Abstract
Background
Many medication adherence metrics are based on refill rates determined from pharmacy claims databases. However, these methods do not incorporate assessment of non-adherence to new prescriptions when those prescriptions are never dispensed (primary non-adherence), or dispensed only once (early non-persistence). As a result, published studies may overestimate adherence, but the extent of overestimation posed by not considering patients with primary non-adherence and early non-persistence has not been assessed.
Objectives
To estimate the magnitude of misestimation in adherence estimates that results from not including patients with primary non-adherence and early non-persistence.
Methods
Retrospective cohort study of 15,417 patients enrolled in an integrated healthcare delivery system newly-ordered an antihypertensive, antidiabetic, or antihyperlipidemic medication. We linked prescription orders to medication dispensings. Based on dispensing and refill rates, we stratified patients into primary non-adherent, early non-persistent and ongoing dispensings groups. Adherence was estimated using the proportion of days covered (PDC). Standardized observation periods were applied across all groups.
Results
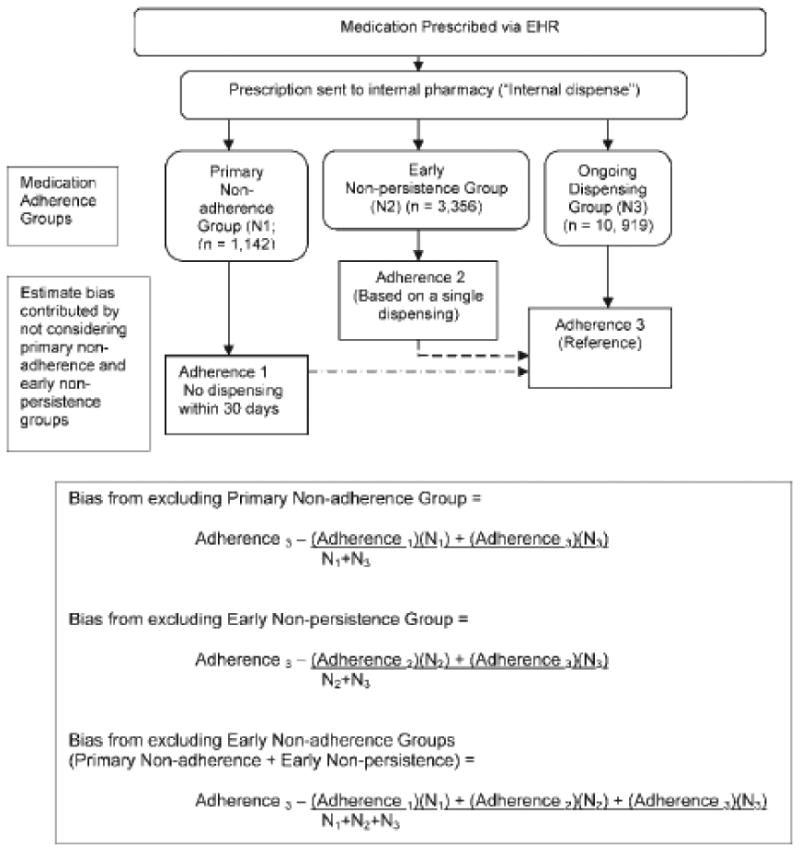
1,142 (7.4%) patients were primarily non-adherent, 3,356 (21.8%) demonstrated early non-persistence and 10,919 (70.8%) patients received ongoing dispensings with a mean PDC of 84%. Not including primarily non-adherent and early non-persistent patients in calculations resulted in adherence estimates overestimated by 9% to 18%.
Conclusions
When medication adherence is estimated from pharmacy claims databases, adherence estimates are substantially inflated because primarily non-adherent and early non-persistent patients are not included in the estimations. An implication of this incorrect estimation is potential distortion of the true relationship between medication adherence and clinical outcomes.
Keywords: medication adherence, primary non-adherence, adherence inflation, early non-persistence
Introduction
Adherence to medications is directly associated with improved clinical outcomes in chronic diseases such as diabetes, heart failure, hyperlipidemia, coronary artery disease, and hypertension.1-7 High adherence is also associated with lower healthcare costs.5, 8 If adherence is inaccurately estimated, results of comparative effectiveness research may not be correctly interpreted as the relationship between medication exposure and clinical outcome is likely to be distorted.
