positive thoughts—accounted for 7% of the overall variance and described thoughts with a positive valence that were not typically about past events.
We projected these components back into subject space by averaging the loadings for each individual. This process describes each individual in terms of how much their thoughts represented each of the ECs. Heat maps describing the ECs are presented in the gray panel in Figure 3. Of these solutions, two (off-task thoughts focused on the future and the modality of thoughts) replicate those observed in prior studies that used a similar approach but a different set of questions (e.g. Medea et al., 2016; Smallwood et al., 2016).
Identifying brain–task relationships
Having determined the latent components describing our participants in terms of their task performance and experience, we next characterized the relationship between the behavioral dimensions and the functional connectivity of different DMN subsystems. We conducted three separate group-level multiple regressions in which individual loadings for each task component were included as between-participant explanatory variables and whole brain connectivity maps for each DMN subdivision at rest were the dependent variables. Automatic outlier detection was applied to minimize the impact of extreme scores in both neural and behavioral data. We examined whole brain differences in the positive and negative connectivity of each DMN subdivision seed region. We used a cluster forming threshold of Z = 2.3, and to minimize Type 1 error rates, we set an alpha value of P < 0.008 FWE. This accounted for: (i) the number of voxels in the brain, (ii) the two-tailed contrasts (positive and negative connectivity) and (iii) the number of separate models (i.e. three—one for each DMN subsystem). Given concerns that the cluster forming thresholds used in fMRI analyses are subject to Type I Errors (Eklund et al., 2016), we re-ran these analyses using a more conservative threshold (Z = 2.6) and all of the observed clusters were significant at this level at the whole brain level (Table 3).
Table 3.
Clusters showing a significant association between task components and functional connectivity of DMN subsystems at rest
| DMN Seed | Cognition component | Cluster center of gravity | Cluster size (voxels) | Region | P value | ||
|---|---|---|---|---|---|---|---|
| Detailed retrieval − | −12 | −92 | −24 | 2322 | Cerebellum extending to the occipital fusiform gyrus | 0.000008*,† | |
| −48 | −72 | −28 | 1104 | Cerebellum extending to the inferior temporal gyms and temporal fusiform cortex | 0.00358* | ||
| −48 | 24 | 34 | 731 | Middle frontal gyrus | 0.0345 | ||
| Social problem solving + | −40 | −82 | 22 | 3117 | Lateral occipital cortex (occipital fusiform gyrus) | 0.0000003† | |
| 34 | −82 | 28 | 696 | Lateral occipital cortex | 0.0433 | ||
| Medial Temporal | Detailed retrieval − | 46 | −70 | −44 | 766 | Cerebellum | 0.0294 |
| Social problem solving + | 34 | 54 | 14 | 798 | Frontal pole | 0.0241 | |
| External engagement + | −4 | −82 | 44 | 875 | Precuneus cortex | 0.015 | |
| 42 | −48 | 60 | 844 | Superior parietal lobule | 0.0181 | ||
| External engagement − | −46 | 48 | 2 | 1809 | Frontal pole | 0.0001*,† | |
| −64 | −26 | −10 | 1286 | Middle temporal gyrus | 0.001*,† | ||
| 2 | 48 | 36 | 1115 | Superior frontal gyrus / dorsal medial pFC | 0.004*,† | ||
| 52 | 40 | −4 | 711 | Frontal pole | 0.0417 | ||
| Dorsal Medial | External engagement − | 8 | −42 | 0 | 805 | Cingulate gyrus | 0.0239 |
Notes: The P-values represent the level of significance after correcting for the number of voxels in the brain. P-values marked with an asterisk identify regions that are significant after correcting for multiple comparisons (P < 0.008), the † identifies regions that are significant at P < 0.008 when thresholding Z = 2.6.
AM, autobiographical memory; SR, semantic retrieval; MEPS, means ends problem solving; TOL, tower of London; RME, reading the mind in the eyes.
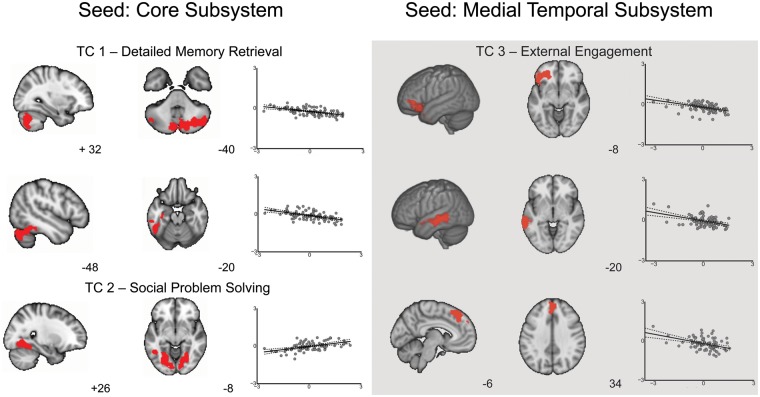
We found six NCs each reflecting modulation of either the connectivity of the core and medial temporal systems of the DMN by individual variation in one of the TCs. These are summarized in Figure 4 and Table 3. Connectivity of the core DMN (Yeo 16) revealed three significant clusters; one cluster in the cerebellum extending into occipital fusiform cortex; another cluster in the inferior temporal gyrus and temporal fusiform cortex and extending into the cerebellum, and the third other in the lateral occipital cortex (in particular, the occipital fusiform gyrus). The first two clusters showed a negative correlation with TC1, indicating that high levels of detail in memory retrieval was related to greater decoupling between these clusters and core regions of the DMN. The third cluster in the fusiform cortex showed a positive correlation with TC2, indicating that connectivity of the core DMN with this cluster was higher for people who were good at social problem solving.
