Abstract
The human brain tracks dynamic changes within the social environment, forming and updating representations of individuals in our social milieu. This mechanism of social navigation builds an increasingly complex map of persons with whom we are familiar and form attachments to guide adaptive social behaviors. We examined the neural representation of known others along a continuum of attachment using fMRI. Heterosexual adults (N = 29, 16 females), in romantic relationships for more than 2 years, made trait judgments for a romantic partner, parent, close friend, familiar acquaintance and self-during scanning. Multivariate analysis, partial least squares, was used to identify whole-brain patterns of brain activation associated with trait judgments of known others across a continuum of attachment. Across conditions, trait judgments engaged the default network and lateral prefrontal cortex. Judgments about oneself and a partner were associated with a common activation pattern encompassing anterior and middle cingulate, posterior superior temporal sulcus, as well as anterior insula. Parent and close friend judgments engaged medial and anterior temporal lobe regions. These results provide novel evidence that mentalizing about known familiar others results in differential brain activity. We provide initial evidence that the representation of adult attachment is a distinguishing feature of these differences.
Keywords: adult attachment, close others, mental representations, default network, salience network
Introduction
We continually update our representations of other individuals and utilize those representations, especially about persons with whom we form attachment relationships, to guide social behaviors. The hallmarks of these unique, close social bonds are feelings of security and concomitant affect-regulatory benefits associated with attachment figures’ presence (Bowlby, 1973; Mikulincer and Shaver, 2007). In infant development, attachment is theorized to play a pivotal role in maintaining proximity to the primary caregiver (Bowlby et al., 1973). Lack of perceived proximity, and accompanying distress, engages attachment representations. These attachment representations in turn provide comfort and security, facilitating exploration, in a constantly shifting system of behavioral dynamics. The extension of this theoretical framework, to explain adult romantic relationships (Hazan and Shaver, 1987), currently serves as a predominant paradigm for understanding the regulatory powers of close social bonds (Hazan et al., 2004; Pietromonaco et al., 2006). The so-called ‘chronic accessibility’ (Andersen and Cole, 1990; Baldwin et al., 1996) of attachment figure mental representations comes about due to learning and conditioning under this inborn system of attachment bonding that is operative across the lifespan.
Recent research demonstrates that attachment figure mental representations serve various functions contributing to health and happiness. Just bringing to mind the cognitive representation of one’s romantic partner, for example, promotes recovery following recollection of upsetting autobiographical memories (Selcuk et al., 2012), provides distress alleviation when giving a public speech (Grewen et al., 2003), decreases the neural response to threat with partner hand-holding (Coan et al., 2006), and reduces the subjective experience of pain (Eisenberger et al., 2011). Importantly, evidence supports the notion that these mental representations are flexible; shifts in cognition, behavior, and patterns of neural activation can be associated with changes in attachment.
Recent neuroimaging results show the involvement of many brain regions, and associated brain networks, in creating, updating, and using mental representations of close others. Several studies highlight the role of the dopaminergic reward system, particularly areas such as the mid-insula, anterior cingulate cortex (ACC), caudate head, ventral tegmental area and putamen, in motivating pair bond formation and maintenance and in the regulation of emotion associated with thinking of a close other (Bartels and Zeki, 2000; Younger et al., 2010; Zeki and Romaya, 2010; Stoessel et al., 2011; Acevedo et al., 2012; see Feldman, 2017). Research utilizing threat-anticipation tasks also links the emotion-regulatory capabilities of close other mental representations with various limbic system regions (Coan et al., 2006).
Others associate activation of regions within the default network, a functionally connected assembly of brain regions broadly implicated in internally directed cognition, with mental representation of social others. Core brain areas within the default network include medial and lateral temporal lobes, medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC) and lateral parietal cortices (Andrews-Hanna et al., 2014). The default network is associated with several aspects of social cognition, including mentalizing (or, making inferences about other people’s mental states) (Andrews-Hanna et al., 2014). Personal experiences are thought to be an important mechanism in the generation of social conceptual knowledge which, in turn, leads to development and implementation of strategic social behavior (Spreng and Mar, 2012).
Neuroimaging research commonly uses trait-judgment paradigms to assess cognition related to social others. Early work by Mitchell et al. (2006) demonstrated the role of the mPFC in differentiating similar vs dissimilar other representations. With respect to studies of close-other cognitive representations, neural regions of interest include the mPFC and PCC (e.g. Gobbini et al., 2004; Heatherton et al., 2006; Platek et al., 2006; Krienen et al., 2010; Tacikowski et al. 2012, 2013; Wang, et al., 2012). Within the literature on mentalizing, studies focus on the role of the default network in differentiating close others from strangers or self. Personal judgments about friends, over and above those about strangers, engage a network of brain regions in mPFC, PCC/retrosplenial cortex, inferior parietal lobe, lateral temporal cortex and medial temporal lobe (Krienen et al., 2010). Recruitment of these regions for friend judgments suggests that the default network plays a role in formation or access of representations of known others. Further, representations of the self are associated with greater engagement of mPFC, over and above representations of intimately known others (Heatherton et al., 2006), suggesting that social proximity or attachment modulates engagement of this region, and the default network more broadly. However, no single study investigates the full spectrum of social proximity.
Here we use fMRI to examine the neural representation of known others along a continuum of attachment. We use a trait-judgment task, requiring participants to make personal judgments about a romantic partner, parent, close friend, familiar acquaintance and the self. Our goal was to determine how mental representations of salient attachment figures and others in our social world are associated with patterns of brain activity, and whether these patterns are modulated by the presence of a primary attachment bond. We predicted that, overall, trait judgments about all known people and the self, relative to a matched control condition, would engage mPFC and PCC. This hypothesis is consistent with literature investigating mentalizing and the default network (Mar, 2011; Andrews-Hanna et al., 2014). We also predicted that neural representations of self and attached romantic partners would be differentiable from representations of parents and friends. Strong evidence exists for the differential behavioral, physiological, cognitive and emotional responses to attachment figures—and, particularly, primary attachment figures—versus less close others (Hazan et al., 2004). As romantic partner representations are more salient than other social representations (e.g. Aron et al., 1991; Pietromonaco et al., 2013), we hypothesized that judgments for self and romantic partners would share a common pattern of brain activity encompassing the salience network, a collection of brain regions implicated in the detection and processing of salient environmental stimuli (Uddin 2015).
Materials and methods
Participants
Participants were 29 healthy, right-handed young adults (16 females, 13 males; M age = 24 years, s.d. = 3.5 years) with normal or corrected-to-normal visual acuity, and no history of psychiatric, neurological or other medical illness that could compromise cognitive functions. Participants gave written informed consent in accordance with the Institutional Review Board of Cornell University. Participants were selected for the scanning procedure based on the study criteria of being in a long-term, committed, exclusive romantic relationship.
Assessment of attachment
We recruited participants on the basis of romantic relationship length and characteristics, asking that participants be in an exclusive and committed relationship for around 2 years or longer. Two years is an important milestone within adult attachment theory, as it is the time around which full-fledged attachment bonds have formed (Hazan and Shaver, 1987). The average participant relationship length (measured in months) was well above 24 (M = 43.97 months, s.d. = 29.37).