Adherence is often calculated using claims-based electronic pharmacy databases.8-12 Pharmacy databases enable adherence monitoring in large populations and assess medication dispensing, the critical first step in the adherence process. Pharmacy databases have also been used to trigger interventions intended to increase medication effectiveness and safety.13 Claims databases are extensively used to estimate adherence because they are relatively inexpensive, efficient, and an accessible source of information about the frequency and timeliness of medication refills in large populations.1, 2 A key limitation to pharmacy databases is that they can only be used to estimate medication possession, not medication consumption. Other tools to measure adherence such as electronic devices, patient self-report, and pill counts each have advantages and disadvantages; no method is considered the gold standard.2
Published adherence literature arising from use of pharmacy databases is based on data from patients who have one or more dispensings of the drug(s) of interest.2, 6, 8, 12, 14-16 By definition, pharmacy claims databases do not contain information about medications ordered but never dispensed (i.e., primary non-adherence). Furthermore, medications dispensed only once but never refilled (i.e., early non-persistence) do not meet the minimum criterion of two dispensings required to calculate indices such as the continuous multiple-interval measure of gaps (CMG, the total number of days for which drug is unavailable within a period) or the continuous multiple-interval measure of medication availability (CMA, the days' supply of medication obtained throughout the period divided by the number of days of participation).14, 17-19 As a result, many adherence studies systematically exclude patients with primary non-adherence or early non-persistence, the two subcategories that together comprise early non-adherence.
In addition, medication ordering and dispensing are generally recorded in separate, unlinked computer systems, thus limiting access to information required to calculate early non-adherence. Prescription orders have seldom been linked to medication dispensings and reconciliation of orders and dispensings has been even less frequent. We must better understand the importance of excluding calculations of early non-adherence and the implications this has on interpreting comparative effectiveness data if we are to achieve the full benefits of adherence and comparative effectiveness initiatives.
We hypothesized that population medication adherence estimates calculated from pharmacy databases are inflated as a result of excluding data from patients who fail to obtain initial prescriptions for chronic medications and patients who obtain only a single dispensing of chronic medications. Our specific objective was to estimate the magnitude of misestimation in adherence estimates that resulted from excluding assessment of patients with early non-adherence. To achieve this objective, we linked prescription orders in an ambulatory electronic health record (EHR) to medication dispensings in a pharmacy information system for three categories of commonly-used oral medication where adherence is directly associated with improved clinical outcomes: antihypertensives, antidiabetics, and/or antihyperlipidemics.3, 6, 7, 20 We then determined the misestimation of adherence that resulted from not including the early non-adherent patients.
Methods
Study Setting and Population
This study was conducted at Kaiser Permanent Colorado (KPCO), a not-for-profit integrated health care system. The study cohort included all KPCO members in the Denver-Boulder area with a newly-initiated (index) order for an oral antihypertensive, antidiabetic, or antihyperlipidemic medication between January 1, 2007 and June 30, 2008. Inclusion criteria were enrollment with a pharmacy benefit for 365 days before and 180 days after the order; no previous order for a drug for the same therapeutic indication within 365 days prior to the initial order; and either at least two coded diagnoses at least one month apart corresponding to an appropriate diagnosis for the order (hypertension ICD9 codes 401.0 – 405.9; diabetes ICD9 codes 250.##, hyperlipidemia ICD9 codes for hyperlipidemia 272.##) or the diagnosis associated with the prescription order. This study was approved by the KPCO Institutional Review Board, and the requirement for informed consent was waived.
Identification of Prescription Orders and Dispensings
Index orders were identified from the EHR medication order table. If a revised order was entered within 30 days of the initial order and the initial order had not been dispensed, the subsequent revised order was chosen as the “definitive” order. Prescriptions clearly not intended for chronic use were excluded (e.g., peri-operative beta-blocker prescriptions for < 30 total days).
