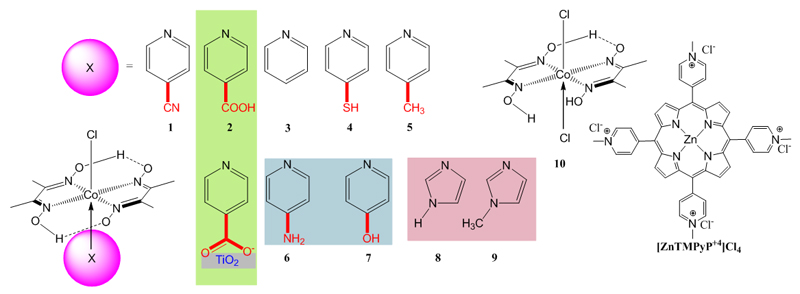
Abstract
We report on the first systematic study of cobaloxime-based hydrogen photoproduction in mixed pH 7 aqueous/acetonitrile solutions and demonstrate that H2 evolution can be tuned through electronic modifications of the axial cobalt ligand or through introduction of TiO2 nanoparticles. The photocatalytic systems consist of various cobaloxime catalysts [Co(dmgH)2(L)Cl] (L = nitrogen-based axial ligands) and a water soluble porphyrin photosensitizer. They were assayed in the presence of triethanolamine as sacrificial electron donor. Optimal turnover numbers related to photosensitizer are obtained with electron-rich axial ligands such as imidazole derivatives (1131 TONs with N-methyl imidazole). Lower stabilities are observed with various pyridine axial ligands (443 TONs for para-methylpyridine), especially for those containing electron-acceptor substituents. Interestingly, when L is the para-carboxylatopyridine the activity of the system is increased from 40 to 223 TONs in the presence of TiO2 nanoparticles
Keywords: Porphyrins, Metallo-porphyrins, Hydrogen production, Photocatalysis, Cobaloxime
Introduction
The development and optimization of methods allowing storing renewable energy sources such as sunlight is a major challenge to tackle both problems of decreasing stocks of fossil fuels and accumulating CO2 greenhouse gas in the atmosphere.1 A fairly promising path for the efficient conversion of solar energy into fuels, so-called solar fuels,2 is to develop efficient photocatalytic systems for hydrogen (H2) production from water,3, which constitutes a great endeavor of artificial photosynthesis. Typical photocatalytic systems contain distinct photosensitizer and catalyst, the former achieving light harvesting and initial charge separation. The system is generally assessed in the presence of a sacrificial electron donor, which allows decoupling the optimization of reductive and oxidative sides of water splitting. Great challenges in the field of photocatalytic hydrogen production include the development of systems based on earth-abundant materials together with the enhancement of their activity and durability.4, 5 Bioinspiration is a key feature in the design of such molecular systems. Water soluble porphyrins and metallated porphyrins, due to their similarity to natural photosynthetic dyes, are excellent candidates for efficient absorption of photons and charge separation in this context.6, 7 In addition cobaloximes gained great attention in the past ten years and have been used as catalysts in photocatalytic systems because of their low-cost, high activity and straightforward synthesis.8–30
Recently our group reported an homogeneous system involving a porphyrin sensitizer and a cobaloxime catalyst [Co(dmgH)2pyCl] (dmgH2 = dimethylglyoxime) for photoinduced hydrogen evolution from water.31 Upon continuous visible irradiation of mixed 50/50 acetonitrile/H2O solutions hydrogen was produced with the best result obtained at pH 7 with a TON of 320 with respect to the porphyrin after 35 h.9, 10, 12, 32 In this study, we use same conditions to examine the influence of different axial ligands on the cobaloxime catalyst.
Results and Discussion
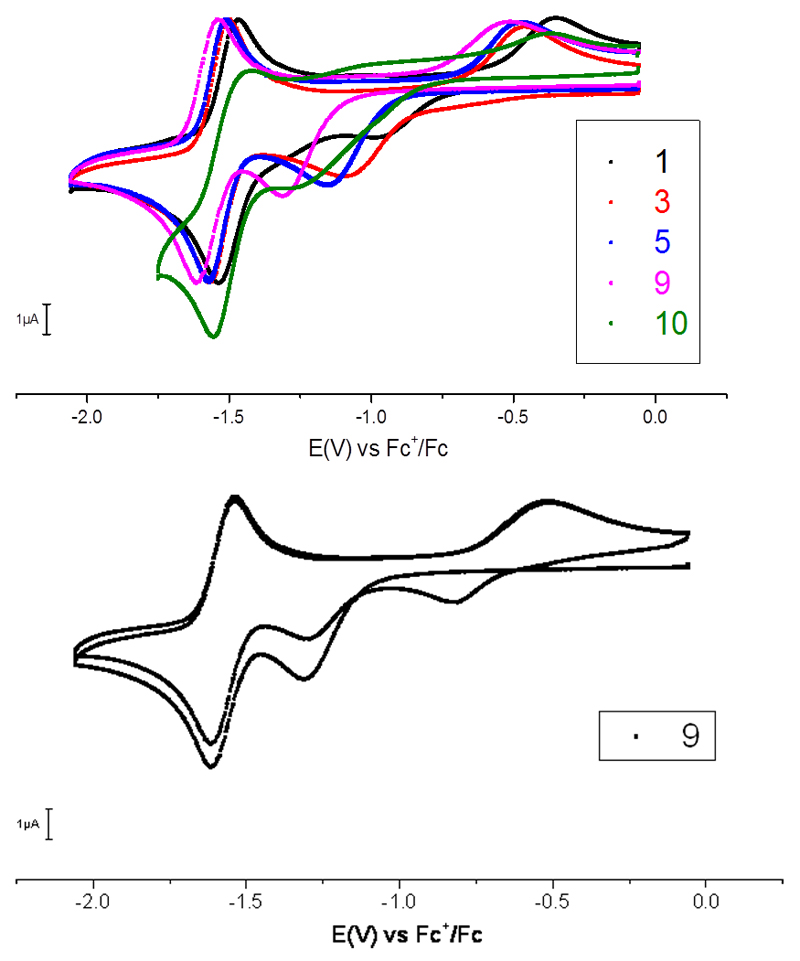
