Exogenous auxin inhibits cell division and elongation of Klebsormidium nitens and causes early induction of an LBD-type transcription factor.
Abstract
The phytohormone auxin regulates many aspects of growth and development in land plants, but the origin and evolution of auxin signaling and response mechanisms remain largely unknown. Indeed, it remains to be investigated whether auxin-related pathways diverged before the emergence of land plants. To address this knowledge deficit, we analyzed auxin responses in the charophyte alga Klebsormidium nitens NIES-2285, whose ancestor diverged from a green algal ancestor during the evolution of land plants. This strain is the same as Klebsormidium flaccidum NIES-2285, for which the draft genome was sequenced in 2014, and was taxonomically reclassified as K. nitens. This genome sequence revealed genes involved in auxin responses. Furthermore, the auxin indole-3-acetic acid (IAA) was detected in cultures of K. nitens, but K. nitens lacks the central regulators of the canonical auxin-signaling pathway found in land plants. Exogenous IAA inhibited cell division and cell elongation in K. nitens. Inhibitors of auxin biosynthesis and of polar auxin transport also inhibited cell division and elongation. Moreover, exogenous IAA rapidly induced expression of a LATERAL ORGAN BOUNDARIES-DOMAIN transcription factor. These results suggest that K. nitens has acquired the part of the auxin system that regulates transcription and cell growth without the requirement for the central players that govern auxin signaling in land plants.
The physiological and morphological effects of auxins have been well studied in land plants. Auxins play crucial roles in regulating many aspects of plant growth and development. For example, auxins are required for root hair initiation and elongation (Takahashi, 2013), formation of all primordia (Gallavotti, 2013), and developmental decisions to initiate various plant tissues such as cotyledons, roots, flowers, and leaves (Vanneste and Friml, 2009). Moreover, auxins regulate cell division and cell expansion during plant growth and development (Perrot-Rechenmann, 2010). Changes in auxin accumulation patterns mediate regulation of tropic growth in response to light and gravity (Adamowski and Friml, 2015). These auxin-dependent plant developmental processes are regulated by the combination of auxin metabolism, transport, and perception/signaling (Sauer et al., 2013).
Recent progress in genome analysis and molecular genetics of model bryophytes has uncovered auxin functions in bryophytes. The genome analysis of the moss Physcomitrella patens revealed the presence of principal gene families involved in auxin homeostasis and signaling (Rensing et al., 2008). In P. patens, auxin induces rhizoid development (Sakakibara et al., 2003). The auxin-signaling pathway is mediated by TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX (TIR1/AFB), and Auxin/INDOLE-3-ACETIC ACID (Aux/IAA) contributes to the chloronema-to-caulonema transition and normal rhizoid development (Prigge et al., 2010). Moreover, analysis of mutants for the auxin-efflux carrier PIN-FORMED (PIN) revealed that PINs in P. patens contribute to the regulation of auxin distribution in the gametophore (Bennett et al., 2014) and to fertility and development of sporophytes (Fujita et al., 2008; Bennett et al., 2014). PIN-mediated auxin transport regulates the chloronema-to-caulonema transition and gametophore leaf development (Viaene et al., 2014). In the liverwort Marchantia polymorpha, no homolog of AUXIN BINDING PROTEIN (ABP1) was found in the genome (Kato et al., 2015), but the M. polymorpha and P. patens genomes contain homologs of the canonical auxin-signaling factors TIR1/AFB, Aux/IAA, and AUXIN RESPONSE FACTOR (ARF; Kato et al., 2015). These auxin-signaling components regulate normal cell elongation and differentiation in M. polymorpha (Flores-Sandoval et al., 2015; Kato et al., 2015). Consequently, auxin-related genes show very few differences between land-plant lineages (Finet and Jaillais, 2012), suggesting that the last common ancestor of land plants had already acquired the core auxin machinery of land plants. However, the origin of the auxin system of land plants remains unclear.
Auxin has been detected in many algae (Niemann and Dörffling, 1980; Cooke et al., 2002; Tarakhovskaya et al., 2007; Stirk et al., 2013). Analyses of various algal genomes indicates that some algae have a subset of auxin biosynthesis genes (De Smet et al., 2011; Le Bail et al., 2010; Finet and Jaillais, 2012), but these genomes do not harbor genes for the components of the central auxin-signaling pathway mediated by TIR1-Aux/IAA-ARF in land plants (Rensing et al., 2008; Riaño-Pachón et al., 2008; Lau et al., 2009). Furthermore, the effects of auxin have been reported in some algae. In the red alga Grateloupia dichotoma, for example, auxin induces the elongation of cut segments and inhibition of the formation of lateral branches (Yokoya and Handro, 1996). In the green alga Chlorella pyrenoidosa, auxin is involved in cell division (Vance, 1987) and promotes enlargement of Chlorella vulgaris cells (Yin, 1937). In the brown algae, the establishment of polarity in developing zygotes is impaired by auxin and auxin transport inhibitors in Fucus distichus (Basu et al., 2002; Sun et al., 2004) and by an auxin transport inhibitor in Fucus vesiculosus (Polevoĭ et al., 2003). In addition, in the brown alga Ectocarpus siliculosus, candidate genes involved in auxin metabolism and signaling have been identified, and auxin has been shown to modify the branching pattern via relaying cell-cell positional information in the sporophyte phase (Le Bail et al., 2010). Red and green algae, which are primary symbiotic algae, diverged long ago from these ancestors; moreover, brown algae are secondary symbiotic algae for which the genetic systems differ greatly from those of primary symbiotic algae. Nevertheless, it is curious that auxin may contribute to growth regulation of some algae. Although more detailed information at the molecular level may be needed to fully understand auxin signaling in algae, the relative lack of genetic information for algal auxin responses makes it difficult to understand not only the responses to auxins in algal cells but also the origin and evolution of auxin signaling in photosynthetic organisms.
The common ancestor of the land plants is believed to be closely related to charophyte algae (Lewis and McCourt, 2004: Leliaert et al., 2012). In fact, auxin has been detected in some charophyte algae (Cooke et al., 2002; Hori et al., 2014), and several auxin responses have been reported. In Micrasterias thomasiana, cell division is induced in the presence of auxin (Wood and Berliner, 1979). Depolymerization of microtubules with auxin was observed in Chara globularis (Jin et al., 2008). Furthermore, PIN-family genes have been identified in the charophyte algae Spirogyra pratenis (De Smet et al., 2011), Klebsormidium flaccidum UTEX321 (Viaene et al., 2013; see below) and Klebsormidium nitens NIES-2285 (Hori et al., 2014; see below) by transcriptome and genome analyses, but their functions in auxin transport remain unclear. The existence of polar auxin transport is supported in several Chara species (Dibb-Fuller and Morris, 1992: Klambt et al., 1992; Boot et al., 2012; Zhang and van Duijn, 2014; Żabka et al., 2016). Therefore, unraveling the auxin system in charophyta—the polyphyletic group that diverged during the evolution of land plants from a green algae ancestor—will be helpful for clarifying the origin and evolution of auxin function and signaling.
