Abstract
Background
Patients on parenteral nutrition require a central venous access and are at risk of catheter-related thrombosis, pulmonary embolism, and vena cava syndrome. Parenteral nutrition guidelines suggest anticoagulation for the primary prevention of catheter-related thrombosis during long-term parenteral nutrition. We conducted a systematic review of the efficacy, safety and feasibility of anticoagulant use for preventing and treating catheter-related thrombosis during parenteral nutrition.
Materials and methods
We searched for interventional and observational studies on adults and children receiving systemic anticoagulants during either short- or long-term parenteral nutrition delivered via central venous access. Primary outcomes were: objectively-confirmed catheter-related thrombosis, pulmonary embolism and bleeding. Secondary outcomes were: heparin-induced thrombocytopenia, prevalence of anticoagulation, and quality of International Normalised Ratio management in vitamin K antagonist-treated patients.
Results
We identified 1,199 studies, of which 23 were included. Seven interventional studies of short-term parenteral nutrition (adult population, n=5) were classified as low-quality: in those, intravenous unfractionated heparin did not prevent catheter-related thrombosis if compared to saline. No interventional studies were conducted in patients on long-term parenteral nutrition. Observational data were sparse, rarely focusing on anticoagulation, and overall of low quality. The reported use of anticoagulants was between 22 and 66% in recent multicentre cohorts.
Discussion
The amount and quality of data in this area are very suboptimal: most studies are outdated and involved heterogeneous populations. Currently, there is insufficient evidence to allow conclusions to be reached regarding the efficacy and safety of anticoagulants in this setting.
Keywords: parenteral nutrition, anticoagulants, central venous catheter, venous thrombosis, systematic review
Introduction
Parenteral nutrition is indicated for patients with intestinal failure unable to maintain their nutritional balance by oral or enteral intake. With this nutrition usually administered via a central venous access, patients are at risk of catheter-related thrombosis, pulmonary embolism, and vena cava syndrome. Recurrent catheter-related thrombosis causes progressive loss of vascular access, leading to inability to continue parenteral nutrition and leaving intestinal transplantation as a last resort solution.
Patients on parenteral nutrition are a very heterogeneous group with different thrombotic risk factors, such as cancer, recurrent bacterial infections, autoimmune and inflammatory bowel diseases, and may have started parenteral nutrition because of acute splanchnic thrombosis. They also share parenteral nutrition-specific risk factors, including hyperosmolar formulation-mediated endothelial injury, need for a long-term venous access, and development of renal and hepatic failure1,2.
National and international parenteral nutrition guidelines suggest or recommend various anticoagulant regimens for the prevention or treatment of catheter-related thrombosis during long-term parenteral nutrition3–9. Overall, recommendations are inconsistent with those presented in the guidelines involving other populations of adult patients with a central venous access (Table I)10–13.
Table I.
Overview of the international guidelines on the prevention and treatment of catheter-related thrombosis.
| PN guidelines | Primary CRT prevention | Treatment of CRT and prevention of recurrent CRT | |
|---|---|---|---|
| DGEM (2009)6 | Low-dose oral prophylactic anticoagulant during long-term PN (grade B) | Urokinase/tPA | - |
| ESPEN (2009)7 | Once-daily LMWH 100 IU/kg in high-risk patients on long-term PNa (grade C) | Removal of the catheter if infected, malpositioned or obstructed (grade B); local/systemic urokinase/tPA for acute symptomatic CRT within 24 h from symptom onset (grade C) | Long-term LMWH or LMWH followed by VKA for subacute symptomatic CRT (grade C); LMWH are preferred (grade C) |
| AuSPEN (2008)5 | - | Low-dose tPA within 3–4 days of symptom onset. Stenting of the partially occluded superior vena cava to enable reinsertion of a CVC (consensus) | In case of CRT resolution, the catheter can remain in place and anticoagulation therapy considered. Long-term warfarin is preferred (consensus) |
| SINPE (2002)9 | Low-dose VKA or LMWH during long-term PNb (grade C) | - | - |
| ASPEN (2002)3 | Low-dose anticoagulant during long-term PNb (grade B) | - | - |
| Other guidelines | Primary CRT prevention | Treatment of CRT and prevention of recurrent CRT | |
| ISTH (2014)10 | No routine CRT prophylaxis/heparin flushes (adult cancer patients) | Anticoagulation with LMWH and no catheter removal in cancer patients; removal if infected or malpositioned; anticoagulation for incidental CRT; anticoagulation over thrombolysis (adult cancer patients) | 3–6 months of anticoagulation; LMWH over warfarin; long-term anticoagulation in patients with persistent need of central venous access (adult cancer patients) |
| ACCP (2012)11 | No routine CRT prophylaxis (grade 2C) | No catheter removal if it is functional (grade 2C); if proximal veins are involved, anticoagulation for 3 months (adults; grade 2C) | After catheter removal, anticoagulation for 3 months (adults; grade 2C); if no catheter removal, long-term anticoagulation (adults; grade 1C) |
| ACCP (2012)47,50 | UFH infusion at 0.5 IU/kg for catheter patency over no prophylaxis in neonates (grade 1A) | Catheter removal; either anticoagulation or radiological monitoring; LMWH or UFH followed-by LMWH for 6 weeks-3 months (neonates and children; grade 2C) | If catheter is still in place on completion of therapeutic anticoagulation, prophylactic anticoagulation until catheter is removed (neonates and children; grade 2C) |
| GCPG (2013)13 | No routine CRT prophylaxis in cancer patients | Anticoagulation for 3 months, no catheter removal if it is functional, LMWH preferred (adult cancer patients) | No recommendations on the duration of anticoagulation (adult cancer patients) |
Patients with cancer, chronic inflammatory disease, or family/personal history of idiopathic venous thrombosis.