A series of cobalt bisdimethylglyoximato complexes [CoIII(dmgH)2(L)Cl] (1-10) with different pyridine and imidazole axial ligands (Figure 1) were synthesized according to the literature procedure by dissolving CoCl2∙6H2O, 2 equivalents of dimethylglyoxime and 1 equivalent of NaOH in 95% ethanol at 70°C.33 Then, 1 equivalent of the appropriate ligand was added. All complexes were isolated in excellent yields after work-up (see Experimental Section for details). Compound [Co(dmgH)(dmgH2)Cl2] (10) was prepared and used as reference.34 Cyclic voltammograms (CVs) were recorded in DMF and displayed an irreversible reduction process assigned to the Co(III)/Co(II) transition and a reversible wave assigned to the Co(II)/Co(I) process. All cobalt complexes show similar behaviors (Table 1). Figure 2 (top) shows cyclic voltammograms of selected compounds in the series. We first note that the potential of the reversible Co(II)/Co(I) process depends on the nature of the axial N ligand. Secondly, on the return scan a novel Co(II) to Co(III) process is observed, the position of which also depends on the nature of the axial ligand. In both cases, a more electron-donating (respectively electron-withdrawing) substituent of the pyridine axial ligand results in a cathodic (respectively anodic) shift of the potential of the redox process. Collectively, these data indicate that the chloride ligand is reductively eliminated at the Co(II) state and that the N-ligand remains coordinated in the Co(II) state and probably also in the Co(I) state since the wave is electrochemically reversible. Multiple scan measurements indicate that the chloride ligand only slowly rebinds to the Co(III) center (Figure 2, bottom).
Figure 1.
Structures of meso-tetrakis (1-methylpyridinium-4-yl) porphyrin chloride ([ZnTMPyP4+]Cl4) and of the series of cobaloxime with different axial ligands 1-10 used in this study.
Table 1.
IR spectral data for catalysts, pKa52 of corresponding axial ligands in water, and redox potentials of their Co(III)/Co(II) (irrev) and Co(II)/Co(I) (rev.) processes.
| Catalyst | pKa | v̄ Co-Naxial (cm–1) | v Co-Cl (cm–1) | E (V vs Fc+/Fc) |
|
|---|---|---|---|---|---|
| Epc Co(III)/Co(II) | (Epc+Epa)/2 Co(II)/Co(I) |
||||
| 1 | 1.90 | 435 | 428 | –0.98 | –1.50 |
| 2 | 4.18 | 441 | 428 | –1.11 | –1.55 |
| 3 | 5.22 | 455 | 420 | –1.10 | –1.53 |
| 42 | 5.30 | 480 | 419 | –1.091 | –1.59 |
| 5 | 6.02 | 483 | 418 | –1.15 | –1.54 |
| 63 | 9.17 | 515 | 416 | –1.08 | –1.61 |
| 72 | 11.0 | 507 | 409 | –1.17 | –1.55 (–1.65)4 |
| 8 | 6.90 | 495 | 413 | –1.26 | –1.58 |
| 9 | 7.40 | 503 | 412 | –1.31 | –1.57 |
| 10 | - | 414 | –1.29 | –1.52 | |
additional processes in this region assigned to thiol/ disulfide transition
low solubilty
low electrochemical response despite good solubility
two reversible processes indicating the presence of isomeric species.
Figure 2.
top: Cyclic voltammograms of selected cobaloxime complexes (1mM, normalized with regards to the Co(II)/Co(I) signal of 3) recorded in DMF (+ 0.1 mol.L–1 n-Bu4NBF4) at glassy carbon electrode (scan rate 100 mV.s–1); bottom: first two cycles recorded under similar conditions as above with cobaloxime complex 9.
Compounds 1-10 were characterized using FTIR spectroscopy. Table 1 gathers the wavenumbers corresponding to the Co-Naxial and Co-Cl stretching vibrations.35 It appears clearly that the Co-Cl bond strength decreases when the Co-Naxial bond strength increases, following an increase of the basicity of the axial N-ligand.