Participants completed a pre-scan survey about their various personal relationships. Participants first provided one name per relationship condition in response to prompts (see Supplementary Appendix S1). This survey included self-report measures of attachment (WHOTO; Hazan et al., 1991; Fraley and Davis, 1997), perceived closeness (Inclusion of Other in Self, IOS; Aron et al., 1992), and relationship length. Additionally, the survey included the Experiences in Close Relationships – Revised Partner Specific (Fraley et al., 2000), Friendship-Based Love Scale (Grote and Frieze, 1994), Passionate Love Scale (Hatfield and Sprecher, 1986), and a partner-specific questionnaire designed to assess relationship quality factors, such as satisfaction, commitment, exclusivity and emotional investment (see Supplementary Appendix S1). We confirmed participants’ attachment relationships and subsequent inclusion in the study based on these self-report variables.
The WHOTO (Hazan et al., 1991; Fraley and Davis, 1997) is an attachment measure that determines the people with whom subjects display attachment relationships. Items are based on four attachment features: proximity seeking, separation distress, safe haven and secure base. Subjects list up to 4 most important figures in their lives for each of the 10 items. The WHOTO can be used in various ways to measure individuals’ attachment to others. In the present study, we used the WHOTO in two distinct ways. First, we utilized it as a continuous measure of attachment with romantic partners, parent, and friends by scoring each item based on the individual’s ranking (highest scores = listed first) and totaling these scores; therefore higher WHOTO total scores were indicative of greater levels of attachment. Second, we examined the presence of primary attachments to either romantic partner or parent by scoring each item on a binary of ‘[partner/parent] listed first?’ = 1 and ‘[partner/parent] not listed first?’ = 0.
We also investigated social cognitive closeness using the IOS scale (Aron et al., 1992). This scale is a single-item pictorial measure of closeness and interconnectedness in dyads. The seven instances of two overlapping circles of the IOS range from mutually exclusive to highly overlapping in appearance. The IOS is a direct self-report measure of perceived closeness with relationship partners, as it is a visual representation of how individuals think of others and themselves.
Task and fMRI design
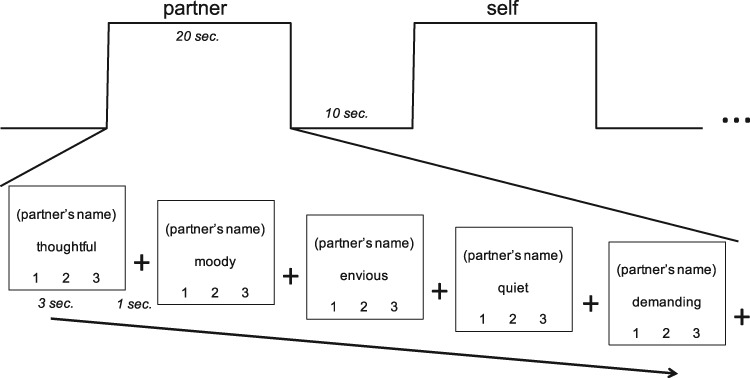
During fMRI scanning, we used a trait-judgment task (cf Grigg and Grady, 2010) in which participants were asked to think about several people in their lives mentioned by name in the pre-scan survey. Each trial contained a trait adjective and a person’s name; participants rated the person on each trait adjective, on a scale of 1 (unlike this person) to 3 (very much like this person). Blocks were composed of five trials in which participants were instructed to hold the person in mind continuously while making each trait judgment about that person. Blocks were interleaved with 10 s of fixation. We also included a motor control condition block, in which participants were prompted with ‘Which number?’, shown a number 1, 2 or 3, and instructed to respond by pushing the button corresponding to that number.
The experiment consisted of 350 trials divided across 5 runs, each consisting of 14 blocks, in turn comprising 5 trials per block. Trials were 3-s long, and a 1-s crosshair fixation screen appeared between each trial. There were two blocks per run for each of the seven conditions (partner, parent, close friend, familiar acquaintance, famous person, self and ‘which number’ motor control). See Figure 1 for behavioral paradigm. The order of conditions within each run was randomized. Each task run lasted 7 min and 40 s. The five runs were then counterbalanced for each participant to eliminate any possibility of ordering effects of the fixed condition order and adjective order.
Fig. 1.
Behavioral paradigm involving trait-judgment task for social others.
Numerous participants reported uncertainty in performing this task for the famous person condition, and the neural results were a multivariate outlier. For these reasons, the famous person condition was excluded from subsequent analyses and interpretation.
Fifty trait adjectives were selected for the study in order to ensure that each word was used exactly once for each condition. The trait adjectives were selected from a list of popularly used personality terms (Anderson, 1968). The trait adjectives were presented in a fixed order across blocks, such that each trait adjective was paired exactly once with each condition.
Magnetic resonance image acquisition
Brain imaging data were acquired using a 3T GE Discovery MR750 MRI scanner with a 32-channel head coil. This MRI scanner was located within the Cornell Magnetic Resonance Imaging Facility in Ithaca, NY, USA. Anatomical scans were acquired using a T1-weighted volumetric MRI magnetization prepared rapid gradient echo (repetition time (TR) = 2530 ms; echo time (TE) = 3.4 ms; inversion time (TI) = 1100 ms; flip angle (FA) = 7°; bandwidth = 195 Hz/pixel; 1.0 mm isotropic voxels, 176 slices). Five 7 min 40 s experimental runs of blood–oxygen level dependent (BOLD) functional scans were acquired with a T2*-weighted multi-echo imaging pulse sequence [TR = 2000 ms; TEs = 12.7, 27.5 and 43 ms; 77° flip angle; 33 axial slices; matrix size = 64 × 64; field of view (FOV) = 240 mm; 33 axial slices; 3.8 mm thick slices].
Preprocessing of magnetic resonance imaging data
BOLD fMRI data were preprocessed to correct for motion, physiological noise and scanner artifacts using Multi-Echo Independent Components Analysis (ME-ICA) with meica.py (Kundu et al., 2012). ME-ICA is a method for de-noising fMRI data based on information about the T2* decay of the BOLD signal, acquired through multi-echo fMRI. Using ME-ICA, multi-echo fMRI datasets can be decomposed into independent components before these components are categorized as BOLD or noise/non-BOLD. ME-ICA robustly de-noises fMRI data by removing all non-BOLD components (Kundu et al., 2012; Lombardo et al., 2016; Kundu et al., 2017). Within the ME-ICA program, the BOLD fMRI images were normalized to the standard space of the MNI template. Subsequently, data were resampled to 2 × 2 × 2-voxel volumetric time-series and smoothed with a 6-mm full width half maximum (FWHM) Gaussian kernel.
fMRI analysis
Partial least squares
Task-based analyses were performed using the multivariate technique partial least squares (PLS), a multivariate functional neuroimaging analysis technique used to identify whole-brain patterns of activity that are correlated with tasks (Krishnan et al., 2011). PLS identifies a set of orthogonal latent variables (LVs) that optimally relate BOLD signal and the experimental design. The statistical significance of the detected patterns is assessed through permutation testing, whereas reliability is determined in an independent step by iterative bootstrap resampling with replacement.