Long-term PN, no contraindication to receive anticoagulants.
PN: parenteral nutrition; DGEM: Deutsche Gesellschaft für Ernährungsmedizin (German Society for Clinical Nutrition); CRT: catheter-related thrombosis; tPA: tissue plasminogen activator; ESPEN: European Society for Clinical Nutrition and Metabolism; LMWH: low-molecular weight heparin; VKA: vitamin K antagonist; AuSPEN: Australasian Society for Parenteral and Enteral Nutrition; CVC: central venous catheter; SINPE: Società Italiana di Nutrizione Artificiale e Metabolismo (Italian Society of Artificial Nutrition and Metabolism); ASPEN: American Society for Parenteral and Enteral Nutrition; ISTH: International Society on Thrombosis and Haemostasis; ACCP: American College of Chest Physicians; UFH: unfractionated heparin; GCPG: Good Clinical Practices Guidelines; IU: international units.
The aim of this systematic review was to evaluate the efficacy and safety of anticoagulants for preventing and managing catheter-related thromboembolic complications in patients on both short- and long-term parenteral nutrition administered via a central access. Prevalence and quality of anticoagulant treatments, as well as heparin-induced thrombocytopenia, are evaluated as secondary outcomes.
Materials and methods
Identification of studies
We systematically searched MEDLINE (January 1966 to November 23, 2015; via PubMed) and EMBASE (January 1980 to November 23, 2015; via OVID). We developed the search strategy without language restrictions after having selected seminal articles for relevant keywords or assigned MeSH subjects (strategy available in the Supplementary Contents). We supplemented this search by manually reviewing reference lists of retrieved articles, nutrition journal databases, relevant review papers, guidelines/guidance documents, and grey literature. Authors were contacted if there was ambiguity about original data. No review protocol was registered. The present review was conducted in accordance with PRISMA statement methodology (available at https://www.prisma-statement.org).
Study selection and outcome definitions
Two reviewers (SB and JJA) selected the studies in duplicate and the inter-observer agreement (Cohen’s κ) was calculated: disagreements were solved by a third reviewer (MC). We included peer-reviewed papers if they met the following criteria:
- population: in- and outpatients (n≥5) on parenteral nutrition requiring a central vascular access. No age restriction was applied. When patients on parenteral nutrition represented a subgroup of a bigger cohort, we excluded the paper if insufficient data were provided on the subgroup.
- Intervention: use of systemic anticoagulant regimens at any specified dose for primary prevention, secondary prevention or treatment of catheter-related thrombosis compared to lower dosage of anticoagulant or no anticoagulant. Heparin locks and heparin-bonded catheters were not considered systemic anticoagulation.
- Primary outcomes (at least one of the following): (i) efficacy outcomes (rates of objectively confirmed first or recurrent catheter-related thrombosis and/or pulmonary embolism); and (ii) safety outcome (rate of major or clinically significant bleeding), expressed as cumulative incidence and/or incidence rate (number of events/patient-time). Accepted diagnostic tests for catheter-related thrombosis included Doppler or compression ultrasonography, venography, and visual central venous catheter inspection after removal in symptomatic patients. Accepted diagnostic tests for pulmonary embolism included computed tomographic angiography, ventilation/perfusion lung scanning, or autopsy findings. Bleeding events were classified according to the definition in the original papers.
- Secondary outcomes: prevalence of anticoagulant administration for any indications reported in either cross-sectional studies or observational longitudinal studies at baseline; pharmacokinetic and pharmacodynamic analyses, quality of anticoagulant treatment (time-in-therapeutic-range of International Normalised Ratio [INR] in patients receiving vitamin K antagonists); rate of heparin-induced thrombocytopenia.
- Study design: no limitations.
Two reviewers (SB and JJA) retrieved the following data in duplicate: author, country, year of publication, patients’ baseline demographics, study setting, study design, catheter type, sample size, pharmacological regimen, duration of follow-up, rate of primary outcomes, and presence of any of the secondary outcomes.
Assessment of study quality and risk of bias
The risk of bias of interventional studies and the validity of observational studies were independently assessed by two reviewers (SB and JJA).