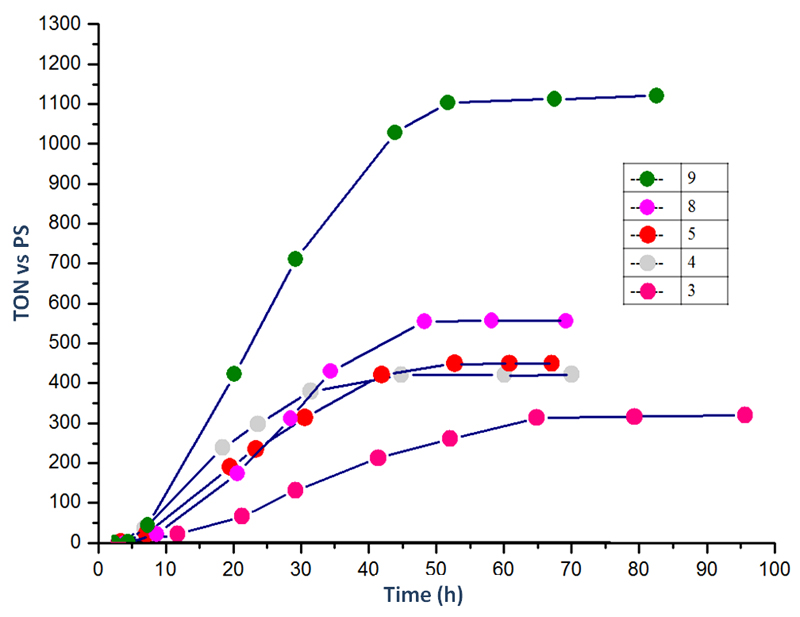
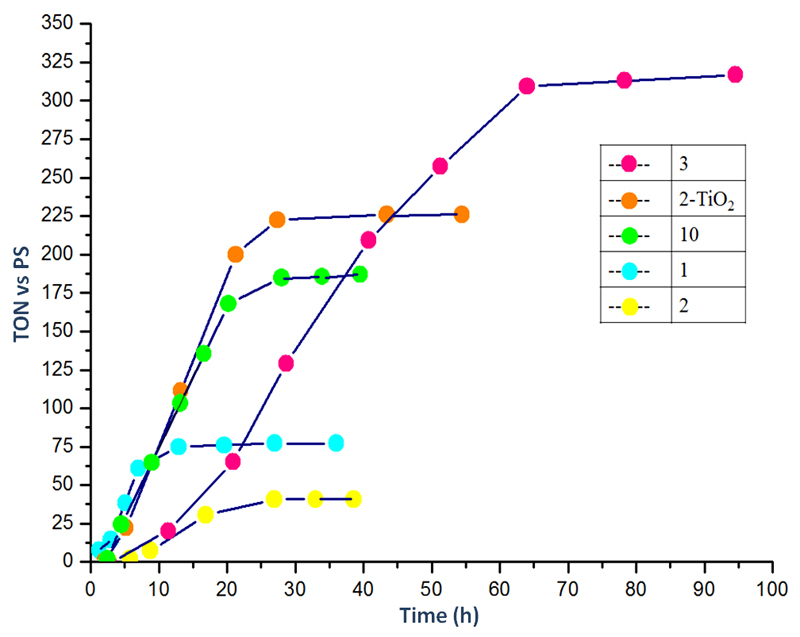
Photocatalytic hydrogen production using 1-10 as catalysts, zinc meso-tetrakis (1-methylpyridinium-4-yl) porphyrin chloride [ZnTMPyP4+]Cl4 as photosensitizer (PS) and triethanolamine (TEOA) as sacrificial donor was measured as a function of time. All experiments were carried out under similar conditions as in our previously published work but with a slightly different setup (detailed conditions are provided in Experimental Section).31 The pH was adjusted to 7 through addition of aqueous HCl. We note that, under such conditions, 10 is likely deprotonated with consequent detachment of (at least) one axial chloride ligand and ligation of neutral solvent molecule(s). Therefore this compound displays a H+-annulated structure similar to other cobaloximes. The activity for each photocatalytic system is measured in turnover number versus photosensitizer (TON), which evolves as a function of time. The results are gathered in Figures 3 and 4. Two photocatalytic behaviors can be distinguished. First cobaloximes containing imidazole or pyridine ligand with donor substituents prove highly active and stable. By contrast, cobaloximes without pyridine ligands (for example with two chloride ligands) or with electron-withdrawing group-substituted pyridine ligands display significantly lower stability. Another difference is seen in the time-profile of H2 evolution: while the less stable systems immediately produce H2 on irradiation, the more stable systems display an S-shaped activity curve with lower initial rates of H2 photoproduction.
Figure 3.
Comparison of photocatalytic H2 production of 3 with catalysts 4, 5, 8 and 9. Conditions used are listed in Table 2 and experimental section.
Figure 4.
Comparison of photocatalytic H2 production of 3 with catalysts 1, 2, and 10. Conditions used are listed in Table 2 and experimental section.
N-methyl imidazole-containing catalyst 9 proved to be the most stable one of the cobaloximes with 1135 turnovers achieved for H2 production after 50 hours of irradiation. Moreover, catalyst 8, containing imidazole axial ligand, exhibits a total TON of 565 under similar conditions. Using pyridine ligands with electron donating groups as axial ligands also yield highly effective catalysts 4-7 exceeding the activity of 3 (TON = 320, Figure 3). Such an activity can be rationalized on the basis of the basicity of these N-based aromatic ligands, resulting in various electron-donor ability, after binding to cobalt. The higher pKa of imidazole derivatives (Table 2) correlates well with the higher stability of the corresponding systems compared to pyridine-based ones. In addition, the performances of systems using 3-5 follow their pKa values: the activity is increased following the order 5 (pKa = 6.02) > 4 (pKa=5.30) > 3 (pKa =5.22).
Table 2.
Photocatalytic Hydrogen Production Using Different Cobaloximes as the Catalysts
| Catalyst | pKa | TON | TOF (h-1) | Time for reaching plateau (h) |
|---|---|---|---|---|
| 1 | 1.90 | 77 | 10 | 11 |
| 2 | 4.18 | 40 | 2 | 26 |
| 2-TiO2 | - | 223 | 9 | 29 |
| 3 | 5.22 | 320 | 8 | 63 |
| 4 | 5.30 | 425 | 12 | 43 |
| 5 | 6.02 | 443 | 9 | 40 |
| 6 | 9.17 | - | - | - |
| 7 | 11.0 | - | - | - |
| 8 | 6.90 | 565 | 11 | 50 |
| 9 | 7.40 | 1135 | 23 | 50 |
| 10 | - | 290 | 10 | 23 |
Difference for the TON with the previously work31 is due to the use of a slightly different setup for the photocatalytic process (a new reactor with 12 positions has been built). Therefore, the irradiation conditions are not exactly the same.
Conditions: Hydrogen production upon irradiation (λ>440nm) of solution (Acetonitrile-10% aqueous solution of TEOA (1:1 v/v) adjusted to pH=7 using HClconc. containing PS (4.0 × 10-5M) and the respective catalyst (4.9 × 10-4 M). The ratio PS / catalyst is 0.082.
