Rice (Oryza sativa), the primary source of calories for more than 2 billion people, is the most sensitive of all cereal crops to soil salinity, which affects more than 20% of irrigated arable land (FAO and ITPS, 2015). Rice paddies are mainly located at the delta of rivers, where they can be inundated with saline floods at any developmental stage. Vegetative and reproductive phases are the most affected by high salinity.
Physiological and molecular studies have uncovered ion channels and transporters involved in mineral nutrition that are also key to plant resilience to salinity. These are the gateway for ions that are necessary for cell function but also are linked to ion toxicity, thus acting on two sides of the same coin. The major quantitative trait loci for salt tolerance in cereals encode members of the Trk/HKT superfamily of transporters. These include rice SKC1/HKT1;5, Triticum aestivum Kna1, and Triticum turgidum ssp. durum Nax2 (Mickelbart et al., 2015).
Unfortunately, salinity tolerance is not achieved by simply up-regulating the expression of HKT1;5 without negative effects on plant growth. This is largely due to the importance of transporter localization within specific cells in the root xylem parenchyma, where it functions to drain Na+ from the xylem into outer root cells during the vegetative phase growth. This localized transport limits movement of ions to the shoot (Hauser and Horie, 2010; Plett et al., 2010). Allelic variation in HKT1;5s within Triticum species has enabled breeding of durum and other wheats with pronounced salt tolerance. Allele variation includes distinctions in site of gene expression as well as protein sequence, likely influencing structure and activity. (Mickelbart et al., 2015; Platten et al., 2013).
It is now recognized that salt tolerance mediated by HKT1-type transporters is more complex than expected. In this context, Chen and coworkers report in this issue of Plant Physiology (Chen et al., 2017) a correlation between Mg2+ transporter OsMGT1 and OsHKT1;5 activity in rice. These new findings renew opportunities for improvement of salinity tolerance in crops.
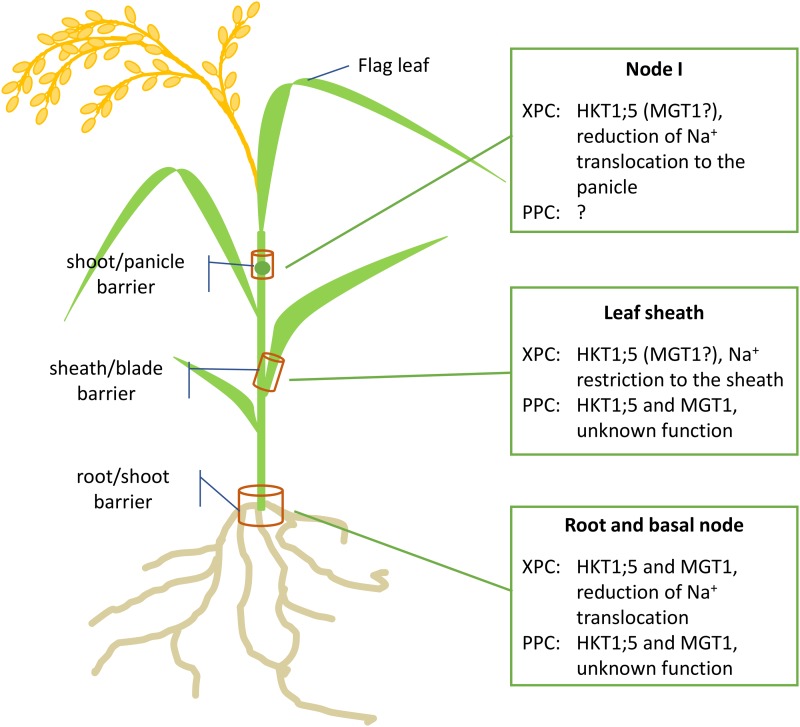
Chen et al. show that OsMGT1 loss-of-function mutants hyperaccumulate Na+ in shoots, a phenotype typical of OsHKT1;5 mutants. Amelioration of this phenotype is achieved by providing Mg2+ at a concentration that limits Mg2+ deficiency, raising the possibility that Mg2+ acts at some level in root-to-shoot Na+ translocation. Moreover, the osmgt1 oshkt1;5 double mutant displayed the same phenotype as each single mutant, supporting the conclusion that the two transporters act in the same pathway. The spatial expression of OsMGT1 in xylem parenchyma cells of roots and in phloem parenchyma cells of basal node and leaf sheath tissue partly overlaps with that of OsHKT1;5 (Kobayashi et al., 2017; Fig. 1). Such refined insight of coexpression and localization of these two transporters aids understanding of the complexity of Na+ regulation at the organismal level.
