Abstract
In patients undergoing major orthopaedic surgery, pre-operative anaemia, peri-operative bleeding and a liberal transfusion policy are the main risk factors for requiring red blood cell transfusion (RBCT). The clinical and economic disadvantages of RBCT have led to the development and implementation of multidisciplinary, multimodal, individualised strategies, collectively termed patient blood management, which aim to reduce RBCT and improve patients’ clinical outcome and safety. Within a patient blood management programme, low pre-operative haemoglobin is one of the few modifiable risk factors for RBCT. However, a survey among Anaesthesia Departments in Spain revealed that, although pre-operative assessment was performed in the vast majority of hospitals, optimisation of haemoglobin concentration was attempted in <40% of patients who may have benefitted from it, despite there being enough time prior to surgery. This indicates that haemoglobin optimisation takes planning and forethought to be implemented in an effective manner. This review, based on available clinical evidence and our experience, is intended to provide clinicians with a practical tool to optimise pre-operative haemoglobin levels, in order to minimise the risk of patients requiring RBCT. To this purpose, after reviewing the diagnostic value and limitations of available laboratory parameters, we developed an algorithm for the detection, classification and treatment of pre-operative anaemia, with a patient-tailored approach that facilitates decision-making in the pre-operative assessment. We also reviewed the efficacy of the different pharmacological options for pre-operative and post-operative management of anaemia. We consider that such an institutional pathway for anaemia management could be a viable, cost-effective strategy that is beneficial to both patients and healthcare systems.
Keywords: anaemia, iron deficiency, intravenous iron, recombinant erythropoietin, orthopaedic surgery
Introduction
Major orthopaedic surgery, especially hip and knee arthroplasties, results in significant peri-operative bleeding, which often exceeds one third of patients’ blood volume1. As a result, a significant proportion of these patients receive allogeneic red blood cell transfusions (RBCT) to treat acute post-operative anaemia, although this practice is widely variable2. At present, orthopaedic surgery departments are responsible for over 7% of all packed red blood cell requests to hospital blood banks3. Population ageing is resulting not only in an increase in primary arthroplasties but also more revision procedures, which entail greater blood losses and transfusion requirements4.
Pre-operative anaemia, peri-operative bleeding and a liberal transfusion policy are the main risk factors for requiring RBCT1. There is now scientific evidence that RBCT is associated with increased post-operative mortality and morbidity, particularly nosocomial infections5,6. The clinical and economic disadvantages of RBCT in this scenario have fuelled a paradigm shift in Transfusion Medicine towards the development and implementation of multidisciplinary, multimodal, individualised strategies, collectively termed patient blood management (PBM), which aim to reduce RBCT and improve clinical outcome and safety7–9. PBM has been endorsed by the World Health Assembly requesting the World Health Organization (WHO) to provide its member states with training and support on the safe, rational use of RBCT and transfusion alternatives10.
Within a PBM programme, low pre-operative haemoglobin (Hb) concentration is one of the few modifiable risk factors for RBCT, but its optimisation takes planning and forethought to be implemented in an effective manner. From pre-operative assessment to post-operative care in post-anaesthesia recovery units, anaesthesiologists play a key role in the implementation of RBCT minimisation strategies11. In Spain, the anaesthesiologist performs a pre-operative evaluation of patients undergoing major surgery in more than 86% of hospitals, and 74% of patients scheduled for major orthopaedic surgery are assessed between 1 week and 2 months prior to surgery12. Thus, major orthopaedic surgery is one of the most appropriate clinical settings for the optimisation of pre-operative Hb, as recommended in the Network of Advancement of Patient Blood Management, Thrombosis and Haemostasis (NATA) guidelines for the detection, assessment, and management of pre-operative anaemia13.
Multidisciplinary collaboration between surgeons, anaesthesiologists, immunohaematologists, physiotherapists and nurses has allowed the implementation of PBM programmes for total arthroplasty of the hip or knee, which have resulted in a shorter time spent in hospital and a reduction in the all-cause readmission rate9,14.
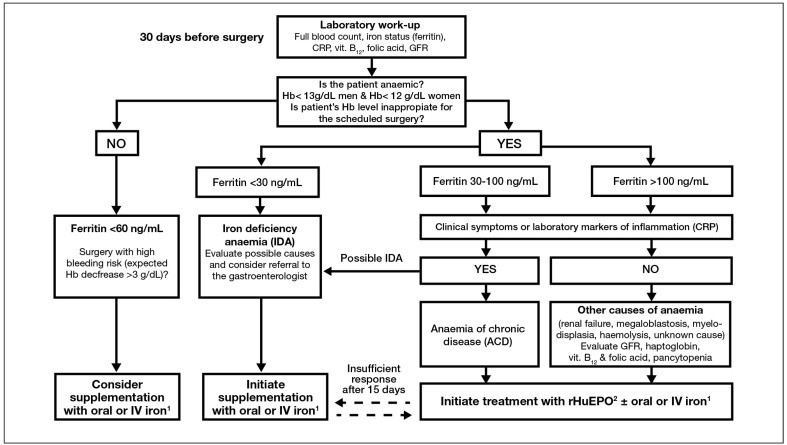
The aim of this review is to provide a practical tool to help clinicians optimise pre-operative Hb, according to the available clinical evidence and our experience, in order to minimise the risk of receiving RBCT15. For this purpose, we have developed a diagnostic and management algorithm (Figure 1), which should facilitate decision-making in the pre-operative assessment, with an approach tailored to the patient who is willing to participate in medical decisions, knowing the risks and benefits of RBCT and its alternatives.
Figure 1.
Consensus algorithm for pre-operative haemoglobin optimisation from the Spanish Best Practice in Peri-operative Anaemia Optimisation Panel from SEDAR (Sociedad Española de Anestesiología y reanimación)30*.
1 If time available for Hb optimisation prior to surgery is short, there is intolerance to oral iron, functional iron deficiency or a poor response to rHuEPO, IV iron should be administered. 2 rHuEPO administration may be an off-label use for some operations, thus requiring a careful risk to benefit evaluation.
Explanatory notes: *the reduction of 1 g/dL in Hb concentration is equivalent to 150 mg iron loss. Thus, considering that 1 ng/mL ferritin is equivalent to 8 mg of stored iron, for an expected Hb decrease of 3–4 g/dL a minimum of 60 ng/mL ferritin is needed to recover from post-operative anaemia.