From the EHR medication orders table we determined whether a prescription was designated for dispensing at a pharmacy internal or external to the KPCO system. Prescription orders intended to be dispensed at an internal pharmacy are routed electronically to the KPCO pharmacy information management system (PIMS) using an established interface. For orders to be dispensed at an external pharmacy, the ordering clinician indicates “external dispense” in the EHR order. During the study period, about 95 percent of orders were routed internally. For orders routed internally, we determined from PIMS whether and when the medication was dispensed. Medication orders and dispensings were linked using unique patient identifiers as well as drug identifiers and date.
For drug identification, a comprehensive drug name listing within each medication class was assembled through a look-up table cross-referenced by drug name and national drug code (NDC). For internal orders, the dispensing dates strength, formulation, instructions for use, days' supply, and NDC were ascertained.
Adherence Assessment
Patients were stratified into patient-drug adherence groups based on the following definitions:
Primary non-adherence: Did not pick up the prescription for the newly-initiated medication at a KPCO pharmacy and did not have it transferred to a pharmacy external to KPCO within 30 days after the order.
Early non-persistence: Picked up the initial prescription for the newly-initiated medication at a KPCO pharmacy within 30 days after the order but did not have it refilled or transferred to a pharmacy external to KPCO within 180 days after initial dispensing.
Ongoing dispensing: Picked up the first prescription for the newly-initiated medication at a KPCO pharmacy within 30 days after the order and had the prescription refilled at least once at a KPCO pharmacy within 180 days after initial dispensing.
For individuals who had prescriptions ordered for external dispensing (or transferred externally), adherence could not be estimated as information about whether and when the prescription was filled was not available. We quantified the number and proportion of patients with prescriptions for external dispensing (n = 756; 5%); these patients were not included in adherence estimates.
Patients were analyzed in only one adherence group (i.e., primary non-adherence, early non-persistence, or ongoing dispensing) and only one therapeutic class (i.e., antidiabetic, antihypertensive, antihyperlipidemic, or multiple drugs). Patients with newly-ordered drugs from more than one therapeutic class (e.g., an antidiabetic and an antihypertensive) at any time during the 18-month study period were classified into the multiple drugs class. Patients in the multiple drugs class were analyzed in the ongoing dispensing group if they had ongoing dispensings of any one of the newly-ordered drugs. Because the study cohort was defined as including only patients who had not had a previous order for a drug in the same therapeutic class within the prior 365 days, if a patient had more than one newly-initiated drug within that therapeutic class during the study period, only the first medication ordered during the study period was included.
Adherence was calculated from KPCO pharmacy databases using the proportion of days covered (PDC) method.17-19 To obtain the PDC, the total days' supply dispensed was divided by the 180 days in the observation period. This value was capped at 1.0 and multiplied by 100 to obtain percent adherence. The PDC was determined at the drug class level so that individuals with a within-drug switch (e.g., brand to generic, different generics, different dosage strengths) or an across-drug switch (e.g., simvastatin to pravastatin) within the observation period were afforded the correct PDC. We calculated adherence both as a continuous measure and categorically, considering patients in the ongoing dispensing group to be non-adherent when the PDC was less than 80 percent. The 80 percent cut-point is commonly used, clinically based, and demonstrates a reasonable balance between sensitivity and specificity.21 The PDC for individuals in the multiple drugs class ongoing dispensing group was calculated based on the most adherent drug. The PDC for individuals classified into the primary non-adherence group could be greater than zero if they picked up their initial prescription, but at some time after the 30 day period used to define placement in the primary non-adherence group.
Other Data Sources, Management, and Statistical Analysis
Existing administrative and clinical databases, the PIMS system, and the EHR were used to ascertain all study data. To assess the extent of misestimation in adherence estimates, patients in the ongoing dispensing group were compared with patients in the primary non-adherence and early non-persistence groups, and differences in characteristics were assessed using a Chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. All data checks and analyses were performed with SAS version 9.1.3.