We previously reported the draft genome sequence of K. nitens NIES-2285, which consists of simple, nonbranching, filamentous cells. K. nitens NIES-2285 is the strain formerly identified as K. flaccidum, the draft genome for which was reported by Hori et al. (2014). This strain was taxonomically reclassified as K. nitens. Moreover, it was also originally the same as K. flaccidum UTEX321. We previously identified gene homologs for several auxin-biosynthesis and auxin signaling-related factors, TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA), YUCCA (YUC) flavin monooxygenase-like proteins, PIN, AUXIN RESISTANT 1/LIKE AUX1, and ABP1, in K. nitens; furthermore, the auxin IAA has been detected in K. nitens (Hori et al., 2014). On the other hand, the draft genome sequence suggested that K. nitens does not have the TIR1-Aux/IAA-ARF-mediated auxin-signaling pathway. Therefore, we investigated the effects of exogenous auxin and auxin inhibitors in K. nitens.
RESULTS
Exogenous Auxin and Auxin Transport Inhibitor Inhibit Growth of K. nitens
To investigate the effect of auxin on growth, K. nitens was cultured in the presence of IAA (0.1–100 µm) in liquid medium with aeration. An increase in exogenous IAA inhibited K. nitens growth (Fig. 1). The intracellular IAA concentration in the absence or presence of 100 µm exogenous IAA was quantified with liquid chromatography-tandem mass spectrometry (Supplemental Fig. S1). The concentration of endogenous IAA was normally several dozen pmol g−1 dry weight. After 1 h of treatment with 100 µm IAA, the intracellular IAA level increased to >1000-fold (nmol order). This IAA level then decreased to approximately one-tenth after 24 h but still remained high at the 72 h time point. The IAA concentration-dependent inhibition of growth was probably affected by the stability of IAA in the medium. This result suggested that treatment of K. nitens with exogenous IAA (100 µm) may be necessary for long-term observation of an IAA effect. Therefore, we treated the cells with 100 µm IAA in subsequent experiments.
Figure 1.
Growth of K. nitens in the presence of IAA. K. nitens was cultured in the presence of 0.1 to 100 µm IAA. A, Cell cultures were photographed at 0, 1, 3, and 7 d. B, Growth curves for K. nitens for each concentration of IAA. Error bars represent sd of values for three replicates.
With respect to K. nitens growth, we also examined the effects of another natural auxin, indole-3-buthylic acid (IBA), as well as the synthetic auxins 2,4-dichlorophenoxyacetic acid (2,4-d) and 1-naphthalenacetic acid (NAA), the polar auxin transport inhibitor 2,3,5-triiodobenzoic acid (TIBA), and N-1-naphthylphthalamic acid (NPA). At 100 µm, each of IBA, NAA, and TIBA clearly inhibited growth, although lesser inhibition was observed when compared with IAA; 2,4-d and NPA did not affect growth (Fig. 2; Supplemental Fig. S2). These observations indicated that these auxin-related compounds inhibit K. nitens growth in dose-dependent manner.
Figure 2.
Growth of K. nitens in the presence of several auxins and auxin transport inhibitor. K. nitens was cultured in the presence of 100 μm IAA, IBA, 2,4-d, NAA, or TIBA. A, Cell cultures were photographed at 0, 1, 3, and 7 d. B, Growth curves for K. nitens for each compound. Error bars represent sd of values for three replicates.
We next tested the effects of certain auxin biosynthesis inhibitors. In land plants, 5-(4-chlorophenyl)-4H-1,2,4-triazole-3-thiol (yucasin), 4-phenoxyphenylboronic acid (PPBo), and 4-biphenylboronic acid (BBo) are potent inhibitors of YUCCA enzymes and inhibit growth of Arabidopsis (Arabidopsis thaliana; Nishimura et al., 2014; Kakei et al., 2015). At 100 µm, each of yucasin, PPBo, and BBo inhibited K. nitens growth (Fig. 3; Supplemental Fig. S3). BBo inhibited growth even at 10 µm and its inhibitory effect increased with its concentration (Supplemental Fig. S3). These results suggested that inhibition of YUCCA function inhibits cell growth.
Figure 3.
Growth of K. nitens in the presence of yucasin, PPBo, and BBo. K. nitens was cultured in the presence of 100 μm yucasin, PPBo, or BBo. A, Cell cultures were photographed at 0, 1, 3, and 7 d. B, Growth curves for K. nitens for each compound. Error bars represent sd of values for three replicates.
Cell Division of K. nitens in the Presence of IAA
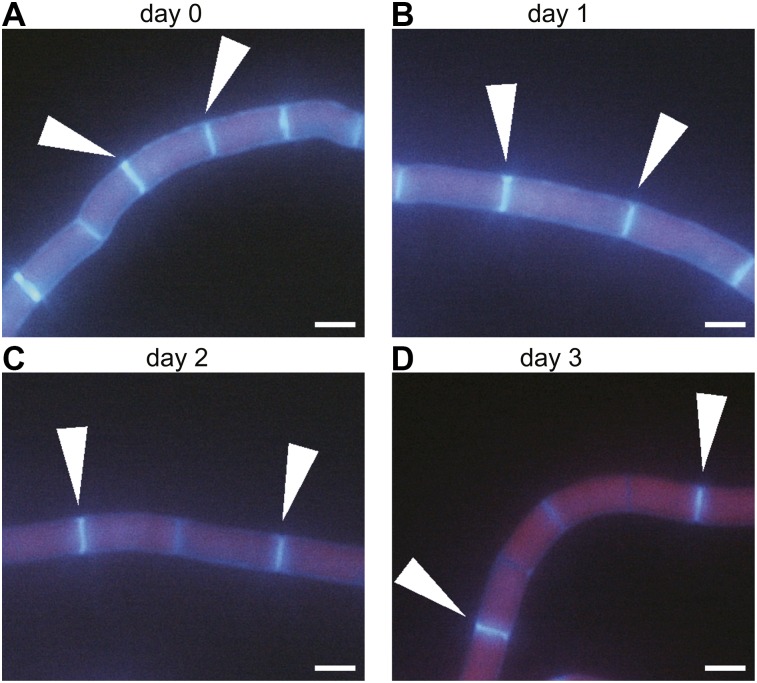
We established a method to observe cell division of K. nitens by transiently staining the cell wall with the fluorescent stain calcofluor white. After removal of the stain, K. nitens was cultured on solid medium. We confirmed that the staining did not affect K. nitens growth (Supplemental Fig. S4). Although the cell wall on day 0 showed strong fluorescence, the fluorescence of de novo synthesized cell wall after staining was very weak. Thus, cell division over time (1–3 d) could be monitored by fluorescence microscopy (Fig. 4; Supplemental Fig. S5) and the effect of 100 µm IAA evaluated (Fig. 5A). By day 3, the untreated control cells had typically divided two times, and the sites of cell division were approximately equally distributed in the filaments of the alga. This suggested that K. nitens does not have specific zones of active cell division, i.e. such as occurs in the meristem of land plants (Fig. 5A; Supplemental Fig. S5A). In the presence of 100 µm IAA, however, almost all the cells did not divide, although the cells were elongated (Fig. 5, A and B; Supplemental Fig. S6). These results indicated that exogenous IAA inhibits cell division of K. nitens.
Figure 4.
