Abstract
We report on an infertile male patient with the predominant 46XX female karyotype. A testicular biopsy revealed widely separated testicular tubules, absence of sperm formation and large numbers of Leydig cells. Chromosome studies, including measurements of the X chromosomes, showed a significant difference between the lengths of the short arm of the 2X chromosomes. This information lends support for an X-Y chromosome interchange as the etiology of this syndrome. The clinical features of this rare syndrome and other theories of etiology of XX male subjects are discussed.
The XX male syndrome consists of azoospermia, underdeveloped genitalia, normal male hair pattern and intelligence, short stature and gynecomastia in 33 per cent of the cases reported. The phenotype of the XX male syndrome resembles Klinefelter's syndrome except for the short stature, normal body hair distribution and normal intelligence. To differentiate between the 2 syndromes cytogenetic studies are necessary.
The incidence of Klinefelter's syndrome is approximately 1 in 500 male births and accounts for about 3 per cent of the cases of infertility in male subjects.1 The XX male syndrome has an incidence of 1 in 9,000 male newborns and would account for approximately 0.2 per cent of the cases of infertile male subjects.2
The first case of the XX male syndrome was reported in 1964 by de la Chapelle and associates.3 Since then, approximately 65 additional cases have been cited in the literature. Since the etiology of this syndrome remains poorly understood, additional case reports are of value to provide more data either to support the current theories or to develop new concepts concerning the cause(s) of this disorder.
Case Report
A 31-year-old white man was referred for fertility evaluation. There was no immediate family history of infertility, short stature or red-green color blindness. He had been married and engaging in unprotected intercourse for 4 years without establishing a pregnancy. There was no reported history of impotence and the patient experienced intercourse with normal erection, adequate vaginal penetration and ejaculation approximately 2 to 3 times a week. When the patient was between 15 and 17 years old he noticed voice and other pubertal changes. Presently, he has a full beard and normal male hair pattern in the pubic, axillary and chest regions.
Physical examination revealed an intelligent, slightly obese but well proportioned white man with an arm span of 168 cm., height 169 cm. and weight 72 kg. No abnormality was found on examination of the head or neck and there was moderate bilateral gynecomastia on the chest. Cardiovascular and abdominal examinations were normal. The penis was 7 cm. in length and 2.5 cm. in diameter. The testes measured 1.5 × 1.0 cm. bilaterally and were positioned normally in the scrotum. Rectal examination showed good anal tone and a normal prostate gland. Neurological examination was unremarkable.
Semen analyses performed 3 times during a 2-year period showed azoospermia. The hemogram and urinalysis were normal. Thyroid uptake and blood chemistry studies also were normal. The blood follicle-stimulating hormone level was 51.0 mU./ml. (normal 5 to 20 mU./ml.) and serum testosterone was 477 ng./100 ml. (normal 300 to 1,200 ng./100 ml.).
The dermatoglyphic analysis showed 5 ulnar loops on the right hand. There were 3 ulnar loops (digits, 1, 3 and 5) and 2 whorls (digits 2 and 4) on the left hand, with a total finger ridge count of 134. The mean ridge count for normal male controls is 145 ± 51.1 and the mean for normal female controls is 127 ± 52.5.4 In 1967 Penrose found the total finger ridge count for Klinefelter's syndrome to be 117.8 ± 49.6, which was lower than either the normal male or female count.5 The a-b ridge count (number of ridges across a-triradius at the base of the second digit and b-triradius at the base of the third digit) was 75 in our patient, which was not appreciably different than the mean established by Holt for normal male (86.7) and normal female (83.8) subjects.4 The maximal atd angle [reflection of degree of distal displacement of the palmar (t) axial triradius] was 46 degrees on the left and 43 degrees on the right sides. There were no simian creases.