We considered the adherence of patients in the ongoing dispensing group as the reference (Figure 1). Equations used to estimate the adherence misestimation associated with omitting patients with early non-adherence from analyses are shown in Figure 1. Estimations were weighted by the proportion of patients in each adherence group. Because the standard errors for the estimates of misestimation must account for the non-independence of the groups being compared, standard errors of the bias were calculated using bootstrap methods. We created the bootstrap replications by re-sampling with replacement, calculating the mean PDC for each group. We then used this distribution for the replicated statistic to calculate the standard error.22
Figure 1. Approach to Assessing Importance of Early Non-Adherence in Medication Adherence Estimates for Patients within an Integrated Healthcare Delivery System.
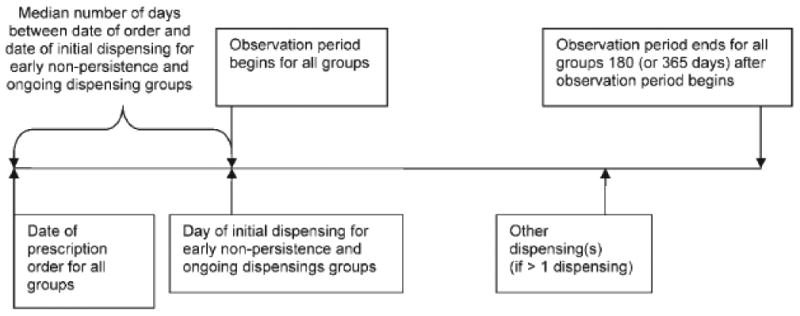
A uniform observation period of 180 days was used for all patients. For patients with ongoing dispensing or early non-persistence, the observation period began on the dispensing date (Figure 2). Because no dispensing date existed for patients with primary non-adherence, the date the observation period began for that group was extrapolated using the median number of days between the date of the order and the date of the dispensing for the patients in the other two adherence groups. The median number of days between the order and dispensing dates for the other two groups -- one day -- was added to the order date for patients in the primary non-adherence group as the day the observation period began for those patients.
Figure 2. Determination of Observation Period to Estimate Adherence for Patients with Primary Non-adherence.
Sensitivity Analyses
As adherence can also be determined using an observation period length greater than 180 days,7, 12, 23 to evaluate the effect that changing the observation period length had on adherence estimates, we additionally determined the PDC using 365 days. Further, because adherence definitions in this study specified dispensing within 30 days after the order, and a 60 day period after the order has also been applied,24 we additionally determined the effect of changing the adherence definition to specify dispensing within 60 days (i.e., reclassifying into a different adherence group the patients who had an initial dispensing between days 31 and 60).
Results
Of 15,417 patients with a newly-initiated prescription for an antihypertensive, antidiabetic, antihyperlipidemic, or multiple medications written to be dispensed at an internal KPCO pharmacy during the study period, 1,142 (7%) patients were primarily non-adherent and 3,356 (22%) were early non-persistent (Table 1). Across early non-persistent patients, the median (5th, 95th percentile) days' supply dispensed was 60 (30, 90). Almost ten percent of individuals newly-prescribed an antidiabetic or antihyperlipidemic and about five percent of patients newly-prescribed an antihypertensive or multiple drugs did not pick up their prescription within 30 days after the order (Table 1). Additional adherence characteristics are shown in Table 1.
Table 1. Adherence Characteristics of Study Population.