Fluorescence micrographs of K. nitens transiently stained with calcofluor white. A, K. nitens was transiently stained with calcofluor white. B to D, The stained K. nitens cells were cultured for 1, 2, or 3 d on solid medium. Red and blue fluorescence indicate chloroplasts (chlorophyll autofluorescence) and cell wall (calcofluor white), respectively. White arrows indicate the cell wall for both sides of the same cell. Scale bars = 5 μm.
Figure 5.
Tracking K. nitens cell division via transient staining with calcofluor white. A, K. nitens transiently stained with calcofluor white was monitored from 0 to 3 d in the absence (left) or presence (right) of 100 μm IAA on solid medium. Red and blue fluorescence indicate chloroplasts (chlorophyll autofluorescence) and cell wall (calcofluor white), respectively. The numerals indicate cell borders at 0 d. Scale bars = 25 μm. B, The frequency of cell division was measured at 1, 2, and 3 d in the absence or presence of 100 μm IAA on solid medium. Bar graph represents the percentage of each frequency class of cell division. The number of samples measured was 105, 138, and 120 for the control cultured for 1, 2, and 3 d, respectively, and 152, 155, and 135 for the IAA treatment group cultured for 1, 2, and 3 d, respectively. This experiment was independently performed three times (Supplemental Fig. S6). C, Schematic of cell division assessment. The frequency of cell division was estimated by counting the number of cells between cell walls stained with calcofluor white at day 0.
Cell Elongation of K. nitens in the Presence of IAA
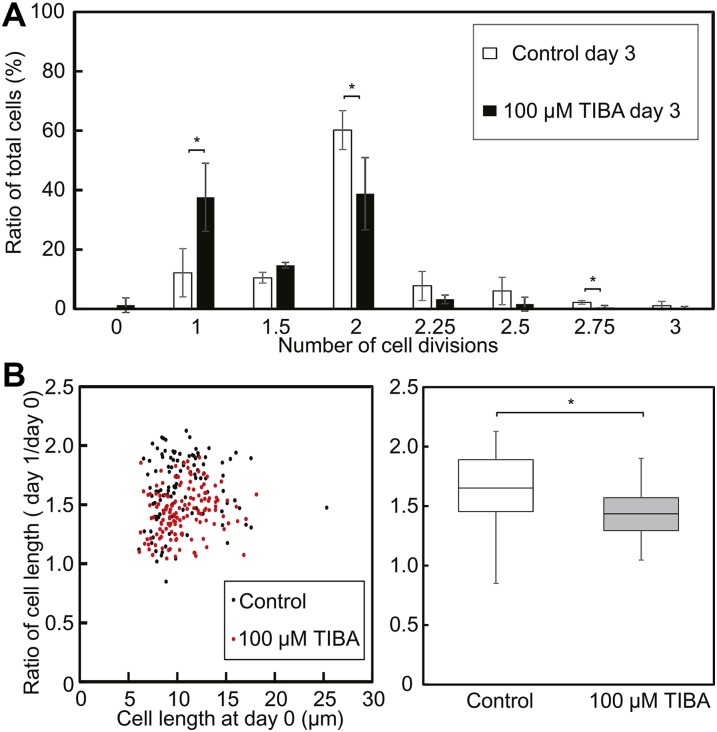
Although IAA clearly inhibited cell division of K. nitens, under the fluorescence microscope, the cells cultured for 5 d in the presence of 100 µm IAA appeared to be longer than control cells (Fig. 6A). The distribution of cell length did not differ significantly between days 0 and 5 in the absence of exogenous IAA. However, the cell length at 5 d in the presence of 100 µm IAA was ∼2 times greater than that at day 0 (Fig. 6B; Supplemental Fig. S7). There are two possibilities for the excessive elongation of the cells in our experiment: (1) IAA promoted cell elongation in K. nitens, or (2) the cells were elongated owing to the inhibition of cell division. Thus, we measured the length of the same undivided cell on day 0 and then again on day 1 post-IAA treatment, and this “elongation ratio” served as a measure of whether 100 µm IAA promoted cell elongation. Comparison of the elongation ratios revealed that exogenous IAA actually suppressed cell elongation (Fig. 6C; Supplemental Fig. S8). Accordingly, cell length in the presence of IAA gradually increased in cells in which cell division was inhibited.
Figure 6.
Elongation of K. nitens cells grown in the presence of IAA. A, K. nitens was cultured for 5 d in the absence or presence of 100 μm IAA on solid medium. Scale bars = 10 µm. B, Distribution of cell length at 5 d in the absence or presence of 100 µm IAA. The number of samples measured was 231 for the control at day 0, 250 for the control at day 5, and 250 for the IAA treatment at day 5. This experiment was independently performed three times (Supplemental Fig. S7). C, Cell elongation ratio (day 1:day 0) represented in dot (left) and box (right) plots. The number of samples measured was 173 (control) and 216 (IAA treatment). *P < 0.01 (Student’s t test). This experiment was independently performed three times (Supplemental Fig. S8).
TIBA Slightly Inhibits Cell Division and Suppresses Cell Elongation
Similarly to the effect of IAA, TIBA also suppressed K. nitens growth (Fig. 2). It has been demonstrated that TIBA inhibits auxin efflux through inhibition of subcellular vesicle trafficking processes, including translocation of PIN by stabilizing actin (Michniewicz et al., 2007; Dhonukshe et al., 2008). It has also been reported that TIBA inhibits the elongation of Arabidopsis root cells (Rahman et al., 2007) and of Nicotiana tabacum cells (Vissenberg et al., 2001). Among basal land plants, e.g. the moss Funaria hygrometrica, TIBA inhibits IAA efflux from protonema and rhizoids (Rose et al., 1983; Rose and Bopp, 1983) and increases IAA accumulation in protoplasts (Geier et al., 1990). Therefore, we also measured cell division and cell elongation in the presence of 100 μm TIBA. The majority of K. nitens cells divided, as expected, two times within 3 d (Fig. 7A). Cell division was delayed, however, in the presence of 100 μm TIBA. Furthermore, the elongation ratio was slightly reduced in the presence of TIBA (Fig. 7B; Supplemental Fig. S9), whereas NPA had no effect on cell division or elongation (Supplemental Fig. S10). These effects of TIBA, although milder, were similar to those of IAA. It has been inferred that the mechanism of action differs between TIBA and NPA (Michniewicz et al., 2007; Dhonukshe et al., 2008). As such, the differential effects of these compounds on K. nitens growth may reflect differences in their intracellular targets. It is expected that polar auxin transport is involved in regulating normal cell division and elongation of K. nitens. Hence, an optimal level and specific distribution of auxin may be required for appropriate growth of K. nitens. However, the possibility cannot be excluded that TIBA affects cellular processes other than auxin transport that cause inhibition of cell division and elongation (Petrásek et al., 2003).
Figure 7.
Cell division and cell elongation of K. nitens grown in the presence of TIBA. A, Frequency of cell division for 3 d in the absence or presence of 100 μm TIBA. Error bars represent sd of values for four replicates. The number of samples analyzed for the four replicates was 157, 162, 138, and 67 (control) and 162, 116, 157, and 94 (TIBA treatment). *P < 0.05 (Student’s t test). B, Cell elongation ratio (day 1:day 0) in dot (left) and box (right) plots. The number of samples measured was 108 (control) and 141 (TIBA treatment). *P < 0.01 (Student’s t test). This experiment was independently performed four times (Supplemental Fig. S9).