Chromosome studies were performed on 3 separate occasions from peripheral blood lymphocytes during a 3-year period. Cytogenetic studies also were performed on fibroblasts in an attempt to detect inter tissue mosaicism, if present. Visual and photographic analysis of 400 early metaphase and metaphase plates revealed a consistent 46XX chromosome complement, confirmed by GTG (G-bands by trypsin using Giemsa's stain), QFQ (Q-bands by fluorescence using quinacrine) and CBG (C-bands by barium hydroxide using Giemsa's stain) banding procedures (see figure). Upon further examination and after performing measurements of the X chromosomes, there was evidence that 1 of the X chromosomes was significantly longer. Detailed cytogenetic findings are published elsewhere.6 The size variation was due to an increased length of the short arm of 1 of the X chromosomes. Various banding procedures were not useful in identifying the extra chromosomal material on the metaphase or early metaphase X chromosome. It was thought that the extra material was the segment of the Y chromosome that has few identifying bands, containing the genes for maleness, that was translocated to the short arm of the X chromosome.7 Karyotypes could not be obtained from the parents for examining this phenomenon.
Figure.
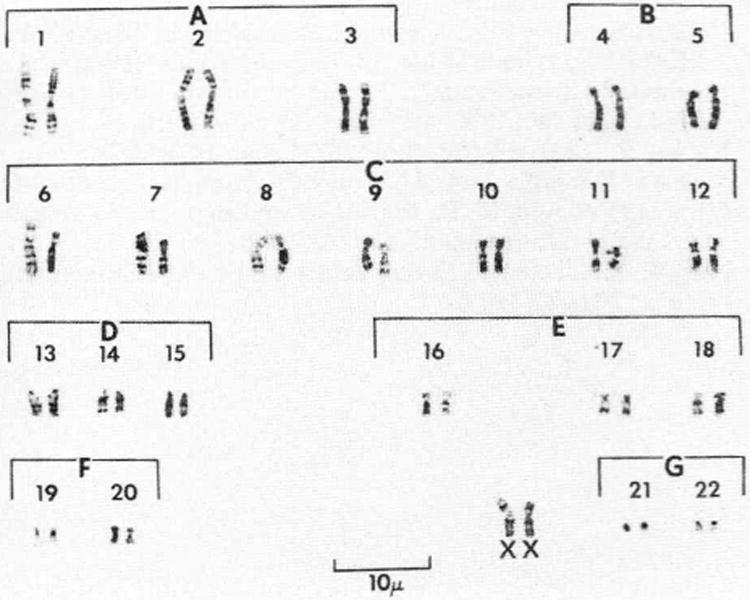
Blood lymphocyte karyotype by GTG-banding technique shows 46XX pattern and X chromosome with possible translocation on left side.
The buccal smear showed 28 per cent X chromatin-positive cells with a normal female control of 30 per cent. There was no evidence of Y chromatin in the buccal cells analyzed by the fluorescent staining technique.
Bilateral testicular biopsies revealed marked and diffuse tubular atrophy, total absence of spermatogenesis with no spermatogonia and few sustentacular cells. There was prominent thickening with fibro-collagenization of the tubular basement membrane. Elsewhere, there was a striking increase in the number of Leydig cells. The elastic and reticulum stains showed total change of the elastic tissue and no abnormal condensation of the reticular fibers. There was no evidence of ovarian tissue.
Discussion
The clinical features in our patient were consistent with those of other case reports of the XX male syndrome, except for a distinct male type of hair distribution consisting of a full beard and hair on the chest. This feature is apparently unusual for the XX male syndrome. The general appearance of the 46XX individual is typically male with a male psychosexual identification. The intelligence is usually higher than individuals with Klinefelter's syndrome. Our patient had bilateral gynecomastia, which is present in a third of XX male individuals. Our patient was also well proportioned but was shorter than typical XXY individuals.
The testicular biopsy showed atrophy with sclerosing tubular degeneration, absence of sperm formation and interstitial cell hyperplasia. The hormone studies were consistent with the testicular histology in showing increased follicle-stimulating and luteinizing hormone levels, and a normal serum testosterone. These findings are similar to XXY individuals.