| Characteristic | Adherence Group1,2 | ||
|---|---|---|---|
|
| |||
| Primary Non-Adherence (n=1,142) | Early Non-Persistence (n=3,356) | Ongoing Dispensing (n=10,919) | |
| Number (%) of Patients by Therapeutic Class | |||
| Diabetes (n = 1,788) | 172 (10) | 267 (15) | 1,349 (75) |
| Hypertension (n = 6,393) | 331 (5) | 1,672 (26) | 4,390 (69) |
| Hyperlipidemia (n = 5,848) | 582 (10) | 1,241 (21) | 4,025 (69) |
| Multiple drugs (n = 1,388) | 57 (4) | 176 (13) | 1,155 (83) |
| Proportion of Days Covered (PDC): 180 days 3 | |||
| mean (sd) | |||
| median (5%,95%) | |||
| Diabetes | 0.14 (0.24) 0.00 (0.00, 0.68) |
0.29 (0.12) 0.33 (0.17, 0.50) |
0.86 (0.19) 0.94 (0.43, 1.00) |
| Hypertension | 0.13 (0.22) 0.00 (0.00, 0.63) |
0.28 (0.11) 0.33 (0.17, 0.50) |
0.84 (0.20) 0.93 (0.33, 1.00) |
| Hyperlipidemia | 0.25 (0.26) 0.17 (0.00, 0.74) |
0.29 (0.11) 0.33 (0.17, 0.50) |
0.84 (0.18) 0.92 (0.49, 1.00) |
| Multiple drugs | 0.18 (0.22) 0.02 (0.00, 0.61) |
0.29 (0.12) 0.33 (0.17, 0.50) |
0.83 (0.20) 0.92 (0.34, 1.00) |
| PDC > 80% by Therapeutic Class: 180 days, n (%) | |||
| Diabetes | 0 (0) | 1 (0) | 983 (73) |
| Hypertension | 2 (1) | 8 (0) | 2,964 (68) |
| Hyperlipidemia | 12 (2) | 0 (0) | 2,751 (68) |
| Multiple drugs | 0 (0) | 2 (1) | 764 (66) |
For persons with newly-initiated drugs from more than one therapeutic class, the most adherent group was assigned (e.g., if they had ongoing dispensings for any of the newly-prescribed drugs, they were placed in the Ongoing group).
Variables compared across columns using Chi-square, Wilcoxon rank sum, or Kruskal-Wallis test. P-values for all <0.001.
PDC is not 0 for the primary non-adherence group because a few patients in this group eventually filled the prescription at some time after the 30 day period that was used to define primary non-adherence. Between days 31 and 60, 14 patients prescribed an antidiabetic, 45 patients prescribed an antihypertensive, 137 patients prescribed an antihyperlipidemic, and 6 patients prescribed drugs from multiple classes filled the prescription.
The misestimation in medication adherence estimates contributed by not considering patients with early non-adherence is shown in Table 2. Adherence estimates were inflated when only the ongoing dispensing group was considered in estimating population adherence: for patients newly-prescribed a drug for diabetes, omitting the early non-adherent patients resulted in an adherence estimate 15 percent higher than if the adherence of the entire population for whom the drugs were ordered had been included. Omitting the patients who were early non-adherent to antihypertensive and antihyperlipidemic medications contributed the greatest inflation to adherence estimates (18% for each), while omitting those initiated on multiple therapeutic classes contributed a lesser, but still substantial, inflation (9%).
Table 2. Misestimation in Medication Adherence Estimates Contributed by Not Considering Early Non-Adherence to Newly-Initiated Medications for Diabetes, Hypertension, and/or Hyperlipidemia.