PLS is sensitive to a distributed voxel response, rather than the activity of individual voxels per se, and assesses the covariance between brain voxels (BOLD signal) and the experimental design to identify a limited number of orthogonal components (LVs) that optimally relate the two. This data-driven approach determines orthogonal whole-brain patterns of activity that covary with the experimental design. Within the PLS framework, brain activity is constrained to examine the covariance between brain activity and task design. In this regard, we are able to examine robust patterns of activity only associated with the experimental conditions. Along these same lines, PLS is capable of analyzing multiple conditions simultaneously to examine covariance of response across conditions. The current study design was optimized for a PLS analysis to assess distributed patterns of activity across conditions.
Activity for each voxel was averaged across blocks for each relationship condition and normalized relative to activity at fixation preceding the trait judgment. The data matrix was expressed as a voxel-by-voxel deviation from the grand mean across the entire experiment, which was decomposed using singular value decomposition to derive the LVs representing task contrasts. Each brain voxel is given a singular value weight, known as a salience (akin to a component loading in principle component analysis), which is proportional to the covariance of voxel activity with the task contrast represented by each LV. Multiplying the salience by the BOLD signal value in that voxel and summing the product across all voxels gives a composite brain activity score for each participant on a given LV. We then used these brain scores to examine similarities and differences in brain activity across conditions and across participants. Greater activity in brain areas with positive (or negative) weights on a specific LV yields positive (or negative) mean brain scores for a given condition. PLS results can be interpreted as identifying co-varying sets of brain regions in which activity is reliably associated with the specific condition-wise contrasts represented by each LV.
The significance of each LV was determined by permutation testing, using 500 permutations with random reordering of the task conditions for each participant. PLS is recalculated for each permutation sample, and the frequency in which the permuted singular value exceeds the observed singular values is determined and expressed as a probability. In a second, independent, step the reliability of the saliences for the brain voxels across participants, characterizing each pattern identified by an LV, was determined by bootstrap resampling with replacement, using 100 iterations, to estimate the standard errors for each voxel. We set a minimum bootstrap ratio (conceptually similar to a Z-score) at 2.58 equivalent to P < 0.01. Because the analysis is performed across voxels in a single step, no correction for multiple comparisons is required.
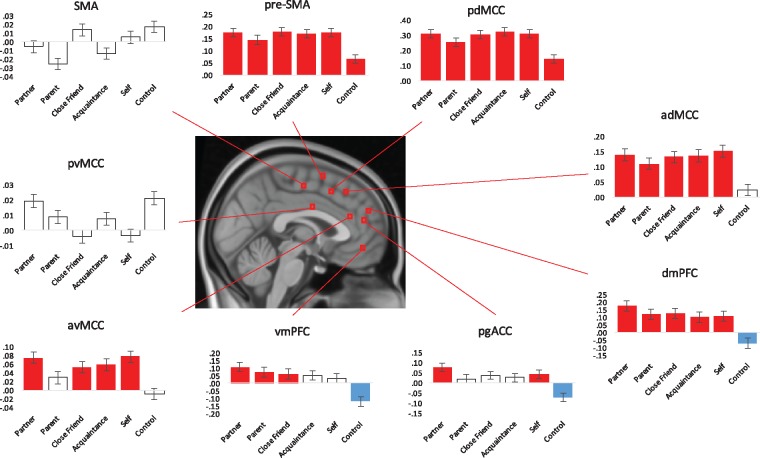
Systematic region of interest (ROI) analyses were conducted for several seed regions within mPFC, utilizing nine peak coordinates from a recent parcellation of this region (de la Vega et al., 2016). ROIs were extracted from the following areas and corresponding MNI coordinates: supplementary motor area (SMA; 0, −14, 54), pre-supplementary motor area (pre-SMA; 0, 4, 62), posterior dorsal midcingulate cortex (pdMCC; 0, 12, 50), anterior dorsal midcingulate cortex (adMCC, 0, 28, 48), posterior ventral midcingulate cortex (pvMCC; 0, −2, 30), anterior ventral midcingulate cortex (avMCC; 0, 36, 24), dorsal mPFC (dmPFC; 0, 50, 28), pregenual ACC (pgACC; 0, 46, 8) and ventral mPFC (vmPFC; 0, 48, −12). Using PLS, we performed a multiple-voxel extraction with a neighborhood size of 1 for each of these coordinates. This analytic approach yielded mean response intensities, averaged across subjects, for each condition. Each region was submitted to a simple t-test to evaluate activation against baseline. We report on this analysis for each of the nine ROIs.
Results
Behavioral results; assessment of attachment
Our first analyses examined two critical measures: reported attachment status (WHOTO) and closeness (IOS) between romantic partners, parents and friends. Descriptive statistics for these measures are in Table 1. We initially conducted repeated measures ANOVA tests across WHOTO total scores and across IOS scores. Results showed a significant difference between means of romantic partner, parent and friend WHOTO scores [F(2, 56) = 22.14, P < 0.001]. Results of non-parametric analyses mirrored these ANOVA results, as a Friedman test yielded significant differences among repeated measures χ2 (2, N = 29) = 40.55, P < 0.001. We conducted this non-parametric test to account for alternative perspectives that consider WHOTO scores as ordinal data. Results also showed a significant difference between means of romantic partner, parent, and friend IOS scores [F(2, 56) = 68.00, P < 0.001].
Table 1.
Descriptive statistics for self-report measures
| Measure | Romantic partner | Parent | Close friend | Acquaintance |
|---|---|---|---|---|
| WHOTO (M, s.d.) | 32.83, 6.80 | 22.31, 8.46 | 6.97, 7.56 | – |
| Out of 40 | ||||
| IOS (M, s.d.) | 4.83, 1.23 | 2.93, 1.39 | 2.97, 1.55 | 1.55, 0.69 |
| Out of 7 | ||||
| Relationship Length (M, s.d.) | 43.97, 29.37 | 274.76, 57.76 | 83.52, 65.82 | 50.35, 48.12 |
| In months | ||||
| ‘How well do you know this person?’ (M, s.d.) | 4.79, 0.63 | 4.43, 0.98 | 4.31, 0.66 | 2.93, 0.88 |
| Out of 5 | ||||
| ‘How familiar are you with this person?’ (M, s.d.) | 4.79, 0.63 | 4.57, 0.95 | 4.45, 0.74 | 3.17, 1.00 |
| Out of 5 |
We ran several post hoc t-tests to clarify the nature of attachment-related differences between specific comparison groups of interest. Results showed that participants reported significantly greater attachment to romantic partners over parents [t(56) = 5.22 P < 0.001] and closeness with partners over parents [t(56) = 5.52, P < 0.001]. Participants also reported significantly greater attachment to romantic partners over friends [t(56) = 13.69, P < 0.001] and closeness with partners over friends [t(56) = 5.08, P < 0.001]. Lastly, participants reported significantly greater attachment to parents over friends [t(56) = 7.28, P < 0.001], but there was no difference in reported closeness between parents and friends [t(56) = −0.09, P = 0.92]. Taken together, these results demonstrate that participants’ romantic partners and parents were attachment figures—with participants showing more attachment to partners than parents—whereas friends were, on average, not attachment figures for the participants. Our general categorization of friends as non-attachment figures is supported by previous theoretical work and behavioral results suggesting young adults’ relationships with friends do not show characteristic features of attachment bonds (Hazan and Zeifman, 1999; Fraley and Davis, 1997).