Fig. 4.
Determining the neuro-cognitive components (NC) associated with task performance. Results of multiple regressions using the task components (TCs) as the independent variables and the functional connectivity of the core of default mode network (Yeo 16) and medial-temporal subsystem (Yeo 15) as the dependent variable. These maps were created using a cluster forming threshold of Z = 2.3, and were corrected for the two tailed nature of our statistics, the number of models (3) and for the number of voxels in the brain yielding an α level of P <0.008 FWE. The scatter plots reflect the relationships between the connectivity with the region indicated in red and the relevant TC.
Connectivity of the medial temporal DMN subsystem also revealed three significant clusters: one cluster in anterior inferior frontal gyrus another cluster in middle temporal gyrus, and the third in superior frontal gyrus (including the dmPFC). These clusters all showed a negative correlation with TC3, suggesting that decoupling between these regions is associated with better external engagement.
Relationships between task and experience components
Our analysis so far has described our sample in terms of components representing descriptions of self-reported experience, performance on the task battery, and the correspondence between task components and the organization of the DMN at rest. Next, we used these components to understand how the DMN contributes to mind-wandering by examining their associations across our sample.
We used a multivariate analysis of variance (MANOVA) to identify whether, at the level of behavior, different combinations of the latent variables describing task performance accounted for the components of experience. In this analysis, the ECs were the dependent variables and the TCs were the independent variables; we examined the main effects of each TC on experience components as well as fully modelling the interactions between the TCs. Two significant interactions were revealed by the MANOVA multivariate tests. First, an interaction between TC1 and TC3: F(4, 69) = 2.97, P =0.025, . Subsequent tests of between-subjects effects showed that this interaction was observed for off-task future thoughts (EC2), F(1, 80) = 4.95, P = 0.029, ). Individuals scoring below the median on TC3 (i.e. people who were less good at tasks with an encoding element and who were therefore potentially less externally engaged) showed a positive correlation between their levels of detail in memory retrieval and spontaneous off-task future thoughts (r = 0.39, P < 0.014) whereas individuals above the median on TC3 did not (r = −0.19, P = 0.240). An interaction between TC1 and TC3 was also observed for positive emotional thoughts (EC4), F(1, 80) = 4.36, P = 0.041, . Individuals scoring above the median for TC1 (detailed memory retrieval) showed a positive correlation between their levels of external engagement (i.e. TC3 scores) and positive emotional thoughts (r = 0.33, P = 0.038) whereas individuals below the median did not (r = −0.19 P = 0.254).
Second, the MANOVA revealed an interaction between TC1 (detailed memory retrieval) and TC2 (social problem solving), F(4, 69) = 2.81, P = 0.032, . Subsequent tests of between-subjects effects indicated that this interaction was observed for positive emotional thinking, F(1, 80) = 8.55, P = 0.005, . Individuals scoring below the mean in detailed memory retrieval (TC1) showed a negative correlation between their levels of social problem solving (TC2) and EC4 (positive emotional thoughts; r = −0.54, P < 0.001) whereas individuals below the median did not (r = 0.18, P = 0.271). These associations are summarized in Figure 5.
Fig. 5.
Scatter plots showing interactions between task components predicting experience components (based on median splits).
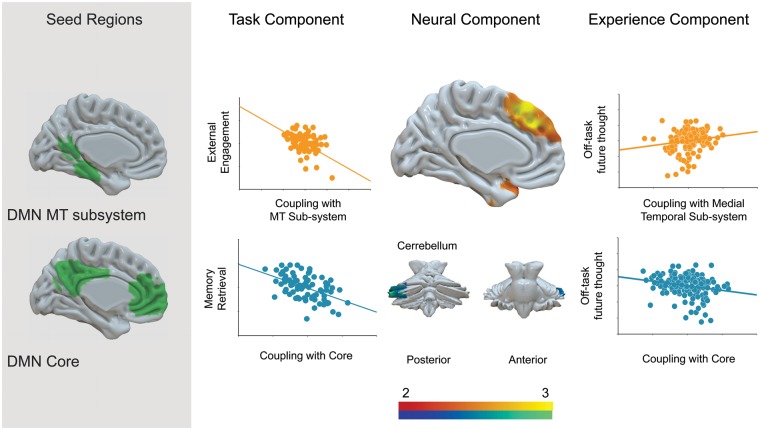
Having demonstrated associations between latent variables that underlie task performance and experience, we next tested whether similar patterns would be reflected in the NCs. We used the average parameter estimates that described the NCs generated by our functional connectivity analyses as independent variables in a MANOVA with the four ECs components as the dependent variables. This analysis was based on the full sample of 157 participants and revealed significant effects for parameter estimates within two clusters for ECs: (i) the cluster comprising the cerebellum extending to the inferior temporal gyrus and temporal fusiform cortex (i.e. the second cluster presented on the left-hand side of Figure 4, and second cluster described in Table 3) [F(4, 147) = 2.44, P = 0.050, ) and 2] the cluster comprising the dmPFC [F(4, 147) = 2.50, P = 0.045, ]. Tests of between-subjects effects showed that greater decoupling between core DMN regions and the cluster comprising the cerebellum was associated with more off-task future thoughts, F(1, 150) = 8.18, P = 0.005, . In contrast, greater coupling between the medial-temporal DMN subsystem and the dmPFC was associated with more off-task future thoughts, F(1, 150) = 4.68, P = 0.029, . Together, these data show that, consistent with the behavioral analysis, the patterns of functional connectivity associated with both TC1 and TC3 (detailed memory retrieval and external engagement, respectively) both predicted the expression of off task future thoughts (these relationships are summarized in Figure 6). We also observed that more immersive self-generated thought (EC1) was linked to greater functional coupling between the medial-temporal subsystem and the dMPC, F(1, 150) = 4.09, P = 0.045, , although since we did not find an association between these variables at the behavioral level we do not interpret this pattern any further.