BBo Suppresses Cell Elongation
BBo inhibited the growth of K. nitens similarly to IAA and TIBA (Fig. 3; Supplemental Fig. S3). Therefore, we also investigated the effect of BBo on cell division and elongation. Cell division was not strongly inhibited at 30 µm BBo (Fig. 8A). The distribution of cell length after 5 d in the presence of BBo was rarely different from the control (Fig. 8B; Supplemental Fig. S11). As with IAA treatment, however, the elongation ratio was clearly suppressed (Fig. 8C; Supplemental Fig. S12). Therefore, it was obvious that inhibition of YUCCA by 30 µm BBo particularly caused suppression of cell elongation. These results suggested that auxin biosynthesis via YUCCA may contribute to cell elongation in K. nitens.
Figure 8.
Cell division, distribution of cell length after 5 d in culture, and cell elongation of K. nitens grown in the presence of BBo. A, Frequency of cell division for 3 d in the absence or presence of 30 μm BBo. Error bars represent sd of values for three replicates. The number of samples analyzed was 102, 163, and 257 (control) and 157, 137, and 189 (BBo treatment). *P < 0.05 (Student’s t test). B, Distribution of cell length at 5 d in the absence or presence of 30 µm BBo. The number of samples measured was 231 for the control at day 0, 241 for the control at day 5, and 257 for the BBo treatment at day 5. This experiment was independently performed three times (Supplemental Fig. S11). C, Cell elongation ratio (day 1:day 0) in dot (left) and box (right) plots. The number of samples was 84 (control) and 124 (BBo treatment). *P < 0.01 (Student’s t test). This experiment was independently performed three times (Supplemental Fig. S12).
Transcriptome Analysis Reveals Auxin-Responsive Genes
The physiological effects of auxin on cell division and elongation suggested the existence of auxin signaling in K. nitens. Therefore, we assessed auxin-mediated regulation of gene expression by analyzing the K. nitens transcriptome. K. nitens was cultured for 10 h, 3 d, and 7 d in the presence or absence of 100 µm IAA. Subsequent analysis revealed 576 differentially expressed genes (DEGs) among all combinations of these six conditions. For comparison, between the presence or absence of IAA, 84, 170, and 106 DEGs were identified at 10 h, 3 d, and 7 d, respectively (Fig. 9A). The 576 DEGs were clustered into 16 groups by quality threshold clustering (Supplemental Table S1), which facilitated the identification of consistently up- or down-regulated genes following exogenous IAA application (Fig. 9B). Subsequently, we investigated the initial expression changes of some genes in the upregulated Cluster11 and downregulated Cluster8 to search for early-response genes; the real-time PCR analysis identified three up-regulated genes and one down-regulated gene (Fig. 10A; Supplemental Fig. S13). Notably, one of three upregulated genes was a LATERAL ORGAN BOUNDARIES-DOMAIN (LBD) transcription factor, which responded to IAA as early as 1 h of treatment (Fig. 10A). In Arabidopsis, some LBD transcription factors are upregulated via auxin signaling during the formation of lateral organs (Okushima et al., 2007; Lee et al., 2009; Berckmans et al., 2011; Goh et al., 2012; Lee et al., 2013; Lee and Kim, 2013). Therefore, we investigated the change in expression of the K. nitens LBD gene (KnLBD1) in more detail by real-time PCR. KnLBD1 expression was upregulated in an IAA concentration-dependent manner (Fig. 10B). Moreover, KnLBD1 responded to IAA in the presence of the translation inhibitor cycloheximide (Fig. 10C), suggesting direct transcriptional regulation by auxin. Although K. nitens does not have clear homologs of the TIR1-Aux/IAA-ARF auxin-signaling pathway components of land plants (Hori et al., 2014), KnLBD1 was induced early by exogenous IAA via some constitutively expressed signal transduction factor(s). Induction of KnLBD1 did not seem to be an indirect effect of the inhibition of cell division and elongation, as KnLBD1 did not respond to TIBA (Fig. 10D).
Figure 9.
Transcriptome analysis of K. nitens grown in the presence of IAA. A, MA plots of mRNAs counted in each of the paired control and IAA treatment groups at 10 h, 3 d, and 7 d of cell culture. Red dots represent each DEG at 10 h (left, 84 DEGs), 3 d (middle, 170 DEGs), and 7 d (right, 106 DEGs). B, Heatmaps of the consistently down-regulated genes (Cluster8) and up-regulated genes (Cluster11) in the presence of IAA. Clusters were abstracted from log2 fold changes (relative to 10 h control) of 576 DEGs by quality threshold clustering.
Figure 10.
Changes in expression of KnLBD1 mRNA. Changes in the expression of KnLBD1 mRNA were analyzed by real-time PCR of samples from cells incubated for (A) 1, 3, and 6 h in the absence or presence of 100 μm IAA, (B) 6 h in the presence of 0.01, 0.1, 1, 10, or 100 μm IAA, (C) 6 h in the absence or presence of 100 μm IAA and 10 µg/mL cycloheximide (CHX), and (D) 6 h in the absence or presence of 100 μm IAA, 100 μm TIBA, or 100 µM NPA. All error bars represent sd of values for three replicates. B and D, Different lowercase letters (a, b) denote a statistically significant difference between treatments. B, P < 0.05; D, P < 0.01 (Tukey’s test). A and C, *P < 0.1; **P < 0.01 (Student’s t test).
DISCUSSION AND CONCLUSION
Our results show that exogenous IAA inhibits cell division and cell elongation and regulates gene expression in K. nitens, suggesting that endogenous auxin likely also regulates cell division/elongation. Concentration-dependent growth inhibition by IAA has been reported in the auxin response of bryophytes. In the moss P. patens, for example, growth of the gametophore is inhibited in the presence of auxin, and increasing the auxin concentration elicits more severe phenotypes (Ashton et al., 1979; Jang and Dolan, 2011; Bennett et al., 2014). In the liverwort M. polymorpha, exogenous auxin promoted abnormal growth phenotypes such as inhibition of thallus growth and rhizoid formation on the dorsal surface and production of many rhizoids (Tarén, 1958; Kaul et al., 1962; Maravolo and Voth, 1966; Otto and Halbsguth, 1976; Maravolo, 1980; Ishizaki et al., 2012). Hence, the inhibition of cell growth in the presence of exogenous IAA is conserved between K. nitens and bryophytes. However, cell differentiation has not been observed in Klebsormidium except for zoosporogenesis (Marchant et al., 1973; Lokhorst, 1991; Rindi et al., 2008; Škaloud and Rindi, 2013). Indeed, all cells in K. nitens filaments appeared to divide equally (Fig. 5; Supplemental Fig. S5), and there was no local area in the filamentous cells of active cell division. These observations suggest that auxin regulates cell division in K. nitens but does not play a role in cellular differentiation. Unlike land plants including bryophytes, the auxin signaling system composed of TIR1-Aux/IAA-ARF has not been found in K. nitens. These components must have emerged after the divergence of the ancestor of K. nitens and probably allowed for more complex auxin signaling in land plants with multiple cell types and differentiated cells.
