Abstract
Dye-sensitized photo-electrochemical cells (DS-PECs) for water splitting hold promises for the large-scale storage of solar energy in the form of (solar) fuels, owing to the low cost and ease to process of their constitutive photoelectrode materials. The efficiency of such systems ultimately depends on our capacity to promote unidirectional light-driven electron transfer from the electrode substrate to a catalytic moiety. We report here on the first noble-metal free and covalent dye-catalyst assembly able to achieve photo-electrochemical visible light-driven H2 evolution in mildly acidic aqueous conditions when grafted onto p-type NiO electrode substrate.
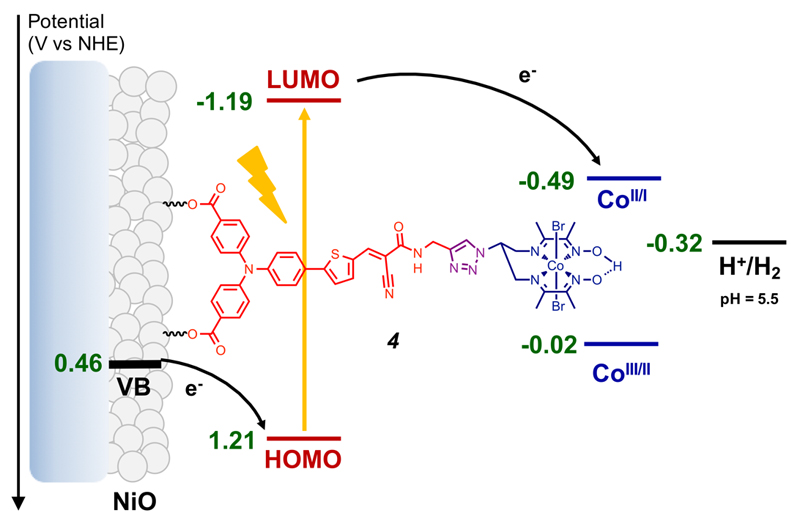
Photosynthesis has inspired for many years the development of water splitting dye-sensitized photo-electrochemical cells (DS-PECs) for hydrogen production.1–3 A key step has been achieved very recently with the report of the first fully operative tandem DS-PEC.4 In such devices, limitation currently arises from the photocathode performances. Therefore different architectures based on the co-grafting4,5 of catalyst and dye onto nickel oxide (NiO), layer-by-layer6 or supramolecular linkage of the catalyst to a grafted dye7 have been investigated. NiO is a p-type transparent conducting oxide specifically suitable for fast hole injection from the highest occupied molecular orbital (HOMO) of the excited dye.8 Then H2 evolution requires that the photogenerated electron is efficiently and rapidly transferred to a catalyst. In that perspect, push-pull organic dyes appear as particularly attractive since they combine large absorptivity in the visible spectrum and spatial charge separation in the excited state that limits the undesired recombination reaction from the reduced dye to the NiO electrode.9,10 The push-pull architecture is also instrumental to foster unilateral electron transfer through direct connection of the acceptor moiety, where the lowest unoccupied molecular orbital (LUMO) is centered and the photogenerated electron located, to the catalyst. We report here the first example of a covalent dye-catalyst molecular assembly integrated in an operative photoelectrode for H2 evolution (Figure 1).
Figure 1.
Energy diagram and working principle of the p-type photocathode based on 4 at pH 5.5. The NiO valence band edge potential at this pH value was estimated from the 0.37V vs NHE value determined at pH 7 in ref. 17.