Fig. 6.
The relationship between the components underlying task performance, experience and the connectivity of the default mode network. Multivariate analysis of variance demonstrated that two of the neuro-cognitive components (NCs) were associated with both components that underpin task performance (TC) and those that explain the experience sampling data (ECs). The scatterplots present the correlations between task and brain and are based on 80 individuals (left), and between the NCs and experience which are based on 157 individuals (right)—scores are residualized.
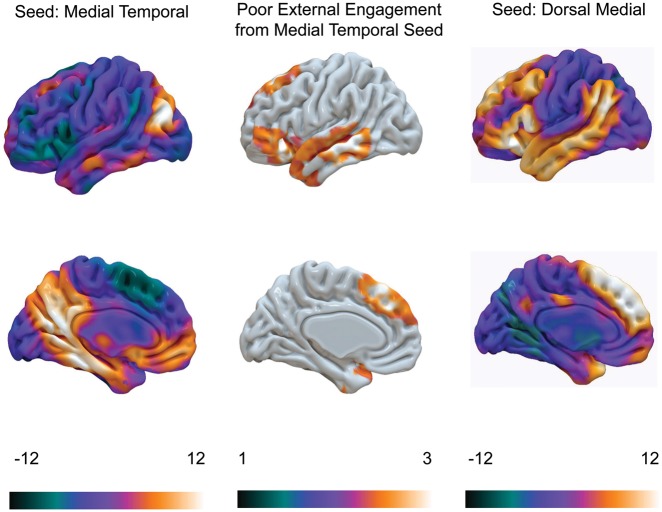
Finally, our analysis suggests that the pattern of poor task engagement is associated with a pattern of functional coupling at rest between the medial-temporal subsystem and regions of inferior frontal cortex, dorsal-medial prefrontal cortex and lateral temporal cortex. These regions are all elements of the dorsal-medial DMN subsystem as defined by Yeo et al. (2011). To quantify this similarity, we examined the spatial overlap between the pattern of functional coupling associated with task disengagement and the functional coupling of the dorsal-medial DMN subsystem (see Figure 7). This analysis reveals that, almost without exception, the pattern of functional connectivity from the medial-temporal subsystem that was associated with task disengagement falls within the connectivity patterns of the dorsal-medial subsystem. This suggests that enhanced communication between these two subsystems of the DMN is linked to reduced capacity to perform tasks that rely on external engagement.
Fig. 7.
Poor external engagement corresponds to coupling between the medial-temporal and dorsal-medial DMN subsystems. Poor performance on tasks with a greater reliance on external engagement is associated with coupling between medial-temporal and dorsal-medial subsystems of the DMN. The left- and right-hand columns represents the unthresholded connectivity pattern for the medial-temporal and dorsal-medial subsystems, respectively. The spatial map in the middle column is thresholded at Z = 2.3 P <0.05 FWE.
Discussion
Our study set out to understand the contribution of the DMN and component cognitive processes to self-generated experiences that naturally occurred during an ongoing task. We used PCA to identify cognitive components that underpin performance on a battery of tasks which captured aspects of memory, social cognition and planning. We also identified patterns of experiences reported while participants performed a simple unrelated cognitive task, identifying a pattern of spontaneous off-task future thoughts which correspond to a major element of the mind-wandering state as measured in multiple studies, and across a range of different cultures (Baird et al., 2011; Iijima and Tanno, 2012; Song and Wang, 2012; Poerio et al., 2013; Ruby et al., 2013a,b). Across individuals, different combinations of task components explained cross-sectional variance in different dimensions of experience. In particular, off-task future thoughts were associated with a pattern of performance characterized by poor external engagement combined with detailed memory retrieval. We also found that these patterns of behavioral covariance were mirrored by patterns of DMN connectivity. Critically, off-task future thoughts were linked to greater coupling between medial-temporal and dorsal-medial DMN subsystems, a neural pattern that predicted poor external engagement. Off-task future thoughts were also associated with decoupling of the DMN core from regions of the cerebellum; a neural pattern that predicted better performance on tasks requiring detailed memory retrieval. These findings have a number of important implications for understanding the role of the DMN in unconstrained experiences, as well as for cognition more generally.
