Abstract
We report a case of tentorial dural arteriovenous fistula (DAVF) with a severe intracranial hemorrhage occurring after Onyx embolization. A 40-year-old man presented with an asymptomatic tentorial DAVF on angiography. Transarterial embolization with Onyx was performed via the middle meningeal artery, and the cast filled the fistula itself and its proximal draining vein. Postoperative angiography confirmed complete occlusion of the DAVF. A computed tomography scan performed immediately after the procedure demonstrated an acute subdural hematoma with the temporal hemorrhage. Emergency craniotomy revealed continuous arterial bleeding from a viable glomus-like vascular structure around the proximal part of the embolized draining vein, fed by a pial artery arising from the posterior cerebral artery. Pathologic findings suggested diagnosis of vascular malformation extending into the subdural space. Tentorial DAVFs can extend to the subdural space along their drainage route, and may be involved in severe hemorrhagic complications of curative endovascular treatment using Onyx, particularly those with pial arterial supply.
Keywords: Dural arteriovenous fistula, endovascular treatment, hemorrhage, Onyx
Introduction
Tentorial dural arteriovenous fistulas (DAVFs) make up approximately 4%–8% of all intracranial DAVFs.1,2 They are universally associated with retrograde leptomeningeal drainage and present with an aggressive clinical course in 79% of patients.3,4 Therefore, surgical or endovascular intervention is recommended even for asymptomatic tentorial DAVFs.2,4–7 With recent advances in endovascular technology, patients with tentorial DAVFs are increasingly treated endovascularly with a high rate of good neurologic outcomes.5,8 Onyx (Medtronic Co., Dublin, Ireland) is a liquid embolic agent that offers increased controllability and deep fistula penetration due to its lower viscosity and delayed precipitation compared with other embolic agents. Endovascular treatment of DAVFs with Onyx is typically performed via a transarterial approach with the aim of obliterating the fistula to prohibit abnormal venous outflow.9,10 Although several studies have reported that the treatment of tentorial DVAFs by intracranial arterial injection of Onyx is safe and effective,5,7,9–16 little is known about the potential for severe hemorrhagic complications after this treatment.
Here, we report the case of a tentorial DAVF treated by transarterial Onyx embolization complicated by a severe intracranial hemorrhage immediately after the procedure. We investigated the relationship between subdural DAVF extension and post-embolization hemorrhage using our intraoperative findings and the histopathological examination.
Case report
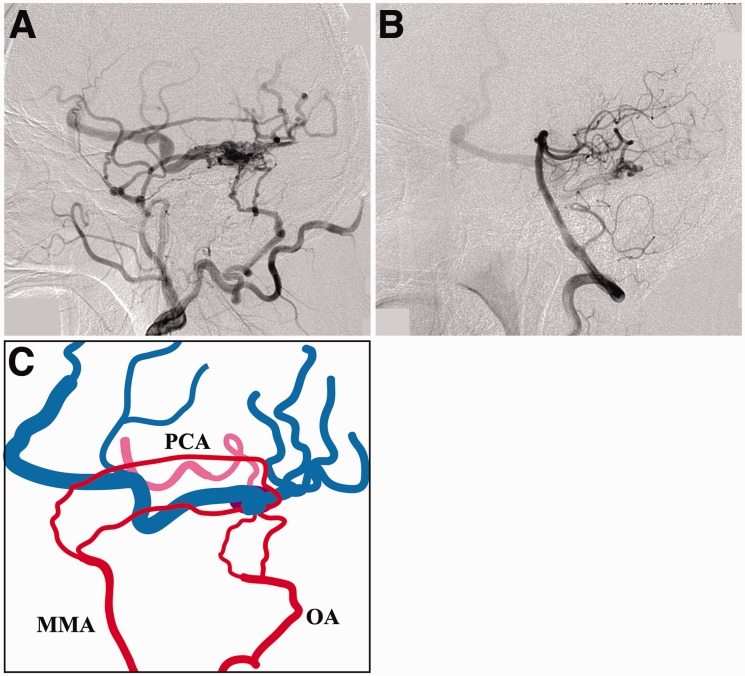
A 40-year-old man presented with an asymptomatic DAVF discovered incidentally during a magnetic resonance imaging examination. Digital subtraction angiography (DSA) showed that the lesion was fed by the posterior branches of the right middle meningeal artery (MMA), mastoid branches of the right occipital artery, and a pial branch arising from the right posterior cerebral artery (PCA) that runs laterally along the tentorium cerebelli. The DAVF drained exclusively into ectatic supratentorial cortical veins (Figure 1). Cross-sectional images revealed that it was located laterally to the point where the tentorium joined the dura of the right middle cranial fossa (Figure 2).