We previously reported that, upon grafting on NiO, an easily affordable push-pull dye based on a triarylamine electron-donor part and an ethyl cyanoacetate electron-acceptor part separated by a thiophene unit generates large photocurrents in the presence of an irreversible electron acceptor in mildly acidic aqueous solution (pH 4-5).11 Cobalt diimine-dioxime complexes are proven catalysts for H2 evolution at low overvoltage.12,13 When grafted onto electrode surfaces, they display sustained activity in pH = 4.5 aqueous solution14 and tolerance to oxygen.15 These features make them particularly attractive for incorporation into dye-sensitized H2-evolving photoelectrodes. To prepare a covalent dye-catalyst assembly, we first synthesized a terminal alkyne derivative (1, Figure 2) of the above-mentioned dye (Scheme S1). We then used Cu-catalyzed azide-alkyne cycloaddition (Cu-AAC) click chemistry to couple 1 with the copper diimine-dioxime complex 2Cu using conditions previously reported by us.16 Metal exchange readily occurs through reaction with CoCl2 in acetone; air bubbling displaced the equilibrium of this reaction towards the stable and inert CoIII derivative.
Figure 2.
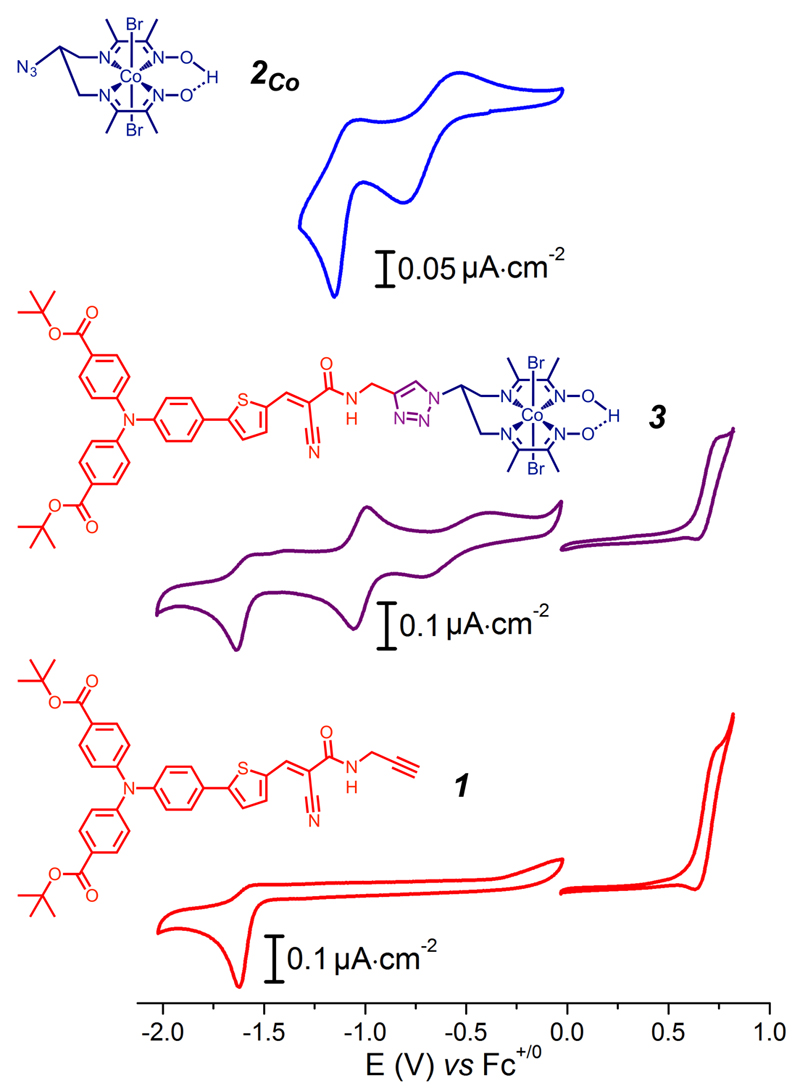
Cyclic voltammograms of 3 (middle) compared with those of 2Co (top) and of the dye precursor 1 (bottom). Measurements were carried out at 100 mV.s-1 at a glassy carbon electrode (reductive scans) or a Pt electrode (oxidative scans) in DMF (0.1 M nBu4NPF6).
