Morphophysiological acclimation to long-term water deficit is linked to the transcriptional response and reflected by gene expression levels measured in intact Arabidopsis.
Abstract
Acclimation to water deficit (WD) enables plants to maintain growth under unfavorable environmental conditions, although the mechanisms are not completely understood. In this study, the natural variation of long-term acclimation to moderate and severe soil WD was investigated in 18 Arabidopsis (Arabidopsis thaliana) accessions using PHENOPSIS, an automated phenotyping platform. Soil water content was adjusted at an early stage of plant development and maintained at a constant level until reproductive age was achieved. The accessions were selected based on the expression levels of ANNEXIN1, a drought-related marker. Severe WD conditions had a greater effect on most of the measured morphophysiological traits than moderate WD conditions. Multivariate analyses indicated that trait responses associated with plant size and water management drove most of the variation. Accessions with similar responses at these two levels were grouped in clusters that displayed different response strategies to WD. The expression levels of selected stress-response genes revealed large natural variation under WD conditions. Responses of morphophysiological traits, such as projected rosette area, transpiration rate, and rosette water content, were correlated with changes in the expression of stress-related genes, such as NINE-CIS-EPOXYCAROTENOID DIOXYGENASE3 and N-MYC DOWNREGULATED-LIKE1 (NDL1), in response to WD. Interestingly, the morphophysiological acclimation response to WD also was reflected in the gene expression levels (most notably those of NDL1, CHALCONE SYNTHASE, and MYB DOMAIN PROTEIN44) in plants cultivated under well-watered conditions. Our results may lead to the development of biomarkers and predictors of plant morphophysiological responses based on gene expression patterns.
Water deficit (WD) is one of the main environmental stress factors affecting crops and global food security. WD is defined as an imbalance between water availability in the soil and evaporative demand due to environmental conditions (Tardieu et al., 2011). Plant responses to WD depend on the WD scenario used in an experiment (Tardieu and Tuberosa, 2010; Tardieu, 2012). Skirycz et al. (2011) compared the responses to severe progressive drought and mild constant WD in several Arabidopsis (Arabidopsis thaliana) mutants and concluded that the results from a particular WD scenario cannot be used to predict the results from another scenario. Plant survival under stress and limited growth under mild WD are not equivalent; thus, the acute response and acclimation need to be considered separately (Tardieu, 1996; Skirycz and Inzé, 2010). Harb et al. (2010) observed biochemical similarities between the response to severe progressive WD and the early response to mild constant WD. However, the response during the acclimation phase was considerably less extensive than that during acute stress. Studies of plant acclimation to WD are labor intensive because they require frequent monitoring and correction of soil water content. Thus, it is essentially impossible to manually perform acclimation experiments on multiple plants at the same time. For plants grown in pots, this problem was solved by the development of plant phenotyping platforms such as PHENOPSIS (Granier et al., 2006) and WIWAM (Skirycz et al., 2011), which enable automatic maintenance of soil water content and automated measurements of multiple plant traits (see also Tisné et al., 2013). These and other phenotyping platforms are capable of supporting reproducible experiments on multiple plants in parallel.
The majority of studies on water deprivation responses in Arabidopsis were performed using the Col-0 accession and derivative mutant lines. Therefore, the question arose whether the gathered knowledge is applicable to other members of the Arabidopsis genus and further to other wild species and crops (Des Marais et al., 2012). Single gene knockout analyses are useful, but for studies of complex multigenic traits, such as growth or WD tolerance, it is often desirable to use sets of natural accessions instead of individual mutant lines (Koornneef et al., 2004). Thousands of natural Arabidopsis accessions are available in public stock centers and, presumably, are adapted to different environmental conditions (Weigel, 2012). In accordance with the large natural genetic variability, the responses to WD were reported to vary greatly between accessions (Granier et al., 2006; Bouchabke et al., 2008; Vile et al., 2012). Natural variation in Arabidopsis has been used in multiple genome-wide association studies aimed at identifying genomic regions linked to the morphophysiological WD response. These studies were carried out using either Arabidopsis accessions (Verslues et al., 2014; Bac-Molenaar et al., 2015, 2016) or recombinant inbred lines derived from genetically distant parental accessions (El-Soda et al., 2015; Lovell et al., 2015) and resulted in the identification of multiple loci associated with responses to WD.
Most of our knowledge regarding Arabidopsis gene expression under WD conditions is derived from small- to medium-scale severe dehydration experiments (for review, see Osakabe et al., 2014). Although automated plant phenotyping platforms facilitate the investigation of constant WD responses, few studies have evaluated the natural variation of the response at the molecular level, and most of them implemented relatively short periods of acclimation (Des Marais et al., 2012; Clauw et al., 2015, 2016).
Most drought-responsive transcript levels in Col-0 change at the start of constant WD, but these responses decline rapidly (Harb et al., 2010). The kinetics of molecular acclimation to WD have not been examined in other Arabidopsis accessions. Using a set of 17 accessions, however, Des Marais et al. (2012) examined WD responses and observed a relationship between changes of gene expression levels during acclimation and ACGT-containing abscisic acid (ABA) response element variants in gene promoters, suggesting that molecular acclimation may be driven largely by the stress hormone ABA.
Advances in whole-transcriptome profiling have allowed the analysis of links between the regulation of gene expression and phenotypic variation (Hansen et al., 2008; Lowry et al., 2013; Lovell et al., 2015). Potential relationships between physiological and molecular responses to WD have been evaluated in different plant species using high-throughput methods such as mRNA sequencing and microarrays (Des Marais et al., 2012; Rengel et al., 2012; Clauw et al., 2016). However, direct correlations between specific transcript abundances and morphophysiological responses have yet to be identified (Clauw et al., 2016). Little is also known about gene expression and its morphophysiological consequences during later phases of acclimation to WD conditions. Knowledge of these processes could generate useful applications, such as gene expression-based markers for assessing plant physiology or predictive models of plant WD responses (Yang et al., 2011; Marchand et al., 2013).
