Abstract
Background
Double lumen balloon catheters (DLBCs) are currently used in the treatment of intracranial aneurysms, especially when involving balloon or stent-assisted coiling. The existing DLBCs allow the delivery of self-expandable stents but do not offer the possibility to deploy flow-diverters. Despite the increasing use and success of flow-diverters, there have been numerous reports of procedural complications such as early in-stent thrombosis or delayed distal embolization. It seems that these complications can be avoided by correct stent positioning and adequate wall apposition, achieved either by manoeuvres with the microguidewire and/or microcatheter or by performing balloon angioplasty following an exchange guidewire manoeuvre.
Objective
Report the use of a new DLBC able to deliver a flow-diverter.
Methods
A 41-year-old woman presented to our hospital with binocular horizontal diplopia for two weeks and reduced visual acuity. A left internal carotid artery aneurysm involving the cavernous and ophthalmic segments was found, with a maximum height of 19 mm and a broad 8 mm neck. It presented extra- and intra-dural components and the parent vessel was significantly narrowed. A decision was made to perform endovascular treatment of the aneurysm with placement of a flow diverter through a DLBC.
Results
Patency and adequate expansion of the flow diverter with evident intra-aneurysmal contrast stasis was observed in the final angiogram. No peri-procedural complications were observed.
Conclusion
This is a technical note demonstrating the feasibility of a new device to deploy a flow diverter, aiming to improve wall apposition and stent configuration without the need of additional devices or exchange manoeuvres.
Keywords: Double lumen balloon catheter, flow diverter, intracranial aneurysm
Background
The double lumen balloon catheter (DLBC) is a recent but already well-established tool in the endovascular armamentarium of the neurointerventionalist. It was initially described as an alternative to the double catheter balloon remodelling technique, aiming to reduce thromboembolic events or possible injuries to the vessel by using one microcatheter instead of two.1–3 It can also be used to deliver a self-expandable stent for stent-assisted coiling. The existing DLBCs are dimethyl sulphoxide compatible and their use has been reported for embolization of arteriovenous malformations and dural fistulas.4 The employment of this device for treating venous sinus thrombosis was also reported.5
The Copernic 2L DLBC (Balt Extrusion, Montmorency, France) presents a larger internal diameter than similar existing devices, allowing the deployment of a flow diverter. If narrowing of the stent or inadequate wall apposition is observed, angioplasty can be performed without the need of additional devices or exchange manoeuvres.
Case presentation
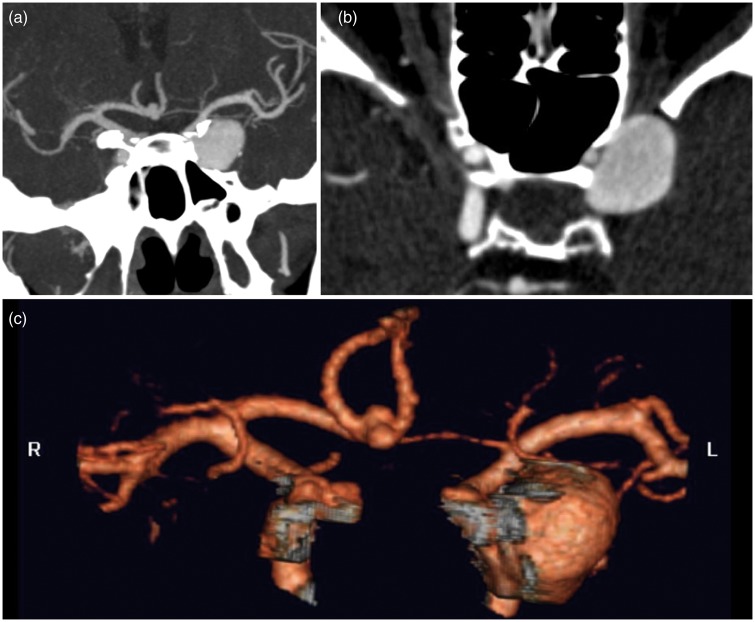
A 41-year-old woman presented to our hospital with binocular horizontal diplopia for two weeks and reduced visual acuity. Neuro-ophthalmology suggested a compressive optic neuropathy and a left partial third nerve palsy. Magnetic resonance imaging study revealed three aneurysms: two internal carotid artery (ICA) aneurysms of both cavernous segments and an anterior communicating artery (AComA) aneurysm (Figure 1). The left ICA aneurysm involved the cavernous and ophthalmic segments, had a maximum height of 19 mm and a broad 8 mm neck. It had both extra-dural and intra-dural location. The parent vessel was significantly narrowed (Figures 1(b) and 2(a)). The right ICA aneurysm was extra-dural, located in the cavernous segment, presenting a maximum height of 5.5 mm. The AComA aneurysm was bi-lobulated with a maximum height of 4 mm. The right ICA and AComA aneurysms were not treated.