Importantly, we further utilized participants’ WHOTO data to determine their primary attachment figures. Most participants listed their romantic partners first across several WHOTO items. This distinction was especially pronounced for the WHOTO items reflecting proximity seeking, separation distress, and safe haven, whereas participants listed parents and partners first at about equal rates for secure base; this finding reflections past observations about the transition of primary attachment figures from parents to partners in early adulthood, with secure base often the final feature to be primarily directed towards partners (Hazan and Zeifman, 1999; Nickerson and Nagle, 2005). Overall, these results provide evidence that romantic partners served as participants' primary attachment figures, whereas parents did not.
Neuroimaging results
The PLS analysis focused on investigating neural activity changes across relationship conditions (partner, parent, friend, acquaintance, self and control). PLS analyses revealed two significant patterns of activity.
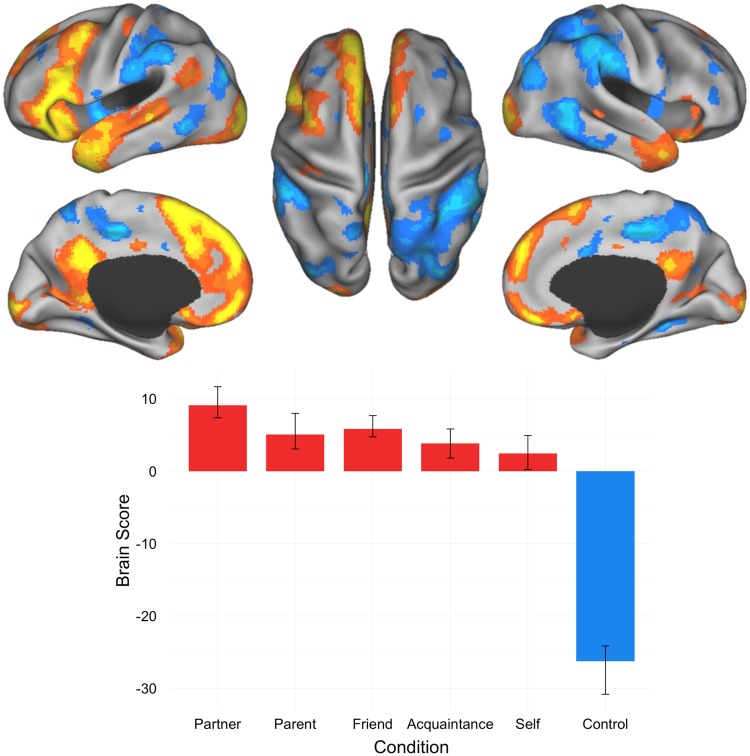
The first significant LV separated all social other—representations from the control (i.e. number matching) condition (P = 0.002; 70.69% covariance explained). This result replicates previous findings implicating the default network in mentalizing about others (Krienen et al., 2010; Mar, 2011). Significant activations for this LV were found within areas of dmPFC, vmPFC and PCC. Other significant activations for this LV were observed in occipital cortex, inferior frontal gyrus, temporal pole, thalamus, superior temporal sulcus (STS), cerebellum, anterior temporal lobe, caudate head, middle cingulate gyrus, basal ganglia, precentral gyrus, and intracalcarine cortex (Figure 2; Table 2).
Fig. 2.
Results of the task PLS analysis contrasting activity across partner, parent, close friend, familiar acquaintance, self and control conditions; LV1 activation map and brain scores with 95% confidence intervals. Brain scores represent cross product of the group result image and the individual subject BOLD response for each given LV. Warm colors on activation maps (red, orange, yellow) correspond to positive brain scores associated with the partner, parent, friend, acquaintance and self conditions, shown by red plotted bars. Cool colors on activation maps (shades of blue) correspond to negative brain scores associated with the control condition, shown by the blue plotted bars. (Left) lateral and medial views of left hemisphere. (Center) dorsal view. (Right) lateral and medial views of right hemisphere.
Table 2.
Peak activation coordinates, LV1
| Region | Coordinates |
BSR | ||
|---|---|---|---|---|
| X | y | z | ||
| Social > Control | ||||
| Medial prefrontal cortex | −10 | 58 | 30 | −11.54 |
| Dorsomedial prefrontal cortex | −8 | 20 | 56 | −11.43 |
| Occipital cortex | −26 | −94 | 4 | −11.21 |
| Inferior frontal gyrus | −58 | 24 | 14 | −11.01 |
| Posterior cingulate cortex | −8 | −50 | 32 | −10.75 |
| Ventromedial prefrontal cortex | −4 | 54 | −16 | −9.52 |
| Temporal pole | −44 | 8 | −38 | −9.01 |
| Occipital cortex | 18 | −102 | −10 | −8.77 |
| Inferior frontal gyrus | 26 | 16 | −22 | −6.56 |
| Thalamus | −8 | −14 | 8 | −6.50 |
| Superior temporal sulcus | −54 | −12 | −8 | −6.08 |
| Cerebellum | 4 | −62 | −40 | −6.05 |
| Anterior temporal lobe | 62 | 0 | −28 | −6.04 |
| Head of caudate | −16 | 10 | 14 | −5.64 |
| Cerebellum | 2 | −58 | −52 | −4.81 |
| Middle cingulate cortex | −2 | −12 | 38 | −4.66 |
| Angular gyrus | −50 | −60 | 30 | −4.64 |
| Superior temporal sulcus | 42 | −34 | 0 | −4.10 |
| Pallidum/basal ganglia | −26 | −8 | −6 | −3.77 |
| Precentral gyrus | −36 | −20 | 52 | −3.60 |
| Intracalcarine cortex | 20 | −66 | 6 | −3.33 |
| Control > social | ||||
| Intraparietal sulcus | 58 | −44 | 46 | 10.47 |
| Posterior middle cingulate | −12 | −28 | 44 | 9.91 |
| MT + | 48 | −54 | 4 | 8.48 |
| Lateral occipital cortex | −42 | −82 | 30 | 8.44 |
| Intraparietal sulcus | −56 | −36 | 52 | 8.43 |
| MT + | −64 | −62 | −4 | 7.77 |
| Mid-insula | −44 | −4 | −2 | 7.16 |
| Supplementary motor area (SMA) | −12 | −10 | 62 | 6.81 |
| Mid-insula | 38 | −12 | −6 | 6.21 |
| Dorsal anterior cingulate | 4 | 10 | 32 | 6.12 |
| Thalamus | 14 | −30 | 2 | 5.59 |
| Dorsolateral prefrontal cortex | 44 | 40 | 0 | 5.41 |
| Posterior superior frontal gyrus | 24 | 12 | 54 | 5.40 |
| Dorsolateral prefrontal cortex | −32 | 32 | 38 | 5.34 |
| Inferior temporal cortex | −62 | −40 | −26 | 5.33 |
| Parahippocampus/brain stem | 16 | −16 | −30 | 5.27 |
| Dorsolateral prefrontal cortex | 40 | 40 | 24 | 4.94 |
| Lingual gyrus | −32 | −42 | −8 | 4.81 |
| Cerebellum | −38 | −44 | −46 | 4.75 |
| Cerebellum | −16 | −74 | −48 | 4.54 |