This study investigated the long-term acclimation to constant WD at the morphophysiological and molecular levels in 18 Arabidopsis accessions isolated from different natural habitats that displayed differences at the transcript level of the drought response-related marker ANNEXIN (ANN1) in the absence of stress treatments. Plants subjected to two severities of constant WD, in addition to well-watered (WW) controls, were phenotyped using the PHENOPSIS phenotyping platform (Granier et al., 2006). We measured a large subset of plant traits related to plant growth and water use to analyze the WD effects. In parallel, we performed expression analyses of a set of 16 genes related to physiological responses to WD. Contrary to most published studies, the transcript levels were analyzed after a long acclimation period using plant material collected before dawn to minimize the effect of circadian oscillations on transcript levels (Baerenfaller et al., 2012). Our results revealed that different accessions displayed different types of acclimation responses to long-term WD and that morphophysiological responses were closely linked to expression levels of stress-response genes. These findings may be useful in the development of expression markers for the morphophysiological WD response.
RESULTS
Response of Arabidopsis Col-0 Accession to WD
Arabidopsis plants were cultivated in individual pots under three watering regimes: WW, moderate water deficit (MWD), and severe water deficit (SWD). In the case of WD-treated plants, soil water content was adjusted at the start of the formation of the first true leaves and was maintained at a constant level until the reproduction phase (Fig. 1). Essentially all morphophysiological traits measured for the Col-0 accession were significantly affected by both WD regimes (Table I). Low soil water content reduced the growth rate by 35% in MWD and 80% in SWD, diminished rosette dry weight by 41% in MWD and 64% in SWD, reduced the transpiration rate by 26% in MWD and 27% in SWD, and decreased rosette water content by 2.4% in MWD and 5.7% in SWD. Leaf rosettes were more compact under WD than under WW conditions; the lamina-petiole ratio increased by 27% in MWD and 48% in SWD. The time interval between germination and flowering increased by 21% in SWD but did not change in MWD. The final number of leaves, leaf temperature, relative water content, and stomatal density were not affected by any of the WD conditions.
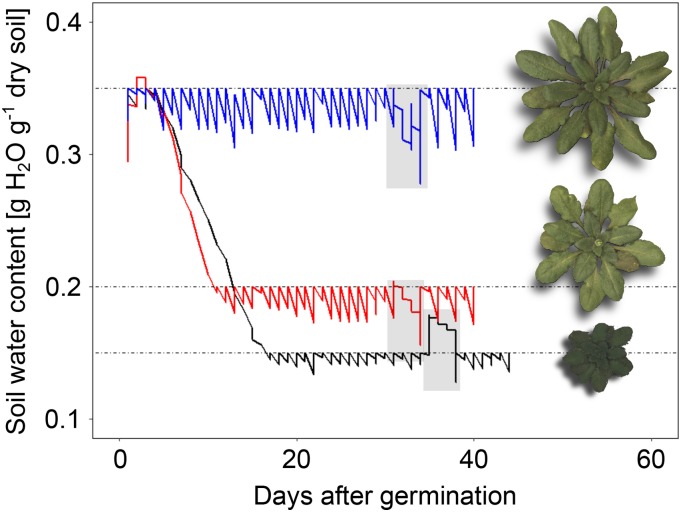
Figure 1.
Changes in soil water content throughout the experiment shown for three individual Col-0 plants. Target soil humidity was set to 0.35 (WW; blue), 0.2 (MWD; red), and 0.15 (SWD; black) mg water g−1 dry soil after the appearance of the first two true leaves. Fluctuations in soil humidity correspond to diurnal water loss via plant transpiration and evaporation from soil. Gray-shaded areas indicate periods of gravimetric water loss measurements (see “Materials and Methods”). Zenithal images of Col-0 rosettes from all three conditions highlight differences in rosette development between conditions at bolting (stage 5.10).
Table I. Morphophysiological trait values of the Arabidopsis Col-0 accession under three watering conditions.
Trait value differences between WD (MWD and SWD) and WW conditions were analyzed with ANOVA and Dunnett’s posthoc test. Significant differences (ns, nonsignificant; **, P < 0.01; and ***, P < 0.001) are indicated. Data are presented as means ± sd. The last column (No.) indicates the number of biological replicates.
| Variable | WW | MWD | SWD | N |
|---|---|---|---|---|
| Rosette dry weight (mg) | 219 ± 33 | 130 ± 23*** | 80 ± 12*** | 4–5 |
| Projected rosette area (cm2) | 3.49 ± 0.16 | 2.33 ± 0.45*** | 0.81 ± 0.42*** | 6–8 |
| Total leaf area (cm2) | 6.12 ± 0.85 | 3.63 ± 0.44*** | 2.07 ± 0.34*** | 4 |
| Growth rate (relative) | 267 ± 28 | 172 ± 38*** | 54 ± 17*** | 8 |
| No. of leaves | 29.8 ± 4.3 | 26.0 ± 2.8ns | 22.3 ± 5.4ns | 4 |
| Lamina-petiole ratio | 3.87 ± 0.24 | 4.92 ± 0.58** | 5.73 ± 0.12*** | 4–5 |
| Transpiration rate (g water cm−2 d−1) | 124 ± 16 | 91 ± 10** | 90 ± 22** | 7–8 |
| Leaf temperature (°C) | 21.1 ± 3.0 | 22.2 ± 2.5ns | 23.3 ± 3.6ns | 8 |
| Water content (%) | 89.1 ± 0.14 | 87 ± 0.86*** | 84 ± 0.82*** | 4–5 |
| Relative water content (%) | 79.9 ± 8.9 | 87.5 ± 1.7ns | 78.3 ± 4.4ns | 4–5 |
| Leaf dry matter content (%) | 8.9 ± 0.98 | 12.0 ± 0.96*** | 12.9 ± 0.52*** | 4–5 |
| Stomatal density (no. mm−2) | 232 ± 50 | 212 ± 46ns | 310 ± 26ns | 3–4 |
| Flowering time (d after germination) | 41 ± 2.5 | 41 ± 3.0ns | 49 ± 3.5*** | 7–8 |
Natural Variation of Responses among Arabidopsis Accessions to WD
To assess the natural variation in WD responses, we chose 18 Arabidopsis accessions from different geographical zones that were selected from 94 available accessions primarily on the basis of their ANN1 expression levels in nontreated seedlings (Supplemental Table S1; Supplemental Fig. S1). The ANN1 gene is a well-established marker of the WD response in Arabidopsis (Konopka-Postupolska et al., 2009; Lu et al., 2015), and its expression was persistently elevated in Col-0 plants grown under conditions similar to those we applied in our previous report (Baerenfaller et al., 2012). In this study, we selected a group of accessions that showed different levels of expression of this stress-related gene in the absence of WD treatment.