Figure 1.
a) The right external carotid artery angiogram demonstrating a tentorial dural arteriovenous fistula (DAVF). (b) The left vertebral artery angiogram. The DAVF is also fed by a pial branch originating from the right posterior cerebral artery (PCA). (c) Schematic drawing of the angioarchitecture showing the feeding arteries arising from the right external carotid artery (red) and from the PCA (pink), and the cortical venous drainage (blue). MMA: middle meningeal artery; OA: occipital artery; PCA: posterior cerebral artery.
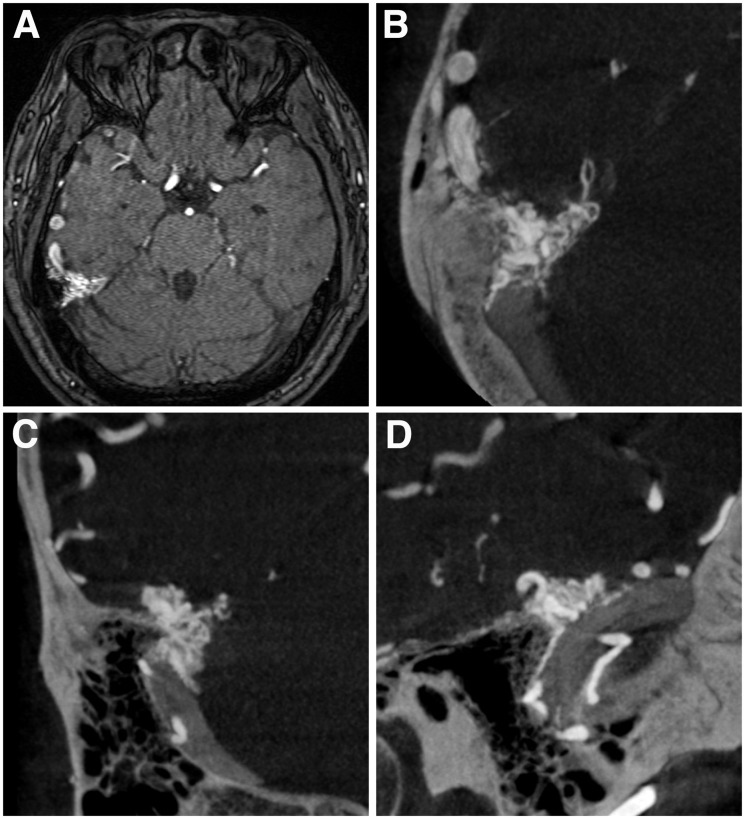
Figure 2.
a) Time-of-flight magnetic resonance image. No parenchymal vascular malformation is seen. (b)–(d) Cone-beam computed tomography scan images ((b) axial; (c) coronal; (d) sagittal) obtained by rotational angiography of the external carotid artery demonstrating a vessel cluster located lateral to the point where the tentorium joins the dura of the right middle cranial fossa and a dilated cortical vein in the right temporal lobe.
The endovascular procedure was performed under general anesthesia and systemic heparinization. A Marathon microcatheter was advanced into the petrosquamous branch of the MMA (Figure 3). Onyx 18 was injected according to the plug-creating technique until it opacified the proximal portion of the draining vein. The microcatheter was withdrawn without complications. Systemic heparinization was reversed by the administration of protamine sulfate.