The transcriptome analysis of K. nitens revealed that IAA treatment rapidly induced KnLBD1 expression (Fig. 10A). Although K. nitens has five KnLBD genes (group I type) (Supplemental Fig. S14), only KnLBD1 responded to IAA. The accumulation of KnLBD1 mRNA was dependent on IAA concentration but independent of translation (Fig. 10, B and C). In Arabidopsis, some LBD genes respond to auxin via Aux/IAA-ARF and regulate lateral root development (Okushima et al., 2007; Lee et al., 2009, 2013; Berckmans et al., 2011; Goh et al., 2012; Lee and Kim, 2013). It is estimated that the LBD genes were acquired from the common ancestor of K. nitens and land plants. Our phylogenetic tree suggests that LBD genes diverged substantially after land colonization of plants (Supplemental Fig. S14). However, the early stage of divergence was unclear because of low support values. In Arabidopsis, LBD16 and LBD29 are directly regulated by the transcription factors ARF7 and ARF19 (Okushima et al., 2007). In contrast, K. nitens has no ARF genes, and therefore other transcription factors probably regulate KnLBD1. The identification of this transcription factor(s) and its target sequence will help clarify the evolutionary relationship between auxin response of K. nitens and Arabidopsis.
Because K. nitens lacks the Aux/IAA-ARF pathway, it remains unclear which signaling mechanism mediates auxin’s effects on K. nitens growth and KnLBD1 transcription. The K. nitens genome contains a candidate homolog of the ABP1 auxin receptor. In Arabidopsis, the physiological importance of ABP1 is unclear because abp1 null mutants show no obvious phenotype (Gao et al., 2015), and previously reported embryonic-lethal abp1 alleles as well as conditional knockdown lines have only off-target effects (Michalko et al., 2015, 2016). In addition, the liverwort M. polymorpha lacks an ABP1 homolog (Kato et al., 2015). However, mutational analysis of the amino acid residues involved in the formation of the auxin-binding pocket of ABP1 supports a role for ABP1 as an auxin receptor during development (Grones et al., 2015). These auxin-binding residues are conserved in the ABP1-like protein encoded in the K. nitens genome (Supplemental Figure S15), indicating that the ABP1 homolog of K. nitens may also be able to bind auxin. Thus, elucidation of the auxin-response mechanism of K. nitens, which lacks TIR1-Aux/IAA-ARF, may help clarify the function of ABP1.
In addition to mediating auxin signaling, polar auxin transport is involved in the normal growth of land plants. Changes in auxin distribution by auxin transporters contribute to tropism and developmental decisions (Tanaka et al., 2006; Vanneste and Friml, 2009). In P. patens, regulation of auxin distribution by PIN auxin efflux carriers plays important roles in the development of protonemata, gametophores, and sporophytes (Fujita et al., 2008; Bennett et al., 2014; Viaene et al., 2014). The K. nitens genome also has a putative PIN homolog (Hori et al., 2014). We investigated the effect of the polar auxin transport inhibitors, TIBA and NPA, on K. nitens growth. TIBA inhibited cell division and suppressed cell elongation (Fig. 7; Supplemental Fig. S9), whereas NPA had no such effects (Supplemental Fig. S10). Although it is expected that auxin transport mediated by the PIN homolog is involved in controlling endogenous auxin levels and thus regulating cell elongation and division in K. nitens, further validation is required.
Furthermore, K. nitens has a homolog of YUCCA, which is a component of the main auxin biosynthesis pathway in land plants (Mashiguchi et al., 2011; Hori et al., 2014). We thus tested the effect of BBo, a potent inhibitor of YUCCA, on cell division and elongation in K. nitens. BBo suppressed cell elongation more so than cell division (Fig. 8), suggesting that suppression of cell elongation was caused by down-regulation of auxin biosynthesis through inhibition of YUCCA function by BBo. It is possible that the basal level of auxin before BBo treatment was sufficient for cell division. If true, then activation of YUCCA-mediated auxin biosynthesis may be required for normal cell elongation in K. nitens, although a higher concentration of IAA (such as that for exogenous IAA in our experiments) may inhibit both cell division and elongation. It is well established that, with respect to plant growth, the optimal concentration of auxin differs depending on organ type and among land plant species. Inhibition of cell elongation of K. nitens—whether with IAA biosynthesis inhibitors or with exogenous IAA treatment—suggests that normal cell elongation requires an optimal concentration of auxin.
Our study suggests that, during evolution, K. nitens acquired a part of the auxin system that regulates cell division and elongation. Although the auxin system of K. nitens may be relatively simple, it probably provided the basis for the evolution of the more complex auxin system that exists in modern plants. It is possible that the acquisition of the primitive auxin-response pathway occurred before the divergence of Klebsormidiophyceae and was one of the important steps that enabled evolution from a simple body plan to a more complex morphology to adapt to harsh terrestrial environments. In future studies, a more comprehensive examination of auxin systems of charophyte algae, including K. nitens, will help elucidate the origin and evolution of the plant auxin system.
MATERIALS AND METHODS
Culture Conditions and Chemicals
The strain Klebsormidium nitens NIES-2285 was found to be the same as Klebsormidium flaccidum NIES-2285, for which the draft genome was reported by Hori et al. (2014). This strain was taxonomically reclassified as K. nitens. K. nitens NIES-2285 was subcultured in 50 mL liquid 0.1% Glc + BCDAT medium (Nishiyama et al., 2000) with aeration at 23°C under 10 to 20 μmol photons m−2 s−1 of light for 2 to 4 weeks. The cells were harvested by centrifugation for 10 min at 130 g (LC-1000 7050-02 swinging-bucket rotor, TOMY, Tokyo, Japan). The loose cell pellet was transferred to a 10-mL centrifuge tube, resuspended in fresh medium, and collected by centrifugation for 3 min at 130g. The pellet was similarly washed again. Finally, the pellet was resuspended in an 8-fold packed cell volume of liquid C medium (Ichimura, 1971), and 1 mL of cell suspension was inoculated into 50 mL of 0.1% Glc + BCDAT medium. IAA, IBA, 2,4-d, NAA, TIBA, and NPA were first dissolved with 1 n NaOH to make 500 mm stock solutions. Yucasin, PPBo, and BBo were first dissolved with dimethyl sulfoxide to make 100 mm stock solutions.
IAA Quantification
IAA was extracted and purified as described (Hori et al., 2014) with some modifications. Samples of K. nitens cells (20–80 mg, dry weight) were used for extraction, which was carried out overnight. Extracts were subjected to purification via a single passage through an Oasis WAX cartridge column (60 mg, 3 mL, Waters). Liquid chromatography-tandem mass spectrometry analysis was performed as described by Kanno et al. (2016).