Figure 1.
(a) Computed tomography angiography shows multiple aneurysms located in the left internal carotid artery cavernous/ophthalmic segment (19 mm × 8 mm), right internal carotid artery cavernous segment (5 mm × 4 mm) and anterior communicating artery (4 mm × 2 mm). Notice the left internal carotid artery narrowing distally to the aneurysm on (b).
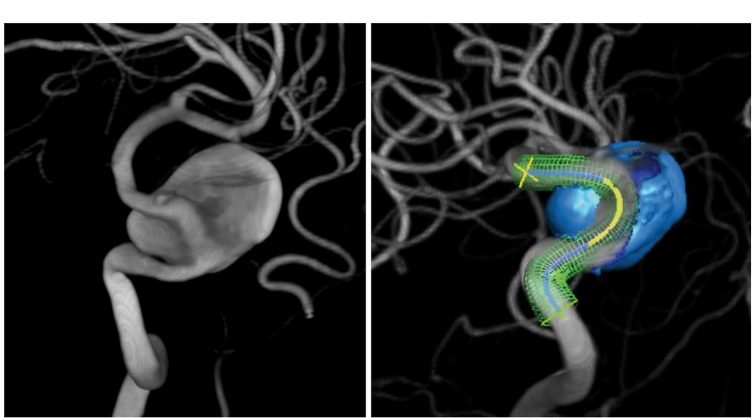
Figure 2.
(a) 3D-digital subtraction angiography images showing the left internal carotid artery aneurysm, with signs of parent vessel narrowing. (b) Working projection with pre-operative planning of the flow diverter position.
The patient was discussed in a multidisciplinary conference. Regarding the symptoms, patient age, presence of intra-dural component and aneurysm size, a decision was made to perform endovascular treatment of the left ICA aneurysm. After explaining the risks of the procedure and the possibility to record/film the case for teaching/research purposes, patient consent was obtained.
Our strategy was to place a flow diverter and (partially) embolize the aneurysm after jailing a microcatheter. As narrowing of the ICA was apparent on computed tomography angiography, we decided to deploy the SILK+ (Balt Extrusion, Montmorency, France) through a Copernic 2L (Balt Extrusion, Montmorency, France) (Figure 2). This strategy would allow for post-deployment angioplasty to be performed if there were angiographic signs of inadequate wall apposition or stent narrowing after high-resolution contrast-enhanced cone-beam computed tomography (VasoCT; Phillips®).
The periprocedural pharmacologic protocol for patients undergoing therapy with a flow diverter in our institution consists of aspirin (325 mg) and clopidogrel (75 mg) starting 10 days prior to the procedure.
The endovascular procedure was performed under general anaesthesia.
After obtaining femoral access with an 8Fr introducer, the patient was anticoagulated with a bolus of intravenous heparin (5000 U). A 5 F Neuron Select Catheter (Penumbra, Alameda, USA) and a Terumo 0.88 mm guidewire (Terumo Medical Corporation, Somerset, USA) were used to navigate a 6 F Shuttle (Cook Medical, Bloomington, USA) into the left cervical ICA. This vessel presented a reduced calibre throughout its course with no apparent vasospasm. In order to avoid vasospasm and facilitate the navigation of the devices, 2 mg of Verapamil was administered. A co-axial system consisting of the 6 F Shuttle and a Navien 6 F intracranial support catheter (ev3/Covidien, Minneapolis, USA) was positioned in the horizontal segment of the petrous ICA.