Hb: haemoglobin; CRP: C-reactive protein; GFR: glomerular filtration rate; IV: intravenous; rHuEPO: recombinant human erythropoietin.
Implementation of patient blood management in Spain: the results of a survey in Anaesthesia Departments
Members from the Haemostasis and Transfusion Medicine section of the Spanish Society of Anaesthesiology and Reanimation (SEDAR) proposed conducting a survey among the heads or PBM key leaders of Anaesthesia Departments (n=91) in order to assess the actual implementation of PBM programmes throughout Spain. The survey consisted of 42 questions related to the characteristics of the pre-operative assessment, the existence of RBCT protocols and the use of alternatives to RBCT in different surgical scenarios, and the main factors limiting the implementation of PBM12.
According to the results yielded by this survey, the time frame between evaluation and surgery is generally long enough (30 days in >75% of patients) to implement an appropriate programme to optimise pre-operative Hb. However, there is wide variability in local strategies for pre-operative management of anaemia among institutions. There is also great variability in transfusion criteria between the different hospitals surveyed. The lack of awareness of the risks associated with anaemia and RBCT among the healthcare staff, which creates barriers to a wider implementation of a comprehensive programme of appropriate RBCT use and treatment of pre-operative anaemia, could be behind the observed variability.
Responders identified the need for a well-established pre-operative pathway, since the lack of involvement or collaboration of the surgical team is the main factor limiting optimal implementation of a PBM programme. Responders further identified lack of support from health authorities and hospital administrators, who should facilitate organisation, as a barrier to the implementation of multidisciplinary, multimodal PBM programmes, while cost issues were considered of lesser relevance.
From our point of view, it would be desirable for health organisations and/or public health services to be aware of the current use of RBCT and its alternatives. This, along with greater knowledge by healthcare professionals of the risks and costs of unnecessary or avoidable RBCT could contribute to greater use of PBM programmes16, with a particular focus on the treatment of pre-operative anaemia, as the first step in avoiding RBCT.
Anaemia in orthopaedic surgery: epidemiology
Pre-operative anaemia is an important independent risk factor for RBCT in operations associated with moderate-to-severe blood loss, such as major orthopaedic surgery17–19. The prevalence of pre-operative anaemia in this population varies according to the type of surgery, age, gender and comorbidities present, but also depends on the criteria used to define anaemia20,21. The prevalence in the majority of series of patients undergoing elective major orthopaedic surgery ranged from 15 to 40%20–22. Up to 10% of women presented with a pre-operative Hb value between 12 and 13 g/dL18, which is considered normal according to WHO definitions. However, these Hb levels should be considered inappropriate for a major surgical procedure in which significant blood loss is anticipated17,19,23. In addition, up to 90% of patients undergoing these procedures develop post-operative anaemia24, which may be aggravated by inflammation-induced blunted erythropoiesis25.
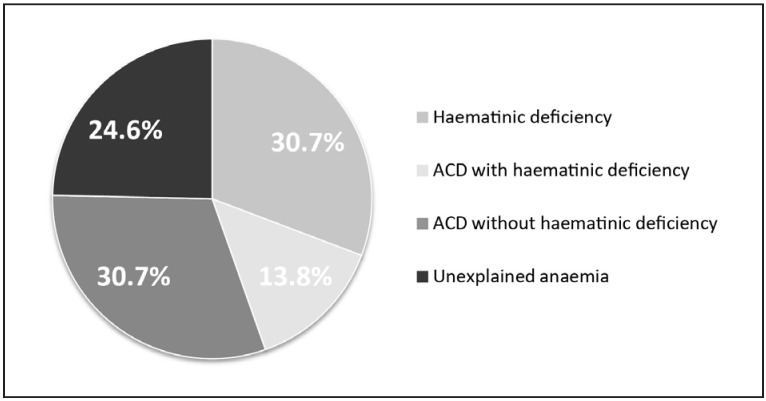
Almost one-third of anaemic orthopaedic patients have haematinic-deficiency anaemia, with iron deficiency being the most commonly observed form21,22, approximately one-third have anaemia of chronic inflammation22,26,27 (with or without chronic kidney disease), and just over one-third have anaemia of mixed cause or unexplained origin (Figure 2).
Figure 2.
Distribution of the main causes of pre-operative anaemia in patients scheduled for major orthopaedic surgery (modified from Bisbe et al.66).
ACD: anaemia of chronic disease.
The prevalence of anaemia of unexplained origin is relatively high among the elderly population28, as is that of major orthopaedic surgery. This anaemia is possibly caused by changes in stem cell physiology, including occult myelodysplasia, in some cases. The impact of changes in oestrogen or testosterone levels with age, multiple drug usage and a variety of medical conditions not classically associated with inflammation (e.g. hypothyroidism), could be other mechanisms. Treatment with erythropoietin has produced very good responses in this type of anaemia29.
Pre-operative management of anaemia: the algorithm30
According to NATA guidelines, all patients scheduled for major orthopaedic surgery should have their Hb and iron status tested at least 1 month prior to surgery in order to detect anaemia and haematinic deficiencies (a GRADE 1C recommendation, according to the Grades of Recommendation Assessment, Development and Evaluation classification). The second recommendation is that anaemia should always be corrected before elective surgery (GRADE 2C)13. This could entail rescheduling a major surgical procedure, when possible.