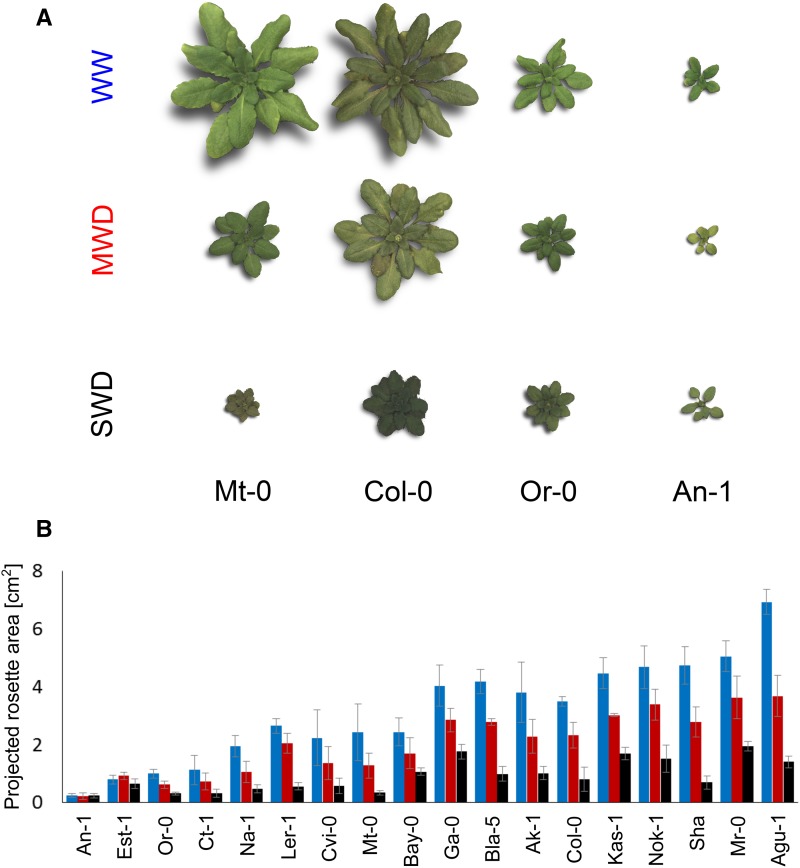
We tested the effects of accession, soil water content, and the accession × soil water content interaction on variance partitioning using all traits and accessions (Table II). We observed that the accession effect was significant for most of the traits, suggesting a high dependence of the trait value in WD on the value under the WW regime. The accession × soil water content interaction was detected for all traits except for leaf temperature and water content, reflecting differences in the WD response between accessions. These differences are well illustrated by the observed variations in the projected rosette area (Fig. 2). Although projected rosette area decreased in essentially all studied accessions, some displayed low-level responses to both WD conditions (e.g. An-1 and Est-1; Fig. 2).
Table II. Partitioning of phenotypic variance among accessions subjected to three watering conditions.
The level of significance (ns, nonsignificant; *, P < 0.05; **, P < 0.01; and ***, P < 0.001) and the percentage of variance explained (SSx/SStotal) are indicated for each parameter (accession and soil water content) and for their interaction (accession × soil water content). Degrees of freedom are indicated in parentheses. Hypotheses were tested using F ratios from type III mean squares for each ANOVA. Nonsignificant terms are reported but not included in the final model.
| Variable | Accession (17) | Soil Water Content (2) | Accession × Soil Water Content (34) | Total |
|---|---|---|---|---|
| Rosette dry weight | 62.49*** | 15.12*** | 6.74** | 84.35 |
| Projected rosette area | 46.46*** | 34.06*** | 12.42*** | 92.93 |
| Total leaf area | 37.61*** | 40.60*** | 11.99*** | 90.21 |
| Growth rate | 13.37*** | 55.18*** | 12.19*** | 80.74 |
| No. of leaves | 82.37*** | 1.23*** | 4.15* | 87.75 |
| Lamina-petiole ratio | 46.29*** | 14.01*** | 11.19** | 71.49 |
| Transpiration rate | 41.28*** | 17.62*** | 12.90*** | 71.80 |
| Leaf temperature | 10.70** | 2.74** | 6.36ns | 13.44 |
| Water content | 46.90*** | 13.59*** | 8.36ns | 60.49 |
| Relative water content | 41.34*** | 18.84*** | 12.05** | 72.23 |
| Leaf dry matter content | 51.68*** | 6.05*** | 12.77** | 70.49 |
| Stomatal density | 29.42*** | 16.14*** | 18.26* | 63.81 |
| Flowering time | 57.80*** | 12.77*** | 7.05*** | 77.62 |
Figure 2.
Effects of different watering conditions on projected rosette area of four accessions (Mt-0, Col-0, Or-0, and An-1) at bolting (stage 5.10). A, Zenithal images of rosettes belonging to four contrasted accessions grown under WW, MWD, and SWD conditions. The four accessions are ordered from left to right by increased tolerance to SWD (i.e. increasing response ratio of projected rosette area to SWD conditions). B, Projected rosette area (means ± sd) in all studied accessions grown under three watering conditions (WW, blue bars; MWD, red bars; and SWD, black bars).