An Excelsior SL-10 microcatheter (Stryker, Kalamazoo, USA) was navigated to the aneurysm sac with a Synchro-14 guidewire (Stryker, Kalamazoo, USA). Maintaining the SL-10 securely placed inside the aneurysm, the Copernic 2L was navigated to the terminal segment of the ICA over a Synchro-14 wire advanced in a J-configuration distally into the middle cerebral artery (MCA) M2 segment (Figure 3(a) and (b)).
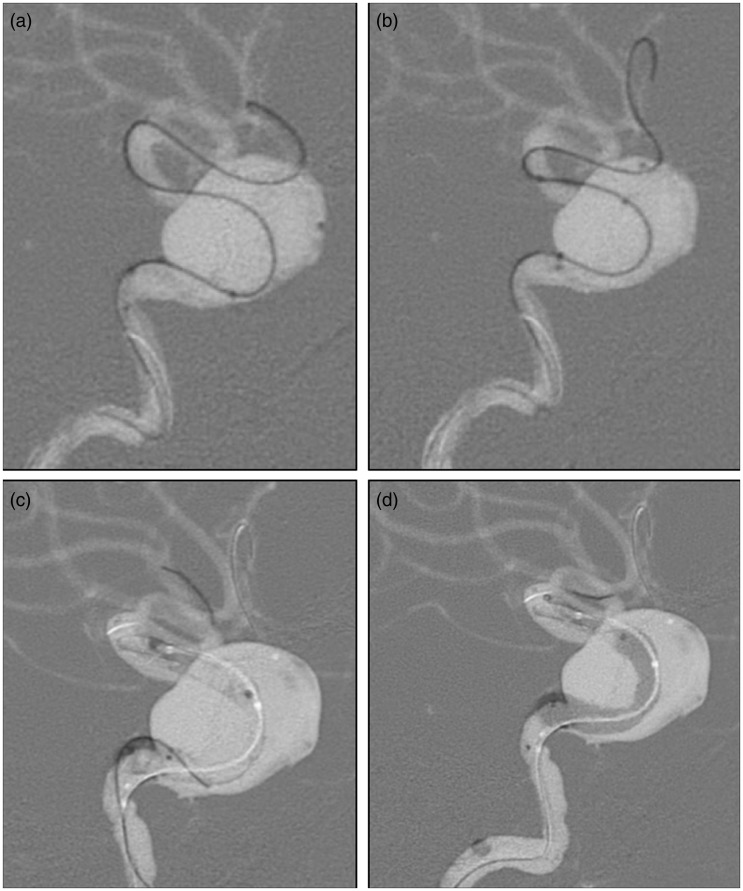
Figure 3.
(a) and (b) Digital subtraction angiography images showing the navigation of the balloon catheter over a Synchro guidewire. It is important to place the wire distally in the middle cerebral artery branches to give enough support, specially in larger broad-neck aneurysms. (c) After deployment, the flow diverter is seen in place but narrowing of its intermediate portion is visible. (d) Balloon angioplasty is performed throughout the stent to improve expansion of the proximal and intermediate portions of the stent.
A 3.5 mm × 35 mm SILK+ was then loaded through the Copernic 2L and deployed just proximal to the anterior choroidal artery origin. For correct positioning, the stent was twice re-sheathed and during one of these manoeuvres the SL-10 microcatheter dislodged from the aneurysm. We ensured coverage of the aneurysm neck with adequate proximal landing zone.
After deployment, narrowing of the stent was noted within the aneurysm (Figure 3(c)). We then proceeded with angioplasty of this segment using the Copernic 2L balloon, which was navigated into the stent over its deployment wire. VasoCT confirmed the patency and adequate expansion of the flow diverter with evident contrast stasis on the final control angiogram (Figure 4).