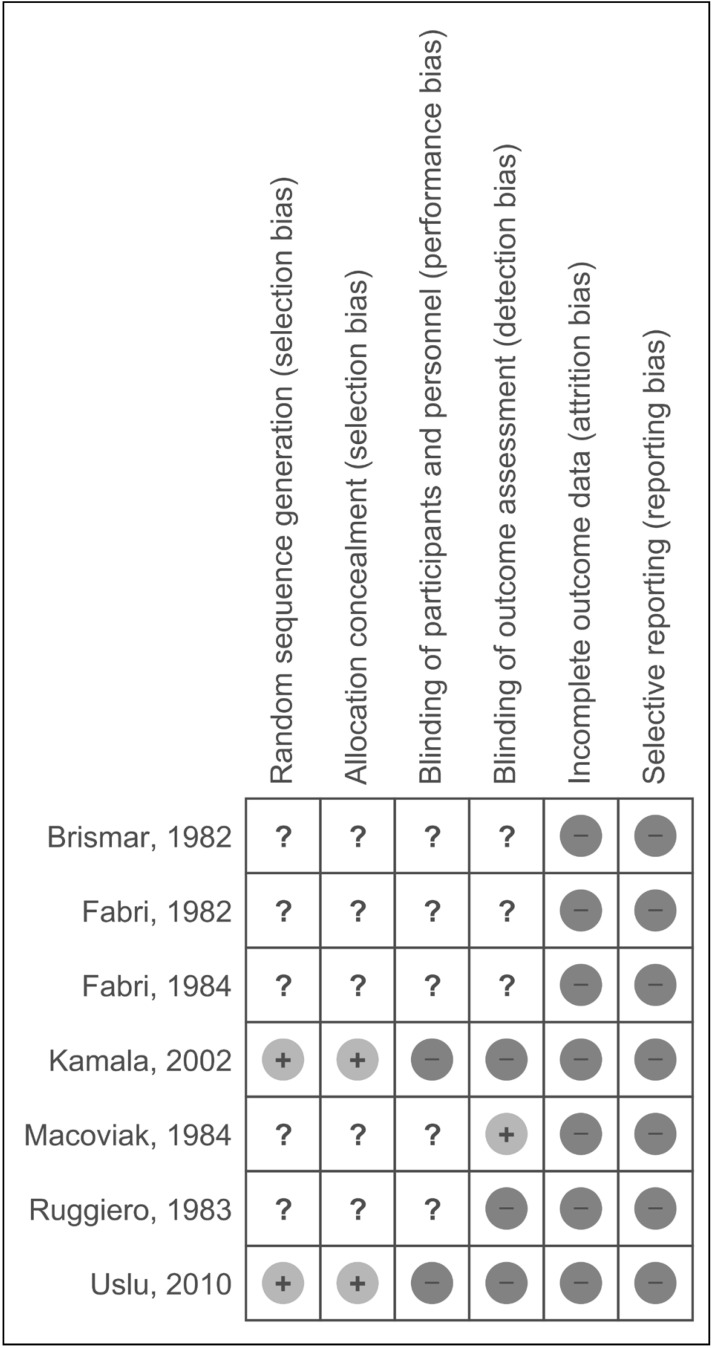
The schemes recommended by the Cochrane Collaboration for Cochrane systematic reviews on interventions served for interventional studies (Figure 1 and Supplementary Figure S2). The assessment comprised a description and a judgment in a “Risk of bias” table, addressing specific features of the study.
Figure 1.
Summary of risk of bias in interventional studies.
The “Risk of bias” figure was drawn on the basis of the indications provided by the Cochrane Collaborations using the model available in RevMan software.
The evaluation of each study is summarised adopting the following symbols: “Low risk” of bias (+), “High risk” of bias (−), or “Unclear risk” of bias (?).
For observational studies, the quality assessment was derived from the Newcastle-Ottawa Scale (www.ohri.ca/programs/clinical_epidemiology/oxford.asp): a “star system” was used to evaluate the selection and comparability of study groups, and the ascertainment of either the exposure or outcome of interest. Studies were considered of high quality standard if they received at least seven stars out of nine. No quality assessment was performed for cross-sectional studies as they were considered only for prevalence of anticoagulant administration.
Statistical methods
We calculated odds ratios and 95% confidence intervals (95% CI) for interventional studies using random-effect Mantel-Haenszel methods. We planned to pool data across intervention studies of adequate quality using the random-effect models due to identifiable reasons for heterogeneity being likely to occur. Heterogeneity of results among studies was tested with the I2 measure, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance (I2 values >50% indicate a substantial level of heterogeneity).
We calculated crude incidence rates and 95% CI for observational studies under the Poisson assumptions on the basis of the available data, if not reported in the original papers: the rates equal the total number of events divided by the population-time-at-risk (patient-year) or catheter life-time-at-risk (catheter-year).
Review Manager 5.0 (Cochrane Collaboration, NL), StatsDirect 2.7.8 (StatsDirect Ltd, Altrincham, UK) and Confidence Interval Analysis 2.0 (Trevor Bryant, University of Southampton, UK) were used for analyses.
Current ongoing studies and grey literature
We made a systematic attempt to search for current ongoing studies on the topic and for the grey literature (abstracts, theses, conferences), exploring the following sites in November 2015: http://www.clinicaltrials.gov, http://www.greylit.org, and http://www.google.com (first 500 results; keywords: “parenteral nutrition” and “anticoagulant”).
Results
Identification and selection of studies
We identified 1,199 studies with our literature search strategy: 392 from MEDLINE and 807 from EMBASE. After first selection on the basis of pre-specified criteria, 31 papers were retrieved in full text and 44 additional studies were obtained from cross-references and relevant reviews/guidelines. After full text evaluation, 23 studies were included and 52 excluded (Supplementary Figure S1). The inter-observer agreement (Cohen’s κ) for the study selection showed an optimal agreement between authors with regard to interventional studies (k=1.00) and a substantial agreement for observational studies (κ=0.80).
Study characteristics
We classified studies into interventional studies (n=7, Table II)14–20 and observational studies (n=16, Supplementary Table SI)21–36. Study populations consisted of adult patients in five interventional14–16,18,19 and ten observational studies22–24,26,27,30,31,33–35, while two observational studies had mixed-age populations25,28. The size of the interventional studies ranged from 34 to 49 adults (total n=206) and from 68 to 239 paediatric patients (total n=307). Observational study sizes ranged from 8 to 1,010 patients.
Table II.