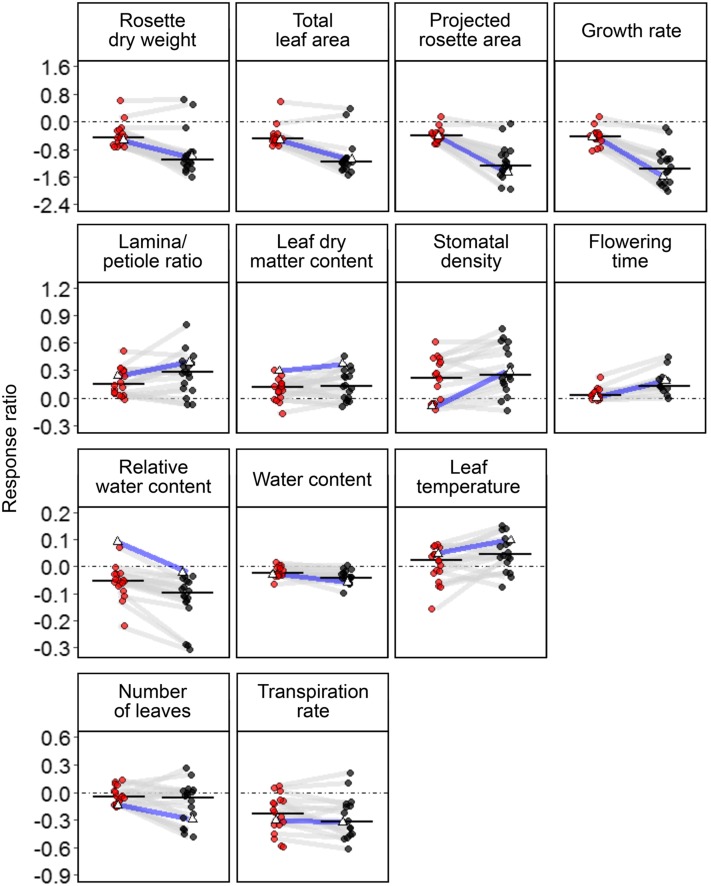
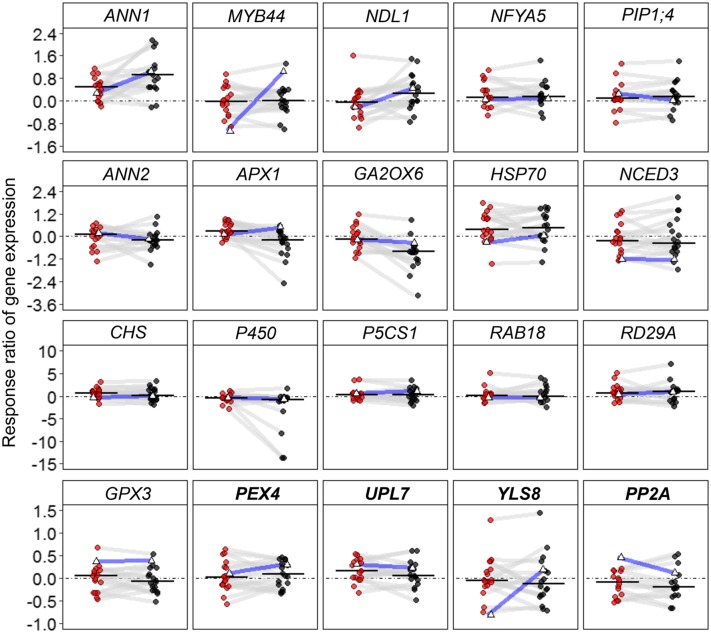
Plant responses were quantified as response ratios (i.e. logarithmized ratios of means under WD and WW conditions), which varied significantly among the 18 accessions and confirmed the effect of accession × soil water content interaction detected in the ANOVAs (Fig. 3). We conducted Wilcoxon signed-rank analyses to test response trends in all accessions by assessing the difference between the median response ratio and zero. Most traits associated with rosette size (rosette dry weight, total leaf area, projected rosette area, and growth rate) tended to decrease under MWD and SWD conditions (P < 0.001). Traits associated with rosette and epidermal architectures (lamina-petiole ratio and stomatal density) tended to increase under MWD and SWD conditions (P < 0.001). Transpiration rate and water content tended to decrease in most accessions under MWD and SWD conditions (P < 0.001). As mentioned earlier, relative water content in Col-0 did not respond to either WD regime. By contrast, it decreased when all accessions were used (PMWD = 0.009 and PSWD < 0.001). Leaf dry matter content increased significantly under MWD and SWD conditions (P < 0.01). Leaf temperature did not change significantly under MWD conditions (P = 0.09) but increased significantly under SWD conditions (P = 0.04). The final number of rosette leaves did not respond to MWD conditions (P = 0.46) but decreased significantly under SWD conditions (P = 0.005). Flowering time tended to increase under MWD conditions (P = 0.01), but the response was substantially higher under SWD conditions (P < 0.001). Overall, SWD had a greater effect on the studied morphophysiological traits.
Figure 3.
Variations of ln response ratios of morphophysiological traits among 18 accessions. Each point corresponds to the mean response ratio of one accession under MWD (red) or SWD (black) conditions. For each morphophysiological trait, solid horizontal lines indicate the median response ratio when considering all accessions. Dashed lines indicate no response [ln(1) = 0] compared with WW conditions. The same accession grown in the two WD conditions is connected with gray lines. Col-0 is indicated with open triangles and blue lines.