Pyridine ligands with electron withdrawing groups and therefore lower pKa values form less effective catalysts 1-2 (Figure 3) as compared to 3. Because of its higher pKa value 2 is expected to produce more H2 than 1. This is however not the case, maybe due to the formation of carboxylate species under the conditions used. Addition of TiO2 nanoparticles into the solution allows the attachment of the catalysts at the surface of the particles26 and the catalytic performance of 2 is improved by a five-fold factor. Upon irradiation of the system up to 223 turnovers of H2 obtained after 29 hours of irradiation. We posit that binding of the carboxylate group of the pyridine ligand to TiO2 forms a mixed organic-inorganic ester26 with lower electron-withdrawing property but we cannot exclude the fact that TiO2 particles partake in catalysis as electron reservoirs as previously demonstrated by Reisner, Durrant and coworkers.28, 36 We note that cobaloxime 6 proves inactive under such conditions. This can be attributed to the amine being protonated at pH=7 (pKa = 9.12) therefore resulting in a strong electron-withdrawing ammonium group.37 The absence of catalytic activity observed with 4-hydroxypyridine derivative (7), may be due to the formation of a 4-pyridone tautomer.38 The above results are in agreement with literature data reported by us18 and by Reisner and coworkers.39 Both studies indeed demonstrated enhanced electrochemical H2 evolution activity for cobaloximes with electron-enriched axial ligands.
Systems containing catalysts 1, 10 and 2-TiO2 rapidly produce H2 immediately upon irradiation, but H2 evolution stops after 10 h. By contrast, the other series of catalysts, achieving higher turnover number, display a significantly lower initial rate for H2 production than the second series. The highest TON is also achieved after a longer irradiation time (~50 hours).
The efficiency and stability of a homogeneous photochemical system for H2 evolution is actually controlled by four main processes: (i) light harvesting, charge separation and generation of a reducing agent; (ii) catalyst reductive activation; (iii) H2 evolution catalysis and (iv) deactivation processes. Under the conditions used, the efficiency of (i), as well as the rate of a possible deactivation of the photosensitizer are likely constant. This is quite different for process (ii). In our previous work involving the same porphyrin photosensitizer,31 we used transient absorption measurements to show that that the triplet excited state of the porphyrin PS* contributes to H2 production. However, the potential of this state can be estimated to –0.45 V vs NHE40 so that only electron transfer to Co(III) generating the Co(II) species is thermodynamically possible. The formation of the Co(I) state of the catalyst can therefore only be possible from the reduced porphyrin photosensitizer PS– (E° ~ –0.85 V vs NHE 40), generated through reductive quenching by TEOA. The catalytic mechanism for H2 production mediated by cobaloximes indeed involves formation of Co(III)-hydride intermediates by protonation of Co(I). Next, Co(III)-hydrides are reduced into Co(II)-hydrides through electron transfer either from the photosensitizer or from Co(I) species.10, 41–43 H2 is then formed through protonation of these Co(II)-H species regenerating the cobaloxime in the Co(II) state.
Comparing redox potentials measured in distinct solvents is always challenging. In the present case, we use a mixture of water and acetonitrile in the presence of a high concentration of TEOA so that extrapolations are almost impossible. It seems however from the comparison of E0(PS–/PS) and E0(CoII/CoI) that the driving force for the formation of Co(I) from PS– is quite small. Under such conditions, small variations of the redox potential of the Co(II)/Co(I) process due to the influence of the axial ligand can control the reduction activation process (ii) and explain the large difference in reactivity observed within the series. In particular, the lower initial rates for H2 evolution observed with the catalysts containing the stronger axial ligands correspond to a lower driving force for electron transfer from the reduced photosensitizer to the Co(II) catalyst.
In addition, the nature of the axial ligand alters the properties of the intermediate Co(III)-hydride derivative, both through a modification of the Co(III)-H/Co(II)-H redox potential and through a modification of the nucleophilicity of the Co(II)-H derivative.18, 44–46 In particular, as said above, electron-donating axial ligands enhance the rate of H2 evolution catalysis (process iii).18, 39 This effect is however not observed here since (ii) is the rate determining process. We note that this behavior contrasts with that described for a related photocatalytic diimine-dioxime cobalt system, in which the introduction of an electron-withdrawing axial ligand capable of stabilizing low oxidation states was observed to slow down the rate of photocatalytic hydrogen evolution.47, 48 In that system, the potential of the Co(II)/Co(I) couple is however more positive than for cobaloxime so that process (iii), not (ii), is rate-determining. Consequently, the electron-withdrawing phosphine ligand decreases the rate of H2 evolution catalysis and initial rates for light-driven H2 evolution.
Factors governing the stability against degradation (process iv) of reduced cobaloxime derivatives, including hydridocobaloximes are still unclear. We recently showed using time-resolved X-ray absorption spectroscopy that, in some cases, Co(I) species that accumulate under photocatalytic conditions22 are not catalytically active. The mechanism for such degradation is still under investigation, although it may be attributed to hydride transfer from the metal center to the ligand, competing catalytic H2 evolution.49, 50 In that perspect, the fastest catalysts are less prone to degradation. The more nucleophilic hydride species indeed preferably reacts with protons, rather than migrating to the ligand, causing deactivation. This results in increased total turnover number, as observed with the stronger axial ligands in this study.39 Such a rational however does not stand for the diimine-dioxime cobalt system,47, 48 in which stabilization of the low valent redox states by electron-withdrawing phosphine ligand is accompanied by an increase of the total turnover number. While more studies are needed to fully understand the reactivity of intermediate species in this popular, though complex, series of catalysts, it seems clear that electron-rich ligands positioned in trans position to the hydride enhance its electron density and therefore its nucleophilicity/hydridicity, favoring protonation and H2 evolution over hydride transfer and resulting in higher stability of the catalytic system.