| Mean PDC (SD) by Adherence Group | |||||
|---|---|---|---|---|---|
|
| |||||
| Ongoing Dispensing | Primary Non-Adherence + Ongoing Dispensing | Early Non-Persistence + Ongoing Dispensing | Ongoing Dispensing + Early Non-Persistence | Primary Non-Adherence + Early Non-Persistence + Ongoing Dispensing | |
| Diabetes | 0.86 (0.19) | 0.77 (0.30) | 0.76 (0.28) | 0.76 (0.28) | 0.70 (0.33) |
| Hypertension | 0.84 (0.20) | 0.79 (0.27) | 0.68 (0.31) | 0.68 (0.31) | 0.65 (0.33) |
| Hyperlipidemia | 0.84 (0.18) | 0.76 (0.27) | 0.71 (0.29) | 0.71 (0.29) | 0.66 (0.32) |
| Multiple Drugs | 0.83 (0.20) | 0.80 (0.24) | 0.76 (0.26) | 0.76 (0.26) | 0.73 (0.28) |
|
| |||||
| Misestimation (SE) | |||||
|
| |||||
| Ongoing Dispensing vs (Primary Non-Adherence + Early Non-Persistence + Ongoing Dispensing) | Ongoing Dispensing vs. (Primary Non-Adherence + Ongoing Dispensing) | Ongoing Dispensing vs. (Early Non-Persistence + Ongoing Dispensing) | (Early Non-Persistence + Ongoing Dispensing) vs. (Primary Non-Adherence + Early Non-Persistence + Ongoing Dispensing) | ||
|
| |||||
| Diabetes | 0.15 (0.01) | 0.08 (0.01) | 0.09 (0.01) | 0.06 (0.01) | |
| Hypertension | 0.18 (0.01) | 0.05 (0.01) | 0.15 (0.01) | 0.03 (0.01) | |
| Hyperlipidemia | 0.18 (0.01) | 0.07 (0.01) | 0.13 (0.01) | 0.05 (0.01) | |
| Multiple Drugs | 0.09 (0.01) | 0.03 (0.01) | 0.07 (0.01) | 0.02 (0.01) | |
Both primary non-adherence and early non-persistence contributed to misestimation (Table 2). Within the individual therapeutic classes the misestimation contributed by omitting patients in the early non-persistence subgroup exceeded that contributed by omitting patients in the primary non-adherence subgroup due to the larger proportion of patients in the early non-persistent subgroup (Table 2).
The misestimation in adherence estimates contributed by not considering early non-adherence was similar when a 365 day observation period was applied, ranging from 9 percent for early non-adherent patients with multiple therapeutic classes to 18 percent for early non-adherent patients prescribed an antihyperlipidemic (not shown in table). Changing the definition of the required dispensing timeframe to 60 days reclassified (from primary non-adherence to a different adherence group) 14 (of 172) prescribed an antidiabetic, 45 (of 331) prescribed an antihypertensive, 137 (of 582) prescribed an antihyperlipidemic, and 6 (of 57) prescribed drugs from multiple classes. The effect of reclassifying these patients was minimal, with misestimation again ranging from 9 percent to 18 percent (not shown in table).
Discussion
The results of this investigation illustrate that medication adherence estimates are substantially inflated when early non-adherent patients are omitted from adherence calculations. In our cohort, adherence estimates were inflated by 9 to 18 percent. This work also demonstrates that nearly one in three patients newly-prescribed a medication for diabetes, hyperlipidemia, and/or hypertension exhibit early non-adherence. Adherence calculations missing patients with early non-adherence are likely to substantially misstate the effects of adherence on associated health outcomes.
Our work also documents that approximately three times as many patients exhibit early non-persistence as primary non-adherence (Table 1). These findings are comparable to prior published research.16, 24-26 Fischer and colleagues found primary non-adherence rates of 28 to 31 percent among patients newly-prescribed medications for hypertension, hyperlipidemia, and diabetes 27 whereas we found primary non-adherence rates of five to ten percent among patients newly-prescribed medications for these same indications. The prevalence of primary non-adherence observed by Fischer et al. is likely overestimated as the authors used cross-linkage of e-prescriptions and paid dispensing claims to identify patients without claims.28 A strength of our study is that we did not rely on paid claims to identify dispensings.
Our findings suggest that patients initiating drugs from different therapeutic classes provide different contributions to a comprehensive adherence picture, thus implying that it may not be appropriate to extrapolate adherence inflation estimates from one patient group or therapeutic class to another. For example, about twice as many patients started on an antidiabetic or antihyperlipidemic medication exhibited primary non-adherence compared with patients initiating an antihypertensive (Table 1). The reasons for this require further study.
Because KPCO is an integrated system caring for a defined population, we could accurately identify, access, and link EHR medication orders and dispensings within internal systems. In conjunction, because the rate of prescription orders transmitted to external pharmacies was defined and low, we present a comprehensive picture of adherence from which we estimate the importance of early non-adherence. As a result, this is one of the first studies to quantify the degree of inflation of adherence estimates when measured using data gleaned from pharmacy claims databases. Some might argue that, because KPCO is an integrated system, the results of this work are only generalizable to similar systems. However, as EMR use becomes more prevalent, other systems can also accurately capture orders and dispensings, and the methods we describe here will allow estimation of early non-adherence in those settings. Further, we believe our results represent a real-world “best case” scenario in that some barriers to prompt prescription dispensing (i.e., no convenient pharmacy, handwritten prescriptions) are absent within our setting. Our results therefore likely underestimate the degree to which misestimation is present when adherence is estimated in less integrated care settings. One additional consideration about underestimating the degree of misestimation is that we classified patients into mutually-exclusive groups; if an individual had a new medication from more than one therapeutic area, we assigned that individual to the ongoing dispensings group if he/she had ongoing dispensings of any of the new medications.