Anaemia management pathway
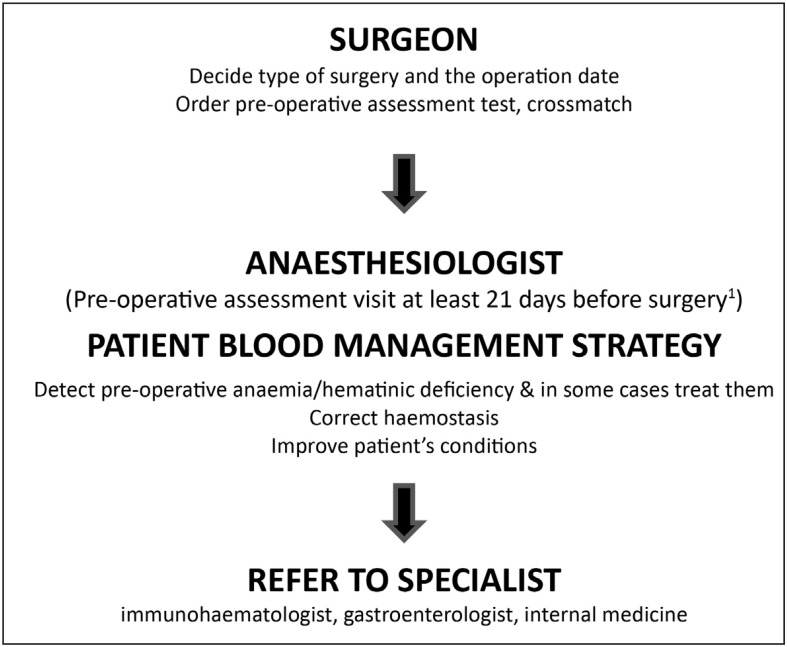
The main objective of a pre-operative assessment is to evaluate a patient’s risks and optimise his or her health status in order to minimise the risks or improve safety. The assessment of RBCT risk within a PBM programme leads to a personalised plan to reduce or avoid exposure to RBCT and improve post-operative outcome. This is clearly a fundamental task of the anaesthesiologist11.
The pre-operative assessment is, therefore, the best moment to decide the most appropriate strategy to minimise the risk of RBCT, based on the type of intervention, the patient’s health status, estimated time to surgery, and available blood-saving techniques31. Ideally, an institutional protocol agreed upon by surgeons, anaesthesiologists and haematologists in the Transfusion Committee should be followed. Such a protocol should also reduce healthcare costs (fewer patient evaluations) and optimise inclusion in the PBM programme.
The first step in the pre-anaesthetic assessment is the evaluation of risk factors for transfusion (Table I)
Table I.
Risk factors for receiving perioperative blood transfusion.
|
Of these factors, a low Hb level is one of the few that can be improved during the pre-operative period. The target Hb value to achieve (generally ≥13 g/dL) will depend on the type of intervention and the patient’s characteristics23. The remaining factors will allow an estimation of transfusion risk in more than 80% of cases, facilitating the planning of complementary strategies.
The second step is to classify and treat pre-operative anaemia and haematinic deficiencies according to a very simple algorithm (Figure 3).
Figure 3.
Pre-operative pathway for patient blood management.
Our goal is optimise the pre-operative Hb level (as the most cost-effective strategy to minimise the exposure of surgical patients to the potential adverse effects of anaemia, RBCT or both) without compromising the patients’ safety. Therefore, if major orthopaedic surgery is elective and the anaemia significant (Hb <10 g/dL) or unexpected, the procedure must be rescheduled and the patient referred to the appropriate specialist for evaluation and treatment of the anaemia. Should the anaemia be of known cause or less severe (Hb ≥10 g/dL), the anaesthesiologist/nurse could proceed to its treatment. To this end, a pre-operative laboratory assessment should include a full blood count as well as an evaluation of iron status (e.g., ferritin) and markers of inflammation (e.g. C-reactive protein), to enable classification of the anaemia and appropriate pharmacological therapy, thus avoiding additional assessments. Alternatively, in some hospitals the laboratory screening is expanded automatically to cover a basic study of anaemia when the pre-operative analysis shows a Hb <12 g/dL in women or <13 g/dL in men. This latter approach will not, however, detect haematinic deficiency without anaemia.
There are a number of questions regarding a patient’s erythropoiesis which need to be addressed before deciding how manage the anaemia (Table II). For example, could the patient be treated by the anaesthesiologist or should he or she be sent to a specialist? What is the target Hb level to achieve to minimise transfusion risk according to the patient’s comorbid conditions, type of surgery, and characteristics? Can this Hb target be attained within a short time (<1 month) or should surgery be postponed?
Table II.
Relevant questions for managing preoperative anaemia.
|
Hb: haemoglobin.
Diagnostic parameters
A pre-operative assessment of iron status is recommended in patients undergoing major orthopaedic surgery, as it can provide the correct indications for iron supplementation and predict the responsiveness to recombinant human erythropoietin (rHuEPO) administration32. The assessment of pre-operative iron status in Europe is not uniform: it is usual in Austria, Spain and Switzerland, unusual in France and the United Kingdom, and restrictively used in the Netherlands and Germany, where it is performed only if extremely low Hb values are found8. However, according to the NATA guidelines, pre-operative assessment of iron status should be considered the standard of care for anaemic patients undergoing major orthopaedic surgery13. Australian guidelines for the evaluation of pre-operative anaemia include determination of ferritin level and transferrin saturation, as well as an assay of C-reactive protein as a marker of inflammation.
It is important to include some iron parameters in the assessment of pre-operative anaemia. Decreased iron availability is the main factor limiting erythropoiesis and may be present in iron-deficiency anaemia after iron deposits have been exhausted and in anaemia of chronic disease, due to inflammation-induced iron sequestration. Functional iron deficiency is also the main cause of an inappropriate response to rHuEPO treatment33.
Biochemical parameters
Measurement of serum or plasma ferritin provides the most useful indirect estimate of body iron stores (1 ng/mL of serum ferritin corresponds to approximately 8 mg of stored iron). It is universally available and well standardised, and the test of choice for detecting both iron deficiency and iron overload. Ferritin levels <30 ng/mL in anaemic patients are highly suggestive of iron-deficiency anaemia. As ferritin is a positive acute phase reactant, in patients of advanced age and those with inflammation or infection ferritin levels up to 60–100 ng/mL are also compatible with iron deficiency34.
As transferrin is the only iron-binding protein involved in iron transport, the transferrin saturation index (%TSAT) reflects the iron transport compartment. Its measurement is universally available and well standardised, although its value is influenced by daily and circadian variability of serum iron levels and transferrin (negative acute phase protein). The transferrin saturation index may be reduced (<20%) in both iron-deficiency anaemia, due to absolute iron deficiency with increased transferrin concentrations, and anaemia of chronic disease, due to iron sequestration in the reticuloendothelial system with normal or decreased transferrin levels.