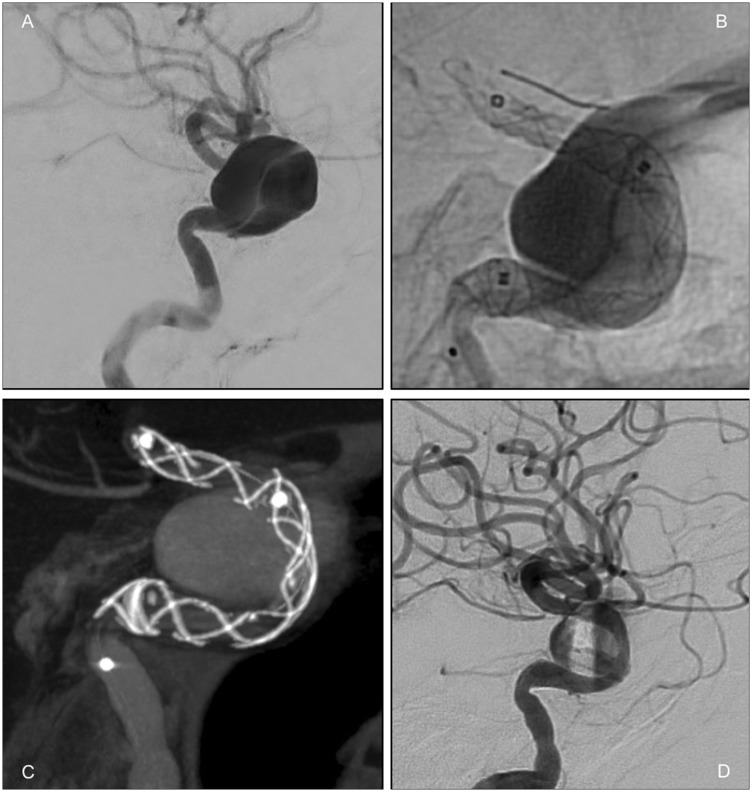
Figure 4.
(a) Initial angiogram prior to stenting. (b) Configuration of the flow diverter after angioplasty, with the Copernic-2L still inside the stent. (c) VasoCT showing good wall apposition and configuration of the stent. (d) Evident contrast stasis in the final control angiogram.
No peri-procedural complications were observed; the patient woke up without new deficits and was discharged the next day. Follow-up two weeks later in our outpatient clinic demonstrated shrinking of the aneurysm, intra-aneurysmal thrombosis and no neurological deficits.
Discussion
To our knowledge, this is the first report of a flow diverter deployment through a double-lumen balloon microcatheter. Full stent deployment and apposition to the vessel wall are known to be critical factors to avoid flow diverter thrombosis and thromboembolic events related to late thrombosis of the aneurysm and to accomplish an adequate scaffold for neo-endothelialization.6 VasoCT has been reported as a powerful tool to confirm good wall apposition.7,8
Copernic 2L is a double-lumen balloon microcatheter with an inner diameter (I.D.) of 0.60 mm (0.024 in.). The outer diameter is 2.5 Fr on the distal tip and 2.7 Fr in the double balloon segment. The balloon comes in three different lengths (10, 20 and 30 mm), always with a maximum diameter of 6 mm.
When we analyse the possibility of delivering other flow diverters through Copernic 2L, we should take into account the I.D. of each flow diverter-associated delivery device. For the Pipeline Flex (ev3/Covidien, Minneapolis, USA), FRED (Microvention Inc., Tustin, USA) and p64 (Phenox GmbH, Bochum, Germany) the recommended microcatheters have an i.d. of 0.69 mm and for this reason we do not recommend using a narrower catheter such as the Copernic 2L. Surpass flow diverter (Stryker, Kalamazoo, USA) is pre-loaded in its own delivery system.