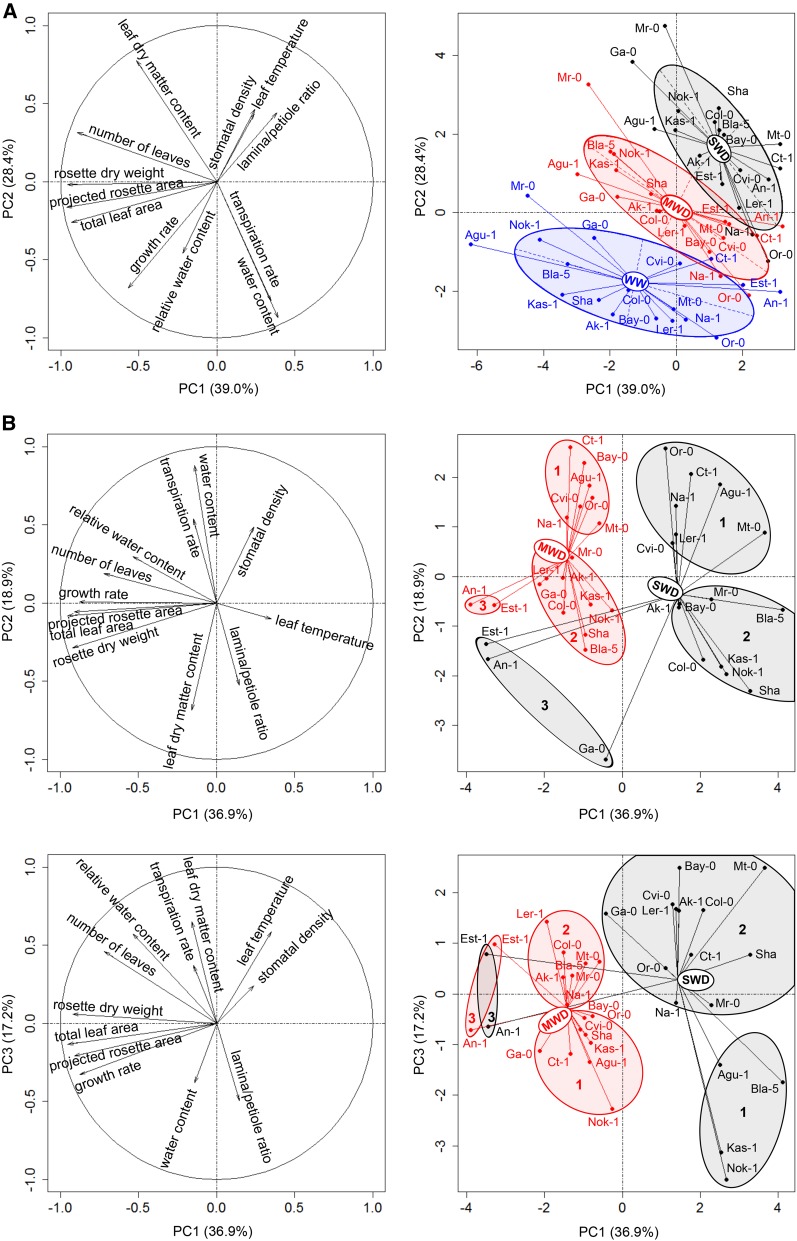
Principal component analysis (PCA) was performed to evaluate how MWD and SWD affect the measured traits globally and to identify relationships between them. The first three principal components explained 39%, 28.4%, and 10% of the total variance when absolute values were analyzed (Fig. 4A). Traits associated with plant size (rosette dry weight, final number of leaves, and projected and total leaf areas) contributed primarily to the principal component 1 (PC1) axis, whereas traits associated with transpiration and water status (transpiration rate, leaf dry matter content, and water content) contributed primarily to the principal component 2 (PC2) axis. Relative water content loaded strongly on the principal component 3 (PC3) axis (see principal component loadings in Supplemental Table S2). The projection of individual accessions displayed significant discrimination between growth conditions (P = 0.001). The centroid positions of the three groups based on watering conditions confirmed that MWD and SWD reduce plant size, growth rate, water content, and transpiration rate and increase leaf dry matter content. Plant traits were more severely affected by SWD than MWD. The absolute values of morphophysiological traits were strongly correlated, including relationships between size-related traits and traits associated with water use, suggesting a considerable allometric scaling effect (Supplemental Fig. S2).
Figure 4.
PCA performed on the morphophysiological traits measured in 18 Arabidopsis accessions grown under three different watering conditions (WW, MWD, and SWD). A, Representation of morphophysiological traits (left) and individual accessions (right) with centers of gravity and lines connected to each accession for each condition. Three variables (i.e. leaf number, leaf dry matter content, and transpiration rate) were ln transformed before analysis to improve the normality of their distributions. Ellipses correspond to inertia ellipses of each group. Each ellipse is centered on the group mean, and its dimensions are 1.5 times the sd of the coordinates on the axes. The covariance sets the slope of the main axis (Thioulouse et al., 1997). B, Representation of traits (left) and individuals (right) from PCA performed on response ratios of morphophysiological traits considering the two WD treatments. Top parts correspond to the PC1:PC2 plane and bottom parts correspond to the PC1:PC3 plane. Ellipses show the results of k-means clustering (k = 3). Numbers in ellipses indicate different clusters obtained for MWD (red ellipses) and SWD (gray ellipses) treatments.
We also conducted PCA on the plant response ratios for MWD and SWD. The first four principal components explained 36.9%, 18.9%, 17.2%, and 7.9% of the total variance (Fig. 4B). Responses of traits associated with plant size contributed primarily to the PC1 axis. Traits associated with leaf water status (water content and leaf dry matter content) contributed to the PC2 and PC3 axes. This result suggests that traits responded consistently within groups related to size and water status but that these groups behaved differently from each other. The projection of the individual accessions displayed high variability, although permutation testing confirmed significant differences between MWD and SWD (P = 0.001). To identify groups of accessions that responded similarly to WD treatments, we performed k-means clustering analysis. Clustering was conducted for the PC1:PC2 and PC1:PC3 planes. The clustering pattern on the PC1:PC2 plane differed only slightly between MWD and SWD. Clusters 1 and 2 were separated from each other primarily on the PC2 axis. WD induced larger reductions in water content and transpiration rate in accessions from cluster 2 than in accessions from cluster 1. Cluster 3 contained An-1 and Est-1 in MWD and An-1, Est-1, and Ga-0 in SWD. Clusters 2 and 3 were primarily separated from each other on the PC1 axis, suggesting that cluster 3 accessions underwent an extremely small decrease in rosette size despite the large reduction in water content. The PC1:PC3 plane displayed similar clustering patterns, but cluster content differed due to the higher weight of relative water content and the final number of leaves in the separation of clusters 1 and 2.
In summary, PCA results suggested that trait responses associated with plant size were generally different from trait responses associated with water management. These two components drove most of the variation. Accessions with similar trait responses were grouped into clusters, which displayed different response strategies to WD in terms of size reduction and the maintenance of rosette water content. We also identified three accessions (An-1, Est-1, and Ga-0) that displayed minimal reductions in shoot size under WD conditions; thus, they can be considered as WD tolerant.