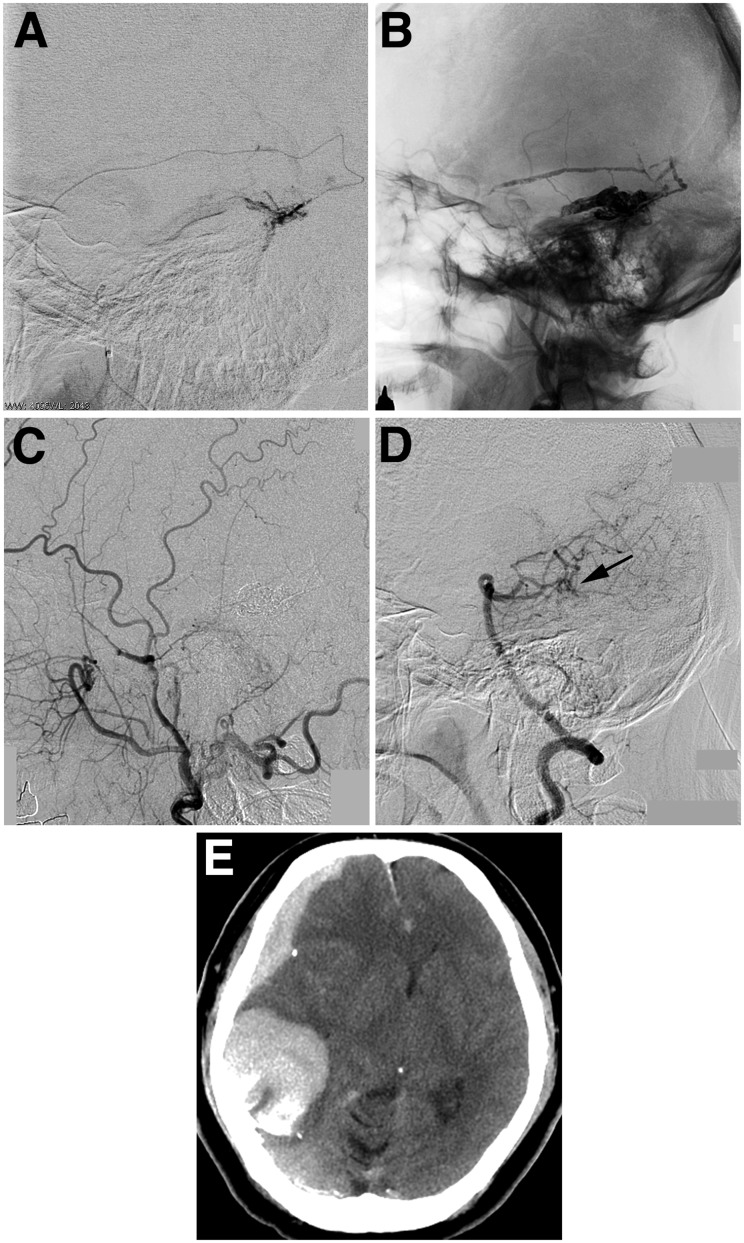
Figure 3.
a) Superselective angiogram of the petrosquamous branch of the middle meningeal artery. (b) Cranial plain radiography showing Onyx cast. The right external carotid artery angiogram (c) and the left vertebral artery angiogram (d) demonstrating the complete disappearance of the dural arteriovenous fistula. The pial feeding artery seems to be occluded (arrow). (e) Computed tomography after endovascular treatment demonstrating a right acute subdural hematoma with a temporal hemorrhage.
DSA performed immediately after the procedure demonstrated the complete disappearance of the tentorial DAVF (Figure 3). Stagnation of the contrast was seen in the pial feeding artery from the right PCA. However, the patient exhibited impaired consciousness and left hemiparesis when waking from the anesthesia. A computed tomography scan revealed an acute subdural hematoma with temporal hemorrhage in the right side (Figure 3). We performed an emergency craniotomy and found continuous arterial bleeding from a viable glomus-like vascular structure around the proximal part of the embolized draining vein and the tentorium, fed by a pial artery arising from the PCA (Figure 4). The hematoma was evacuated, the patent pial feeding artery was coagulated and cut, and the viable abnormal vascular structure was resected. Histological findings suggested diagnosis of vascular malformation extending into the subdural space (Figure 5).