The levels of soluble transferrin receptor (sTfR) reflect the degree of iron availability to bone marrow cells. Upon depletion of functional iron, sTfR concentrations increase progressively while Hb concentration begins to fall. Circulating levels of sTfR are hardly influenced by inflammation or chronic disease, allowing the diagnosis of iron deficiency in patients of advanced age who have normal ferritin values27. However, sTfR levels also reflect increased erythropoietic activity (haemolytic anaemia, chronic lymphocytic leukaemia, therapy with rHuEPO). The ferritin index (sTfR/log ferritin) has a better discriminatory capacity than ferritin or sTfR separately. However, measurement of sTfR is expensive, not universally available, and the method is not standardised, further limiting a wider utilisation of this parameter.
Red cell variables
A routine complete blood count yields red cell indices, such as mean corpuscular volume, mean corpuscular Hb and red cell distribution width, which are useful for classifying simple cases of anaemia. These indices are also useful in assessing trends over periods of weeks or months, but have no use in assessing acute changes in iron availability.
There are, however, important haematological indices, such as the reticulocyte Hb content (CHr) and percentage of hypochromic red blood cells (%Hypo), which may help in the diagnosis of functional iron deficiency, although they can only be measured by specific haematology analysers. %Hypo is the best-established parameter for the identification of functional iron deficiency and seems to be superior to measurements of sTfR35. %Hypo values are related to iron status in the preceding 2–3 months36, because of the long circulating life-span of mature erythrocytes. CHr is the next most established option but this test provides an early direct measurement of iron supply to red blood cell production (<7 days). Both haematological indices are direct indicators of functional iron deficiency in contrast to the majority of biochemical markers35. These parameters are also good predictors of response to intravenous (IV) iron administration37.
The reticulocyte count is an important adjunct to CHr for evaluating response to treatment. While reticulocytes indicate the adequacy of red blood cell production in response to erythropoietin, the CHr level indicates actual red blood cell Hb content. If iron stores are low, any reticulocytes produced may have a low Hb content.
Treatment of pre-operative anaemia and haematinic deficiencies
The purpose of pharmacological treatment of pre-operative anaemia is to achieve, at least, normal Hb values, as defined by the WHO13. However, in the context of major orthopaedic surgery, the target Hb to minimise the risk of requiring a transfusion should be customised according to the type of surgery and the patient’s characteristics. Thus, for women scheduled for hip or knee arthroplasty, it would be desirable to achieve a Hb value ≥13 g/dL to minimise the risk of transfusion23, whereas for revision of total hip arthroplasty the Hb target may be ≥14 g/dL, since peri-operative bleeding usually exceeds 30% of the patient’s blood volume4.
In one-third of anaemic patients undergoing major orthopaedic surgery, the anaemia may be effectively corrected before surgery by pharmacological treatment such as iron, folic acid, and vitamin B12. In iron-deficiency anaemia, choice regarding which iron preparation to administer and by what route are not simple. Oral administration of iron may be the route of choice for anaemic patients whose surgery is not urgent, due to its low cost and simplicity. However, elderly patients taking multiple drugs (e.g. proton pump inhibitors) are often intolerant of, or unresponsive to, oral iron. In addition, the time required to correct anaemia and replenish iron stores make the oral route unhelpful for most patients.
If the time before surgery is short or the patient does not tolerate oral iron or has a poor response to it, IV iron supplementation is indicated as a safe and effective alternative for the treatment of pre-operative anaemia: it has fewer side effects and adherence to this treatment is better than that for oral iron38,39. IV supplementation is the route of choice in functional iron deficiency. Should the response to IV iron not be optimal in 2–3 weeks, a dose of subcutaneous rHuEPO may be administered.
Most IV iron formulations available in Europe (Table III) are safe and effective at correcting absolute or functional iron deficiency in anaemic patients undergoing major orthopaedic surgery, and produce an average Hb increase of 1 to 3 g/dL in 1 month40,41. It is important to stress that administration of IV iron alone never results in supra-physiological Hb levels and will not, therefore, increase the risk of thromboembolic complications.
Table III.
Some characteristics of IV iron formulations.
| Carbohydrate shell | Carboxymaltose | Sucrose | Isomaltoside-1000 | Low molecular weight dextran | Sodium ferric gluconate |
|---|---|---|---|---|---|
| Complex type | Type I Robust and strong |
Type II Semi-robust and strong |
Type I Robust and strong |
Type I Robust and strong |
Type III Labile and weak |
|
| |||||
| Maximal single dose | 1,000 mg | 200 mg | 20 mg/kg | 20 mg/kg | 125 mg |
|
| |||||
| Administration time | 15 min | 30 min | >15 min (up to 1,000 mg) ≥30 min (>1,000 mg) |
4–6 h | 1 h |
|
| |||||
| Maximal weekly dose | 1,000 mg | 600 mg | 20 mg/kg | 20 mg/kg | 125 mg |
|
| |||||
| Presentation | |||||
| Vial volume | 2,10 and 20 mL | 5 mL | 1,5 and 10 mL | 2 mL | 5 mL |
| Concentration | 50 mg/mL | 20 mg/mL | 100 mg/mL | 50 mg/mL | 12.5 mg/mL |
Newer IV iron formulations, such as ferric carboxymaltose, low molecular weight iron dextran or iron isomaltoside-1000, are more robust and stable, allow rapid administration of up to 1,000 mg in a single dose, and result in prompt, effective pre-operative iron replacement. These newer IV iron formulations may, therefore, facilitate treatment and be more convenient both for the patient (fewer venepunctures, less time off work) and for the healthcare system (shorter administration time, fewer day-hospital assessments and fewer ambulance transfers, which may offset their higher acquisition cost)40. Finally, it should be emphasised that iron deficiency without anaemia also needs correction, since it may compromise both the optimisation of pre-operative Hb levels and the recovery from post-operative anaemia secondary to haemorrhage.
Elderly patients with anaemia of unknown cause, kidney disease or mild myelodysplastic syndrome show a good response to rHuEPO administration, although adequate iron supply should always be ensured, preferably via the IV route42. The dose of IV iron should be calculated according to the desired increase in Hb, without regard to replenishment of iron stores. For example, if an 80-year old patient scheduled for total knee arthroplasty with a Hb of 11 g/dL, ferritin of 150 ng/mL and no comorbidity or contraindication to treatment were to be prescribed two doses of rHuEPO (2×40,000 IU) in order to raise the Hb level to 13 g/dL (a 2 g/dL increment), co-administration of 500 mg IV would be sufficient to meet iron requirements for erythropoiesis.