First, our data provide convincing evidence in support of component process accounts of the mind-wandering state. These theoretical accounts propose that experiences such as mind-wandering are not the consequence of a single process, but emerge through the ‘interaction of discrete functional elements that serve specified cognitive processes’ (Smallwood, 2013b, p. 545). In particular, the process of attentional decoupling is hypothesized to lead to two consequences during mind-wandering: (i) it explains the association between the occurrence of mind-wandering and poor performance on tasks requiring external engagement and (ii) it allows cognition to focus on information that is generated from memory without retrieval being constrained by external input (Smallwood, 2013a; Smallwood and Schooler, 2015). Consistent with these accounts, our functional connectivity analysis demonstrates that the contributions of both attentional decoupling and memory retrieval to the mind-wandering state have unique neural patterns. High levels of off-task future thoughts were independently associated with the patterns of cerebellar decoupling linked to memory retrieval, and a pattern of within-DMN connectivity was linked to poor external engagement. Moreover, our behavioral analysis demonstrated that neither poor external engagement, nor detailed memory retrieval, in isolation, were predictive of individual differences in mind-wandering. Instead, higher detail in memory retrieval was associated with greater off-task future thoughts only in individuals who performed poorly on tasks requiring external engagement. Together, these patterns of dissociation at the level of brain and behavior confirm two predictions of component process accounts of the mind-wandering state: (i) that off-task future thought is related to multiple dissociable NCs and (ii) that decoupling provides a mechanism that allows memory retrieval to contribute to the unconstrained experiences that occur during mind-wandering.
Second, our findings suggest that the associations between mind-wandering and poor external engagement depend on patterns of integration within the DMN; specifically, a pattern of heightened coupling between the dorsal-medial and medial-temporal subsystems of the DMN. This pattern is consistent with a prior study which found that the coupling between the temporal pole (a region in the dorsal-medial subsystem) and the core of the DMN was linked to individual variations in greater off-task thought (e.g. Smallwood et al., 2016). Although our data suggest a link between these aspects of the DMN and poor external engagement, these regions have well-documented connections with the cortical input streams important for task performance. For example, regions of the temporal lobe that are implicated in semantic memory have been linked to both the DMN core and visual cortex via the ventral visual stream (e.g. Binney et al., 2010; Visser et al., 2012); similarly, retrosplenial cortex is linked to the DMN core and also receives input from visual regions (e.g. Vann et al., 2009). These dorsal-medial and medial-temporal subsystems of the DMN network can, therefore, act in response to external input (during encoding for episodic memory, and during object recognition and verbal comprehension in the semantic domain). However, our data suggest that they can also integrate information from each other, and that when they do so they may create a pattern of cortical organization that underpins the negative impact that mind-wandering can have on external task performance.
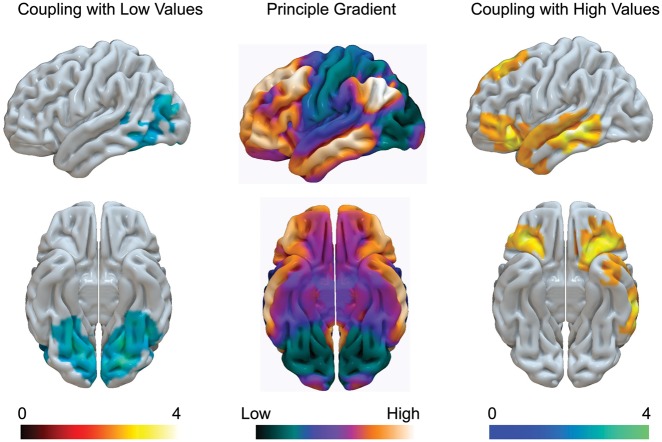
Third, our results suggest that the DMN can contribute to a range of different types of cognition by forming distinct modes of connectivity that are distinguished by their location on the principle gradient of connectivity (Margulies et al., 2016; see Figure 7, middle). One mode of connectivity reflects integration from regions of cortex specialized in unimodal representations of information into the DMN. In our study, participants who were better at identifying and sequencing steps to achieve a social goal showed stronger connectivity between the core of the DMN and regions in the occipital lobe/fusiform gyrus. These regions of cortex are close to the unimodal end of the principle gradient (for an illustration see Figure 8) and are important in the representation of people, places and scenes (Kanwisher and Yovel, 2006; Martin, 2007; Grill-Spector and Weiner, 2014). This pattern suggests that one type of functional behavior that the DMN can exhibit at rest entails coupling with regions that are more closely linked to the input stream of the cortex and hence support forms of cognition that are ‘tethered’ to either perception or behaviour (Buckner and Krienen, 2013). This process of integration may help to explain how the DMN can contribute to situations when external input is important for cognition (e.g. Konishi et al., 2015; Spreng et al., 2015; Vatansever et al., 2015). Our data also suggest that the DMN can form patterns of coupling that emphasize regions of cortex that are less connected to perception or action. Participants who were worse at tasks requiring external engagement exhibited the most coupling between regions located towards the heteromodal end of the functional gradient, such as the anterior temporal lobe and the ventrolateral prefrontal cortex. This pattern was associated with greater off-task future thoughts, indicating a potential role for integration at heteromodal ends the gradient when cognitive processes are reliant on memory generation such as during mind-wandering or mental time travel (Mason et al., 2007; Schacter and Addis, 2007; Christoff et al., 2009). Together these patterns of connectivity suggest that the contribution of DMN to different forms of cognition (e.g. those depending to a lesser or greater extent on internal vs external information processing) may emerge through the flexibility with which it can engage in different modes of cortical integration. At present, this interpretation is tentative because our data reflect individual differences in functional connectivity at rest rather than reliable connectivity changes during active cognitive processing, changes which may more accurately reflect responses to variations in the internal or external environment. Future research might address our interpretation more directly by examining the capacity of the DMN to flexibly change its patterns of connectivity with different regions along the principle gradient during task-based paradigms.