Several theories have been proposed to explain the XX male syndrome, including autosomal mutation,8 X-Y interchange9 and mosaicism with an undetected XXY cell line.10
According to the autosomal gene theory, the male sex determining factors are located on chromosomes other than the Y chromosome. A gonad under the influence of an autosomal gene for maleness would develop into a testis but, without the Y chromosome to exercise a regulatory function, the testis differentiation would not be normal. There are 2 mammalian models (the Sxr mouse11 and the polled goat12) that have produced evidence of autosomal genes causing XX individuals to be phenotypic male subjects. In support of this theory in man de la Chapelle and associates reported 2 XX male subjects in 1 family whom they concluded were the result of an autosomal gene.13
The X-Y interchange theory hypothesizes the translocation of a portion of the Y chromosome to the X. The X and Y chromosomes pair end-to-end at meiotic prophase with the short arms of the X and Y being in close association.14 The observation of the inheritance of the blood group Xga in 2 male subjects with a 46XX karyotype, who were Xga-negative like their mothers while their fathers were Xga-positive, supports this hypothesis. Other evidence includes the use of the chromosome banding procedures in which Madan and Walker reported the terminal pale band (p22) of the short arm of 1 X chromosome of an XX male subject to be longer than the other X chromosome in 80 per cent of the analyzed cells.15 This difference was significant and was interpreted as a possible exchange between the X and Y chromosome.16 Additional cytogenetic evidence of this theory also was found by Pescia and Jotterand,17 and Wachtel and associates.18 Thus, we report an additional case with cytogenetic findings to support the X-Y interchange theory.
Undetected mosaicism is the third possible etiology. Kaiser and associates,19 and Miró and associates20 reported on XX male subjects with a mosaic XXY cell line. Other authorities theorize the existence of a cell line containing a Y chromosome that was present during an early stage of development but was subsequently lost. Also, there has been immunologic evidence that the Y chromosome, or its male determining part, is present in XX individuals through the detection of the H-Y antigen, which is necessary for testicular differentiation.18 Therefore, in our patient the presence of testicular tissue would be evidence of the effect of the H-Y antigen on gonadal cells for testes formation.18 The recent H-Y antigen analysis by Noël and Tous supports the X-Y interchange and autosomal theories, not the mosaicism theory, as the etiology in the XX male subjects studied.21
Therefore, we concluded that our 46XX male patient had a translocation of a Y chromosome segment containing the genes for maleness to the X, which occurred during meiosis. It is important to identify these individuals and to manage properly the infertility. The management of the infertile XX male subject consists of counseling the married couple on the alternatives of adoption or artificial insemination by donor. The wife of our patient delivered triplets after a single artificial insemination attempt.
It is our hope that additional cases of the 46XX male syndrome will be studied carefully with the X-Y interchange theory in mind. By so doing, the relative frequency of X-Y interchange as the cause of this syndrome would be determined.