In a recent study43, patients scheduled for knee or hip arthroplasty who had a Hb <13 g/dL, without classification of the type of anaemia, were initially treated with one dose of IV iron (1,000 mg) and subcutaneous rHuEPO (40,000 IU) if there were no contraindications. They also received pre-operative supplementation with parenteral vitamin B12 (1 mg) and oral folic acid (5 mg/day) to treat or prevent any functional or absolute deficiencies. After 14 days, patients who were still anaemic (15%) received a second dose of IV iron, rHuEPO and vitamin B12. All treated patients presented with normal Hb levels (≥13 g/dL) on the day of surgery, resulting in a reduction of RBCT rate. Although we believe that treatment should be targeted to the type of anaemia, this pragmatic approach achieved the goal of correcting pre-operative anaemia and reducing RBCT in a very simple way and with few adverse effects44.
In contrast, a recent randomised controlled trial showed that administration of four doses of rHuEPO plus oral iron, starting 3 weeks prior to the scheduled procedure, significantly decreased RBCT rates in anaemic patients (Hb <13 g/dL) undergoing lower limb arthroplasty, but not the number of transfused units and at an unacceptably high cost (€ 7,300 per avoided transfusion) and with a trend to a higher rate of cardiovascular adverse events45. Thus, routine, uncontrolled use of four doses of rHuEPO in patients undergoing major orthopaedic surgery should be seriously questioned.
Treatment of post-operative anaemia
Post-operative anaemia after major orthopaedic surgery is primarily caused by peri-operative bleeding, although it is exacerbated or maintained by the inflammatory response to surgical injuries25. A common treatment of acute, severe post-operative anaemia is RBCT, administered in order to avoid tissue hypoxia-related morbidity and mortality. Available evidence shows that a restrictive transfusion threshold (Hb 7–8 g/dL) does not increase morbidity or mortality, even in elderly patients with cardiovascular risk46–48. Application of restrictive transfusion triggers has resulted in many patients being discharged with significant post-operative anaemia which may be accompanied by iron deficiency. Both anaemia and iron deficiency could hamper early rehabilitation and return to daily activities49.
Thus, as part of the first pillar of PBM, treatment of post-operative anaemia is aimed at avoiding RBCT and improving post-operative outcome7, physical performance and quality of life50, and should not be neglected.
Peri-operative bleeding implies considerable iron loss (150–200 mg of iron for each gram of Hb decrease). Moreover, as Van Iperen et al. demonstrated, surgical injuries induce a state of hypoferraemia in the presence of adequate iron stores, due to inflammation25. The degree of this transient form of functional iron deficiency is related to the extent of the surgery. This could explain why post-operative oral iron is not useful for accelerating the recovery from anaemia or reducing the RBCT rate following major surgical procedures and is not, therefore, recommended51 (GRADE 1B). In contrast, IV iron could play an important role in this context15. However, published studies on the efficacy of postoperative IV iron are scant and mostly observational, retrospective, and focused on the reduction of RBCT rate52,53, rather than on the improvement of anaemia and its effects on physical performance and quality of life. It should also be noted that IV iron was administered peri-operatively in most studies (some doses of iron were administered just before surgery) and not selectively targeted to anaemic patients. In addition, some patients with Hb <13 g/dL received a single dose of rHuEPO53. However, in a prospective randomised study of 200 patients with hip fracture, Serrano-Trenas et al. highlighted that peri-operative monotherapy with IV iron resulted in a significant reduction of RBCT only among patients presenting with a Hb >12 g/dL or subcapital fracture, and did not influence post-operative morbidity54. Based on the evidence supplied by these studies, the Seville Consensus Document suggests short-term peri-operative treatment with IV iron in anaemic patients subjected to major orthopaedic surgery in order to reduce the RBCT rate (GRADE 2B)15. Similarly, United Kingdom guidelines for the management of pre-operative anaemia recommend that, when surgery is urgent, whatever time is available before the operation should still be used to investigate anaemia and initiate treatment (GRADE 1C)55.
One of the first studies on the effectiveness of IV iron for the treatment of post-operative anaemia was conducted in a small sample of children with moderate-to-severe anaemia after spinal surgery (Hb <9 g/dL); the Hb increased 0.36 g/dL per day in patients given IV iron compared to 0.25 g/dL per day in those treated with oral iron56. In a more recent study of adult patients undergoing major orthopaedic surgery, post-operative administration of 600 mg IV iron to anaemic patients (Hb <10 g/dL) reduced the RBCT rate from 84 to 46%; however, the study was retrospective and included patients who underwent total hip arthroplasty for hip fracture repair52.
In a recently published clinical trial50, patients who underwent total knee arthroplasty and had a Hb <12 g/dL the day after surgery were given oral iron for 30 days or a single dose of ferric carboxymaltose IV (825±125 mg). Compared to oral iron, IV iron resulted in higher rates of correction of anaemia (42 vs 23%) and iron deficiency (87 vs 35%) on day 30 after surgery. The primary outcome variable of this study was not RBCT, as Hb was optimised before surgery in all anaemic patients and the expected RBCT rate was very low (5%). However, differences in Hb level and some quality of life scores on post-operative day 30 were significant only for patients with low pre-operative iron stores and/or moderate-to-severe pre-operative anaemia. Similar results were observed for patients with low iron stores (ferritin <100 ng/mL) who received 400 mg iron sucrose 1–2 days before total knee arthroplasty57. In contrast, Karkouti et al.58 observed that post-operative IV iron administration, either alone or in combination with rHuEPO, did not affect Hb concentrations during the first week after surgery, thus scarcely the influencing RBCT rate. Once again, the study sample was too small (10 patients per group) to make firm conclusions and patients underwent different types of surgery (major orthopaedic surgery and on-pump cardiac surgery).
Treatment of pre-operative anaemia from an economic perspective
Current economic constrictions are challenging the sustainability of the public healthcare system. Therefore, in addition to issues concerning patients’ safety, the cost-benefit balance of any therapeutic intervention is of paramount importance. In this regard, rational use of RBCT16 and its alternatives, such as optimisation of pre-operative Hb, may offer significant cost savings to the healthcare system, despite an initially perceived cost increment due to acquisition of drugs and devices59.