Fig. 8.
The default mode network has different modes of connectivity that correspond to different states and that can be organized along the principle gradient. Coupling between the core of the DMN and regions in fusiform/lateral visual cortex were associated with better performance on a task of social problem solving (left). In contrast, decoupling between the medial-temporal subsystem and regions of lateral temporal and prefrontal regions was associated with poor external engagement, but greater propensity for off task future thoughts (right). These correspond to patterns of connectivity that are either focused on integrating information from the unimodal end of the principle gradient into the core or coupling with regions at the heteromodal end of the gradient. The data in the middle column comes from Margulies et al. (2016). The data in the left- and right-hand columns comes from the current study and is thresholded at Z = 2.3 P <0.05 FWE.
Our study focused on the role of the DMN in mind-wandering in terms of its selection of seed regions, and many of our whole brain results highlight regions that fall within the broader DMN. These data confirm the important role that this network plays in spontaneous states such as mind-wandering, a conclusion supported by a number of previous individual difference studies (Bernhardt et al., 2014; Smallwood et al., 2016; Karapanagiotidis et al., 2017) as well as the majority of online experience sampling studies (Stawarczyk et al., 2011; Allen et al., 2013; Tusche et al., 2014 although see Christoff et al., 2009). More recent work suggests that mind-wandering also engages regions outside the DMN, in particular regions important for the executive network such as the dorso-lateral prefrontal cortex (Fox et al., 2015; Christoff et al., 2016). These regions are thought to be important at different times in the genesis of mind-wandering (see for example, Ellamil et al., 2016) so it is possible that our individual difference method of analysis is insensitive to subtle temporal features of the experience emphasized by these accounts. Alternatively, it may be that our experience sampling battery fails to capture important aspects of the experience that these systems support. Consistent with this possibility, recent work demonstrates that regions of the executive system show stronger communication with regions of the DMN for participants who engage in particularly deliberate forms of mind-wandering (Golchert et al., 2017).
Finally, our study highlights a pattern of reduced coupling between the core of the DMN and regions of lateral occipital cortex/cerebellum that was linked to detailed memory retrieval, as well as to spontaneous off-task future thinking. Although component process accounts predict a close link between the functional behavior of the DMN core during processes such as episodic and semantic memory retrieval and states of off-task thought, based on our current findings, it is difficult to determine the precise psychological meaning associated with these patterns of neural coupling. Decoupling from the lateral occipital cortex may reflect a process of separation from input, hypothesized to support better memory retrieval (Huijbers et al., 2009). Previous research has also found spatial differences within the cerebellum in terms of patterns of coupling with the DMN for autobiographical memory retrieval (Addis et al., 2016). In our study the cerebellar cluster falls within a regions showing reduced connectivity with the DMN core (see the Neurovault collection associated with this study). To fully understand the role of decoupling from the cerebellum and the lateral occipital, it will be necessary to record online neural data during both the process of autobiographical memory retrieval, and during mind-wandering, and examine how different features of these states are related to common and distinct patterns of functional decoupling between the DMN and these regions.
Although our findings provide important insights into how the DMN contributes to mind-wandering, there are a number of limitations that should be taken into account. First, to understand the component processes of self-generated thought, our study exploited individual differences in neurocognitive functioning; that is, we examined relationships at the trait rather than state level, relying on intrinsic rather than task based functional connectivity. This approach is warranted since previous research has shown that mind-wandering often shows similar patterns of associations using either approach. For example, similar trait and state results have been obtained in the domain of event related potentials (e.g. Barron et al., 2011; Baird et al., 2014) and mood (Smallwood and O'Connor, 2011; Poerio et al., 2013, 2015). Other work also highlights the overlap between task-based and intrinsic functional connectivity (Smith et al., 2009; Krieger-Redwood et al., 2016). Nonetheless, there may be patterns that our study cannot capture, so future work should explore similarities between neural processing within the DMN as it occurs during both task-related states such as memory retrieval as well as during off-task states such as mind-wandering. Second, our decomposition failed to highlight a distinct component associated with social cognition. This may reflect our task selection or it may indicate the close association between memory and social cognition that has been identified by several meta-analyses of neuroimaging data (e.g. Spreng et al., 2009). Given the potential social functions of mind wandering (Poerio and Smallwood, 2016), future research might profit from a closer examination of the role of social cognitive processes in the mind-wandering state, using a more comprehensive battery of tasks that include measures of visual perspective taking (Surtees et al., 2013) and measures that distinguish the self from others (Macrae et al., 2004). Finally, one general concern with experience sampling studies is that the act of measurement may alter the nature of the underlying state, known as reactivity (e.g. Wheeler and Reis, 1991). However, we collected resting state data before any of the experience sampling measures were recorded, ensuring that the functional behavior of the DMN could not be affected by the act of monitoring experience. Thus, our demonstration of the relationship at the level of brain, behavior and experience is unlikely to be accounted for by the hypothesis that the pattern is a consequence of thought monitoring (Konishi and Smallwood, 2016).