Conclusions
In this study we have for the first time investigated different cobaloximes with various axial ligands as catalysts in photocatalytic hydrogen production. This systematic study shows a subtle influence of axial ligands on the performances of the system, both in terms of initial catalytic rate and overall catalytic stability. Weak axial ligands display rapid initial catalysis but result in low stability. By contrast, stronger axial ligands induce a reduced initial rate, but a better long-term stability. Further studies are still needed to fully understand the nature of the degradative process. In that prospect, the use of various techniques such as time-resolved X-ray absorption spectroscopies20–22 may be very valuable to identify all species at work during catalysis.
Experimental Section
Materials
All chemicals were purchased from the usual commercial sources and used as received.
Spectroscopy
1H NMR spectra were recorded on Bruker AMX-500 MHz and Bruker DPX-300 MHz spectrometers as solutions in deuterated solvents by using the solvent peak as the internal standard. UV-Vis absorption spectra were measured on a Shimadzu UV-1700 spectrophotometer using 10 mm path-length cuvettes. IR spectra were recorded on Bruker IFS 66 spectrometer.
Electrochemistry
Cyclic voltammetry experiments were carried out at room temperature using an SP-300 Bio-Logic potentiostat. All measurements were carried out in DMF with a solute concentration of ca. 1.0 mM in the presence of tetrabutylammonium tetrafluoroborate (0.1 M) as supporting electrolyte. A three-electrode cell setup was used with a glassy carbon working electrode, a silver/silver chloride reference electrode, and a platinum wire as counter electrode. All potentials are reported versus the ferrocene/ferricinium couple added in the electrolyte at the end of each measurement.
Synthesis of the Photosensitizer
The dye [ZnTMPyP]4+Cl-4 (PS) were synthesized according to the literature.51
General synthesis of catalysts 1-10
The general synthesis of cobalt complexes with dimethylglyoxime, [Co(dmgH)2(L)(L’)] is described in the literature.33 CoCl2∙6H2O (500 mg, 2.15 mmol), dimethylglyoxime (551 mg, 4.70 mmol), and NaOH (86.0 mg, 2.15 mmol) were dissolved in 95% ethanol (20 mL) and heated to 70°C. Ligand (L) (2.15 mmol) was then added and the resulting solution cooled to room temperature. A stream of air was then passed through the solution for 30 min, which caused precipitation of a brown solid. The suspension was stirred for 1 h and filtered. The precipitate was successively washed with water (5 mL), ethanol (2 x 5 mL), and diethyl ether (3 x 5 mL). The product was then extracted with acetone. Removal of the solvent from the extracts yielded pure complex.
Synthesis of the [Co(dmgH)2(4-CN-py)Cl] (1)
4-cyano-pyridine used as the axial ligand yielding the desired complex with 87%. Anal. Calcd for C14H18ClCoN6O4: C, 39.22; H, 4.23; N, 19.60. Found: C, 39.14; H, 4.28; N, 19.53. 1H NMR (500 MHz, CDCl3): δ= 18.12 (broad, OH), 8.53 (d, J = 6.7 Hz, 2H), 7.47 (d, J = 6.7 Hz, 2H), 2.41 (s, 12H). 13C NMR (125 MHz, CDCl3): δ=153.27, 152.51, 127.32, 123.73, 114.57, 13.37.
Synthesis of [Co(dmgH)2(4-COOH-py)Cl] (2)
4-carboxy-pyridine used as the axial ligand yielding the desired complex with 58%. Anal. Calcd for C14H19ClCoN5O6: C, 37.56; H, 4.28; N, 15.64. Found: C, 37.44; H, 4.35; N, 15.72. 1H NMR (300 MHz, DMSO): δ=18.44 (s, 2H, OH), 14.14 (s, 1H, OH), 8.21 (d, J = 6.1 Hz, 2H), 7.85 (d, J = 6.0 Hz, 2H), 2.31 (s, 12H). 13C NMR (75 MHz, DMSO): δ= 164.61, 152.73, 151.37, 141.17, 125.17, 12.70.
Synthesis of [Co(dmgH)2(py)Cl] (3)
Pyridine used as the axial ligand yielding the desired complex with 52%. Anal. Calcd for C13H19ClCoN5O4: C, 38.68; H, 4.74; N, 17.35. Found: C, 38.74; H, 4.61; N, 17.30. 1H NMR (300 MHz, CDCl3): δ=18.20 (broad, OH), 8.27 (d, J = 5.4 Hz, 2H), 7.70 (t, J = 7.5 Hz, 1H), 7.22 (d, J = 7.0 Hz, 2H), 2.40 (s, 12H). 13C NMR (75 MHz, CDCl3): δ=152.73, 151.21, 139.03, 125.78, 13.24.
Synthesis of [Co(dmgH)2(4-SH-py)Cl] (4)
4-thiolo-pyridine used as the axial ligand yielding the desired complex with 61%. Anal. Calcd for C13H19ClCoN5O4S: C, 35.83; H, 4.39; N, 16.07. Found: C, 35.93; H, 4.23; N, 16.12. 1H NMR (300 MHz, DMSO): δ= 18.25 (broad, 4H), 14.45 (broad, 1H), 8.12 (d, J = 5.9 Hz, 2H), 7.52 (d, J = 7.1 Hz, 2H), 2.25 (s, 12H). 13C NMR (75 MHz, DMSO): δ=173.88, 150.33, 149.25, 127.36, 12.38.
Synthesis of [Co(dmgH)2(4-CH3-py)Cl] (5)
4-methyl-pyridine used as the axial ligand yielding the desired complex with 89%. Anal. Calcd for C14H21ClCoN5O4: C, 40.25; H, 5.07; N, 16.77. Found: C, 40.32; H, 4.99; N, 16.65. 1H NMR (300 MHz, CDCl3): δ=18.30 (broad, OH), 8.07 (d, J = 6.1 Hz, 2H), 7.01 (d, J = 5.8 Hz, 2H), 2.36 (d, J = 15.1 Hz, 12H), 2.32 (s, 3H). 13C NMR (75 MHz, CDCl3): δ=152.56, 151.47, 150.34, 126.77, 21.00, 13.20.