Enko et al.26 compared pre-operative anaemia treatment implemented according to an algorithm vs conventional practice in patients undergoing major orthopaedic surgery. The overall therapeutic costs of the group that received preoperative IV iron and/or rHuEPO were cost-neutral compared with those for the untreated group (€ 35.7 and € 36, respectively) because of less RBCT use in the former group.
A correct economic assessment should also take into account “transfusion costs”, which may be three to four times higher than the “acquisition costs”60, as well as costs derived from RBCT-associated early and delayed reactions and adverse events.
When comparing the costs of blood-saving strategies, such as treatment of pre-operative anaemia, with those of RBCT, a number of issues should be considered. These include not only the costs of adverse effects for which a causal relationship can be established (e.g. wrong blood episodes, transmission of infectious diseases, transfusion-related acute lung injury), but also those derived from the worse outcomes of patients receiving RBCT (e.g. increased rates of nosocomial infections, kidney failure, thromboembolic events, prolonged mechanical ventilation), which lead to longer stays in hospital and higher readmission rates61. Using the American College of Medical Quality database, Morton et al. quantified a $ 17,000 increment in health spending for each transfused patient62.
In the context of major orthopaedic surgery (knee and hip arthroplasties), treatment of pre-operative anaemia with a comprehensive PBM programme results in significant economic savings, despite the increment in direct costs for pharmaceuticals and reinfusion drain devices. In a study by Kotze et al.9, the application of PBM in 281 patients undergoing major orthopaedic surgery was estimated to have saved € 160,000, since reduction of RBCT-associated morbidity shortened the time spent in hospital by 0.7 days and decreased the readmission rate by 5%.
It should be noted that even the particular IV iron formulation used to treat pre-operative anaemia can affect the cost-benefit balance. In major orthopaedic surgery, the administration of 1,000 mg ferric carboxymaltose, instead of iron sucrose, resulted in € 63 savings per patient, despite the drug acquisition cost for the former being more than € 100 higher40. The full dosage of ferric carboxymaltose can be administered in one session, instead of the five required to give iron sucrose, and with a shorter infusion time (15 min/infusion vs 30 min/infusion, respectively) (Table III). This saving only took into account the decrease in administration costs (day hospital, infusion drip, saline, nursing time, etc.), but savings in travel expenses and loss of work should also be added. Similarly, using a cost-minimisation analysis, Calvet et al.63 found that pre-operative ferric carboxymaltose infusion was less costly than iron sucrose infusion or oral iron replacement and appeared to reduce the total costs in patients with iron-deficient anaemia and colon cancer.
Muñoz et al. published a cost-analysis study on post-operative treatment with two IV iron preparations following total knee or hip arthroplasty. The results showed that IV treatments were effective at reducing the percentage of patients receiving allogeneic transfusions and the number of transfused units and were cost-neutral in the different cost scenarios evaluated64.
In a more comprehensive manner, the Government of Ontario quantified that implementation of PBM programmes for four surgical procedures (cardiac surgery, radical prostatectomy, knee and hip arthroplasty) resulted in $ 10.5 million savings in packed red cells and $ 39 million savings in health care, whereas the costs of implementing PBM were $ 3 million65.
Key messages
- Pre-operative anaemia is frequent among patients undergoing major orthopaedic surgery and is, in itself, a risk factor for poor clinical outcome.
- Pre-operative anaemia is also one of the strongest predisposing factors for peri-operative transfusion, which in turn increases post-operative morbidity, mortality and costs.
- Patients undergoing major orthopaedic surgery should have a complete blood cell count, and assessment of iron status and a markers of inflammation, preferably 30 days before the scheduled procedure.
- Unexpected or severe pre-operative anaemia should be considered an indication for rescheduling any elective major orthopaedic surgery until evaluation and treatment are completed, when possible.
- Patients undergoing major orthopaedic surgery with pre-operative iron deficiency anaemia may benefit from oral iron therapy provided that it is tolerated, that there are no contraindications, and that there is sufficient time prior to surgery.
- IV iron supplementation should be considered if there is poor absorption or poor tolerance of oral iron, ongoing blood loss or an accelerated response to treatment is required.
- When using rHuEPO, it would be advisable to adjust the dose individually to the target Hb, ensure iron supply to the bone marrow (administering adjuvant iron, preferably IV), and provide adequate pharmacological thromboembolic prophylaxis.
- Peri-operative or post-operative IV iron administration, with or without rHuEPO, is suggested for anaemic or iron-deficient patients undergoing major orthopaedic surgery when the time to surgery is short.
- An institutional algorithm for the detection, diagnosis and proper treatment of anaemia and haematinic deficiencies within a PBM programme may be a feasible, cost-effective strategy that is beneficial for both patients and healthcare systems.
Appendix
Spanish Best practice in perioperative anaemia optimisation panel (in alphabetical order):
- Misericordia Basora Macaya (H. Clínic de Barcelona, Barcelona);
- Elvira Bisbe Vives (H. del Mar, Barcelona);
- Óscar Díaz Cambronero (H. Universitari i Politècnic La Fe, Valencia);
- Mª José Colomina (H. Vall d’Hebron, Barcelona);
- Natalia García Claudio (H. Universitari i Politècnic La Fe, Valencia);
- Núria García Gregorio (H. Universitari i Politècnic La Fe, Valencia);
- Eduardo García Pascual (H. Galdakao-Usansolo, Galdakao, Vizcaya);
- Elena Gredilla Díaz (H. Universitario La Paz, Madrid);
- Salomé Matose Jaén (H. Universitari i Politècnic La Fe, Valencia);
- Jorge Molins Espinosa (H. Universitari i Politècnic La Fe, Valencia);
- Luís Moltó (H. del Mar, Barcelona);
- Gabriel Yanes Vidal (H. Universitario Virgen del Rocío, Sevilla).
Footnotes
Disclosure of conflicts of interest
EB, MB and MJC have received speaker honoraria and travel support from Vifor Pharma.