This study was not designed to assess patient factors associated with adherence. We did not attempt to identify reasons for early non-adherence or describe characteristics that differentiated early non-adherers from those with continued refills. Medication intolerance or adverse events, with subsequent medication discontinuation, likely occurred among some early non-adherers. This would be reflected in estimates of early non-persistence and would affect our estimates if the patient was subsequently initiated on a different medication for the same indication within the 180 day observation period.
Two other sources of overestimation are relevant to early non-persistent individuals. First, the PDC assumes the patient ingests the full dispensed days' supply; if this is not the case the bias introduced would be most pronounced in PDC estimates among those with early non-persistence. For example, if a patient with early non-persistence stopped taking the medication after only a few days, the calculated PDC would reflect a higher PDC than the “truth.” Second, PDC is sensitive to days' supply. In this work, the median days' supply was 60. If it had been 30 days, the median PDC estimates in the early non-persistent group would have been lower. If a patient who initiated therapy had a hospital or nursing home admission within the 180 day follow-up period, the PDC could have been either under- or overestimated.
Primary non-adherence and adherence to ongoing medications might represent different patient behaviors. It could be argued that measurements such as the PDC are not intended to measure primary non-adherence and that incorporating primary non-adherence data to assess misestimation in adherence estimates is not appropriate. While we acknowledge this perspective, it does not lessen the importance of the results of our work.
This study also was not designed to assess either the contributions of provider-patient interactions or clinical outcomes. Such process and outcomes assessments will be crucial to establishing the clinical importance of our results.
In summary, this study extends our understanding of medication adherence by addressing how omitting early non-adherence information contributes to misleading adherence estimations. Through linking medication orders and dispensings we add to knowledge of the accuracy and completeness of dispensing databases, of proportions of patients with primary non-adherence and early non-persistence, and of adherence estimates based on pharmacy claims. We also provide preliminary information about the importance of early non-adherence that is useful to clinicians, researchers, and those who work in healthcare information technology as they strive to increase the efficiency of the data infrastructure for comparative effectiveness studies.
Acknowledgments
The work presented in this manuscript was supported by an internal grant from the Kaiser Permanente Colorado Institute for Health Research. Dr. Schroeder is also funded by Training Grant 5 T32 DK007446 from the National Institute of Diabetes and Digestive and Kidney Diseases.
We thank Capp Luckett for programming efforts. We also thank Brandy McGinnis, PharmD and Susan M. Shetterly, MS for contributing to study design.
This work has not been previously presented and is not under consideration by any other journal.
Footnotes
The authors report no conflicts of interest related to this work.
Contributor Information
Marsha A. Raebel, Investigator in Pharmacotherapy, Institute for Health Research, Kaiser Permanente Colorado, PO Box 378066, Denver, CO 80237, Phone: (303) 614-1260, Fax: (303) 614-1265, and, Clinical Professor, School of Pharmacy, University of Colorado, Aurora, Colorado.
Nikki M. Carroll, Biostatistician, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado.
Jennifer L. Ellis, Manager of Analytic Resources/Biostatistician, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado.
Emily B. Schroeder, Research Fellow, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado, and, Endocrinology Fellow, University of Colorado, School of Medicine, Aurora, Colorado.
Elizabeth A. Bayliss, Clinician Investigator, Institute for Health Research, Kaiser Permanente Colorado, Denver, Colorado, and, Associate Clinical Professor, University of Colorado, School of Medicine, Aurora, Colorado.