In summary, our study demonstrates a dissociable role of the DMN in both decoupling and retrieval from memory, both processes that are hypothesized to be important in the mind-wandering state (Smallwood, 2013; Smallwood and Schooler, 2015). Our study highlights patterns of heightened functional communication between subsystems of the DMN that supports disengagement of attention from the external environment, and a pattern of cerebellar decoupling that affords greater detail in memory retrieval. In combination, these patterns provide the basis of a rich internal context that simultaneously takes account of episodic details from the past, and factual knowledge of the world gained through experience and which allows detailed retrieval of information from memory to be deployed on information unrelated to an ongoing task.
Acknowledgements
We would like to thank Theo Karapanagiotidis for neuroimaging data collection; Irene De Caso, Zacharria Cotter and Charlotte Murphy for their assistance with collecting data on self-generated thought; Giovanna Mollo and Mahiko Konishi for their help with the semantic and mind-wandering tasks, respectively; to Robin Dunbar, Kevin Madore, Nathan Spreng, and Roland Zahn for sharing materials for the perspective taking task, the autobiographical memory interview, the tower of London task, and the social semantic task, respectively. We are also grateful to Merete Stene and Julia Stietz for their significant help with data coding. The authors declare no competing financial interests.
Conflict of interest. None declared.
Funding
E.J. was supported by grants from BBSRC (BB/J006963/1) and the European Research Council (SEMBIND—283530). J.S. was supported by the European Research Council (WANDERINGMINDS—646927) and the Volkswagen Foundation (Wandering Minds—89440 and 89439). This publication was also made possible through the support of a grant from the John Templeton Foundation, ‘Prospective Psychology Stage 2: A Research Competition’ to Martin Seligman. The opinions expressed in this publication are those of the author(s) and do not necessarily reflect the views of the John Templeton Foundation.
Footnotes
Due to the relatively lower reliability rates for the effectiveness and specificity scores we re-ran the PCA analyses excluding these two measures. We obtained three similar components with and without these variables and the components from each analyses were highly correlated, (component 1, r = 0.95, p < 0.001, component 2, r = 0.66, p < 0.001, component 3 r = 0.98, p < 0.001). Given these high correlations we report analysis using the more comprehensive PCA in this paper.
References
- Addis D.R., Moloney E.E., Tippett L.J., Roberts R.P., Hach S. (2016). Characterizing cerebellar activity during autobiographical memory retrieval: ALE and functional connectivity investigations. Neuropsychologia, 90, 80–93. [DOI] [PubMed] [Google Scholar]
- Addis D.R., Wong A.T., Schacter D.L. (2008). Age-related changes in the episodic simulation of future events. Psychological Science, 19, 33–41. [DOI] [PubMed] [Google Scholar]
- Allen M., Smallwood J., Christensen J., et al. (2013). The balanced mind: the variability of task-unrelated thoughts predicts error monitoring. Frontiers in Human Neuroscience, 7, 743.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7, 268–77. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Huang C., Buckner R.L. (2010a). Evidence for the default network's role in spontaneous cognition. Journal of Neurophysiology, 104, 322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010b). Functional-anatomic fractionation of the brain's default network. Neuron, 65, 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Lutz A., Schooler J.W. (2014). The decoupled mind: mind-wandering disrupts cortical phase-locking to perceptual events. Journal of Cognitive Neuroscience, 26, 2596–607. [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Mrazek M.D., Kam J.W., Franklin M.S., Schooler J.W. (2012). Inspired by distraction: mind wandering facilitates creative incubation. Psychological Science, 23, 1117–22. [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Schooler J.W. (2011). Back to the future: autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition, 20, 1604–11. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Bowen D.C., Holt R.J., et al. (2015). The “reading the mind in the eyes” test: complete absence of typical sex difference in ∼400 men and women with autism. PLoS One, 10, e0136521.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron E., Riby L.M., Greer J., Smallwood J. (2011). Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychological Science, 22, 596–601. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Smallwood J., Tusche A., et al. (2014). Medial prefrontal and anterior cingulate cortical thickness predicts shared individual differences in self-generated thought and temporal discounting. Neuroimage, 90, 290–7. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19, 2767–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney R.J., Embleton K.V., Jefferies E., Parker G.J., Ralph M.A. (2010). The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cerebral Cortex, 20, 2728–38. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M. (2013). The evolution of distributed association networks in the human brain. Trends in Cognitive Sciences, 17, 648–65. [DOI] [PubMed] [Google Scholar]
- Christoff K., Gordon A.M., Smallwood J., Smith R., Schooler J.W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of United States of America, 106, 8719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Irving Z.C., Fox K.C., Spreng R.N., Andrews-Hanna J.R.. 2016. Mind-wandering as spontaneous thought: a dynamic framework. Nature Reviews Neuroscience, 17, 718–31. [DOI] [PubMed] [Google Scholar]
- D'Zurilla T.J., Goldfried M.R. (1971). Problem solving and behavior modification. Journal of Abnormal Psychology, 78, 107–26. [DOI] [PubMed] [Google Scholar]
- Davey J., Cornelissen P.L., Thompson H.E., et al. (2015). Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. Journal of Neuroscience, 35, 15230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of United States of America, 113, 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil M., Fox K.C., Dixon M.L., et al. (2016). Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage, 136, 186–96. [DOI] [PubMed] [Google Scholar]