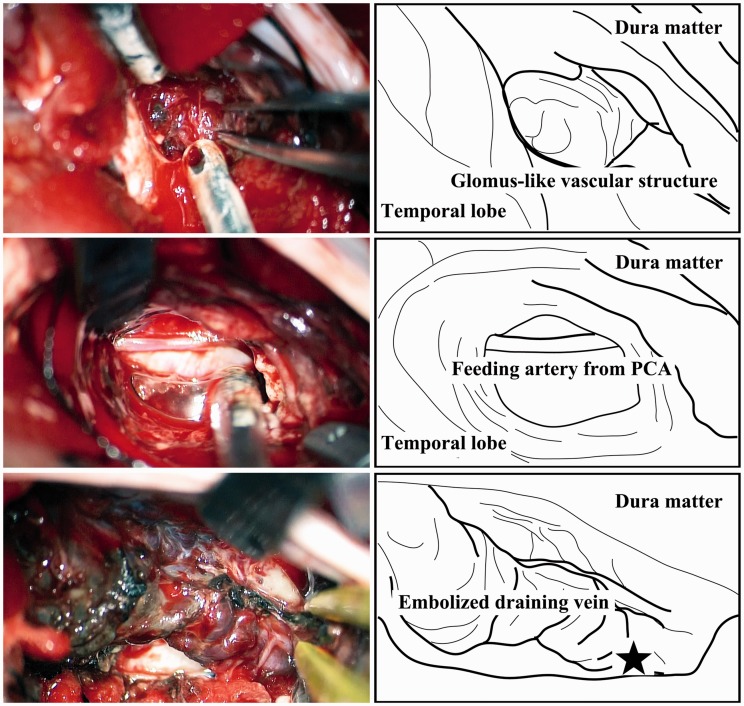
Figure 4.
Operative photographs (left) and corresponding illustrations (right) show a viable glomus-like vascular structure protruding from the inner surface of the dura into the subdural space (upper row), a pial feeding artery originating from the right posterior cerebral artery and traveling laterally along the superior surface of the tentorium (middle row), and the hypervascularized tentorium and vasa vasorum around the embolized draining vein (lower row). The asterisk indicates the location where the viable glomus-like vascular structure was found.
Figure 5.
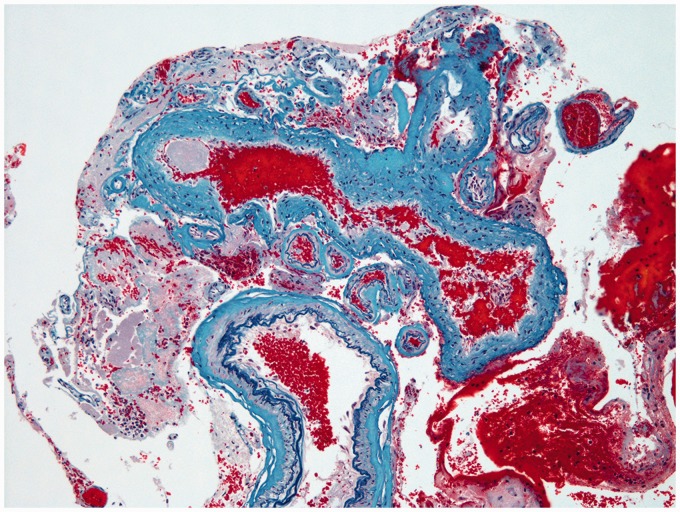
Histological images demonstrating an abnormal aggregation of vascular structures, including vessels that have an irregularly thickened wall caused by elastic fibrosis (corresponding to arterialized veins), and vessels with internal elastic lamina and regular smooth muscle (corresponding to normal arteries). Modified Verhoeff Elastic-Masson Trichrome Stain. Original magnification × 100.
The patient’s postoperative course was favorable, and he showed clinical improvement. He was discharged home with quadrantanopsia and mild left-side agnosia (Glasgow Outcome Scale score of 4). DSA performed six months postoperatively again demonstrated the complete resolution of the tentorial DAVF.