References
- 1.Grumbach MM, Blanc WA, Engle ET. Sex chromatin pattern in seminiferous tubule dysgenesis and other testicular disorders: relationship to true hermaphroditism and to Klinefelter's syndrome. J Clin Endocr. 1957;17:703. doi: 10.1210/jcem-17-6-703. [DOI] [PubMed] [Google Scholar]
- 2.de la Chapelle A. Analytic review: nature and origin of males with XX sex chromosomes. Amer J Hum Genet. 1972;24:71. [PMC free article] [PubMed] [Google Scholar]
- 3.de la Chapelle A, Hortling H, Niemi M, Wennström J. XX sex chromosomes in a human male. First case. Acta Med Scand. 1964;175(412):25. doi: 10.1111/j.0954-6820.1964.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 4.Holt SB. Quantitative genetics of finger-print patterns. Brit Med Bull. 1961;17:247. doi: 10.1093/oxfordjournals.bmb.a069917. [DOI] [PubMed] [Google Scholar]
- 5.Penrose LS. Finger-print pattern and the sex chromosomes. Lancet. 1967;1:298. doi: 10.1016/s0140-6736(67)91237-8. [DOI] [PubMed] [Google Scholar]
- 6.Butler MG, Walzak MP, Sanger WG, Todd CT. Additional evidence for X-Y chromosome interchange in a 46,XX male. Nebraska Med J. 1980;65:330. [PMC free article] [PubMed] [Google Scholar]
- 7.Rary JM, Cummings DK, Jones HW, Jr, Rock JA. Assignment of the H-Y antigen gene to the short arm of chromosome Y. J Heredity. 1979;70:78. doi: 10.1093/oxfordjournals.jhered.a109197. [DOI] [PubMed] [Google Scholar]
- 8.Hamerton JL. Significance of sex chromosome derived hetero-chromatin in mammals. Nature. 1968;219:910. doi: 10.1038/219910a0. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson-Smith MA. X-Y chromosomal interchange in the aetiology of true hermaphroditism and of XX Klinefelter's syndrome. Lancet. 1966;2:475. doi: 10.1016/s0140-6736(66)92778-4. [DOI] [PubMed] [Google Scholar]
- 10.Lindsten J, Berstrand CG, Tillinger KG, Schwarzacher HG, Tiepolo L, Muldal S, Hokfelt B. A clinical and cyto-genetical study of three patients with male phenotype and apparent XX sex chromosome constitution. Acta Endocr. 1966;52:91. doi: 10.1530/acta.0.0520091. [DOI] [PubMed] [Google Scholar]
- 11.Cattanach BM, Pollard CE, Hawkes SG. Sex-reversed mice: XX and XO males. Cytogenetics. 1971;10:318. doi: 10.1159/000130151. [DOI] [PubMed] [Google Scholar]
- 12.Hamerton JL, Dickson JM, Pollard CE, Grieves SA, Short RV. Genetic intersexuality in goats. J Reprod Fertil. 1969;(7):25. [PubMed] [Google Scholar]
- 13.de la Chapelle A, Schroder J, Murros J, Tallqvist G. Two XX males in one family and additional observations bearing on the etiology of XX males. Clin Genet. 1977;11:91. doi: 10.1111/j.1399-0004.1977.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 14.Pearson PL, Bobrow M. Definite evidence for the short arm of the Y chromosome associating with the X chromosome during meiosis in the human male. Nature. 1970;226:959. doi: 10.1038/226959a0. [DOI] [PubMed] [Google Scholar]
- 15.Madan K, Walker S. Possible evidence for Xp+ in an XX male. Lancet. 1974;1:1223. doi: 10.1016/s0140-6736(74)91028-9. [DOI] [PubMed] [Google Scholar]
- 16.Madan K. Chromosome measurements on an XXp+ male. Hum Genet. 1976;32:141. doi: 10.1007/BF00291496. [DOI] [PubMed] [Google Scholar]
- 17.Pescia G, Jotterand M. Possible evidence of X-Y interchange in an XX male. Letter to the Editor. Lancet. 1977;1:550. doi: 10.1016/s0140-6736(77)91416-7. [DOI] [PubMed] [Google Scholar]
- 18.Wachtel SS, Koo GC, Breg WR, Thaler HT, Dillard GM, Rosenthal IM, Dosik H, Gerald PS, Saenger P, New M, Lieber E, Miller OJ. Serologic detection of Y-linked gene in XX males and XX true hermaphrodites. New Engl J Med. 1976;295:750. doi: 10.1056/NEJM197609302951403. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser P, Gerhard-Ratschow K, Zabel B, Gey W. A new case of XX-male (XX/XXY mosaic) Hum Genet. 1977;39:131. doi: 10.1007/BF00273164. [DOI] [PubMed] [Google Scholar]
- 20.Miro R, Caballin MR, Marina S, Egozcue J. Mosaicism in XX males. Hum Genet. 1978;45:103. doi: 10.1007/BF00277581. [DOI] [PubMed] [Google Scholar]
- 21.Noël B, Tous J. Sexual determination of XX men. J Genet Hum. 1978;26:287. [PubMed] [Google Scholar]