| Precentral gyrus | 38 | −4 | 52 | 4.34 |
| Posterior superior frontal gyrus | −22 | 8 | 58 | 4.24 |
| Frontal pole | 34 | 56 | −16 | 3.84 |
| Medial orbital sulcus | 16 | 34 | −20 | 3.64 |
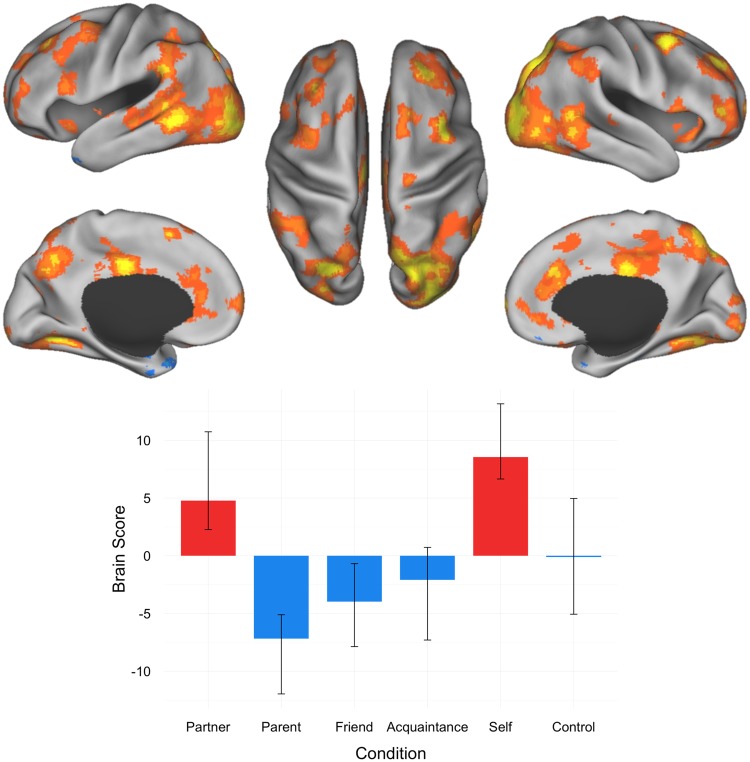
A second significant LV was observed, central to our hypothesis regarding differentiation of attached romantic partner representations versus parent and friend representations (P = 0.018; 13.91% covariance explained). This LV dissociated brain activity for partner and self from parent and friend. Anterior insula, anterior and middle cingulate, and posterior STS were associated with partner and self-representations. Activations in frontal gyrus, occipital fusiform gyrus, cerebellum, precuneus, frontal pole, supramarginal gyrus, anterior superior frontal sulcus, occipital cortex, thalamus, precentral gyrus, posterior dmPFC were also associated with partner and self-representations. In contrast, parent and friend judgments engaged left temporal pole and parahippocampal gyrus. Acquaintance and motor control conditions did not contribute to the multivariate pattern (Figure 3; Table 3).
Fig. 3.
Results of the task PLS analysis contrasting activity across partner, parent, close friend, familiar acquaintance, self and control conditions; LV2 Activation map and brain scores with 95% confidence intervals. Brain scores represent cross product of the group result image and the individual subject BOLD response for each given LV. Warm colors on activation maps (red, orange, yellow) correspond to positive brain scores associated with the partner and self conditions, shown by red plotted bars. Cool colors on activation maps (shades of blue) correspond to negative brain scores associated with the parent and friend conditions, shown by the blue plotted bars. (Left) lateral and medial views of left hemisphere. (Center) dorsal view. (Right) lateral and medial views of right hemisphere.
Table 3.
Peak activation coordinates, LV2
| Region | Coordinates |
BSR | ||
|---|---|---|---|---|
| X | y | z | ||
| Partner, self > parent, friend, acquaintance | ||||
| Middle frontal gyrus | 40 | 10 | 56 | 6.99 |
| Occipital fusiform gyrus | −28 | −66 | −2 | 6.64 |
| Cerebellum | −42 | −52 | −40 | 6.34 |
| Occipital fusiform gyrus | 22 | −84 | −2 | 6.30 |
| Dorsal precuneus | 16 | −64 | 48 | 6.18 |
| Dorsal precuneus | −22 | −76 | 50 | 6.06 |
| Frontal pole | 16 | 68 | 2 | 6.06 |
| Posterior superior temporal sulcus | 48 | −52 | 4 | 5.60 |
| Middle cingulate gyrus | −4 | −18 | 36 | 5.50 |
| Supramarginal gyrus | −70 | −42 | 30 | 5.15 |
| Anterior superior frontal sulcus | 24 | 42 | 34 | 4.90 |
| Anterior cingulate cortex | 10 | 32 | 26 | 4.52 |
| Occipital cortex | −30 | −100 | 18 | 4.47 |
| Thalamus | 22 | −26 | −2 | 4.34 |
| Superior frontal gyrus | 14 | 20 | 58 | 4.23 |
| Precuneus | −10 | −66 | 40 | 4.20 |
| Ventral anterior cingulate cortex | 10 | 30 | −8 | 4.20 |
| Precentral gyrus | −40 | 0 | 46 | 4.16 |
| Cerebellum | −16 | −60 | −62 | 4.07 |
| Anterior superior frontal sulcus | −18 | 42 | 28 | 4.07 |
| Cerebellum | −36 | −90 | −34 | 4.06 |
| Cerebellum | −56 | −66 | −40 | 4.01 |
| Posterior dorsomedial prefrontal cortex | −10 | 12 | 56 | 3.95 |
| Frontal pole | 42 | 46 | 0 | 3.89 |
| Middle frontal gyrus | −32 | 30 | 40 | 3.88 |
| Cerebellum | 40 | −60 | −40 | 3.85 |
| Superior temporal sulcus | −56 | −22 | −2 | 3.85 |
| Frontal pole | −20 | 70 | 4 | 3.82 |
| Cerebellum | −46 | −68 | −56 | 3.68 |
| Cerebellum | 12 | −54 | −46 | 3.67 |
| Rostral anterior cingulate | −4 | 40 | 10 | 3.67 |
| Precuneus | 6 | −46 | 54 | 3.66 |
| Inferior frontal gyrus | −60 | 20 | 14 | 3.51 |
| Inferior frontal gyrus | 52 | 16 | 2 | 3.43 |
| Rostral anterior cingulate | 0 | 48 | −4 | 3.41 |
| Inferior precentral sulcus | 62 | 12 | 12 | 3.36 |
| Superior frontal gyrus | −12 | 24 | 56 | 3.25 |
| Anterior insula | 28 | 18 | −10 | 3.23 |
| Anterior insula | −36 | 14 | −12 | 3.07 |
| Parent, friend > partner, self | ||||
| Temporal pole | −34 | 10 | −34 | −5.19 |
| Parahippocampal cortex | −24 | −2 | −42 | −3.54 |
For our ROI analyses, we conducted simple t-tests (α = 0.05) comparing each condition’s voxel intensity response against baseline. Distinct patterns of response were observed for specific areas within mPFC. Most notably, results showed significant positive activation intensity for dmPFC and adMCC across all of the social cognitions (partner, parent, friend, acquaintance and self). This pattern was similar for vmPFC, with partner, parent and friend showing significant positive intensities, avMCC, with partner, friend, acquaintance and self-showing significant positive intensities, and pgACC, with partner and self-showing significant positive intensities (Figure 4).