References
- 1.Gombotz HRP, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:150–6. doi: 10.1111/j.1537-2995.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 2.Gombotz H, Rehak PH, Shander A, Hofmann A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54:2646–57. doi: 10.1111/trf.12687. [DOI] [PubMed] [Google Scholar]
- 3.Bosch MA, Contreras E, Madoz P, et al. The epidemiology of blood component transfusion in Catalonia, Northeastern Spain. Transfusion. 2011;51:105–16. doi: 10.1111/j.1537-2995.2010.02785.x. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TS, Palmer J, Watson D, et al. Multicentre cohort study of red blood cell use for revision hip arthroplasty and factors associated with greater risk of allogeneic blood transfusion. Br J Anaesth. 2012;108:63–71. doi: 10.1093/bja/aer326. [DOI] [PubMed] [Google Scholar]
- 5.Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283–92. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 6.Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–8. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 7.Spahn DR, Moch H, Hofmann A, Isbister JP. Patient blood management: the pragmatic solution for the problems with blood transfusions. Anesthesiology. 2008;109:951–3. doi: 10.1097/ALN.0b013e31818e3d75. [DOI] [PubMed] [Google Scholar]
- 8.Shander A, Van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012;109:55–68. doi: 10.1093/bja/aes139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotze A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–52. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 10.Pan American Health Organization - World Health Organization. 146th Session of the Executive Committee CE146/INF/7. Resolution WHA63.12. [Accessed on 21/05/2010]. Available at: http://apps.who.int/iris/bitstream/10665/169232/1/CE146-INF-7-e.pdf.
- 11.Llau JV, Bisbe E. [Transfusion and hemostasis: unavoidable commitment for modern anesthesiology]. Rev Esp Anestesiol Reanim. 2003;50:495–7. [In Spanish.] [PubMed] [Google Scholar]
- 12.Colomina MJ, Basora Macaya M, Bisbe Vives E. [Implementation of blood sparing programs in Spain: results of a survey of departments of anesthesiology and resuscitation]. Rev Esp Anestesiol Reanim. 2015;62(Suppl 1):3–18. doi: 10.1016/S0034-9356(15)30002-5. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 13.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jans O, Jorgensen C, Kehlet H, Johansson PI Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group. Role of preoperative anemia for risk of transfusion and postoperative morbidity in fast-track hip and knee arthroplasty. Transfusion. 2014;54:717–26. doi: 10.1111/trf.12332. [DOI] [PubMed] [Google Scholar]
- 15.Leal-Noval SR, Muñoz M, Asuero M, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthes E. Evidence-based medicine: save blood, save lives. Nature. 2015;520:24–6. doi: 10.1038/520024a. [DOI] [PubMed] [Google Scholar]
- 17.Saleh E, McClelland DB, Hay A, et al. Prevalence of anaemia before major joint arthroplasty and the potential impact of preoperative investigation and correction on perioperative blood transfusions. Br J Anaesth. 2007;99:801–8. doi: 10.1093/bja/aem299. [DOI] [PubMed] [Google Scholar]
- 18.Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–69. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 19.Salido JA, Marin LA, Gomez LA, et al. Preoperative hemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors. J Bone Joint Surg Am. 2002;84-A:216–20. doi: 10.2106/00004623-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Shander A, Knight K, Thurer R, et al. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):58S–69S. doi: 10.1016/j.amjmed.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113:482–95. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 22.Bisbe E, Castillo J, Sáez M, et al. Prevalence of preoperative anemia and hematinic deficiencies in patients scheduled for elective orthopedic surgery. Transfus Alternatives Transfus Med. 2009;10:166–73. [Google Scholar]
- 23.Basora M, Tio M, Martin N, et al. Should all patients be optimized to the same preoperative hemoglobin level to avoid transfusion in primary knee arthroplasty? Vox Sang. 2014;107:148–52. doi: 10.1111/vox.12147. [DOI] [PubMed] [Google Scholar]
- 24.Lasocki S, Krauspe R, von Heymann C, et al. PREPARE: the prevalence of perioperative anaemia and need for patient blood management in elective orthopaedic surgery: a multicentre, observational study. Eur J Anaesthesiol. 2015;32:160–7. doi: 10.1097/EJA.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 25.Van Iperen CE, Kraaijenhagen RJ, Biesma DH, et al. Iron metabolism and erythropoiesis after surgery. Br J Surg. 1998;85:41–5. doi: 10.1046/j.1365-2168.1998.00571.x. [DOI] [PubMed] [Google Scholar]
- 26.Enko D, Wallner F, von-Goedecke A, et al. The impact of an algorithm-guided management of preoperative anemia in perioperative hemoglobin level and transfusion of major orthopedic surgery patients. Anemia. 2013;2013:641876. doi: 10.1155/2013/641876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basora M, Deulofeu R, Salazar F, et al. Improved preoperative iron status assessment by soluble transferrin receptor in elderly patients undergoing knee and hip replacement. Clin Lab Haematol. 2006;28:370–5. doi: 10.1111/j.1365-2257.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 29.Bisbe E, Castillo J, Nomen N, et al. [Preoperative erythropoietin as blood conservation technique for elderly patients in elective orthopedic surgery]. Med Clin (Barc) 2004;123:413–5. doi: 10.1016/s0025-7753(04)74536-4. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 30.Bisbe E, Basora M. [Algorithm for treating preoperative anemia]. Rev Esp Anestesiol Reanim. 2015;62(Suppl 1):27–34. doi: 10.1016/S0034-9356(15)30004-9. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 31.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basora Macaya M, Bisbe Vives E. [The first pillar of patient blood management. Types of anemia and diagnostic parameters]. Rev Esp Anestesiol Reanim. 2015;62(Suppl 1):19–26. doi: 10.1016/S0034-9356(15)30003-7. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 33.Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96:823–33. [PubMed] [Google Scholar]
- 34.Guyatt GH, Patterson C, Ali M, et al. Diagnosis of iron-deficiency anemia in the elderly. Am J Med. 1990;88:205–9. doi: 10.1016/0002-9343(90)90143-2. [DOI] [PubMed] [Google Scholar]