After purification through silica-gel flash chromatography and precipitation in NaBr-saturated aqueous solution, compound 3 was obtained and its identity was confirmed by 1H and 13C NMR spectroscopy, high resolution electro-spray ionization mass spectrometry (HR ESI-MS) and elemental analysis (see the Supporting Information).
The cyclic voltammogram (CV) of 3 in DMF displays four events (Figure 2, middle). In the anodic regime, a reversible wave is observed at 0.68 V vs Fc+/0. We assigned this wave to the oxidation of the triarylamine moiety, by comparison with the electrochemical signature of the dye precursor 1 (Figure 2, bottom).18 In the cathodic window, the CV of 3 features a first wave at −0.55 V vs Fc+/0 and a second reversible wave at −1.02 V vs Fc+/0, assigned to the CoIII/II and CoII/I couples, respectively, by analogy with the cobalt diimine-dioxime compound 2Co (Figure 2, top). An irreversible cathodic wave monitored at Ep = −1.64 V vs Fc+/0 (Figure 2, purple line) is assigned to a reduction of the electron accepting group on the dye (Figure 2, red line).18,19 The UV-visible spectrum of 3 (Figure S2) corresponds to the sum of the spectra of 2Co and 1. Compound 3 displays two CT processes characterized by bands at 349 and 431 nm (ε= 33 600 and 31 800 M−1∙cm−1, respectively), the second one corresponding to the HOMO-LUMO transition on the dye unit. The retention of the electrochemical and UV-visible signatures of 2Co and 1 building blocks indicates electronic decoupling of light-harvesting and electrocatalytic components in the covalent dyad 3. Upon excitation at 430 nm, emission of 3 is observed with maximum intensity at 608 nm (figure S3). Comparison with the spectrum of 1 reveals 30% fluorescence quenching, suggesting electronic transfer from the excited push-pull moiety to the Co center.
From these data, we could estimate an E0-0 value of 2.40 eV leading to a redox potential of −1.72 V vs Fc+/0 for the 3*/3+ couple (3* is the excited state of 3). Generation of the CoI state is thus thermodynamically feasible from either 3* (oxidative quenching) or 3− (resulting from reductive quenching of 3* by a sacrificial electron donor or from hole injection from 3* into NiO valence band), with driving forces of 0.70 and 0.62 eV, respectively.
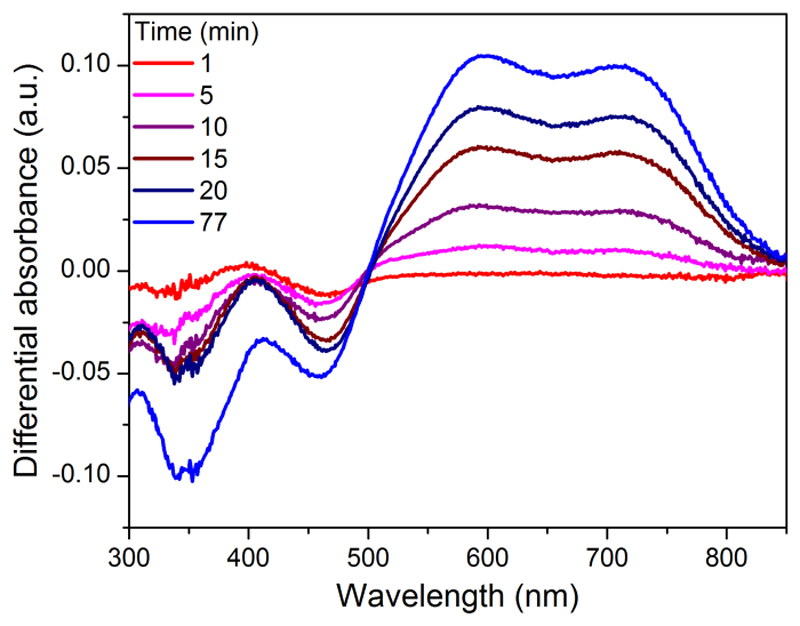
Photolysis of 3 in CH3CN in the presence of triethanolamine (TEOA) as electron donor was monitored by UV-visible spectroscopy. Upon irradiation with visible light, the CoI species builds up within minutes as evidenced by the appearance of the characteristic two-band spectrum with maxima at 595 and 710 nm (Figure 3).20
Figure 3.