- Engert V., Smallwood J., Singer T. (2014). Mind your thoughts: associations between self-generated thoughts and stress-induced and baseline levels of cortisol and alpha-amylase. Biological Psychology, 103, 283–91. [DOI] [PubMed] [Google Scholar]
- Fox K.C., Spreng R.N., Ellamil M., Andrews-Hanna J.R., Christoff K. (2015). The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage, 111, 611–21. [DOI] [PubMed] [Google Scholar]
- Gerlach K.D., Spreng R.N., Madore K.P., Schacter D.L. (2014). Future planning: default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience, 9, 1942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchert J., Smallwood J., Jefferies E., et al. (2017). Individual variation in intentionality in the mind-wandering state is reflected in the integration of the default-mode, fronto-parietal, and limbic networks. Neuroimage, 146, 226–35. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Weiner K.S. (2014). The functional architecture of the ventral temporal cortex and its role in categorization. Nature Reviews Neuroscience, 15, 536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren K.A. (2012). Computing inter-rater reliability for observational data: an overview and tutorial. Tutorials in Quantitative Methods for Psychology, 8, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A.J., Bisby J.A., Bush D., Lin W.J., Burgess N. (2015). Evidence for holistic episodic recollection via hippocampal pattern completion. Nature Communications, 6, 7462.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W., Pennartz C.M., Cabeza R., Daselaar S.M. (2009). When learning and remembering compete: a functional MRI study. PLOS Biology, 7, e1000011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima Y., Tanno Y. (2012). [The effect of cognitive load on the temporal focus of mind wandering]. Shinrigaku Kenkyu, 83, 232–6. [DOI] [PubMed] [Google Scholar]
- Jackson R.L., Hoffman P., Pobric G., Lambon Ralph M.A. (2016). The semantic network at work and rest: differential connectivity of anterior temporal lobe subregions. Journal of Neuroscience, 36, 1490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., Yovel G. (2006). The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361, 2109–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapanagiotidis T., Bernhardt B.C., Jefferies E., Smallwood J. (2017). Tracking thoughts: exploring the neural architecture of mental time travel during mind-wandering. Neuroimage, 147, 272–81. [DOI] [PubMed] [Google Scholar]
- Killingsworth M.A., Gilbert D.T. (2010). A wandering mind is an unhappy mind. Science, 330, 932.. [DOI] [PubMed] [Google Scholar]
- Klinger E., Cox W.M. (1987). Dimensions of thought flow in everyday life. Imagination, Cognition and Personality, 7, 105–28. [Google Scholar]
- Konishi M., McLaren D.G., Engen H., Smallwood J. (2015). Shaped by the past: the default mode network supports cognition that is independent of immediate perceptual input. PLoS One, 10, e0132209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Smallwood J. (2016). Shadowing the wandering mind: how understanding the mind-wandering state can inform our appreciation of conscious experience. Wiley Interdisciplinary Reviews: Cognitive Science, 7, 233–46. [DOI] [PubMed] [Google Scholar]
- Krieger-Redwood K., Jefferies E., Karapanagiotidis T., et al. (2016). Down but not out in posterior cingulate cortex: deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition. Neuroimage, 141, 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Redwood K., Teige C., Davey J., Hymers M., Jefferies E. (2015). Conceptual control across modalities: graded specialisation for pictures and words in inferior frontal and posterior temporal cortex. Neuropsychologia, 76, 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Jefferies E., Patterson K., Rogers T.T. (2017). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18, 42–55. [DOI] [PubMed] [Google Scholar]
- Leech R., Braga R., Sharp D.J. (2012). Echoes of the brain within the posterior cingulate cortex. Journal of Neuroscience, 32, 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. (2011). Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. Journal of Neuroscience, 31, 3217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Svoboda E., Hay J.F., Winocur G., Moscovitch M. (2002). Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology and Aging, 17, 677–89. [PubMed] [Google Scholar]
- Macrae C.N., Moran J.M., Heatherton T.F., Banfield J.F., Kelley W.M. (2004). Medial prefrontal activity predicts memory for self. Cerebral Cortex, 14, 647–54. [DOI] [PubMed] [Google Scholar]
- Madore K.P., Gaesser B., Schacter D.L. (2014). Constructive episodic simulation: dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. Journal of Experimental Psychology: Learning, Memory, and Cognition, 40, 609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D.S., Ghosh S.S., Goulas A., et al. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences, 113, 12574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. (2007). The representation of object concepts in the brain. Annual Review of Psychology, 58, 25–45. [DOI] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315, 393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay J.C., Kane M.J. (2009). Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35, 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medea B., Karapanagiotidis T., Konishi M., et al. (2016). How do we decide what to do? Resting-state connectivity patterns and components of self-generated thought linked to the development of more concrete personal goals. Experimental Brain Research, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittner M., Hawkins G.E., Boekel W., Forstmann B.U. (2016). A neural model of mind wandering. Trends in Cognitive Sciences, 20, 570–8. [DOI] [PubMed] [Google Scholar]
- Moscovitch M., Cabeza R., Winocur G., Nadel L. (2016). Episodic memory and beyond: the hippocampus and neocortex in transformation. Annual Review of Psychology, 67, 105–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K., Nestor P.J., Rogers T.T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience, 8, 976–87. [DOI] [PubMed] [Google Scholar]
- Platt J.J., Spivack G.. 1975. Manual for the Mean-end Problem-solving Procedure (MEPS): A Measure of Interpersonal Cognitive Problem-solving Skill. Hahnemann Community Mental Health/Mental Retardation Center, Hahnemann Medical College and Hospital.