- 35.Thomas DW, Hinchliffe RF, Briggs C, et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161:639–48. doi: 10.1111/bjh.12311. [DOI] [PubMed] [Google Scholar]
- 36.Urrechaga E, Borque L, Escanero JF. Biomarkers of hypochromia: the contemporary assessment of iron status and erythropoiesis. Biomed Res Int. 2013;2013:603786. doi: 10.1155/2013/603786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basora M, Colomina MJ, Tio M, et al. Optimizing preoperative haemoglobin with intravenous iron. Br J Anaesth. 2013;110:488–90. doi: 10.1093/bja/aes587. [DOI] [PubMed] [Google Scholar]
- 38.Bisbe E. Role of iron replacement in the management of preoperative anemia. Transfus Alternatives Transfus Med. 2012;12:150–6. [Google Scholar]
- 39.Muñoz M, Garcia-Erce JA, Cuenca J, et al. AWGE (Spanish Anaemia Working Group) On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012;10:8–22. doi: 10.2450/2011.0061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bisbe E, Garcia-Erce JA, Diez-Lobo AI, Muñoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–8. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz M, Garcia-Erce JA, Diez-Lobo AI, et al. [Usefulness of the administration of intravenous iron sucrose for the correction of preoperative anemia in major surgery patients]. Med Clin (Barc) 2009;132:303–6. doi: 10.1016/j.medcli.2008.04.011. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 42.Weber EW, Slappendel R, Hemon Y, et al. Effects of epoetin alfa on blood transfusions and postoperative recovery in orthopaedic surgery: the European Epoetin Alfa Surgery Trial (EEST) Eur J Anaesthesiol. 2005;22:249–57. doi: 10.1017/s0265021505000426. [DOI] [PubMed] [Google Scholar]
- 43.Theusinger OM, Kind SL, Seifert B, et al. Patient blood management in orthopaedic surgery: a four-year follow-up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus. 2014;12:195–203. doi: 10.2450/2014.0306-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muñoz M, Gomez-Ramirez S, Garcia-Erce JA. Implementing patient blood management in major orthopaedic procedures: orthodoxy or pragmatism? Blood Transfus. 2014;12:146–9. doi: 10.2450/2014.0050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.So-Osman C, Nelissen RG, Koopman-van Gemert AW, et al. Patient blood management in elective total hip- and knee-replacement surgery (part 2): a randomized controlled trial on blood salvage as transfusion alternative using a restrictive transfusion policy in patients with a preoperative hemoglobin above 13 g/dl. Anesthesiology. 2014;120:852–60. doi: 10.1097/ALN.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 46.Carless PA, Henry DA, Carson JL, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2010;10:CD002042. doi: 10.1002/14651858.CD002042.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carson JL, Terrin ML, Jay M. Anemia and postoperative rehabilitation. Can J Anaesth. 2003;50(6 Suppl):S60–4. [PubMed] [Google Scholar]
- 50.Bisbe E, Molto L, Arroyo R, et al. Randomized trial comparing ferric carboxymaltose vs oral ferrous glycine sulphate for postoperative anaemia after total knee arthroplasty. Br J Anaesth. 2014;113:402–9. doi: 10.1093/bja/aeu092. [DOI] [PubMed] [Google Scholar]
- 51.Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92:265–9. doi: 10.2106/JBJS.I.00883. [DOI] [PubMed] [Google Scholar]
- 52.Muñoz M, Naveira E, Seara J, Cordero J. Effects of postoperative intravenous iron on transfusion requirements after lower limb arthroplasty. Br J Anaesth. 2012;108:532–4. doi: 10.1093/bja/aes012. [DOI] [PubMed] [Google Scholar]
- 53.Muñoz M, Gomez-Ramirez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54:289–99. doi: 10.1111/trf.12195. [DOI] [PubMed] [Google Scholar]
- 54.Serrano-Trenas JA, Ugalde PF, Cabello LM, et al. Role of perioperative intravenous iron therapy in elderly hip fracture patients: a single-center randomized controlled trial. Transfusion. 2011;51:97–104. doi: 10.1111/j.1537-2995.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- 55.Kotze A, Harris A, Baker C, et al. British Committee for Standards in Haematology guidelines on the identification and management of pre-operative anaemia. Br J Haematol. 2015;171:322–31. doi: 10.1111/bjh.13623. [DOI] [PubMed] [Google Scholar]
- 56.Berniere J, Dehullu JP, Gall O, Murat I. [Intravenous iron in the treatment of postoperative anemia in surgery of the spine in infants and adolescents]. Rev Chir Orthop Reparatrice Appar Mot. 1998;84:319–22. [In Spanish.] [PubMed] [Google Scholar]
- 57.Garcia-Erce JA, Cuenca J, Martinez F, et al. Perioperative intravenous iron preserves iron stores and may hasten the recovery from post-operative anaemia after knee replacement surgery. Transfus Med. 2006;16:335–41. doi: 10.1111/j.1365-3148.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 58.Karkouti K, McCluskey SA, Ghannam M, et al. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Can J Anaesth. 2006;53:11–9. doi: 10.1007/BF03021522. [DOI] [PubMed] [Google Scholar]
- 59.Hofmann A, Ozawa S, Farrugia A, et al. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013;27:59–68. doi: 10.1016/j.bpa.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 61.Bisbe Vives E. [Treatment of anemia in patient blood management from an economic perspective]. Rev Esp Anestesiol Reanim. 2015;62(Suppl 1):80–5. doi: 10.1016/S0034-9356(15)30013-X. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 62.Morton J, Anastassopoulos KP, Patel ST, et al. Frequency and outcomes of blood products transfusion across procedures and clinical conditions warranting inpatient care: an analysis of the 2004 healthcare cost and utilization project nationwide inpatient sample database. Am J Med Qual. 2010;25:289–96. doi: 10.1177/1062860610366159. [DOI] [PubMed] [Google Scholar]
- 63.Calvet X, Gene E, AngelRuiz M, et al. Cost-minimization analysis favours intravenous ferric carboxymaltose over ferric sucrose or oral iron as preoperative treatment in patients with colon cancer and iron deficiency anaemia. Technol Health Care. 2016;24:111–20. doi: 10.3233/THC-151074. [DOI] [PubMed] [Google Scholar]
- 64.Muñoz M, Gomez-Ramirez S, Martin-Montanez E, et al. Cost of post-operative intravenous iron therapy in total lower limb arthroplasty: a retrospective, matched cohort study. Blood Transfus. 2014;12:40–9. doi: 10.2450/2013.0088-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freedman J. The ONTraC Ontario program in blood conservation. Transfus Apher Sci. 2014;50:32–6. doi: 10.1016/j.transci.2013.12.010. [DOI] [PubMed] [Google Scholar]