Gene Expression Responses to WD
For gene expression analyses, in addition to standard dehydration markers (RESPONSIVE TO ABA18 [RAB18] and RESPONSIVE TO DESICCATION29A [RD29A]; Harb et al., 2010; Rasheed et al., 2016), we selected genes associated with different types of stress response, namely redox processes (ASCORBATE PEROXIDASE1 [APX1] and GLUTATHIONE PEROXIDASE3 [GPX3]; Miao et al., 2006; Koussevitzky et al., 2008), protection of cellular components (HEAT SHOCK PROTEIN70 [HSP70]; Wang et al., 2004), metabolism of biologically active molecules (DELTA1-PYRROLINE-5-CARBOXYLATE SYNTHASE1 [P5CS1], NINE-CIS-EPOXYCAROTENOID DIOXYGENASE3 [NCED3], CHALCONE SYNTHASE [CHS], and GIBBERELLIN 2-OXIDASE6 [GA2OX6]; Besseau et al., 2007; Székely et al., 2008; Harb et al., 2010; Colebrook et al., 2014), response to sugar starvation (N-MYC DOWNREGULATED-LIKE1 [NDL1] and CYTOCHROME P450 [P450]; Mudgil et al., 2009, 2013; Hummel et al., 2010), water management (MYB DOMAIN PROTEIN44 [MYB44], NUCLEAR FACTOR Y SUBUNIT A5 [NFYA5], and PLASMA MEMBRANE INTRINSIC PROTEIN1;4 [PIP1;4]; Li et al., 2008; Alexandersson et al., 2010; Jaradat et al., 2013), and calcium fluxes (ANN1 and ANN2; Cantero et al., 2006; Konopka-Postupolska et al., 2009; Wang et al., 2015). Reference genes for quantitative PCR (qPCR) analyses were chosen from the set of superior genes for transcript normalization proposed by Czechowski et al. (2005), and four genes (PROTEIN PHOSPHATASE2A SUBUNIT A3, PEROXIN4 [PEX4], UBIQUITIN-PROTEIN LIGASE7, and YELLOW-LEAF-SPECIFIC GENE8) were finally selected for normalization of transcript levels.
Under our experimental conditions, changes in gene expression levels varied greatly among accessions. The levels of most transcripts were either increased or decreased under WD conditions, depending on the accession (Fig. 5). We used Wilcoxon signed-rank tests to identify significant trends in gene expression. Only a few transcripts displayed consistent behavior in response to WD. The expression level of ANN1 increased in most accessions under MWD and SWD conditions (PMWD < 0.001 and PSWD < 0.001) and provided the clearest trend with respect to the response to both WDs. SWD attenuated the expression levels of GA2OX6 (P < 0.001) and P450 (P = 0.0013). APX1 expression increased under MWD (P = 0.0019) but not SWD conditions. We also observed slight but significant up-regulation of HSP70 (PMWD = 0.024 and PSWD = 0.027), CHS (PMWD = 0.027), and NFYA5 (PMWD = 0.043). Reference genes displayed relatively stable expression between accessions and watering regimes.
Figure 5.
Variations of the ln-response ratios of gene expression under MWD and SWD conditions among 18 accessions. Each point corresponds to the response ratio of one accession (MWD, red; SWD, black). For each transcript, solid horizontal lines indicate the median response ratio when considering all accessions; dashed lines indicate no response [ln(1) = 0] compared with WW conditions. The same accessions from two WD conditions are connected with gray lines. Col-0 is indicated with open triangles and blue lines. Reference genes used are shown in boldface.
Relationships between Gene Expression and Morphophysiological Responses to WD
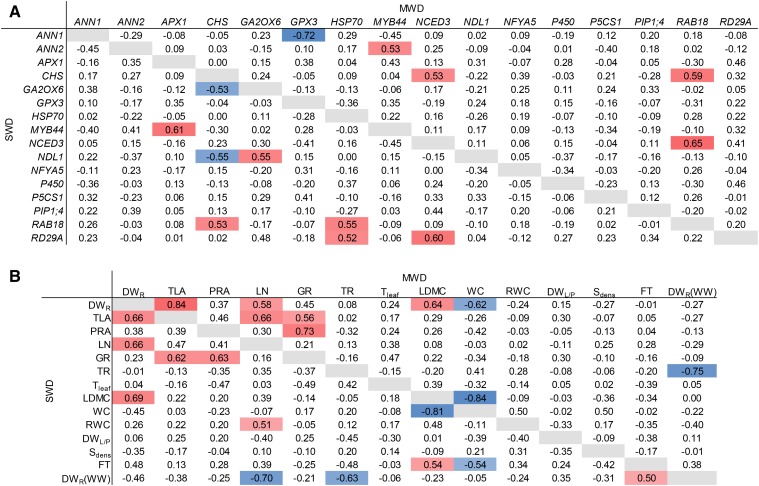
We performed coexpression analyses to evaluate correlations between gene expression responses under MWD and SWD conditions (Fig. 6A). The strongest bivariate correlation was observed for ANN1 and GPX3 expression (ρ = −0.72). Correlations also were observed for NCED3, which is involved in ABA biosynthesis and was coexpressed with RAB18 under MWD conditions (ρ = −0.65) and with RD29A under both MWD and SWD conditions (ρMWD = −0.41 and ρSWD = −0.60). Both transcripts are known to be highly responsive to ABA treatment. The responses of RAB18 and CHS expression also were positively correlated under MWD and SWD conditions (ρMWD = 0.59 and ρSWD = 0.53). The gene expression responses of CHS, GA2OX6, and NDL1 to SWD were moderately correlated with each other (ρ ≤ −0.53 or ρ ≥ 0.55).
Figure 6.
Bivariate correlations between response ratios of gene expression (A) and morphophysiological traits (B) under MWD and SWD conditions. Numbers represent Spearman’s ρ for each pair of variables. Correlations with ρ ≥ 0.5 (red) or ρ ≤ −0.5 (blue) are highlighted. For trait abbreviations, see Supplemental Table S4. In B, the last column and last row represent correlations with rosette dry weight from WW conditions [DWR(WW)].