Observation of Cell Division and Measurement of Cell Elongation by Calcofluor White Staining
K. nitens was cultured in liquid C medium with aeration at 23°C under 10 to 20 µmol photons m−2 s−1 of light for 4 d. A 1-mL aliquot of culture was plated on solid C medium. K. nitens cultured for 7 d on solid medium was transferred to liquid C medium and stained for 1 h with calcofluor white (final concentration: 10 µg/mL). The cells were pelleted in a mini-centrifuge for ∼10 to 20 s (Force Mini Centrifuge). The pellet was resuspended with C medium, left to stand for 10 min and then collected by centrifugation. This washing procedure was repeated twice. The cell suspension was plated on solid C medium in the absence or presence of 100 µm IAA, TIBA, NPA, or BBo. The cultures were observed by fluorescence microscopy (ECLIPSE 80i equipped with a V-2A filter, Nikon). To calculate the cell elongation ratio, the length of individual K. nitens cells was measured at day 0 (control) and day 1 posttreatment using ImageJ (Abramoff et al., 2004). For analysis of cell-length distribution, unstained K. nitens was cultured and stained with calcofluor white just before fluorescence imaging.
RNA Extraction
Total RNA was extracted from K. nitens cells grown in liquid 0.1% Glc + BCDAT medium in the absence or presence of 100 μm IAA. Cells were harvested by vacuum filtration through a nitrocellulose transfer membrane (Protran, 0.45 µm pore size, Whatman). The pelleted sample was frozen in liquid nitrogen and ground to a fine powder with a mortar and pestle. At least three volumes of RNA extraction buffer (0.8% sodium dodecyl sulfate, 25 mm Tris-HCl, pH 7.6, 25 mm MgCl2, 25 mm KCl):acid phenol (1:1) was added, and the samples were further ground. The aqueous phase was extracted three times by adding an equal volume of acid phenol:chloroform (1:1). The RNA was precipitated by adding an equal volume of isopropanol, and the precipitate was rinsed with 70% ethanol. After the ethanol was removed, the RNA was treated with DNase I at 37°C for 30 min and extracted twice with acid phenol:chloroform (1:1). The RNA was precipitated by adding 2.5 volumes of l00% ethanol. The RNA pellet was dissolved in RNase-free water and used for real-time PCR. For RNA sequencing, the RNA was precipitated by adding one-third volume of 10 m lithium chloride, incubating for >1 h at −20°C, and centrifuging at 10,000g for 10 min. The RNA pellet was washed with a buffer containing 2 m LiCl and 50 mm EDTA. The dissolved RNA was further purified using the RNeasy Mini or Midi kit (Cat. No. 74104/75142, Qiagen).
RNA Sequencing and Data Analysis
The cDNA library for RNA sequencing was prepared following the protocol of the Illumina TruSeq RNA Sample Preparation kit v2. Sequencing of 76-bp single reads was performed on the Illumina GAIIx platform. The sequencing data were deposited in the DNA Data Bank of Japan Sequence Read Archive under BioProject accession number PRJDB4958. Reads were mapped to K. nitens transcriptome reference v1.1 (http://www.plantmorphogenesis.bio.titech.ac.jp/∼algae_genome_project/klebsormidium/) using Bowtie2 ver2.2.5 (Langmead et al., 2009). The read counts were extracted from the output file and normalized using R package TCC (Tag count comparison) ver1.2.0 (Sun et al., 2013). DEGs among all combinations of the six conditions were identified using TCC with a false discovery rate < 0.1. Clusters were abstracted from log2 fold changes (relative to 10 h of control) of DEGs by quality threshold clustering (Heyer et al., 1999) using MeV ver4.9.0 (Saeed et al., 2003).
Real-Time PCR
First-strand cDNA was synthesized using oligo(dT) and SuperScript II Reverse Transcriptase (Invitrogen). Real-time PCR was performed using the Thermal Cycler Dice Real-Time System II and SYBR Premix Ex Taq II (Tli RNaseH Plus; TaKaRa) under the following conditions: 95°C for 10 s, followed by 45 cycles of 95°C for 5 s, 52.5°C for 5 s, and 72°C for 30 s. Gene expression was calculated with the ΔΔCT method and normalized to the citrate synthase homolog as the housekeeping gene (kfl00009_0420). The following primers were used for KnLBD1 (kf100147_0060): forward primer CTGTGTATGGGTGCGTAGGG, reverse primer ACCGCTGGGTTTTCGTG; for the citrate synthase homolog (kfl00009_0420): forward primer GCAGGAACTGACCAAGGAGAAGA, reverse primer TCCGGCAGATGCTTGAGTG; for kfl00295_0090: forward primer GGAGATGGTCGCTGAGATGG, reverse primer TGAGCGTTGTAGGCGGAGTT; for kfl00812_0030: forward primer CGATGCGGGTGGTTACA, reverse primer AGCGGTTGTTCCTGGCTGT; and for kfl00396_0100: forward primer CATACGTCGAGAGCGATACCA, reverse primer AACGCCCAAACCGGAAA.
Accession Numbers
The Illumina transcriptome sequencing data were deposited in the DNA Data Bank of Japan Sequence Read Archive under BioProject Accession number PRJDB4958. GenBank accession numbers for ABP1 proteins are as follows: Arabidopsis (NP_192207), Populus trichocarpa (XP_006386838), Oryza sativa (XP_015620272), O. sativa (ABA99317), Selaginella moellendorffii (XP_002979034), P. patens (XP_001782753), K. nitens (GAQ82792), Chlamydomonas reinhardtii (XP_001692979), Chlorella variabilis (XP_005850997), and C. variabilis (XP_005844798). KnLBD1 (kf100147_0060) is located in scaffold00147 (GenBank accession number DF237096) within KFL_001470060 locus. The citrate synthase homolog of K. nitens as the housekeeping gene (kfl00009_0420) is located in scaffold00009 (GenBank accession number DF236958) within KFL_000090420 locus. kfl00295_0090 is located in scaffold00295 (GenBank accession number DF237244) within KFL_002950090 locus. kfl00812_0030 is located in scaffold00812 (GenBank accession number DF237761) within KFL_008120030 locus. kfl00396_0100 is located in scaffold00396 (GenBank accession number DF237345) within KFL_003960100 locus.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Quantification of the IAA concentrations of K. nitens in the absence or presence of exogenous 100 µm IAA.
Supplemental Figure S2. Growth of K. nitens in the presence of NPA.
Supplemental Figure S3. Growth of K. nitens in the presence of BBo.
Supplemental Figure S4. Growth of K. nitens in the presence of calcofluor white.
Supplemental Figure S5. Measurement of the frequency of cell division in K. nitens by calcofluor white staining.
Supplemental Figure S6. Measurement of the frequency of cell division in K. nitens by calcofluor white staining in the presence of IAA.
Supplemental Figure S7. Distribution of cell length at 5 d in the absence or presence of 100 µm IAA.
Supplemental Figure S8. Cell elongation ratio as calculated in the absence or presence of 100 µm IAA.
Supplemental Figure S9. Cell elongation ratio as calculated in the absence or presence of 100 µm TIBA.
Supplemental Figure S10. Cell division and cell elongation of K. nitens grown in the presence of NPA.
Supplemental Figure S11. Distribution of cell length at 5 d in the absence or presence of 30 µm BBo.
Supplemental Figure S12. Cell elongation ratio as calculated in the absence or presence of 30 µm BBo.
Supplemental Figure S13. The initial expression changes of certain mRNAs in the downregulated Cluster8 and upregulated Cluster11.
Supplemental Figure S14. Phylogenetic tree analysis of LBD genes.
Supplemental Figure S15. Multiple sequence alignment of ABP1 homologs.