Baseline characteristics of interventional studies in short-term parenteral nutrition.
| Study | Population, n | Venous access | Intervention | Comparator | Outcome | Follow-up |
|---|---|---|---|---|---|---|
| Fabri, 198215 | 24 (adults) | Subclavian CVC | UFH 3,000 IU/L PN solution | No UFH | (a)symptomatic CRT | Not available |
| Brismar, 198214 | 49 (adults) | External jugular CVC | UFH 5,000 IU/6 hours | No UFH | (a)symptomatic CRT | <8 weeks |
| Ruggiero, 198319 | 34 (adults) | Subclavian CVC | UFH 1,000 IU/L PN solution | No UFH | (a)symptomatic CRT | <8 weeks |
| Fabri, 198416 | 40 (adults) | Subclavian CVC | UFH 3,000 IU/L PN solution | No UFH | (a)symptomatic CRT | <8 weeks |
| Macoviak, 198418 | 37 (adults) | Subclavian CVC | UFH 1 IU/mL PN solution | No UFH | (a)symptomatic CRT | <8 weeks |
| Kamala, 200217 | 68 (neonates) | PICC | UFH 1 IU/mL PN solution | No UFH | (a)symptomatic CRT; IVH | <6 weeks |
| Uslu, 201020 | 239 (neonates) | PICC | UFH 0.5 IU/kg/h PN solution | No UFH | (a)symptomatic CRT; IVH | <4 weeks |
All the studies evaluated UFH for primary prevention of asymptomatic CRT. PICC: peripherally inserted central venous catheter; PN: parenteral nutrition; RCT: randomised controlled trial; aPTT: activated partial thromboplastin time; IVH: intraventricular haemorrhage; CVC: central venous catheter; CRT: central venous catheter thrombosis; UFH: unfractionated heparin sodium; DIC: disseminated intravascular coagulation; AC: anticoagulant; ICU: intensive care unit.
At least one primary efficacy or safety outcome was reported in all interventional studies14–20 and in nine out of the 16 observational studies22,24–28,32,35,36.
The types of central venous access varied largely among studies, consistent with the time when the studies were conducted. These are summarised in Table II and Supplementary Table SI, together with the duration of follow-up and patients’ main baseline characteristics. The indications for parenteral nutrition in each study are presented in Supplementary Table SII.
Risk of bias and study quality assessment
All the interventional studies were classified as low quality because of their high or unclear risk of bias14–20 (Figure 1 and Supplementary Figure S2): therefore, no meta-analysis of results was performed. Importantly, the external validity of all the older studies is limited, as some of the described protocols of catheter, anticoagulant and parenteral nutrition management are outdated14–16,18,19,22,25,27,28,32,35. Only one prospective observational study in children29 was classified as a high-quality study according to the Newcastle-Ottawa Scale (7 or more stars) and evaluated one of the secondary outcomes (quality of anticoagulant treatment). Due to their cross-sectional design, the Newcastle-Ottawa Scale could not be applied to three studies21,31,34.
Primary outcomes
Catheter-related thrombosis
Five interventional studies in adults and two in neonates randomised patients on short-term parenteral nutrition to receive either intravenous prophylactic intravenous unfractionated heparin or no anticoagulant14–20. None of the studies demonstrated a statistical difference between groups (Table III). All studies were of low quality and therefore no further meta-analysis was performed.
Table III.
Primary outcomes in interventional studies including patients on short-term parenteral nutrition.
| Study | CRT (UFH group) | CRT (saline) | Odds ratio (95%CI) | Bleeding* (UFH group) | Bleeding* (saline) | Odds ratio (95%CI) |
|---|---|---|---|---|---|---|
| Neonatal populations | ||||||
| Kamala17 | 5/36 (13.9%) | 7/32 (21.9%) | 0.58 (0.16–2.04) | 4/23 (17.4%) | 4/20 (20%) | 0.84 (0.18–3.92) |
| Uslu20 | 2/118 (1.7%) | 5/121 (4.1%) | 0.40 (0.08–2.10) | 21/118 (17.8%) | 23/121 (19%) | 0.92 (0.48–1.78) |
| Adult populations | ||||||
| Brismar14 | 0/23 (0.0%) | 1/26 (3.8%) | 0.36 (0.01–9.32) | - | - | - |
| Fabri15 | 2/24 (8.3%) | 7/22 (31.8%) | 0.19 (0.04–1.17) | - | - | - |
| Ruggiero19 | 0/20 (0.0%) | 0/20 (0.0%) | Not estimable | - | - | - |
| Fabri16 | 0/17 (0.0%) | 0/17 (0.0%) | Not estimable | - | - | - |
| Macoviak18 | 2/17 (11.8%) | 1/20 (5%) | 2.53 (0.21–30.68) | - | - | - |
Bleeding: developing/worsening intraventricular haemorrhage in neonates admitted tot the intensive care unit.
CRT: central venous catheter thrombosis; UFH: unfractionated heparin; 95% CI: 95% confidence interval.
For most of the observational studies, the strategies of warfarin or parenteral nutrition management and the definitions of study outcomes are not comparable to the current standards of care, or the study design had major limitations: therefore, no useful information could be retrieved.
Only one observational study in children36 had a (partly) prospective design and aimed to compare the effect of nadroparin or acenocumarol treatment vs no treatment for the prevention of catheter-related thrombosis (n=32). Anticoagulation was associated with a decrease of catheter-related thrombosis rate from 0.12 events/patient-year (95% CI, 0.06–0.23) to 0.03 events/patient-year (95% CI, 0.001–0.14) over time, improving the cumulative thrombosis-free survival at 5 years (93% vs 48%, p=0.047).
Pulmonary embolism
In one retrospective longitudinal study25, six symptomatic (17.6%) and four asymptomatic pulmonary embolism events (11.8%) were recorded in 32 children on long-term parenteral nutrition. The authors concluded that unfractionated heparin did not prevent pulmonary embolism: however, the data analysis was not reported in the original paper and therefore no conclusions can be drawn25.