Synthesis of [Co(dmgH)2(4-NH2-py)Cl] (6)
4-amino-pyridine used as the axial ligand yielding the desired complex with 28%. Anal. Calcd for C13H20ClCoN6O4: C, 37.29; H, 4.81; N, 20.07. Found: C, 37.20; H, 4.94; N, 19.99. 1H NMR (300 MHz, DMSO): δ=19.33 (broad, OH), 7.61 (s, 2H), 6.74 (s, 2H), 6.33 (s, 2H, NH2), 2.47 (s, 12H). 13C NMR (75 MHz, DMSO): δ= 165.53, 152.73, 150.57, 115.11, 12.67.
Synthesis of [Co(dmgH)2(4-OH-py)Cl] (7)
4-hydroxy-pyridine used as the axial ligand yielding the desired complex with 26%. Anal. Calcd for C13H19ClCoN5O5: C, 37.20; H, 4.56; N, 16.69. Found: C, 37.12; H, 4.65; N, 16.65. 1H NMR (300 MHz, DMSO): δ=18.49 (s, 2H, OH), 11.80 (s, 1H, OH), 7.65 (s, 2H), 6.79 (s, 2H), 2.32 (s, 12H). 13C NMR (75 MHz, DMSO): δ=166.60, 152.18, 150.68, 114.17, 12.55.
Synthesis of [Co(dmgH)2(imidazole)Cl] (8)
Imidazole used as the axial ligand yielding the desired complex with 77%. Anal. Calcd for C11H18ClCoN6O4: C, 33.64; H, 4.62; N, 21.40. Found: C, 33.52; H, 4.73; N, 21.30. 1H NMR (300 MHz, DMSO): δ=18.67 (s, 2H, OH), 12.78 (s, 1H), 7.28 (s, 1H), 7.08 (s, 1H), 6.43 (s, 1H), 2.30 (s, 12H). 13C NMR (75 MHz, DMSO): δ=151.40, 136.21, 125.84, 118.98, 12.42.
Synthesis of [Co(dmgH)2(N-methyl-imidazole)Cl] (9)
N-methyl-imidazole used as the axial ligand yielding the desired complex with 78%. Anal. Calcd for C12H20ClCoN6O4: C, 35.44; H, 4.96; N, 20.66. Found: C, 35.36; H, 4.98; N, 20.55. 1H NMR (300 MHz, CDCl3): δ=18.46 (broad, OH), 6.69 (d, J = 15.7 Hz, 2H), 3.60 (s, 3H), 2.38 (s, 12H). 13C NMR (75 MHz, CDCl3): δ=151.93, 137.89, 128.60, 122.05, 35.20, 13.06.
Synthesis of [Co(dmgH2)(dmgH)Cl2] (10)
CoCl2∙6H2O (500 mg, 2.15 mmol) dissolved in acetone (17mL). Dimethylglyoxime (551 mg, 4.70 mmol) was then added and the resulting solution stirred at room temperature for 20 minutes and filtered. The product was then extracted with acetone. Removal of the solvent from the extracts yielded pure 10 with 72%. Anal. Calcd for C8H14Cl2CoN4O4: C, 26.69; H, 3.92; N, 15.56. Found: C, 26.79; H, 3.84; N, 15.42. 1H NMR (300 MHz, DMSO): δ=7.98 (broad, OH), 2.34 (s, 12H). 13C NMR (75 MHz, DMSO): δ=149.60, 12.31.
Hydrogen production measurements
For photoinduced hydrogen evolution, each sample was prepared in a 30 mL Schlenck flask. Prior to sample preparation, an aqueous solution of TEOA (10% vol) was adjusted to pH=7 using conc.HCl. The components were then dissolved in 10 mL of a 1:1 (v/v) mixture of acetonitrile= and the aqueous TEOA (10%) solution. The sample flask was sealed with a Suba-seal septum and degassed by bubbling nitrogen through the solution for 20 min at room temperature. The samples were irradiated with a 500 W Xenon lamp using a cutoff filter designed to remove all light with λ < 440 nm. The amounts of hydrogen evolved were determined by gas chromatography (external standard technique) using a Shimadzu GC-2010 plus chromatograph with a TCD detector and a molecular sieve 5 Å column (30m-0.53 mm). Control experiments were run under the same conditions as the hydrogen evolution experiments with the removal of one of the components of the hydrogen generating samples.
For some control experiments a filter assembly was used consisting of a narrow bandpass filter at 572nm (bandwidth about 10nm FWHM) and a color-glass filter KG5. The color-glass filter transmits almost everything between 330 and 700nm but blocks the deep UV and IR radiation of the lamp. Extra care was taken to prevent stray UV light from reaching the sample by covering the area around the filter assembly with a large black carton.
An excess of mercury (ca 30eq) was added to our in order to investigate the effect of mercury during hydrogen production. More particularly, hydrogen production was not affected by the presence of mercury in the reaction mixture indicating that metallic cobalt particles are not formed as active catalytic species during the experiment. Metallic mercury remains shiny without forming an amalgam.
For the hydrogen evolution experiments the conditions were as follows: Hydrogen production upon irradiation (λ>440nm) of solution (Acetonitrile-TEOAaq (10%, pH 7) (1:1 v/v) containing 1 (4.0 × 10-5M) and the respective catalyst (4.9 × 10-4M). In the case of the experiments containing TiO2 the procedure was the following. A solution of 2 (0.1 μmol in 0.5mL of TEOA, 10% v/v, pH 7) was added to a stirred dispersion of TiO2 (20mg in 1mL TEOA). The mixture was left stirring for 1 h, was then centrifuged, and the filtered, clear supernatant was then analysed by UV-vis spectrophotometry. The amount of 2 adsorbed to TiO2 was quantified by the absorbance difference at 244 nm before and after exposure to TiO2 nanoparticles. The amount adsorbed to TiO2 particles after 1 h was 89 ± 2 %.26
Supplementary Material
Supporting Information: Additional photocatalytic measurements and spectroscopic data.