UV-visible monitoring (differential spectra) of the formation of the CoI derivative of 3 (1.8 10-5 M) in CH3CN/TEOA (90:10 v/v) upon visible light irradiation.
Reaction of 3 with TFA for 4 hours resulted in the quantitative hydrolysis of tert-butyl carboxylic esters and isolation of 4 (see the Supporting Information for characterization of 4). Then, F108-templated21,22 1 µm-thick NiO electrodes were prepared and sensitized through soaking in a CH3CN solution of 4 for 24 hours. Chenodeoxycholic acid (CDCA) is a classical co-adsorbent utilized in dye-sensitized solar cells (DSSCs) to improve photovoltaic performance.23,24 We therefore used the typical 4/1 CDCA:dyemolar ratio to avoid aggregation of molecules of 4 on the NiO surface.24 The presence of the CoIII centers at the surface of the electrode was confirmed by XPS analysis (Figure S4). From the UV-visible absorption spectra (Figure S5), we estimated 8.2 and 7.2 ±0.1 nmol∙cm–2 values for surface concentration of 4 immobilized in the absence and in the presence of CDCA, respectively. Such values are similar to those previously measured for 1 on similar electrodes.11 The presence of CDCA as co-adsorbent only slightly impedes the grafting of 4 onto NiO.
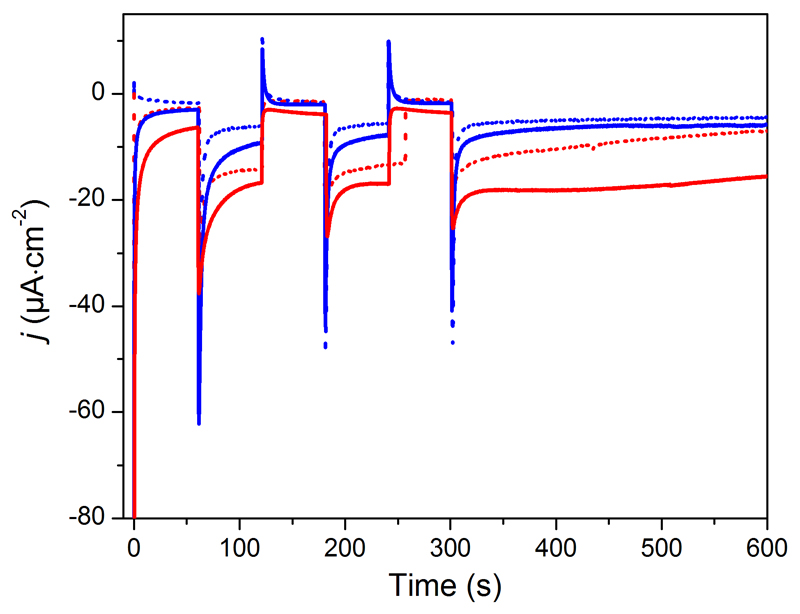
The resulting molecular photocathodes were assessed for photo-electrochemical H2 evolution in a fully aqueous pH 5.5 2-(N-morpholino)ethanesulfonic acid (MES) buffer. They develop cathodic photocurrents starting from ca 0.93 V vs RHE (Figure S6) and sustained over the scanned potential range. Two broad processes are observed in the dark around 0.83 V and 0.61 V vs RHE and we assigned them to capacitive charging of the NiO film.25 Chronoamperometric measurements at more negative potentials (0.54, 0.34 and 0.14 V vs RHE) clearly show steady-state cathodic photocurrents (Figure 4, S7 and Table S1). Control experiments on pristine and 1/CDCA co-sensitized NiO electrodes (Figure S7) confirm that the photocurrent is enhanced in the presence of the cobalt diimine-dioxime H2-evolving catalyst. We therefore ascribe such photocurrents to electron transfer from the NiO valence band to the cobalt diimine-dioxime moiety in 4 via the light-promoted excited state of the photosensitizer unit. Such a process is similar to the one monitored under homogeneous conditions (Figure 3) but with the NiO electrode acting as the electron supplier instead of TEOA. Eventually, the electrons are used by the cobalt diimine-dioxime moiety to reduce protons into H2. An energy-level diagram of the resulting NiO photocathode sensitized with 4 is shown in Figure 1. The magnitude of the photocurrent proved superior at the most cathodic potential (0.14 V vs RHE) with a current density of ca –15 µA·cm-2. We posit that this trend is a result from recombination pathways with the NiO surface slowed down at less positive potentials, as proposed by Hammarström and co-workers.26 The presence of CDCA as co-adsorbent does not modify the initial photocurrent value but significantly improves its stability during continuous irradiation.27
Figure 4.