Bleeding
Interventional studies in adults did not report bleeding rates14–16,18,19. No difference in developing/worsening of intraventricular haemorrhage was found in two randomised trials comparing intravenous prophylactic unfractionated heparin to saline in neonates (Table III)17,20.
No bleeds were reported in an observational paediatric study (0 events/39 patient-years in the anticoagulated group vs 0/74.2 patient-years in controls)36 and in a retrospective study including adults with acquired immunodeficiency syndrome (0 events/5.2 patient-years in the warfarin group vs 0/19.4 patient-years in controls)26.
Secondary outcomes
Prevalence of anticoagulant administration
The prevalence of patients receiving systemic anticoagulation for any indication was reported in 15 observational studies (Table IV)21–28,30–36. The proportion of patients receiving systemic anticoagulants varied considerably among studies, ranging from 1 to 100% in cohort studies, and from 22 to 42% in cross-sectional studies. A recent multicentre prospective study published in 2015 showed that 41 out of 62 patients on home parenteral nutrition (66%) were receiving various anticoagulant regimens at the start of parenteral nutrition (low-dose vitamin K antagonist 45%; INR 2.0–2.5-adjusted vitamin K antagonist 8%; low molecular weight heparin 13%) with differences among countries24.
Table IV.
Proportion of patients receiving anticoagulation in cohort and cross-sectional studies.
| Study | Population (n) | Type of anticoagulant | Proportion (%) |
|---|---|---|---|
| Ladefoeged, 198128 | Children and adults (70) | UFH, P | 100 |
| Imperial, 198227 | Adults (1,010) | UFH | 98 |
| Bern, 1986a,22 | Adults (23) | UFH flushes | 100 |
| W | 56 | ||
| Schmidt, 198932 | Children and adults (35) | UFH | 100 |
| Dollery, 199425 | Children (34) | UFH | 25 |
| Veerabagu, 199535 | Adults (90) | UFH | 100 |
| W | 49 | ||
| Andrew, 1995b,21 | Children (12) | W | 42 |
| Duerksen, 199626 | Adults (47) | W | 19 |
| Cowl, 200023 | Adults (102) | UFH flushes | 100 |
| Other systemic AC | 1 | ||
| Van Gossum, 2001b,34 | Adults (228) | Therapeutic-dose AC | 26 |
| Prophylactic-dose AC | 12 | ||
| Vegting, 201236 | Children (32) | LMWH or acenocoumarol | 25 |
| Puiggrós, 2012b,31 | Adults (49) | UFH flushes | 69 |
| Systemic AC | 22 | ||
| Olthof, 2014c,30 | Adults (212) | Systemic AC | 53–55 |
| Tourè, 2014c,33 | Adults (196) | VKA or LMWH | 25–26 |
| Cuerda, 201524 | Adults (62) | Low-dose VKA | 45 |
| VKA (INR target 2.0–2.5) | 8 | ||
| LMWH | 13 | ||
| Total | 66 |
Patients receiving other AC regimens were excluded.
Cross-sectional.
Prevalence of AC administration per catheter.
UFH: unfractionated heparin; P: phenprocoumon; W: warfarin; AC: anticoagulant, LMWH: low-molecular-weight heparin; VKA: vitamin K antagonist; INR: International Normalised Ratio.
Pharmacological studies and quality of anticoagulant treatment
A prospective observational study in eight children on home parenteral nutrition29 analysed the mean dose of warfarin required to achieve a target INR of either 2.0 to 3.0 (0.33 mg/kg/day; range: 0.125–0.65 mg/kg/day) or 1.3 to 2.0 (0.26 mg/kg/day; range: 0.16–0.37 mg/kg/day). The percentage of INR tests in which the target therapeutic range was achieved was 51.9% in the first group (INR target: 2.0–3.0) and 69.4% in the second group (INR target: 1.3–2.0). The median duration of treatment was 817 days (range, 186–1,025 days) and INR was monitored every 6.6 days on average.
Heparin-induced thrombocytopenia
No studies evaluated the proportion of patients developing heparin-induced thrombocytopenia during parenteral nutrition. To our knowledge, only five cases of medium-high probability heparin-induced thrombocytopenia have been described37–41.
Current ongoing studies and grey literature
The grey literature did not provide further data and we did not identify any protocols of ongoing interventional studies on anticoagulants. A crossover phase I study has just concluded the enrolment of patients with short bowel syndrome treated with at least 3 consecutive months of parenteral nutrition and exposed to the direct oral anticoagulants dabigatran etexilate and rivaroxaban (NTR4192).
Discussion
Our results indicate that there is a crucial knowledge gap regarding the efficacy, safety and feasibility of anticoagulation for the prevention (and treatment) of catheter-related thrombosis in adults and children on parenteral nutrition. Interventional studies conducted in adults on short-term parenteral nutrition are of low quality and cannot provide adequate evidence (Figure 1 and Supplementary Figure S2). Two recent interventional studies indicate a non-significant protective effect of prophylactic unfractionated heparin in neonates on short-term parenteral nutrition17,20, but their quality with respect to this outcome precludes any objective interpretation (Figure 1 and Supplementary Figure S2). We were not able to retrieve any interventional studies evaluating patients on long-term parenteral nutrition or patients with acute catheter-related thrombosis. Since up to 22–66% of patients on parenteral nutrition receive systemic anticoagulation, the subject of the present review represents an urgent and relevant issue to address24,30,31,33,34.