Acknowledgments
This work was supported by the European Commission's Seventh Framework Programme (FP7/2007-2013) under grant agreement n.° 229927 (FP7-REGPOT-2008-1, Project BIOSOLENUTI) and ERC Grant Agreement n.306398 (Project photocatH2ode), Heraklitos grant from Ministry of Education, Special Research Account of the University of Crete, GSRT and the French National Research Agency (Labex program, ARCANE, ANR-11-LABX-0003-01). The COST Action CM1202 PERSPECT-H2O is also acknowledged for support via a short term scientific mission to France for AP.
References
- 1.Lewis NS, Nocera DG. Proceedings of the National Academy of Sciences. 2006;103:15729–15735. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thapper A, Styring SA, Saracco G, Rutherford AW, Robert B, Magnuson A, Lubitz W, Llobet AA, Kurz P, Holzwarth AR, Fiechter S, et al. Green. 2013;3:43–57. [Google Scholar]
- 3.Cook TR, Dogutan DK, Reece SY, Surendranath Y, Teets TS, Nocera DG. Chemical Reviews. 2010;110:6474–6502. doi: 10.1021/cr100246c. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Sun L. ChemSusChem. 2010;3:551–554. doi: 10.1002/cssc.201000062. [DOI] [PubMed] [Google Scholar]
- 5.Du P, Eisenberg R. Energy & Environmental Science. 2012;5:6012–6021. [Google Scholar]
- 6.Ladomenou K, Natali M, Iengo E, Charalampidis G, Scandola F, Coutsolelos AG. Coordination Chemistry Reviews [Google Scholar]
- 7.Darwent JR, Douglas P, Harriman A, Porter G, Richoux M-C. Coordination Chemistry Reviews. 1982;44:83–126. [Google Scholar]
- 8.Artero V, Chavarot-Kerlidou M, Fontecave M. Angewandte Chemie International Edition. 2011;50:7238–7266. doi: 10.1002/anie.201007987. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Goeb S, Ji Z, Pogulaichenko NA, Castellano FN. Inorganic Chemistry. 2011;50:705–707. doi: 10.1021/ic101731j. [DOI] [PubMed] [Google Scholar]
- 10.Lazarides T, McCormick T, Du P, Luo G, Lindley B, Eisenberg R. Journal of the American Chemical Society. 2009;131:9192–9194. doi: 10.1021/ja903044n. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Wang M, Li C, Li X, Dong J, Sun L. Chemical Communications. 2010;46:8806–8808. doi: 10.1039/c0cc03154b. [DOI] [PubMed] [Google Scholar]
- 12.Du P, Schneider J, Luo G, Brennessel WW, Eisenberg R. Inorganic Chemistry. 2009;48:4952–4962. doi: 10.1021/ic900389z. [DOI] [PubMed] [Google Scholar]
- 13.Guttentag M, Rodenberg A, Bachmann C, Senn A, Hamm P, Alberto R. Dalton Transactions. 2013;42:334–337. doi: 10.1039/c2dt31699d. [DOI] [PubMed] [Google Scholar]
- 14.Peuntinger K, Lazarides T, Dafnomili D, Charalambidis G, Landrou G, Kahnt A, Sabatini RP, McCamant DW, Gryko DT, Coutsolelos AG, Guldi DM. The Journal of Physical Chemistry C. 2012;117:1647–1655. [Google Scholar]
- 15.Fihri A, Artero V, Razavet M, Baffert C, Leibl W, Fontecave M. Angewandte Chemie International Edition. 2008;47:564–567. doi: 10.1002/anie.200702953. [DOI] [PubMed] [Google Scholar]
- 16.Fihri A, Artero V, Pereira A, Fontecave M. Dalton Transactions. 2008:5567–5569. doi: 10.1039/b812605b. [DOI] [PubMed] [Google Scholar]
- 17.Baffert C, Artero V, Fontecave M. Inorganic Chemistry. 2007;46:1817–1824. doi: 10.1021/ic061625m. [DOI] [PubMed] [Google Scholar]
- 18.Razavet M, Artero V, Fontecave M. Inorganic Chemistry. 2005;44:4786–4795. doi: 10.1021/ic050167z. [DOI] [PubMed] [Google Scholar]
- 19.Valdez CN, Dempsey JL, Brunschwig BS, Winkler JR, Gray HB. Proceedings of the National Academy of Sciences. 2012;109:15589–15593. doi: 10.1073/pnas.1118329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolentsev G, Guda A, Zhang X, Haldrup K, Andreiadis ES, Chavarot-Kerlidou M, Canton SE, Nachtegaal M, Artero V, Sundstrom V. The Journal of Physical Chemistry C. 2013;117:17367–17375. doi: 10.1021/jp4010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolentsev G, Guda AA, Janousch M, Frieh C, Jud G, Zamponi F, Chavarot-Kerlidou M, Artero V, van Bokhoven JA, Nachtegaal M. Faraday Discuss. 2014;171:259–273. doi: 10.1039/c4fd00035h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smolentsev G, Cecconi B, Guda A, Chavarot-Kerlidou M, van Bokhoven JA, Nachtegaal M, Artero V. Chemistry – A European Journal. 2015 doi: 10.1002/chem.201502900. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dempsey JL, Brunschwig BS, Winkler JR, Gray HB. Acc Chem Res. 2009;42:1995–2004. doi: 10.1021/ar900253e. [DOI] [PubMed] [Google Scholar]