Figure 1.
Colocalization of OsGMT1 and OsHKT1;5 in xylem parenchyma cells (XPC) and phloem parenchyma cells (PPC) in a rice plant. Regulation of the activity of both transporters might orchestrate barriers preventing indiscriminate Na+ accumulation in young leaves and reproductive organs.
The functional connection between OsMGT1 and OsHKT1;5 in tuning Na+ content in the xylem sap opens new perspectives in engineering salt-tolerant crops. Phloem transport redistributes solutes and ions throughout the plant, but it was thought to play a marginal role in Na+ tolerance (Maathuis et al., 2014). The colocalization of the two transporters in the phloem companion cells of basal nodes and sheaths points out a role in Na+ redistribution in shoots, to reduce toxic effects.
From the new findings, along with those of Kobayashi et al. (2017), one can deduce that nodes and sheaths play a vital role as barriers for uncontrolled Na+ movement (Fig. 1), thus avoiding ion toxicity in sensitive tissues particularly in reproductive organs. Interestingly, Na+ restriction to older leaves, a typical salt tolerance mechanism (Cotsaftis et al., 2012), is lost in osmgt1 mutants (Chen et al., 2017). This suggests that OsMGT1 contributes to the fine-tuning of HKT1;5 activity. It would be valuable to know more of the cell-specific expression and role of OsMGT1 during later developmental stages, as OsHKT1;5 expression is higher during the reproductive stage, in particular in node I (Kobayashi et al., 2017; Fig. 1).
Electrophysiology of OsHKT1;5 by Chen and colleagues endorses the role of Mg2+ in enhancing Na+ flux, although the mechanism is completely unknown. A similar current potentiation was reported for the carrot K+ channel KDC1 in the presence of Zn2+ (Picco et al., 2004). Histidines on external loops of KDC1 mediate the Zn2+ coordination promoting conformational changes that potentiate the flux. To address if a similar potentiation mechanism is involved in modulation of OsHKT1;5 activity by Mg2+, site-directed mutagenesis on specific amino acids on the external side could be performed.
Open questions are indeed left when we consider that OsMGT1 should mediate the transport of Mg2+ to the cytosol, while HKT1;5 activity seems to be modulated by high external (apoplastic) [Mg2+]. Further investigations should address this point.
From Chen et al. (2017) and Kobayashi et al. (2017), it is evident that nodes act as barriers where OsMGT1 regulates the gating of OsHKT1;5 in a spatio-temporal manner to limit ion toxicity. More experiments are required to better define the dynamics of such barriers in view of future biotechnological applications.
References
- Chen ZC, Yamaji N, Horie T, Che J, Li J, An G, Ma JF (2017) A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physiol 174: 1837–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsaftis O, Plett D, Shirley N, Tester M, Hrmova M (2012) A two-staged model of Na+ exclusion in rice explained by 3D modeling of HKT transporters and alternative splicing. PLoS One 7: e39865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, ITPS (2015) Status of the World’s Soil Resources (SWSR): Main Report. Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils, Rome [Google Scholar]
- Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K(+)/Na(+) ratio in leaves during salinity stress. Plant Cell Environ 33: 552–565 [DOI] [PubMed] [Google Scholar]
- Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T, et al. (May 10,2017) OsHKT1;5 mediates Na(+) exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J 10.1111/tpj.13595 [DOI] [PubMed] [Google Scholar]
- Maathuis FJ, Ahmad I, Patishtan J (2014) Regulation of Na(+) fluxes in plants. Front Plant Sci 5: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelbart MV, Hasegawa PM, Bailey-Serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet 16: 237–251 [DOI] [PubMed] [Google Scholar]
- Picco C, Bregante M, Naso A, Gavazzo P, Costa A, Formentin E, Downey P, Lo Schiavo F, Gambale F (2004) Histidines are responsible for zinc potentiation of the current in KDC1 carrot channels. Biophys J 86: 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten JD, Egdane JA, Ismail AM (2013) Salinity tolerance, Na+ exclusion and allele mining of HKT1;5 in Oryza sativa and O. glaberrima: many sources, many genes, one mechanism? BMC Plant Biol 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett D, Safwat G, Gilliham M, Skrumsager Møller I, Roy S, Shirley N, Jacobs A, Johnson A, Tester M (2010) Improved salinity tolerance of rice through cell type-specific expression of AtHKT1;1. PLoS One 5: e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]