The dramatic improvement in the management of parenteral nutrition observed over the past 20 years involved catheter size, type and technique for placement, characteristics of parenteral nutrition solutions, and strategies for preventing infectious complications. These improvements are probably the main factors reducing the risk of catheter-related thrombosis, contributing to a decrease in its rate of almost two orders of magnitude (from about 0.2–1.0 events of catheter-related thrombosis per patient-year in 1980–199514–16,18,19,22,27,28,35,42 to 0.01–0.04 events per patient-year in recent studies24,36,43). For this reason, the results presented in older papers cannot be considered valid and applicable anymore: vice versa, evolving strategies have drastically improved the survival of patients requiring parenteral nutrition and will, it is to be hoped, contribute to reduce the heterogeneity among studies.
Parenteral nutrition-focused guidelines on catheter-related thrombosis have been published between 2002 and 2009 (Table I), but are mostly based on papers published at least 20 years ago. Given the lack of evidence in this setting, studies and evidence-based guidelines on anticoagulants for catheter-related thrombosis prevention in other (non-parenteral nutrition) adult and children populations might be considered (Table I). In adult cancer patients, both heparins and vitamin K antagonists vs placebo or no anticoagulant might prevent only asymptomatic catheter-related thrombosis44 and it is not clear whether this corresponds to an overall clinical benefit. In neonates, prophylactic intravenous unfractionated heparin (0.5 IU/kg/hour) did not significantly alter the risk of thrombosis of peripherally inserted central catheters, but was associated with a prolonged duration of catheter patency45. Only one single study reported imprecise effects of low-molecular-weight heparin on the risk of catheter-related thrombosis in children with central venous catheters46, while no effects of systemic anticoagulation on the risk of (a)symptomatic thromboembolic events were demonstrated in paediatric cancer patients with a tunnelled catheter47.
Interventional studies on PN patients did not report bleeding rates, which appeared to be low in two observational studies26,36. No data are available on heparin-induced thrombocytopenia. In the absence of adequate data on harm and demonstrated efficacy, routine primary thromboprophylaxis does not seem justified. Updates are urgently required and there is a strong need for multicentre, prospective, interventional studies involving both parenteral nutrition experts and coagulation specialists. Future studies need to include bleeding events as study outcomes, as well as less frequent complications, including pulmonary embolism, vena cava syndrome, and heparin-associated complications.
There are several issues regarding the pharmacokinetics and pharmacodynamics of anticoagulants during parenteral nutrition, especially when patients with short bowel syndrome receive oral compounds. Erratic vitamin K antagonist and vitamin K absorption, bacterial overgrowth, vitamin K supplementation contributed from fat emulsion in the feeding solution, interfering concomitant medications and hepatic impairment are important factors complicating the management of vitamin K antagonists42. The adverse effects of long-term intravenous unfractionated heparin include osteoporosis and the formation of precipitates with lipids2, while the use of low-molecular-weight heparin in patients on parenteral nutrition is not recommended in those with concomitant severe renal function and the subcutaneous route of administration could reduce patients’ compliance. Two recent reports of four patients with short bowel syndrome treated with rivaroxaban suggest that this direct oral anticoagulant could represent a therapeutic alternative in selected patients48,49. A randomised, crossover phase I study has just concluded enrolment of patients with short bowel syndrome exposed to the direct oral anticoagulants dabigatran etexilate and rivaroxaban (NTR4192).
Conclusions
There is insufficient evidence to allow conclusions to be drawn regarding the efficacy, safety, and feasibility of anticoagulant treatment used to prevent (and treat) catheter-related thrombosis50 in subjects on short- and long-term parenteral nutrition. Well-designed studies are urgently needed.
Supplementary Contents
Footnotes
Authorship contributions
SB: conception and design of the study, acquisition, analysis and interpretation of data, drafting of the manuscript, and statistical analysis; JJA: acquisition and interpretation of data, critical revision of the manuscript, final approval; MC, MJS, SM: interpretation of data, critical revision of the manuscript, final approval.
Disclosure of conflicts of interest
SB received an educational travel grant from Daiichi Sankyo; his work has been supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503) since August 01, 2015, and by the University of Pavia (Italy) before May 15, 2015. JJA has no relevant conflicts to disclose. MC received consulting and lecturing fees from Boehringer Ingelheim, Daiichi Sankyo, the alliance of Bristol-Myers Squibb and Pfizer and Sanquin Blood Supply, and research support from Boehringer Ingelheim and Sanquin Blood Supply. MJS received consulting fees from Fresenius Kabi and research support from Baxter and TEFA Mediq. SM received consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Daiichi-Sankyo, and research support from Glaxo SmithKline, Aspen, Bristol-Meyers Squibb/Pfizer and Sanquin Blood Supply. The present work was not funded.