We observed numerous relationships between different morphophysiological responses to WD (Fig. 6B). The response ratios of size-related traits (rosette dry weight, total leaf area, and final number of leaves) were strongly correlated with each other. The response ratios of water content and leaf dry matter content also were strongly correlated with each other (ρ ≤ −0.81) and with the response of rosette dry weight under MWD and SWD conditions (ρ ≤ −0.45 or ρ ≥ 0.64). The response ratio of flowering time was moderately correlated with those of leaf dry matter content and water content under SWD conditions (ρLDMC = 0.54 and ρWC = −0.54). These results suggest that accessions that did not show a large reduction in rosette size under WD were affected by a more pronounced decrease in rosette water content, which might have resulted in the postponement of flowering.
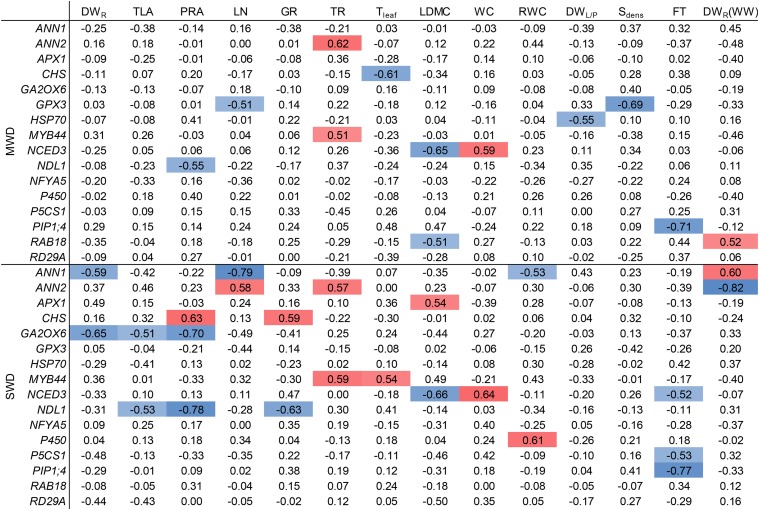
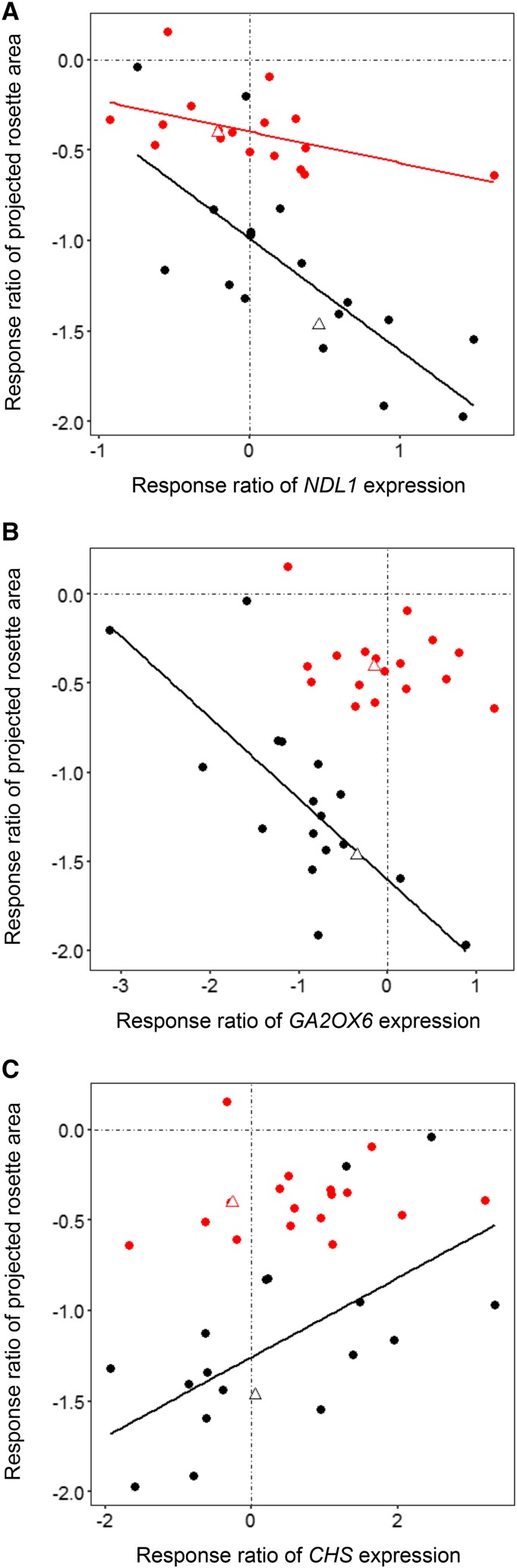
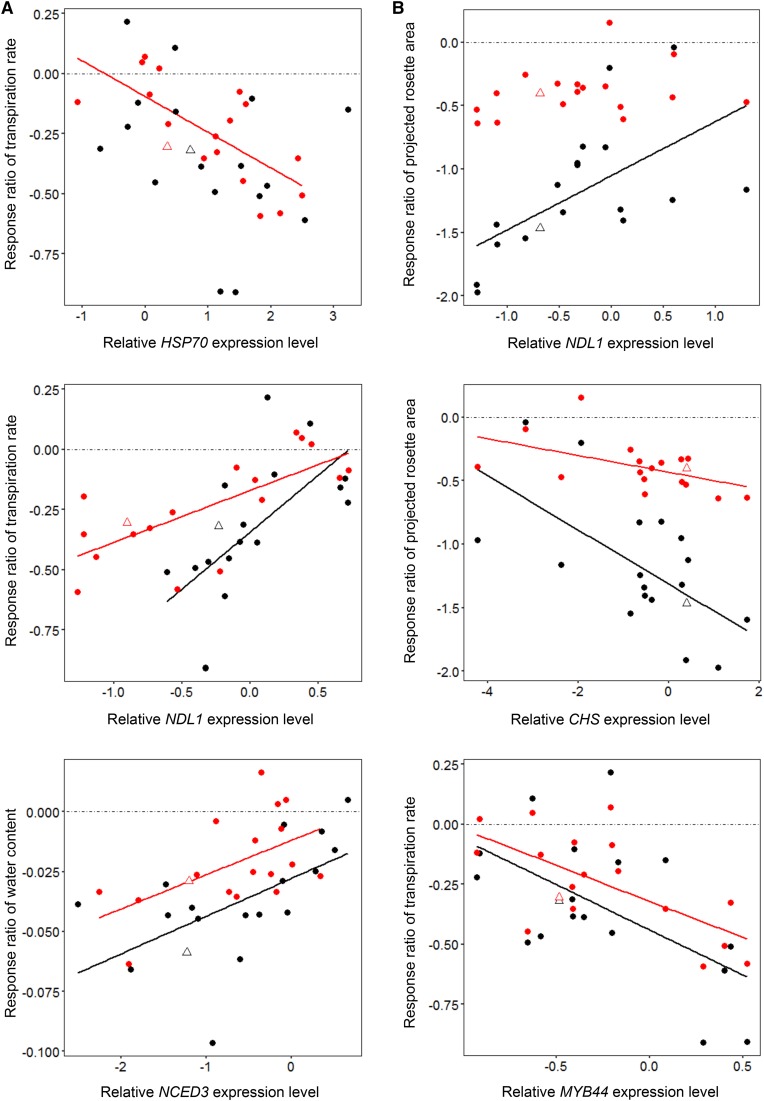
All correlations between gene expression changes and morphophysiological responses to MWD and SWD are presented in Figure 7. The correlation matrices obtained for MWD and SWD differed, with more relationships observed in the response to SWD. To decrease the number of comparisons, we selected correlations in Pearson’s tests that involved three morphophysiological traits: projected rosette area as an indicator of plant size, and transpiration rate and water content as measures of water management. We focused on transcript/trait pairs that displayed relatively strong relationships based on Spearman’s rank correlation coefficient. The response ratio of projected rosette area was highly correlated with those of CHS, GA2OX6, and NDL1 expression (Fig. 8). Under MWD conditions, the correlation was statistically