Discussion
This case demonstrates the risk of curative endovascular treatment using Onyx for tentorial DAVFs. The tentorial DAVF in our case was complicated not only by arteriovenous shunts in the dural leaflet but by glomus-like angioarchitecture around the subdural drainage vein and a hypervascularized tentorium with a pial arterial supply. We attempted transarterial Onyx embolization of both the fistula itself and the proximal draining vein. Although the postoperative angiogram showed the fistula had disappeared, the residual glomus-like vascular malformation produced continuous arterial bleeding. These findings highlight that subdural extension of a tentorial DAVF is possible. Further, this case illustrates the need for extreme caution when planning treatment for a DAVF accompanied by additional angioarchitectural defects, especially when a pial arterial supply is also present.
Intracranial DAVFs have been defined as abnormal connections between feeding arteries and a dural venous sinus or leptomeningeal vein, with the point of fistulization located within the dural leaflets. It is important to distinguish between DAVFs that drain directly into a dural sinus and those with leptomeningeal venous drainage because the latter are not protected by a dural envelope.17 In some tentorial DAVFs, especially those with high arteriovenous shunt flow, the exact site of the fistula is not always apparent because there are a variety of fine dural arteries and the tentorium is hypervascularized.17 Venous hypertension around the fistula may promote the growth of microscopic arteriovenous shunts, which are found within the vasa vasorum of normal pachymeninges.18 Furthermore, although most of the feeding arteries are thought to be dural arteries, tentorial DAVFs may still be fed by pial arteries. 2,19 DAVFs with pial arterial supply, also called mixed or acquired pial-dural arteriovenous fistula by some investigators, have rarely been reported.20,21 Their etiology and pathogenesis are unclear, but treatment with simple venous outflow disconnection may be insufficient and risky. Additional procedures, including the obliteration and removal of the subdural component or the individual catheterization and occlusion of pial feeding arteries, may be required for the treatment of this type of DAVF.19,21
Serious complications related to the Onyx embolization of DAVFs are infrequent and occur almost exclusively in those with leptomeningeal drainage.11,14 These complications include intracranial hemorrhage, which has been reported in cases with complete obliteration of DVAFs.7,9 Intracranial hematoma immediately after curative endovascular treatment usually occurs near the fistulous site rather than distal to the occluded draining veins or the previously ruptured venous varix.2,7,9,19 These hemorrhagic complications may be secondary to the restriction of venous outflow, which is similar to the mechanism of hemorrhage during the procedure for cerebral arteriovenous malformations. In our case, high arteriovenous shunt flow and the complex angioarchitecture of the DAVF may have made the retrograde filling of pial feeding arteries and the subdural component of the DAVF impossible, resulting in hemorrhage. Staged embolization or surgical fistula disconnection combined with embolization may be safer than a complete single-session Onyx embolization. At a minimum, major pial feeding arteries, and if possible the entire pial network, should be obliterated before performing curative embolization.
Conclusion
Tentorial DAVFs can extend to the subdural space along their drainage route. Those with pial arterial supply may be involved in hemorrhagic complications of curative endovascular treatment using Onyx. A precise understanding of the angioarchitecture and shunt flow dynamics may be required for the safe and effective treatment of DAVFs.