A French multicentre retrospective study of 65 consecutive patients treated with SILK+ considers poor wall apposition to be a main concern.9 It reports stent misdeployment in nine out of 63 stents (12.6%), ultimately resulting in occlusion of the parent artery in 9.3% of cases. Another study focusing on delayed complications following the use of SILK+ and the Pipeline Embolization Device (PED; ev3/Covidien, Minneapolis, USA) reports that configuration and location changes (like stent creeping or tapering) were rare but occurred exclusively with the former.6 In-stent stenosis was more frequent (38% for SILK+ and 39% for PED) but the authors reinforce that patients with no signs of in-stent stenosis in the first angiogram did not develop stenosis on the second angiogram. The Copernic 2L allows the operator to deliver the flow diverter and immediately perform an angioplasty, if needed, without having to use any other device, such as an angioplasty balloon or a low-pressure balloon, and without having to perform an exchange guidewire manoeuvre.
The first operator (TK) reported that navigating the Copernic 2L was very straightforward as it tracked very well over the micro-guidewire; however, the deployment of the SILK+ was slightly more challenging as the DLBC is stiffer than the VASCO + 21 (Balt Extrusion, Montmorency, France). This could also be attributed to the broad-neck morphology of this specific aneurysm. During the inflation, the balloon was stable and showed no tendency to move.
Our initial strategy included partial coiling of the aneurysm but this was not feasible due to slipping of the jailed microcatheter during deployment of the flow diverter. We think this technical shortcoming can be prevented by delivering some loops of the first coil into the aneurysm sac before flow diverter deployment. This would help to support the microcatheter in the aneurysm sac and would act as an anchor to replace the microcatheter into position if it slipped.
Our case demonstrates that flow diverter delivery through a DLBC is technically feasible and represents an efficient strategy to improve wall apposition and stent configuration without the need of additional devices or exchange manoeuvres. We think that in cases of parent vessel narrowing, the use of Copernic 2L may represent a valid option. It is more simple, less risky than and equally effective as using an over-the-wire balloon.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Mustafa W, Kadziolka K, Leautaud A, et al. Double lumen remodelling balloon: A new technique for treatment of bifurcation aneurysms. J Neuroradiol 2013; 38: 183–186. [DOI] [PubMed] [Google Scholar]
- 2.Pukenas B, Albuquerque F, Weigele J, et al. Use of a new double-lumen balloon catheter for single-catheter balloon-assisted coil embolization of intracranial aneurysms: Technical note. Neurosurgery 2011; 69(1 Suppl. Operative): ons8–12. [DOI] [PubMed]
- 3.Gory B, Kessier I, Nakiri G, et al. Initial experience of intracranial aneurysm embolization using the balloon remodeling technique with Scepter C, a new double-lumen balloon. Interv Neuroradiol 2012; 18: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paramasivam S, Niimi Y, Fifi J, et al. Onyx embolization using dual-lumen balloon catheter: Initial experience and technical note. J Neuroradiol 2013; 40: 294–302. [DOI] [PubMed] [Google Scholar]
- 5.Tecle N, Patel B, Ahmadieh T, et al. Novel use of a double lumen balloon catheter for venous sinus thrombolysis and venoplasty. J Clin Neurosci 2015; 22: 1018–1020. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Gomori J, Moscovici S, et al. Delayed complications after flow-diverter stenting: Reactive in-stent stenosis and creeping stents. J Clin Neurosci 2014; 1116–1122. [DOI] [PubMed]
- 7.Van der Marel K, Gounis MJ, Weaver JP, et al. Grading of Regional Apposition after Flow-diverter Treatment (GRAFT) – a comparative evaluation of VasoCT and intravascular OCT. J Neurointerv Surg 2016; 8: 847–852. [DOI] [PubMed] [Google Scholar]
- 8.Kizilkilic O, Kocer N, Metaxas G, et al. Utility of VasoCT in the treatment of intracranial aneurysm with flow-diverter stents. J Neurosurg 2012; 117: 45–49. [DOI] [PubMed] [Google Scholar]
- 9.Berge J, Biondi A, Machi P, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. Am J Neuroradiol 2012; 33: 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]