- 24.Marinescu SC, Winkler JR, Gray HB. Proceedings of the National Academy of Sciences. 2012;109:15127–15131. doi: 10.1073/pnas.1213442109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempsey JL, Winkler JR, Gray HB. Journal of the American Chemical Society. 2010;132:1060–1065. doi: 10.1021/ja9080259. [DOI] [PubMed] [Google Scholar]
- 26.Lakadamyali F, Reisner E. Chemical Communications. 2011;47:1695–1697. doi: 10.1039/c0cc04658b. [DOI] [PubMed] [Google Scholar]
- 27.Lv Y, Willkomm J, Leskes M, Steiner A, King TC, Gan L, Reisner E, Wood PT, Wright DS. Chemistry – A European Journal. 2012;18:11867–11870. doi: 10.1002/chem.201201827. [DOI] [PubMed] [Google Scholar]
- 28.Lakadamyali F, Reynal A, Kato M, Durrant JR, Reisner E. Chemistry – A European Journal. 2012;18:15464–15475. doi: 10.1002/chem.201202149. [DOI] [PubMed] [Google Scholar]
- 29.Natali M, Argazzi R, Chiorboli C, Iengo E, Scandola F. Chemistry – A European Journal. 2013;19:9261–9271. doi: 10.1002/chem.201300133. [DOI] [PubMed] [Google Scholar]
- 30.Natali M, Orlandi M, Chiorboli C, Iengo E, Bertolasi V, Scandola F. Photochemical & Photobiological Sciences. 2013;12:1749–1753. doi: 10.1039/c3pp50184a. [DOI] [PubMed] [Google Scholar]
- 31.Lazarides T, Delor M, Sazanovich IV, McCormick TM, Georgakaki I, Charalambidis G, Weinstein JA, Coutsolelos AG. Chemical Communications. 2013;50:521–523. doi: 10.1039/c3cc45025b. [DOI] [PubMed] [Google Scholar]
- 32.McCormick TM, Calitree BD, Orchard A, Kraut ND, Bright FV, Detty MR, Eisenberg R. J Am Chem Soc. 2010;132:15480–15483. doi: 10.1021/ja1057357. [DOI] [PubMed] [Google Scholar]
- 33.Schrauzer GN, Parshall GW, Wonchoba ER. Inorganic Syntheses. Editon edn. John Wiley & Sons, Inc; 2007. pp. 61–70. [Google Scholar]
- 34.Ramesh P, SubbiahPandi A, Jothi P, Revathi C, Dayalan A. Acta Crystallographica Section E. 2008;64:m300–m301. doi: 10.1107/S1600536807068407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutherford PE, Thornton DA. Spectrochimica Acta Part A: Molecular Spectroscopy. 1979;35:711–714. [Google Scholar]
- 36.Reynal A, Willkomm J, Muresan NM, Lakadamyali F, Planells M, Reisner E, Durrant JR. Chem Commun. 2014;50:12768–12771. doi: 10.1039/c4cc05143b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaljurand I, Kütt A, Sooväli L, Rodima T, Mäemets V, Leito I, Koppel IA. The Journal of Organic Chemistry. 2005;70:1019–1028. doi: 10.1021/jo048252w. [DOI] [PubMed] [Google Scholar]
- 38.Chen MWC, Yeh A, Tsai TYR. Inorg Chim Acta. 1998:81–86. [Google Scholar]
- 39.Wakerley DW, Reisner E. Physical Chemistry Chemical Physics. 2014;16:5739–5746. doi: 10.1039/c4cp00453a. [DOI] [PubMed] [Google Scholar]
- 40.Kalyanasundaram K, Neumann-Spallart M. The Journal of Physical Chemistry. 1982;86:5163–5169. [Google Scholar]
- 41.Muckerman JT, Fujita E. Chem Commun. 2011;47:12456–12458. doi: 10.1039/c1cc15330g. [DOI] [PubMed] [Google Scholar]
- 42.Solis BH, Hammes-Schiffer S. Inorg Chem. 2011;50:11252–11262. doi: 10.1021/ic201842v. [DOI] [PubMed] [Google Scholar]
- 43.Artero V, Chavarot-Kerlidou M, Fontecave M. Angew Chem Int Ed Engl. 2011;50:7238–7266. doi: 10.1002/anie.201007987. [DOI] [PubMed] [Google Scholar]
- 44.Benniston AC. Physical Chemistry Chemical Physics. 2007;9:5739–5747. doi: 10.1039/b708166a. [DOI] [PubMed] [Google Scholar]
- 45.Solis BH, Yu Y, Hammes-Schiffer S. Inorg Chem. 2013;52:6994–6999. doi: 10.1021/ic400490y. [DOI] [PubMed] [Google Scholar]
- 46.Solis BH, Hammes-Schiffer S. Journal of the American Chemical Society. 2011;133:19036–19039. doi: 10.1021/ja208091e. [DOI] [PubMed] [Google Scholar]
- 47.Kaeffer N, Chavarot-Kerlidou M, Artero V. Accounts of Chemical Research. 2015;48:1286–1295. doi: 10.1021/acs.accounts.5b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang P, Jacques P-A, Chavarot-Kerlidou M, Wang M, Sun L, Fontecave M, Artero V. Inorg Chem. 2012;51:2115–2120. doi: 10.1021/ic2019132. [DOI] [PubMed] [Google Scholar]
- 49.Simándi LI, Budó-Záhonyi É, Szeverényi Z. Inorganic and Nuclear Chemistry Letters. 1976;12:237–241. [Google Scholar]
- 50.Simándi LI, Szeverényi Z, Budó-Záhonyi É. Inorganic and Nuclear Chemistry Letters. 1975;11:773–777. [Google Scholar]
- 51.Christensen PA, Harriman A, Porter G, Neta P. Journal of the Chemical Society, Faraday Transactions 2: Molecular and Chemical Physics. 1984;80:1451–1464. [Google Scholar]
- 52.Brown HC, Mihm XR. Journal of the American Chemical Society. 1955;77:1723–1726. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.