Acknowledgments
We thank Dr Mika Watanabe (Department of Pathology, Tohoku University Hospital) for her contribution to the histological study. We would like to thank Editage (www.editage.jp) for English language editing.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Hiramatsu M, Sugiu K, Hishikawa T, et al. Epidemiology of dural arteriovenous fistula in Japan: Analysis of Japanese Registry of Neuroendovascular Therapy (JR-NET2). Neurol Med Chir (Tokyo) 2014; 54: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller TR, Gandhi D. Intracranial dural arteriovenous fistulae: Clinical presentation and management strategies. Stroke 2015; 46: 2017–2025. [DOI] [PubMed] [Google Scholar]
- 3.Bulters DO, Mathad N, Culliford D, et al. The natural history of cranial dural arteriovenous fistulae with cortical venous reflux—the significance of venous ectasia. Neurosurgery 2012; 70: 312–318. [DOI] [PubMed] [Google Scholar]
- 4.Youssef PP, Schuette AJ, Cawley CM, et al. Advances in surgical approaches to dural fistulas. Neurosurgery 2014; 74: 532–541. [DOI] [PubMed] [Google Scholar]
- 5.Cannizzaro D, Brinjikji W, Rammos S, et al. Changing clinical and therapeutic trends in tentorial dural arteriovenous fistulas: A systematic review. AJNR Am J Neuroradiol 2015; 36: 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross BA, Du R. The natural history of cerebral dural arteriovenous fistulae. Neurosurgery 2012; 71: 594–602. [DOI] [PubMed] [Google Scholar]
- 7.Jiang C, Lv X, Li Y. Endovascular treatment of high-risk tentorial dural arteriovenous fistulas: Clinical outcomes. Neuroradiology 2009; 51: 103–111. [DOI] [PubMed] [Google Scholar]
- 8.Daniels DJ, Vellimana AK, Zipfel GJ, et al. Intracranial hemorrhage from dural arteriovenous fistulas: Clinical features and outcome. Neurosurg Focus 2013; 34: E15. [DOI] [PubMed] [Google Scholar]
- 9.Cognard C, Januel AC, Silva NA, Jr, et al. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: New management using Onyx. AJNR Am J Neuroradiol 2008; 29: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanlandingham M, Fox B, Hoit D, et al. Endovascular treatment of intracranial dural arteriovenous fistulas. Neurosurgery 2014; 74(Suppl 1): S42–S49. [DOI] [PubMed] [Google Scholar]
- 11.Abud TG, Nguyen A, Saint-Maurice JP, et al. The use of Onyx in different types of intracranial dural arteriovenous fistula. AJNR Am J Neuroradiol 2011; 32: 2185–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandra RV, Leslie-Mazwi TM, Mehta BP, et al. Transarterial Onyx embolization of cranial dural arteriovenous fistulas: Long-term follow-up. AJNR Am J Neuroradiol 2014; 35: 1793–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Q, Xu Y, Hong B, et al. Use of Onyx in the management of tentorial dural arteriovenous fistulae. Neurosurgery 2009; 65: 287–292. [DOI] [PubMed] [Google Scholar]
- 14.Lv X, Jiang C, Zhang J, et al. Complications related to percutaneous transarterial embolization of intracranial dural arteriovenous fistulas in 40 patients. AJNR Am J Neuroradiol 2009; 30: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maimon S, Nossek E, Strauss I, et al. Transarterial treatment with Onyx of intracranial dural arteriovenous fistula with cortical drainage in 17 patients. AJNR Am J Neuroradiol 2011; 32: 2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puffer RC, Daniels DJ, Kallmes DF, et al. Curative Onyx embolization of tentorial dural arteriovenous fistulas. Neurosurg Focus 2012; 32: E4. [DOI] [PubMed] [Google Scholar]
- 17.Heros RC. Tentorial AVMs. J Neurosurg 1995; 82: 1098–1100. [DOI] [PubMed] [Google Scholar]
- 18.Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg 1997; 87: 267–274. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Zhang XS, Wang HD, et al. Onyx embolization for tentorial dural arteriovenous fistula with pial arterial supply: Case series and analysis of complications. World Neurosurg 2016; 92: 58–64. [DOI] [PubMed] [Google Scholar]
- 20.Kato N, Tanaka T, Suzuki Y, et al. Multistage indocyanine green videoangiography for the convexity dural arteriovenous fistula with angiographically occult pial fistula. J Stroke Cerebrovasc Dis 2012; 21: 918.e1–918.e5. [DOI] [PubMed] [Google Scholar]
- 21.Jimbo H, Ikeda Y, Izawa H, et al. Mixed pial-dural arteriovenous malformation in the anterior cranial fossa—two case reports. Neurol Med Chir (Tokyo) 2010; 50: 470–475. [DOI] [PubMed] [Google Scholar]