Supplemental References. A list of supplemental references.
Supplemental Table S1. The 576 differentially expressed genes between all combinations of six conditions in the presence or absence of IAA.
Supplemental Table S2. Datasets for phylogenetic analysis.
Acknowledgments
IAA measurements were supported by the Japan Advanced Plant Science Research Network.
Footnotes
This research was partially supported by Grant-in-Aid for Scientific Research (B) 15H04393 from the Japan Society for the Promotion of Science and by the Core Research for Evolutional Science and Technology program of the Japan Science and Technology Agency.
Articles can be viewed without a subscription.
References
- Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image Processing with ImageJ. Biophoton Int 11: 36–42 [Google Scholar]
- Adamowski M, Friml J (2015) PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 27: 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton NW, Grimsley NH, Cove DJ (1979) Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta 144: 427–435 [DOI] [PubMed] [Google Scholar]
- Basu S, Sun H, Brian L, Quatrano RL, Muday GK (2002) Early embryo development in Fucus distichus is auxin sensitive. Plant Physiol 130: 292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett TA, Liu MM, Aoyama T, Bierfreund NM, Braun M, Coudert Y, Dennis RJ, O’Connor D, Wang XY, White CD, et al. (2014) Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr Biol 24: 2776–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berckmans B, Vassileva V, Schmid SPC, Maes S, Parizot B, Naramoto S, Magyar Z, Alvim Kamei CL, Koncz C, Bögre L, et al. (2011) Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell 23: 3671–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot KJM, Libbenga KR, Hille SC, Offringa R, van Duijn B (2012) Polar auxin transport: An early invention. J Exp Bot 63: 4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke TJ, Poli D, Sztein AE, Cohen JD (2002) Evolutionary patterns in auxin action. Plant Mol Biol 49: 319–338 [PubMed] [Google Scholar]
- De Smet I, Voss U, Lau S, Wilson M, Shao N, Timme RE, Swarup R, Kerr I, Hodgman C, Bock R, et al. (2011) Unraveling the evolution of auxin signaling. Plant Physiol 155: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hašek J, Paciorek T, Petrásek J, Seifertová D, Tejos R, et al. (2008) Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA 105: 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb-Fuller JE, Morris DA (1992) Studies on the evolution of auxin carriers and phytotropin receptors: Transmembrane auxin transport in unicellular and multicellular Chlorophyta. Planta 186: 219–226 [DOI] [PubMed] [Google Scholar]
- Finet C, Jaillais Y (2012) Auxology: When auxin meets plant evo-devo. Dev Biol 369: 19–31 [DOI] [PubMed] [Google Scholar]
- Flores-Sandoval E, Eklund DM, Bowman JL (2015) A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha. PLoS Genet 11: e1005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Sakaguchi H, Hiwatashi Y, Wagstaff SJ, Ito M, Deguchi H, Sato T, Hasebe M (2008) Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evol Dev 10: 176–186 [DOI] [PubMed] [Google Scholar]
- Gallavotti A. (2013) The role of auxin in shaping shoot architecture. J Exp Bot 64: 2593–2608 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y (2015) Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci USA 112: 2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier U, Werner O, Bopp M (1990) Indole-3-acetic acid uptake in isolated protoplasts of the moss Funaria hygrometrica. Physiol Plant 80: 584–592 [Google Scholar]
- Goh T, Joi S, Mimura T, Fukaki H (2012) The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139: 883–893 [DOI] [PubMed] [Google Scholar]
- Grones P, Chen X, Simon S, Kaufmann WA, De Rycke R, Nodzyński T, Zažímalová E, Friml J (2015) Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J Exp Bot 66: 5055–5065 [DOI] [PubMed] [Google Scholar]
- Heyer LJ, Kruglyak S, Yooseph S (1999) Exploring expression data: Identification and analysis of coexpressed genes. Genome Res 9: 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Maruyama F, Fujisawa T, Togashi T, Yamamoto N, Seo M, Sato S, Yamada T, Mori H, Tajima N, et al. (2014) Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat Commun 5: 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T. (1971) Sexual cell division and conjugation-papilla formation in sexual reproduction of Closterium strigosum. In Proceedings of the Seventh International Seaweed Symposium. University of Tokyo Press, Tokyo, pp 208–214 [Google Scholar]
- Ishizaki K, Nonomura M, Kato H, Yamato KT, Kohchi T (2012) Visualization of auxin-mediated transcriptional activation using a common auxin-responsive reporter system in the liverwort Marchantia polymorpha. J Plant Res 125: 643–651 [DOI] [PubMed] [Google Scholar]
- Jang G, Dolan L (2011) Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1and PpRSL2 in Physcomitrella patens. New Phytol 192: 319–327 [DOI] [PubMed] [Google Scholar]
- Jin Q, Scherp P, Heimann K, Hasenstein KH (2008) Auxin and cytoskeletal organization in algae. Cell Biol Int 32: 542–545 [DOI] [PubMed] [Google Scholar]
- Kakei Y, Yamazaki C, Suzuki M, Nakamura A, Sato A, Ishida Y, Kikuchi R, Higashi S, Kokudo Y, Ishii T, et al. (2015) Small-molecule auxin inhibitors that target YUCCA are powerful tools for studying auxin function. Plant J 84: 827–837 [DOI] [PubMed] [Google Scholar]
- Kanno Y, Oikawa T, Chiba Y, Ishimaru Y, Shimizu T, Sano N, Koshiba T, Kamiya Y, Ueda M, Seo M (2016) AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nat Commun 7: 13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Ishizaki K, Kouno M, Shirakawa M, Bowman JL, Nishihama R, Kohchi T (2015) Auxin-mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha. PLoS Genet 11: e1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul KN, Mitra GC, Tripathi BK (1962) Responses of marchantia in aseptic culture to well-known auxins and antiauxins. Ann Bot 26: 447–466 [Google Scholar]
- Klambt D, Knauth B, Dittmann I (1992) Auxin dependent growth of rhizoids of Chara globularis. Physiol Plant 85: 537–540 [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Shao N, Bock R, Jürgens G, De Smet I (2009) Auxin signaling in algal lineages: Fact or myth? Trends Plant Sci 14: 182–188 [DOI] [PubMed] [Google Scholar]
- Le Bail A, Billoud B, Kowalczyk N, Kowalczyk M, Gicquel M, Le Panse S, Stewart S, Scornet D, Cock JM, Ljung K, et al. (2010) Auxin metabolism and function in the multicellular brown alga Ectocarpus siliculosus. Plant Physiol 153: 128–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Kim J (2013) EXPANSINA17 up-regulated by LBD18/ASL20 promotes lateral root formation during the auxin response. Plant Cell Physiol 54: 1600–1611 [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim MJ, Kim NY, Lee SH, Kim J (2013) LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J 73: 212–224 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Park JW, Lee HW, Kim J (2009) Genome-wide analysis of the auxin-responsive transcriptome downstream of iaa1 and its expression analysis reveal the diversity and complexity of auxin-regulated gene expression. J Exp Bot 60: 3935–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF, De Clerck O (2012) Phylogeny and molecular evolution of the green algae. Crit Rev Plant Sci 31: 1–46 [Google Scholar]