Chopped-light chronoamperometric measurements at 4-sensitized NiO electrodes poised at 0.14 (red traces) and 0.54 V (blue traces) vs RHE in the absence (dotted) and in the presence of CDCA (plain) under visible-light irradiation (400-800 nm, 65 mW∙cm–2, 1 sun) in pH 5.5 MES 0.1 M/NaCl 0.1 M supporting electrolyte.
Photo-electrochemical H2 generation was confirmed by long-term electrolysis at 0.14 V vs RHE under visible-light irradiation (1 sun). During the course of the photoelectrolysis, the magnitude of the photocurrent displayed by 4/CDCA co-sensitized NiO electrodes slowly decreases from 15 to 6 µA·cm-2 (Table S1). Gas chromatographic analysis of the headspace confirmed the evolution of hydrogen during the chronoamperometric measurements with 8-10% faradaic yields reproducibly and independently measured after 2 and 3 hours. Such values are conservative, as they do not take into account H2 present in solution. Importantly, no H2 was detected when pristine and 1/CDCA co-sensitized NiO electrodes were measured under similar conditions, despite the passage of 23 and 40 mC.cm-2, respectively. To understand this behavior, we recorded the XPS spectra of the photoelectrode at the Ni 2p energy level before and after 2h photo-electrochemical H2 evolution (Figure S8). The 2p3/2 core region spectrum of the as-prepared electrode contains signals assigned to NiO (853.8 eV and satellite at 862.7 eV) and Ni(OH)2/NiOOH (855.6 eV).28,29 After photo-electrocatalysis, the principal peak corresponding to NiO shifts to 853.5 eV. This 0.3 eV shift indicates increased amounts of Ni0 (binding energy of nickel metal 2p3/2 core region is reported at 852.7-853.1 eV).28,29 A slight shift of the other peaks is also observed, which can be assigned to a change in the balance between NiIII and NiII ions as previously observed in a similar context.30 Formation of metallic nickel and change of redox balance within the NiO film26,30 provide reasonable explanations for the low faradaic yield for H2 evolution measured in this work, although we cannot exclude that this also derives from reductive degradation31 of the dye-catalyst assembly. We also note that the faradaic yields for H2 evolution reported in previous works describing NiO-based photoelectrodes in aqueous media are generally far from unity,4,6,7 which suggests that formation of Ni0 may be quite frequent in this context. Further studies are urgently required to understand this process and develop strategies limiting its extent.
In conclusion, we designed the first covalent and noble metal free dye-catalyst dyad and immobilized it on benchmark F108-templated NiO electrodes. The resulting photocathode based on a cobalt diimine-dioxime catalyst proved functional for light-driven H2 evolution from fully aqueous solution. We found that co-grafting of CDCA, a classical co-adsorbent in DSSCs but introduced here for the first time in DS-PEC research, has a strong positive influence on the photocurrent stability. In addition, post-mortem XPS analysis revealed that competitive reduction of the bulk NiO electrode substrate decreases the faradaic yield for H2 evolution. These observations open new horizons for gaining deeper understanding of surface confined light-driven electron transfer processes and overcoming the current performance limitations of DS-PEC photocathodes.