References
- 1.Dudley J, Rogers R, Sealy L. Renal consequences of parenteral nutrition. Pediatr Nephrol. 2014;29:375–85. doi: 10.1007/s00467-013-2469-9. [DOI] [PubMed] [Google Scholar]
- 2.Staun M, Pironi L, Bozzetti F, et al. ESPEN Guidelines on Parenteral Nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr. 2009;28:467–79. doi: 10.1016/j.clnu.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26:1SA–138SA. [PubMed] [Google Scholar]
- 4.Dreesen M, Foulon V, Vanhaecht K, et al. Guidelines recommendations on care of adult patients receiving home parenteral nutrition: a systematic review of global practices. Clin Nutr. 2012;31:602–8. doi: 10.1016/j.clnu.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Gillanders L, Angstmann K, Ball P, et al. AuSPEN clinical practice guideline for home parenteral nutrition patients in Australia and New Zealand. Nutrition. 2008;24:998–1012. doi: 10.1016/j.nut.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Koletzko B, Jauch KW, Verwied-Jorky S, et al. Guidelines on Parenteral Nutrition from the German Society for Nutritional Medicine (DGEM) - overview. Ger Med Sci. 2009;7 doi: 10.3205/000086. Doc27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittiruti M, Hamilton H, Biffi R, et al. ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications) Clin Nutr. 2009;28:365–77. doi: 10.1016/j.clnu.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 8.National Collaborating Centre for Acute Care-NICE Clincial Guideline. Nutrition Support for Adults Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. 2006. [Accessed on 05/02/2016]. Available at: https://www.nice.org.uk/guidance/cg32/resources/guidance-nutrition-support-in-adults-pdf. [PubMed]
- 9.SINPE. [Complications of central venous access for parenteral nutrition]. Riv Ital Nutr Parenter Enterale. 2002;S5:29–33. [In Italian.] [Google Scholar]
- 10.Zwicker JI, Connolly G, Carrier M, et al. Catheter-associated deep vein thrombosis of the upper extremity in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:796–800. doi: 10.1111/jth.12527. [DOI] [PubMed] [Google Scholar]
- 11.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11:71–80. doi: 10.1111/jth.12071. [DOI] [PubMed] [Google Scholar]
- 14.Brismar B, Hardstedt C, Jacobson S, et al. Reduction of catheter-associated thrombosis in parenteral nutrition by intravenous heparin therapy. Arch Surg. 1982;117:1196–9. doi: 10.1001/archsurg.1982.01380330054013. [DOI] [PubMed] [Google Scholar]
- 15.Fabri PJ, Mirtallo JM, Ruberg RL, et al. Incidence and prevention of thrombosis of the subclavian vein during total parenteral nutrition. Surg Gynecol Obstet. 1982;155:238–40. [PubMed] [Google Scholar]
- 16.Fabri PJ, Mirtallo JM, Ebbert ML, et al. Clinical effect of nonthrombotic total parenteral nutrition catheters. JPEN J Parenter Enteral Nutr. 1984;8:705–7. doi: 10.1177/0148607184008006705. [DOI] [PubMed] [Google Scholar]
- 17.Kamala F, Boo NY, Cheah FC, Birinder K. Randomized controlled trial of heparin for prevention of blockage of peripherally inserted central catheters in neonates. Acta Paediatr. 2002;91:1350–6. doi: 10.1111/j.1651-2227.2002.tb02833.x. [DOI] [PubMed] [Google Scholar]
- 18.Macoviak JA, Melnik G, McLean G, et al. The effect of low-dose heparin on the prevention of venous thrombosis in patients receiving short-term parenteral nutrition. Curr Surg. 1984;41:98–100. [PubMed] [Google Scholar]
- 19.Ruggiero RP, Aisenstein TJ. Central catheter fibrin sleeve--heparin effect. JPEN J Parenter Enteral Nutr. 1983;7:270–3. doi: 10.1177/0148607183007003270. [DOI] [PubMed] [Google Scholar]
- 20.Uslu S, Ozdemir H, Comert S, et al. The effect of low-dose heparin on maintaining peripherally inserted percutaneous central venous catheters in neonates. J Perinatol. 2010;30:794–9. doi: 10.1038/jp.2010.46. [DOI] [PubMed] [Google Scholar]
- 21.Andrew M, Marzinotto V, Pencharz P, et al. A cross-sectional study of catheter-related thrombosis in children receiving total parenteral nutrition at home. J Pediatr. 1995;126:358–63. doi: 10.1016/s0022-3476(95)70448-5. [DOI] [PubMed] [Google Scholar]
- 22.Bern MM, Bothe A, Bistrian B, et al. Prophylaxis against central vein thrombosis with low-dose warfarin. Surgery. 1986;99:216–21. [PubMed] [Google Scholar]
- 23.Cowl CT, Weinstock JV, Al-Jurf A, et al. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin Nutr. 2000;19:237–43. doi: 10.1054/clnu.2000.0103. [DOI] [PubMed] [Google Scholar]
- 24.Cuerda C, Joly F, Corcos O, et al. Prospective study of catheter-related central vein thrombosis in home parenteral nutrition patients with benign disease using serial venous Doppler ultrasound. Clin Nutr. 2016;35:153–7. doi: 10.1016/j.clnu.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Dollery CM, Sullivan ID, Bauraind O, et al. Thrombosis and embolism in long-term central venous access for parenteral nutrition. Lancet. 1994;344:1043–5. doi: 10.1016/s0140-6736(94)91707-8. [DOI] [PubMed] [Google Scholar]
- 26.Duerksen DR, Ahmad A, Doweiko J, et al. Risk of symptomatic central venous thrombotic complications in AIDS patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr. 1996;20:302–5. doi: 10.1177/0148607196020004302. [DOI] [PubMed] [Google Scholar]
- 27.Imperial J, Bistrian BR, Bothe A, et al. Limitation of central vein thrombosis in total parenteral nutrition by continuous infusion of low-dose heparin. J Am Coll Nutr. 1983;2:63–73. doi: 10.1080/07315724.1983.10719910. [DOI] [PubMed] [Google Scholar]
- 28.Ladefoged K, Efsen F, Krogh CJ, Jarnum S. Long-term parenteral nutrition. II. Catheter-related complications. Scand J Gastroenterol. 1981;16:913–9. doi: 10.3109/00365528109181822. [DOI] [PubMed] [Google Scholar]