- Poerio G.L., Smallwood J. (2016). Daydreaming to navigate the social world: what we know, what we don't know, and why it matters. Social and Personality Psychology Compass, 10, 605–18. [Google Scholar]
- Poerio G.L., Totterdell P., Emerson L.M., Miles E. (2015). Love is the triumph of the imagination: daydreams about significant others are associated with increased happiness, love and connection. Consciousness and Cognition, 33, 135–44. [DOI] [PubMed] [Google Scholar]
- Poerio G.L., Totterdell P., Emerson L.M., Miles E. (2016a). Helping the heart grow fonder during absence: daydreaming about significant others replenishes connectedness after induced loneliness. Cognition & Emotion, 30, 1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poerio G.L., Totterdell P., Emerson L.M., Miles E. (2016b). Social daydreaming and adjustment: an experience-sampling study of socio-emotional adaptation during a life transition. Frontiers in Psychology, 7, 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poerio G.L., Totterdell P., Miles E. (2013). Mind-wandering and negative mood: does one thing really lead to another? Consciousness and Cognition, 22, 1412–21. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby F.J., Smallwood J., Engen H., Singer T. (2013a). How self-generated thought shapes mood—the relation between mind-wandering and mood depends on the socio-temporal content of thoughts. PLoS One, 8, e77554.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby F.J., Smallwood J., Sackur J., Singer T. (2013b). Is self-generated thought a means of social problem solving? Frontiers in Psychology, 4, 962.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R. (2007). The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 362, 773–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S.B., Rotarska-Jagiela A., Fink G.R., Vogeley K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17, 457–67. [DOI] [PubMed] [Google Scholar]
- Smallwood J. (2013a). Distinguishing how from why the mind wanders: a process-occurrence framework for self-generated mental activity. Psychological Bulletin, 139, 519–35. [DOI] [PubMed] [Google Scholar]
- Smallwood J. (2013b). Searching for the elements of thought: reply to Franklin, Mrazek, Broadway, and Schooler (2013). Psychological Bulletin, 139, 542–7. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Karapanagiotidis T., Ruby F., et al. (2016). Representing representation: integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS One, 11, e0152272.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J., O'Connor R.C. (2011). Imprisoned by the past: unhappy moods lead to a retrospective bias to mind wandering. Cognition & Emotion, 25, 1481–90. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J.W. (2015). The science of mind wandering: empirically navigating the stream of consciousness. Annual Review of Psychology, 66, 487–518. [DOI] [PubMed] [Google Scholar]
- Smeekens B.A., Kane M.J. (2016). Working memory capacity, mind wandering, and creative cognition: an individual-differences investigation into the benefits of controlled versus spontaneous thought. Psychology of Aesthetics, Creativity, and the Arts, 10, 389–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., et al. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of United States of America, 106, 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Wang X. (2012). Mind wandering in Chinese daily lives—an experience sampling study. PLoS One, 7, e44423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Gerlach K.D., Turner G.R., Schacter D.L. (2015). Autobiographical planning and the brain: activation and its modulation by qualitative features. Journal of Cognitive Neuroscience, 27, 2147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22, 1112–23. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., Schacter D.L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage, 53, 303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D., Majerus S., Maquet P., D'Argembeau A. (2011). Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One, 6, e16997.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller J., Dunbar R.I. (2007). Perspective-taking and memory capacity predict social network size. Social Networks, 29, 93–104. [Google Scholar]
- Surtees A., Apperly I., Samson D. (2013). Similarities and differences in visual and spatial perspective-taking processes. Cognition, 129, 426–38. [DOI] [PubMed] [Google Scholar]
- Tusche A., Smallwood J., Bernhardt B.C., Singer T. (2014). Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. Neuroimage, 97, 107–16. [DOI] [PubMed] [Google Scholar]
- Vann S.D., Aggleton J.P., Maguire E.A. (2009). What does the retrosplenial cortex do? Nature Reviews Neuroscience, 10, 792–802. [DOI] [PubMed] [Google Scholar]
- Vatansever D., Menon D.K., Manktelow A.E., Sahakian B.J., Stamatakis E.A. (2015). Default mode dynamics for global functional integration. Journal of Neuroscience, 35, 15254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M., Jefferies E., Embleton K.V., Lambon Ralph M.A. (2012). Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. Journal of Cognitive Neuroscience, 24, 1766–78. [DOI] [PubMed] [Google Scholar]
- Weissman D.H., Roberts K., Visscher K., Woldorff M. (2006). The neural bases of momentary lapses in attention. Nature Neuroscience, 9, 971–8. [DOI] [PubMed] [Google Scholar]
- Wheeler L., Reis H.T. (1991). Self‐recording of everyday life events: origins, types, and uses. Journal of Personality, 59, 339–54. [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R., Moll J., Krueger F., Huey E.D., Garrido G., Grafman J. (2007). Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences of United States of America, 104, 6430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]