References
- 1.Balkrishnan R. The importance of medication adherence in improving chronic-disease related outcomes: what we know and what we need to further know. Med Care. 2005;43:517–520. doi: 10.1097/01.mlr.0000166617.68751.5f. [DOI] [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 3.Walker EA, Molitch M, Kramer MK, et al. Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care. 2006;29:1997–2002. doi: 10.2337/dc06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JR, Moser DK, DeJong MJ, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am Heart J. 2009;157:285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 6.Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med. 2008;168:271–276. doi: 10.1001/archinternmed.2007.72. [DOI] [PubMed] [Google Scholar]
- 7.McGinnis BD, Olson KL, Delate TMA, Stolcpart RS. Statin adherence and mortality in patients enrolled in a secondary prevention program. Am J Manag Care. 2009;15:689–695. [PubMed] [Google Scholar]
- 8.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff. 2011;30:91–99. doi: 10.1377/hlthaff.2009.1087. [DOI] [PubMed] [Google Scholar]
- 9.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing Adherence and Persistence Across 6 Chronic Medication Classes. J Manag Care Pharm. 2009;15:728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon J, Ettner SL. Cost-Sharing and Adherence to Antihypertensives for Low and High Adherers. Am J Manag Care. 2009;15:833–840. [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan DV, Keene MS, Eaddy M, Krulewicz S, Kraus JE, Carpenter DJ. Difference in adherence and healthcare resource utilization patterns: older versus newer antidepressant agents in patients with depression and/or anxiety disorders. CNS Drugs. 2008;22:963–973. doi: 10.2165/00023210-200822110-00005. [DOI] [PubMed] [Google Scholar]
- 12.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacother. 2008;28:437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raebel MA, Charles J, Dugan J, et al. Randomized trial to improve prescribing safety among ambulatory elderly patients. J Am Geriatr Soc. 2007;55:977–985. doi: 10.1111/j.1532-5415.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 14.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 15.Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs,a nd all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011;17:25–39. doi: 10.18553/jmcp.2011.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raebel MA, Delate T, Ellis JL, Bayliss EA. Effects of reaching the drug benefit threshold on Medicare members' healthcare utilization during the first year of Medicare Part D. Med Care. 2008;46:1116–1122. doi: 10.1097/MLR.0b013e318185cddd. [DOI] [PubMed] [Google Scholar]
- 17.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 18.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: A proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 19.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 20.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 21.Hansen RA, Kim MM, Song L, Tu W, Jingwei W, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 22.Barker N. Proceedings of PhUse 2005. Heidelberg, Germany: Oct 10-12, 2005. [June 17, 2011]. A practical introduction to the bootstrap using the SAS system. http://www.lexjansen.com/phuse/2005/pk/pk02.pdf. [Google Scholar]
- 23.Chan DC, Shrank WH, Cutler D, et al. Patient, physician, and payment predictors of statin adherence. Med Care. 2010;48:196–202. doi: 10.1097/MLR.0b013e3181c132ad. [DOI] [PubMed] [Google Scholar]
- 24.Karter AJ, Parker MM, Moffet HH, Ahmed AM, Schmittdiel JA, Selby JV. New prescription medication gaps: A comprehensive measure of adherence to new prescriptions. Health Services Research. 2009;44:1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hertz RP, Unger AN, Lustik MB. Adherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insurance. Clinical Therapeutics. 2005;27:1064–1073. doi: 10.1016/j.clinthera.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Shah NR, Hirsch AG, Zacker C, Taylor S, Wood GC, Stewart WF. Factors associated with first-fill adherence rates for diabetic medications: a cohort study. J Gen Intern Med. 2009;24:233–237. doi: 10.1007/s11606-008-0870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer MA, Stedman MR, Lii J, et al. Primary Medication Non-Adherence: Analysis of 195,930 Electronic Prescriptions. J Gen Intern Med. 2010;25:284–290. doi: 10.1007/s11606-010-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karter AJ, Parker MM, Adams AS, et al. Primary non-adherence to prescribed medications. J Gen Intern Med. 2010;25:763. doi: 10.1007/s11606-010-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