Fig. 4.
Results of ROI analysis examining nine seed regions within mPFC. Significance is shown through the different colors within the bar graphs; red plotted bars correspond with significant positive response intensities, blue plotted bars correspond with significant negative response intensities, and clear plotted bars correspond with non-significance.
Discussion
The present study aimed to determine how mental representations of other individuals in our social world reflect underlying patterns of neural activity and, more specifically, how our brains represent others with whom we share attachment bonds. First, we successfully replicated findings on mentalizing (Heatherton et al. 2006; Krienen et al., 2010; Mar, 2011), as all social conditions and the self-condition engaged regions of the default network including dmPFC, vmPFC and PCC, in addition to lateral PFC. Second, our results showed that presence of a primary attachment bond modulated neural activation associated with the mental representation of others. Specifically, ACC, anterior insula and posterior STS were associated with representations of attached romantic partner and self vs less-close others. Lastly, our ROI analysis results highlighted the role of vmPFC and dmPFC in representing social others and the self, with rostral cingulum regions showing robust activation for romantic partner.
In line with our predictions, results replicated the role of the default network in social cognitive processing, including mentalizing (Mar, 2011; Andrews-Hanna et al., 2014). Increased activation within dmPFC, vmPFC, and PCC was associated with all of the conditions involving mentalizing, a critical component of social cognition (Mar, 2011), versus the control condition. Default network brain regions have been associated with attributional decisions, judgments of others’ emotional states (Haas et al., 2015), and imagining the experiences of others (Krienen et al., 2010; Hassabis et al., 2014). Activations outside the default network were also observed. Recruitment of lateral PFC has been observed in studies of social cognitive reasoning (Mar, 2011), consistent with the role of maintaining social information online (McKinnon and Moscovitch, 2007). The left lateralization is also consistent with verbally mediated processes (Nagel et al., 2013). Activity in thalamus, basal ganglia and caudate could indicate recruitment of the limbic system for emotional regulation in response to social cues (Coan et al., 2006). Occipital cortex activity, often related to visual object recognition (Malach et al., 1995), was possibly recruited in imagining images of social others.
A second significant pattern dissociated brain activity for judgments about partner and self from parent and friend. Increased activation in anterior cingulate, anterior insula and posterior STS was observed in mentalizing about partner and self. These regions have been implicated in social cognitive processes crucial for close bond formation, such as empathy (Decety and Jackson, 2004). The pattern of brain activity associated with partner and self-judgment is consistent with the topology of the salience network (Seeley et al., 2007). The salience network, including regions such as anterior insula and anterior cingulate, is thought to coordinate responses to environmental stimuli that are most important to an individual (see Uddin, 2015, for review), such as representations of primary attachment figures and self, suggesting that this network may differentiate representations of self and romantic partners serving as primary attachment figures from other social representations. These results demonstrate that nuanced differences between neural representations of salient social figures (partner, parent, friend and self) are likely associated with differences in attachment status. Engagement of the salience network dichotomized our attachment schema, showing greater activation during mentalizing about one’s self or a romantic partner versus one’s parent or a friend.
Contrary to previous work suggesting the default and salience networks work in opposition (Hermans et al., 2014), we found these networks are recruited together to represent romantic partners and the self. The antagonistic relationship observed between these networks in past research may have more to do with the tasks used, which do not assess personal significance as we do in the present work. Our results confirm these networks work in concert, similar to research showing both competition and interdependence between default network function and salience network integrity (Bonnelle et al., 2012). Research in moral cognition also suggests an interactive role for these two networks (Sevinc et al., 2017). Salience network regions such as anterior insula detect both internal and external salient events, interacting with the default network to process internal events specifically. We observed this interaction in the unique neural response to attached romantic partners and the self, wherein the default network is engaged in construction and utilization of social representations and the salience network is selectively attuned to the most meaningful of these representations.
Since cognitive representations of attachment figures are chronically accessible and serve emotion-regulatory functions, they are understood to be different in content and use from representations of less-close others (Pietromonaco et al., 2006; Mikulincer and Shaver, 2007). The mental representations we form of close others are composed of perceptually salient social memories and, yet, are differentiable depending on the specific person about whom we are thinking. As a pair bond forms, a romantic partner becomes integrated into one’s sense of self—into one’s head (Aron et al., 1991) and ‘under [one’s] skin’ (Pietromonaco et al., 2013). Recent work in neurobiology highlights biobehavioral synchrony as a characteristic of pair bonds (Feldman, 2017). In many cases, the presence of intrinsically rewarding contact comfort and sexual activity also enables romantic attachment relationships that are uniquely intimate in nature (Zayas et al., 2015). Our finding that representations of romantic attachment partners and of the self have common neural underpinnings confirmed these theoretical principles.
In early development, parents serve as our primary attachment figures, a role often supplanted by romantic partners in young adulthood (Hazan and Zeifman, 1999; Nickerson and Nagle, 2005). Both our self-report and neuroimaging results note this social-psychological distinction; romantic partners were predominately nominated by participants as primary attachment figures. Furthermore, we observed that overall romantic partner attachment was higher than parent attachment, closeness to a parent as measured by the IOS was no different from closeness to a friend, and friend and parent brain activity covaried together. Our findings indicate that, although both romantic partners and parents categorically served as attachment figures for participants, there are subtle differences in attachment status across these categories—observable in both brain and behavior—that require further exploration. We assert these differences are related to the distinctive status of primary attachment figures and, relatedly, to the unique physical and emotional intimacy of romantic relationships. These differences could be better understood by asking participants additional questions about the nature of their romantic relationships, such as ‘Do you currently share a home with this person?’
Left temporal pole and parahippocampal gyrus showed increased activity for parent and friend over partner and self. Recent work suggests that parahippocampal regions play a critical role in judgments of trustworthiness and uncertainty (Bhatt et al., 2012). Parahippocampal involvement may reflect visual input related to the task or the retrieval of previous experiences with the person about whom the participant was making a judgment (Aminoff et al., 2013). The temporal pole is thought to integrate social conceptual knowledge, enabling processes like empathy (Pehrs et al., 2015) and sharing others’ embarrassment (Paulus et al., 2014; Müller-Pinzler et al., 2016). Within the context of our experimental paradigm, these neural regions may uniquely contribute to trait judgment for these individuals of varied closeness. These activity patterns could be associated with differences in cognitive processing necessary to access more distal social conceptual knowledge for others who are close to us but not primary attachment figures, for whom judgments are more readily retrieved.