- Lewis LA, McCourt RM (2004) Green algae and the origin of land plants. Am J Bot 91: 1535–1556 [DOI] [PubMed] [Google Scholar]
- Lokhorst GM. (1991) Synopsis of genera of Klebsormidiales and Ulotrichales. Crypt Bot 2/3: 274–288 [Google Scholar]
- Maravolo NC. (1980) Control of development in hepatics. Bull Torrey Bot Club 107: 308–324 [Google Scholar]
- Maravolo NC, Voth PD (1966) Morphogenetic effects of three growth substances on Marchantia gemmalings. Bot Gaz 127: 79–86 [Google Scholar]
- Marchant HJ, Pickett-Heaps JD, Jacobs K (1973) An ultrastructural study of zoosporogenesis and the mature zoospore of Klebsormidium flaccidum. Cytobios 8: 95–107 [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 108: 18512–18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalko J, Dravecká M, Bollenbach T, Friml J (2015) Embryo-lethal phenotypes in early abp1 mutants are due to disruption of the neighboring BSM gene. F1000 Res 4: 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalko J, Glanc M, Perrot-Rechenmann C, Friml J (2016) Strong morphological defects in conditional Arabidopsis abp1 knock-down mutants generated in absence of functional ABP1 protein. F1000 Res 5: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Brewer PB, Friml JÍ (2007) Polar auxin transport and asymmetric auxin distribution. Arabidopsis Book 5: e0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann DI, Dörffling K (1980) Growth inhibitors and growth promoters in Enteromorpha compressa (Chlorophyta). J Phycol 16: 383–389 [Google Scholar]
- Nishimura T, Hayashi K, Suzuki H, Gyohda A, Takaoka C, Sakaguchi Y, Matsumoto S, Kasahara H, Sakai T, Kato J, et al. (2014) Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J 77: 352–366 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Hiwatashi Y, Sakakibara I, Kato M, Hasebe M (2000) Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res 7: 9–17 [DOI] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto KR, Halbsguth W (1976) Die Förderung der Bildung von Primärrhizoiden an Brutkörpern Von Marchantia polymorpha L. durch Licht und IES (Stimulation of primary rhizoid formation on gemmae of Marchantia polymorpha L. as caused by light and IAA). Z Pflanzenphysiol 80: 197–205 [Google Scholar]
- Perrot-Rechenmann C. (2010) Cellular responses to auxin: division versus expansion. Cold Spring Harb Perspect Biol 2: a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Cerná A, Schwarzerová K, Elckner M, Morris DA, Zazímalová E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131: 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polevoĭ VV, Tarakhovskaia ER, Maslov IuI, Polevoĭ AV (2003) [Role of auxin in induction of polarity in zygotes of Fucus vesiculosus L]. Ontogenez 34: 432–437 [PubMed] [Google Scholar]
- Prigge MJ, Lavy M, Ashton NW, Estelle M (2010) Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr Biol 20: 1907–1912 [DOI] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50: 514–528 [DOI] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69 [DOI] [PubMed] [Google Scholar]
- Riaño-Pachón DM, Corrêa LG, Trejos-Espinosa R, Mueller-Roeber B (2008) Green transcription factors: a chlamydomonas overview. Genetics 179: 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi F, Guiry MD, López-Bautista JM (2008) Distribution, morphology, and phylogeny of Klebsormidium (Klebsormidiales, Charophyceae) in urban environments in Europe(1). J Phycol 44: 1529–1540 [DOI] [PubMed] [Google Scholar]
- Rose S, Rubery PH, Bopp M (1983) The mechanism of auxin uptake and accumulation in moss protonemata. Physiol Plant 58: 52–56 [Google Scholar]
- Rose S, Bopp M (1983) Uptake and polar transport of indoleacetic acid in moss rhizoids. Physiol Plant 58: 57–61 [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. (2003) TM4: A free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Nishiyama T, Sumikawa N, Kofuji R, Murata T, Hasebe M (2003) Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 130: 4835–4846 [DOI] [PubMed] [Google Scholar]
- Sauer M, Robert S, Kleine-Vehn J (2013) Auxin: Simply complicated. J Exp Bot 64: 2565–2577 [DOI] [PubMed] [Google Scholar]
- Škaloud P, Rindi F (2013) Ecological differentiation of cryptic species within an asexual protist morphospecies: A case study of filamentous green alga Klebsormidium (Streptophyta). J Eukaryot Microbiol 60: 350–362 [DOI] [PubMed] [Google Scholar]
- Stirk WA, Ördög V, Novák O, Rolčík J, Strnad M, Bálint P, van Staden J (2013) Auxin and cytokinin relationships in 24 microalgal strains(1). J Phycol 49: 459–467 [DOI] [PubMed] [Google Scholar]
- Sun H, Basu S, Brady SR, Luciano RL, Muday GK (2004) Interactions between auxin transport and the actin cytoskeleton in developmental polarity of Fucus distichus embryos in response to light and gravity. Plant Physiol 135: 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Nishiyama T, Shimizu K, Kadota K (2013) TCC: An R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics 14: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H. (2013) Auxin biology in roots. Plant Root 7: 49–64 [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J (2006) Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell Mol Life Sci 63: 2738–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physiol 54: 163–170 [Google Scholar]
- Tarén N. (1958) Factors regulating the initial development of gemmae in Marchantia polymorpha. Bryologist 61: 191–204 [Google Scholar]
- Vance BD. (1987) Phytohormone effects on cell division in Chlorella pyrenoidosa chick (TX-7-11-05) (Chlorellaceae). J Plant Growth Regul 5: 169–173 [Google Scholar]
- Vanneste S, Friml J (2009) Auxin: A trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Viaene T, Delwiche CF, Rensing SA, Friml J (2013) Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci 18: 5–10 [DOI] [PubMed] [Google Scholar]
- Viaene T, Landberg K, Thelander M, Medvecka E, Pederson E, Feraru E, Cooper ED, Karimi M, Delwiche CF, Ljung K, et al. (2014) Directional auxin transport mechanisms in early diverging land plants. Curr Biol 24: 2786–2791 [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Feijó JA, Weisenseel MH, Verbelen JP (2001) Ion fluxes, auxin and the induction of elongation growth in Nicotiana tabacum cells. J Exp Bot 52: 2161–2167 [DOI] [PubMed] [Google Scholar]
- Wood NL, Berliner MD (1979) Effects of indoleacetic acid on the desmid Micrasterias thomasiana. Plant Sci Lett 16: 285–289 10.1016/0304-4211(79)90040-3 [Google Scholar]
- Yin HC. (1937) Effect of auxin on Chlorella vulgaris. Proc Natl Acad Sci USA 23: 174–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoya NS, Handro W (1996) Effects of auxins and cytokinins on tissue culture of Grateloupia dichotoma (Gigartinales, Rhodophyta). Hydrobiologia 326/327: 393–400 [Google Scholar]
- Żabka A, Polit JT, Winnicki K, Paciorek P, Juszczak J, Nowak M, Maszewski J (2016) PIN2-like proteins may contribute to the regulation of morphogenetic processes during spermatogenesis in Chara vulgaris. Plant Cell Rep 35: 1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, van Duijn B (2014) Cellular auxin transport in algae. Plants (Basel) 3: 58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]