Supporting Information
Synthesis procedures, experimental details and additional spectroscopic and photo-electrochemical data. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This work was supported by the French National Research Agency (Labex program, ARCANE, ANR-11-LABX-0003-01) and the European Research Council under the European Union’s Seventh Framework Program (FP/2007-2013)/ERC Grant Agreement n.306398. The authors thanks J.-F. Lefebvre (DPM, Grenoble) and D. Imbert (INAC, Grenoble) for 500 MHz NMR and fluorescence measurements, respectively.
Footnotes
Notes
The authors declare no competing financial interests.
References
- (1).Ashford DL, Gish MK, Vannucci AK, Brennaman MK, Templeton JL, Papanikolas JM, Meyer TJ. Chem Rev. 2015;115:13006–49. doi: 10.1021/acs.chemrev.5b00229. [DOI] [PubMed] [Google Scholar]
- (2).Yu Z, Li F, Sun L. Energy Environ Sci. 2015;8:760–775. [Google Scholar]
- (3).Queyriaux N, Kaeffer N, Morozan A, Chavarot-Kerlidou M, Artero V. J Photochem Photobiol C. 2015;25:90–105. [Google Scholar]
- (4).Li F, Fan K, Xu B, Gabrielsson E, Daniel Q, Li L, Sun L. J Am Chem Soc. 2015;137:9153–9159. doi: 10.1021/jacs.5b04856. [DOI] [PubMed] [Google Scholar]
- (5).Fan K, Li F, Wang L, Daniel Q, Gabrielsson E, Sun L. Phys Chem Chem Phys. 2014;16:25234–25240. doi: 10.1039/c4cp04489d. [DOI] [PubMed] [Google Scholar]
- (6).Gross MA, Creissen CE, Orchard KL, Reisner E. Chem Sci. 2016;7:5537–5546. doi: 10.1039/c6sc00715e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ji Z, He M, Huang Z, Ozkan U, Wu Y. J Am Chem Soc. 2013;135:11696–11699. doi: 10.1021/ja404525e. [DOI] [PubMed] [Google Scholar]
- (8).Wood CJ, Summers GH, Clark CA, Kaeffer N, Braeutigam M, Carbone LR, D'Amario L, Fan K, Farre Y, Narbey S, Oswald F, et al. Phys Chem Chem Phys. 2016;18:10727–10738. doi: 10.1039/c5cp05326a. [DOI] [PubMed] [Google Scholar]
- (9).Qin P, Zhu HJ, Edvinsson T, Boschloo G, Hagfeldt A, Sun LC. J Am Chem Soc. 2008;130:17629–17629. doi: 10.1021/ja8001474. [DOI] [PubMed] [Google Scholar]
- (10).Mishra A, Fischer MKR, Bauerle P. Angew Chem Int Ed. 2009;48:2474–2499. doi: 10.1002/anie.200804709. [DOI] [PubMed] [Google Scholar]
- (11).Massin J, Bräutigam M, Kaeffer N, Queyriaux N, Field MJ, Schacher FH, Popp J, Chavarot-Kerlidou M, Dietzek B, Artero V. Interface Focus. 2015;5:20140083. doi: 10.1098/rsfs.2014.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kaeffer N, Chavarot-Kerlidou M, Artero V. Acc Chem Res. 2015;48:1286–1295. doi: 10.1021/acs.accounts.5b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Jacques P-A, Artero V, Pécaut J, Fontecave M. Proc Natl Acad Sci USA. 2009;106:20627–20632. doi: 10.1073/pnas.0907775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Andreiadis ES, Jacques PA, Tran PD, Leyris A, Chavarot-Kerlidou M, Jousselme B, Matheron M, Pecaut J, Palacin S, Fontecave M, Artero V. Nat Chem. 2013;5:48–53. doi: 10.1038/nchem.1481. [DOI] [PubMed] [Google Scholar]