Results of our ROI analyses confirmed the role played by dorsal and ventral areas of mPFC in social cognition. We found activity in dmPFC and vmPFC was robustly associated with thinking about all social others and the self. Our results support the functional separation noted in de la Vega et al.’s (2016) tripartite mPFC parcellation. These subregions fall within the ‘anterior zone’ and, in the present study, also fit within the functional profile of this zone, important for social cognition, affect, decision-making and episodic memory (de la Vega et al., 2016). We note, consistent with the second LV, that rostral cingulum regions demonstrated a robust response to romantic partner.
Overall, these findings enhance our understanding of neural representations of known others and how attachment modulates these representations. As our experimental stimuli included names of real individuals, highly relevant to each participant, our findings provide ecologically valid evidence that mentalizing about close others is associated with different patterns of brain activation depending on social proximity and attachment. Unlike previous studies, which involved more passive tasks (e.g. viewing photos of known others), our paradigm required active mentalizing about personalized, social targets. Our initial findings provided validation of this paradigm by replicating previous findings of default network activation associated with mentalizing about known others and provided the first evidence that this association is consistent across multiple levels of social closeness. The second pattern of activity provided unique insights with respect to modulation of these neural representations by attachment. The covariance of partner and self, showing activity within the salience network, suggests that we form highly overlapping neural representations for ourselves and romantic attachment figures. Interpretation of our results provides a potential mechanistic explanation for the differentiated neural response to close others; while the default network more broadly supports social cognition for known others and the self, recruitment of the salience network is critical for capturing the nuanced representations, and their significance, of our most ‘intimate’ adult relationships: romantic partners serving as primary attachment figures and the self.
Our findings provide empirical evidence to support recent work on the topic of attachment indicating that human attachment representations recruit cortical and subcortical networks for processes such as mentalization and reward (Feldman, 2017). Future work should further consider the role of attachment in dissociable patterns of brain activity. The neural correlates of relationship quality factors—specifically, attachment styles (e.g. secure, anxious, avoidant)—remain undiscovered. Further investigations could examine individual differences in attachment styles and neural foundations of mentalizing processes. In the current study, we leveraged valid and reliable self-report measures that reflect participants’ potential attachments. Our set of measures provides extensive information about participants’ thoughts, behaviors, and emotions within the context of their close relationships. For future studies involving known others, we recommend the administration of these measures as a standardized battery to assess the construct of adult attachment.
With this study, we introduce a common framework across disciplines to inform investigations of the neural basis of attachment as a deeper view of social cognitive neuroscience. Attachment figure mental representations play a powerful role in assisting individuals with emotion regulation and navigation of their social environments. Further investigations could provide significant insight into how the brain represents attachment, perhaps the most important consequence of real-world social interactions. Our findings support the hypothesis that mentalizing about different attachment figures—individuals from whom we seek proximity, security and comfort—may engage unique brain response patterns. By utilizing adult attachment as one end of a spectrum of personal closeness, we can begin to disentangle some of the important functional areas and networks of the brain recruited in social cognition. Attachment styles and patterns of behavior are well-studied within social psychology; attachment theory may therefore provide neuroscientists with behavioral constructs at a high level of specificity with respect to social proximity. Here we provide preliminary support for the idea that, by utilizing attachment criteria, we can implement more directed empirical studies to differentiate how social relationships are represented in the brain.
Funding
This project was supported in part by NIH grant 1S10RR025145.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
References
- Acevedo B.P., Aron A., Fisher H.E., Brown L.L. (2012). Neural correlates of long-term intense romantic love. Social Cognitive and Affective Neuroscience, 7, 145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. (2013). The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences, 17, 379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N.H. (1968). Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology, 9, 272.. [DOI] [PubMed] [Google Scholar]
- Andersen S.M., Cole S.W. (1990). "Do I know you?": The role of significant others in general social perception. Journal of Personality and Social Psychology, 59, 384–99. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna, J. R., Smallwood, J., Spreng, R. N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A., Aron E.N., Smollan D. (1992). Inclusion of other in the self scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology, 63, 596–612. [Google Scholar]
- Aron A., Aron E.N., Tudor M., Nelson G. (1991). Close relationships as including other in the self. Journal of Personality and Social Psychology, 60, 241. [Google Scholar]
- Baldwin M.W., Keelan J.P.R., Fehr B., Enns V., Koh-Rangarajoo E. (1996). Social-cognitive conceptualization of attachment working models: availability and accessibility effects. Journal of Personality and Social Psychology, 71, 94. [Google Scholar]
- Bartels, A., Zeki, S. (2000). The neural basis of romantic love. Neuroreport, 11, 3829–34. [DOI] [PubMed] [Google Scholar]
- Bhatt M.A., Lohrenz T., Camerer C.F., Montague P.R. (2012). Distinct contributions of the amygdala and parahippocampal gyrus to suspicion in a repeated bargaining game. Proceedings of the National Academy of Sciences, 109(22), 8728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V., Ham T.E., Leech R., et al. (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proceedings of the National Academy of Sciences, 109, 4690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby, J. (1973). Attachment and loss: Separation (Vol. 2). New York: Basic Books. [Google Scholar]
- Coan J.A., Schaefer H.S., Davidson R.J. (2006). Lending a hand: social regulation of the neural response to threat. Psychological Science, 17, 1032–9. [DOI] [PubMed] [Google Scholar]
- Decety, J., Jackson, P. L. (2004). The functional architecture of human empathy. Behavioral and cognitive neuroscience reviews, 3, 71–100. [DOI] [PubMed] [Google Scholar]
- de la Vega A., Chang L.J., Banich M.T., Wager T.D., Yarkoni T. (2016). Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. The Journal of Neuroscience, 36, 6553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Master S.L., Inagaki T.K., et al. (2011). Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences, USA, 108, 11721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, R. (2017). The neurobiology of human attachments. Trends in Cognitive Sciences, 21, 80–99. [DOI] [PubMed]
- Fraley R.C., Davis K.E. (1997). Attachment formation and transfer in young adults' close friendships and romantic relationships. Personal Relationships, 4, 131–44. [Google Scholar]
- Fraley R.C., Waller N.G., Brennan K.A. (2000). An item-response theory analysis of self-report measures of adult attachment. Journal of Personality and Social Psychology, 78, 350–65. [DOI] [PubMed] [Google Scholar]
- Gobbini M.I., Leibenluft E., Santiago N., Haxby J.V. (2004). Social and emotional attachment in the neural representation of faces. Neuroimage, 22, 1628–35. [DOI] [PubMed] [Google Scholar]
- Grewen K.M., Anderson B.J., Girdler S.S., Light K.C. (2003). Warm partner contact is related to lower cardiovascular reactivity. Behavioral Medicine, 29, 123–30. [DOI] [PubMed] [Google Scholar]
- Grigg O., Grady C.L. (2010). Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One, 5, e13311.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote N., Frieze I.H. (1994). The measurement of friendship-based love in intimate relationships. Personal Relationships, 1, 275–300. [Google Scholar]
- Haas B.W., Anderson I.W., Filkowski M.M. (2015). Interpersonal reactivity and the attribution of emotional reactions. Emotion, 15, 390.. [DOI] [PubMed] [Google Scholar]
- Hassabis, D., Spreng, R. N., Rusu, A. A., Robbins, C. A., Mar, R. A., Schacter, D. L. (2014). Imagine all the people: how the brain creates and uses personality models to predict behavior. Cerebral Cortex, 24, 1979–87. [DOI] [PMC free article] [PubMed]
- Hatfield E., Sprecher S. (l986). Measuring passionate love in intimate relations. Journal of Adolescence, 9, 383–4l0. [DOI] [PubMed] [Google Scholar]
- Hazan C., Gur-Yaish N., Campa M. (2004). What does it mean to be attached? In: Rholes W.S., Simpson J.A. editors. Adult Attachment: New Directions and Emerging Issues. New York: Guilford Press, 55–85. [Google Scholar]
- Hazan C., Hutt M.J., Sturgeon J., Bricker T. (1991). The process of relinquishing parents as attachment figures. Paper presented at the biennial meetings of the Society for Research in Child Development, Seattle, WA.