- 29.Newall F, Barnes C, Savoia H, et al. Warfarin therapy in children who require long-term total parenteral nutrition. Pediatrics. 2003;112:e386. doi: 10.1542/peds.112.5.e386. [DOI] [PubMed] [Google Scholar]
- 30.Olthof ED, Versleijen MW, Huisman-de WG, et al. Taurolidine lock is superior to heparin lock in the prevention of catheter related bloodstream infections and occlusions. PLoS One. 2014;9:e111216. doi: 10.1371/journal.pone.0111216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puiggros C, Cuerda C, Virgili N, et al. [Catheter occlusion and venous thrombosis prevention and incidence in adult home parenteral nutrition (HPN) programme patients]. Nutr Hosp. 2012;27:256–61. doi: 10.1590/S0212-16112012000100033. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 32.Schmidt-Sommerfeld E, Snyder G, Rossi TM, Lebenthal E. Catheter-related complications in 35 children and adolescents with gastrointestinal disease on home parenteral nutrition. JPEN J Parenter Enteral Nutr. 1990;14:148–51. doi: 10.1177/0148607190014002148. [DOI] [PubMed] [Google Scholar]
- 33.Toure A, Duchamp A, Peraldi C, et al. A comparative study of peripherally-inserted and Broviac catheter complications in home parenteral nutrition patients. Clin Nutr. 2015;34:49–52. doi: 10.1016/j.clnu.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Van Gossum A, Vahedi K, Abdel M, et al. Clinical, social and rehabilitation status of long-term home parenteral nutrition patients: results of a European multicentre survey. Clin Nutr. 2001;20:205–10. doi: 10.1054/clnu.2000.0380. [DOI] [PubMed] [Google Scholar]
- 35.Veerabagu MP, Tuttle-Newhall J, Maliakkal R, et al. Warfarin and reduced central venous thrombosis in home total parenteral nutrition patients. Nutrition. 1995;11:142–4. [PubMed] [Google Scholar]
- 36.Vegting IL, Tabbers MM, Benninga MA, et al. Prophylactic anticoagulation decreases catheter-related thrombosis and occlusion in children with home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2012;36:456–62. doi: 10.1177/0148607111416482. [DOI] [PubMed] [Google Scholar]
- 37.Bigsby E, Chaudhuri A, Whiteway A. Mesenteric and coeliac occlusions following heparin-induced thrombocytopenia after aortobiprofunda bypass surgery. Ann R Coll Surg Engl. 2010;92:W15–7. doi: 10.1308/147870810X476629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun F, Platz KP, Faendrich F, et al. Management of venous access problems before and after intestinal transplantation: case reports. Transplant Proc. 2004;36:392–3. doi: 10.1016/j.transproceed.2004.01.083. [DOI] [PubMed] [Google Scholar]
- 39.Lee E, Lee JO, Lim Y, et al. Thrombocytopenia caused by low-dose heparin supplementation of parenteral nutrition solution. Blood Res. 2013;48:160–3. doi: 10.5045/br.2013.48.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranze O, Ranze P, Magnani HN, Greinacher A. Heparin-induced thrombocytopenia in paediatric patients--a review of the literature and a new case treated with danaparoid sodium. Eur J Pediatr. 1999;158:S130–3. doi: 10.1007/pl00014338. [DOI] [PubMed] [Google Scholar]
- 41.Ranze O, Rakow A, Ranze P, et al. Low-dose danaparoid sodium catheter flushes in an intensive care infant suffering from heparin-induced thrombocytopenia. Pediatr Crit Care Med. 2001;2:175–7. doi: 10.1097/00130478-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Kearns PJ, Jr, O’Reilly RA. Bioavailability of warfarin in a patient with severe short bowel syndrome. JPEN J Parenter Enteral Nutr. 1986;10:100–1. doi: 10.1177/0148607186010001100. [DOI] [PubMed] [Google Scholar]
- 43.Cotogni P, Barbero C, Garrino C, et al. Peripherally inserted central catheters in non-hospitalized cancer patients: 5-year results of a prospective study. Support Care Cancer. 2015;23:403–9. doi: 10.1007/s00520-014-2387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akl EA, Ramly EP, Kahale LA, et al. Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst Rev. 2014;7:CD006468. doi: 10.1002/14651858.CD006468.pub5. [DOI] [PubMed] [Google Scholar]
- 45.Shah PS, Shah VS. Continuous heparin infusion to prevent thrombosis and catheter occlusion in neonates with peripherally placed percutaneous central venous catheters. Cochrane Database Syst Rev. 2008;2:CD002772. doi: 10.1002/14651858.CD002772.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandao LR, Shah N, Shah PS. Low molecular weight heparin for prevention of central venous catheterization-related thrombosis in children. Cochrane Database Syst Rev. 2014;3:CD005982. doi: 10.1002/14651858.CD005982.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Schoot RA, Kremer LC, van de Wetering MD, van Ommen CH. Systemic treatments for the prevention of venous thrombo-embolic events in paediatric cancer patients with tunnelled central venous catheters. Cochrane Database Syst Rev. 2013;9:CD009160. doi: 10.1002/14651858.CD009160.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christensen LD, Vinter-Jensen L, Rasmussen HH, et al. Rivaroxaban as anticoagulant therapy in short bowel syndrome. Report of three cases. Thromb Res. 2015;135:568–70. doi: 10.1016/j.thromres.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Douros A, Schlemm L, Bolbrinker J, et al. Insufficient anticoagulation with dabigatran in a patient with short bowel syndrome. Thromb Haemost. 2014;112:419–20. doi: 10.1160/TH14-02-0104. [DOI] [PubMed] [Google Scholar]
- 50.Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S–801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.