- (15).Kaeffer N, Morozan A, Artero V. J Phys Chem B. 2015;119:13707–13. doi: 10.1021/acs.jpcb.5b03136. [DOI] [PubMed] [Google Scholar]
- (16).Queyriaux N, Andreiadis ES, Torelli S, Pécaut J, Veldkamp B, Margulies E, Wasielewski M, Chavarot-Kerlidou M, Artero V. submitted for publication. [DOI] [PMC free article] [PubMed]
- (17).Natu G, Hasin P, Huang Z, Ji Z, He M, Wu Y. ACS Appl Mater Interfaces. 2012;4:5922–9. doi: 10.1021/am301565j. [DOI] [PubMed] [Google Scholar]
- (18).Ji Z, Natu G, Huang Z, Wu Y. Energy Environ Sci. 2011;4:2818–2821. [Google Scholar]
- (19).Yen Y-S, Chen W-T, Hsu C-Y, Chou H-H, Lin JT, Yeh M-CP. Org Lett. 2011;13:4930–4933. doi: 10.1021/ol202014x. [DOI] [PubMed] [Google Scholar]
- (20).Smolentsev G, Cecconi B, Guda A, Chavarot-Kerlidou M, van Bokhoven JA, Nachtegaal M, Artero V. Chem Eur J. 2015;43:15158–15162. doi: 10.1002/chem.201502900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Li L, Gibson EA, Qin P, Boschloo G, Gorlov M, Hagfeldt A, Sun LC. Adv Mater. 2010;22:1759–1762. doi: 10.1002/adma.200903151. [DOI] [PubMed] [Google Scholar]
- (22).Sumikura S, Mori S, Shimizu S, Usami H, Suzuki E. J Photochem Photobiol A. 2008;199:1–7. [Google Scholar]
- (23).Ito S, Miura H, Uchida S, Takata M, Sumioka K, Liska P, Comte P, Pechy P, Gratzel M. Chem Commun. 2008:5194–6. doi: 10.1039/b809093a. [DOI] [PubMed] [Google Scholar]
- (24).Favereau L, Warnan J, Pellegrin Y, Blart E, Boujtita M, Jacquemin D, Odobel F. Chem Commun. 2013;49:8018–20. doi: 10.1039/c3cc44232b. [DOI] [PubMed] [Google Scholar]
- (25).Dini D, Halpin Y, Vos JG, Gibson EA. Coord Chem Rev. 2015;304–305:179–201. [Google Scholar]
- (26).D'Amario L, Antila LJ, Pettersson Rimgard B, Boschloo G, Hammarstrom L. J Phys Chem Lett. 2015;6:779–83. doi: 10.1021/acs.jpclett.5b00048. [DOI] [PubMed] [Google Scholar]
- (27).Addition of CDCA slightly impacts the dark current values
- (28).Hüfner S, editor. Photoelectron Spectroscopy: Principles and Applications. Springer; Berlin Heidelberg: 1996. [Google Scholar]
- (29).Renaud A, Chavillon B, Cario L, Pleux LL, Szuwarski N, Pellegrin Y, Blart E, Gautron E, Odobel F, Jobic S. J Phys Chem C. 2013;117:22478–22483. [Google Scholar]
- (30).Sahara G, Abe R, Higashi M, Morikawa T, Maeda K, Ueda Y, Ishitani O. Chem Commun. 2015;51:10722–10725. doi: 10.1039/c5cc02403j. [DOI] [PubMed] [Google Scholar]
- (31).Kaeffer N, Morozan A, Fize J, Martinez E, Guetaz L, Artero V. ACS Catalysis. 2016;6:3727–3737. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.