- Hazan C., Shaver P.R. (1987). Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology, 52, 511–24. [DOI] [PubMed] [Google Scholar]
- Hazan, C., Zeifman, D. (1999) Pair bonds as attachments. Handbook of Attachment: Theory, Research, and Clinical Applications. New York: Guilford Press, 336–54. [Google Scholar]
- Heatherton T.F., Wyland C.L., Macrae C.N., Demos K.E., Denny B.T., Kelley W.M. (2006). Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience, 1, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J., Henckens M.J., Joëls M., Fernández G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences, 37, 304–14. [DOI] [PubMed] [Google Scholar]
- Krienen F.M., Tu P.C., Buckner R.L. (2010). Clan mentality: evidence that the medial prefrontal cortex responds to close others. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 13906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. (2011). Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage, 56, 455–75. [DOI] [PubMed] [Google Scholar]
- Kundu P., Inati S.J., Evans J.W., Luh W.M., Bandettini P.A. (2012). Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage, 60, 1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu, P., Voon, V., Balchandani, P., Lombardo, M.V., Poser, B.A., Bandettini, P. (2017). Multi-Echo fMRI: A Review of Applications in fMRI Denoising and Analysis of BOLD Signals. Neuroimage, doi: 10.1016/j.neuroimage.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Auyeung B., Holt R.J., et al. (2016). Improving effect size estimation and statistical power with multi-echo fMRI and its impact on understanding the neural systems supporting mentalizing. Neuroimage, 142, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R., Reppas J.B., Benson R.R., et al. (1995). Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceedings of the National Academy of Sciences, 92, 8135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R.A. (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–34. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R. (1999). Mapping cognition to the brain through neural interactions. Memory, 7, 523–48. [DOI] [PubMed] [Google Scholar]
- McKinnon M.C., Moscovitch M. (2007). Domain-general contributions to social reasoning: theory of mind and deontic reasoning re-explored. Cognition, 102, 179–218. [DOI] [PubMed] [Google Scholar]
- Mikulincer M., Shaver P.R. (2007). Attachment in Adulthood: Structure, Dynamics, and Change. New York: Guilford Press. [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. (2006). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron, 50, 655–63. [DOI] [PubMed] [Google Scholar]
- Müller-Pinzler L., Rademacher L., Paulus F.M., Krach S. (2016). When your friends make you cringe: social closeness modulates vicarious embarrassment-related neural activity. Social Cognitive and Affective Neuroscience, 11, 466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel B.J., Herting M.M., Maxwell E.C., Bruno R., Fair D. (2013). Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain and Cognition, 82, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson A.B., Nagle R.J. (2005). Parent and peer attachment in late childhood and early adolescence. The Journal of Early Adolescence, 25, 223–49. [Google Scholar]
- Paulus F.M., Müller-Pinzler L., Jansen A., Gazzola V., Krach S. (2014). Mentalizing and the role of the posterior superior temporal sulcus in sharing others' embarrassment. Cerebral Cortex, 25, 2065–75. [DOI] [PubMed] [Google Scholar]
- Pehrs C., Zaki J., Schlochtermeier L.H., Jacobs A.M., Kuchinke L., Koelsch S. (2015). The temporal pole top-down modulates the ventral visual stream during social cognition. Cerebral Cortex, bhv226. [DOI] [PubMed] [Google Scholar]
- Pietromonaco P.R., DeBuse C.J., Powers S.I. (2013). Does attachment get under the skin? Adult romantic attachment and cortisol responses to stress. Current Directions in Psychological Science, 22, 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco P.R., Feldman Barrett L., Powers S. (2006). Adult attachment theory and affective reactivity and regulation In: Snyder D.K., Simpson J.A., Hughes J.N., editors. Emotion Regulation in Families: Pathways to Dysfunction and Health. Washington, DC: American Psychological Association, 57–74. [Google Scholar]
- Platek S.M., Loughead J.W., Gur R.C., et al. (2006). Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping, 27, 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W.W., Menon, V., Schatzberg, A.F. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27, 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcuk E., Zayas V., Günaydin G., Hazan C., Kross E. (2012). Mental representations of attachment figures facilitate recovery following upsetting autobiographical memory recall. Journal of Personality and Social Psychology, advance online publication, doi:10.1037/a0028125. [DOI] [PubMed] [Google Scholar]
- Sevinc, G., Gutvit, I.H, Spreng, R.N. (2017). Salience network engagement with the detection of morally laden information. Social Cognitive and Affective Neuroscience. https://doi.org/10.1093/scan/nsx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A. (2012). I remember you: a role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Research, 1428, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe L.A., Waters E. (1977). Attachment as an organizational construct. Child Development, 48, 1184–99. [Google Scholar]
- Stoessel C., Stiller J., Bleich S., et al. (2011). Differences and similarities on neuronal activities of people being happily and unhappily in love: a functional magnetic resonance imaging study. Neuropsychobiology, 64, 52–60. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Brechmann A., Marchewka A., Jednorog K., Dobrowolny M., Nowicka A. (2012). Is it about the self or the significance? An fMRI study of self-name recognition. Social Neuroscience, 6, 98–107. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Brechmann A., Nowicka A. (2013). Cross-modal pattern of brain activations associated with the processing of self- and significant other's name. Human Brain Mapping, 34, 2069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16, 55–61. [DOI] [PubMed] [Google Scholar]
- Wang G., Mao L., Ma Y., et al. (2012). Neural representations of close others in collectivistic brains. Social Cognitive and Affective Neuroscience, 7, 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J., Aron A., Parke S., Chatterjee N., Mackey S. (2010). Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS One, 5, e13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas V., Merrill S.M., Hazan C. (2015). Fooled around and fell in love: the role of sex in adult romantic attachment formation In: Attachment Theory and Research: New Directions and Emerging Themes. New York: The Guilford Press, 68–96. [Google Scholar]
- Zeki S., Romaya J.P. (2010). The brain reaction to viewing faces of opposite- and same-sex romantic partners. PLoS One 5, e15802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.