significant only for NDL1 expression (r = −0.50, P = 0.034; Fig. 8A). Under SWD conditions, the correlation was significant for NDL1 (r = −0.75, P < 0.001), GA2OX6 (r = −0.77, P < 0.001; Fig. 8B), and CHS (r = 0.62, P = 0.0059; Fig. 8C) expression. It is worth noting that these three genes code for proteins previously reported to be involved in auxin and GA signaling (Besseau et al., 2007; Mudgil et al., 2009; Colebrook et al., 2014); thus, they are likely to be involved in the regulation of plant growth. We observed linear relationships for these and other transcripts despite the fact that accessions displayed both positive and negative responses to WD.
Figure 7.
Bivariate correlations between response ratios of morphophysiological traits and gene expression under MWD and SWD conditions. The last column represents correlations with the rosette dry weight from WW conditions [DWR(WW)]. Numbers represent Spearman’s ρ for each pair of variables. Correlations with ρ ≥ 0.5 (red) or ρ ≤ −0.5 (blue) are highlighted. For trait abbreviations, see Supplemental Table S4.
Figure 8.
Scatterplots demonstrating the relationships between the response ratios of projected rosette area and NDL1 (A), GA2OX6 (B), and CHS (C) expression to MWD (red) and SWD (black) conditions. Each point corresponds to one accession. Col-0 is indicated with open triangles. Solid lines display statistically significant correlations (Pearson’s test).
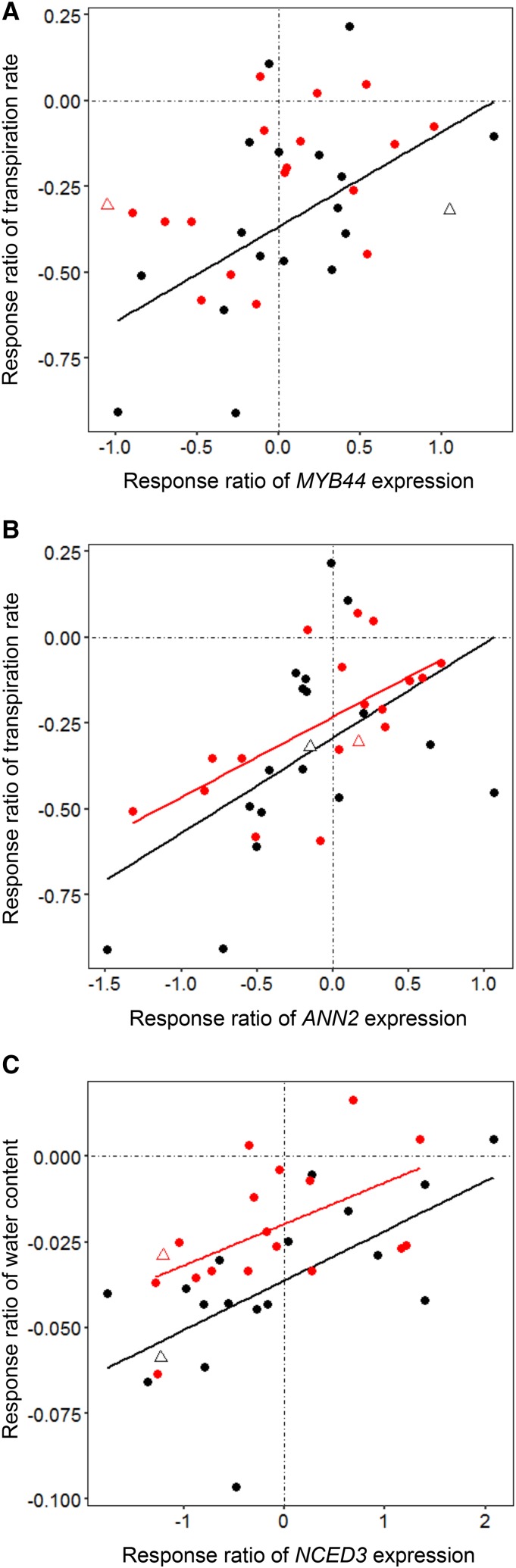
Changes in the transpiration rate were positively correlated with the response ratio of MYB44 and ANN2 expression (Fig. 9). Although the correlation between transpiration rate and MYB44 expression was significant only under SWD conditions (r = 0.53, P = 0.024; Fig. 9A), it was relatively strong, but insignificant, under MWD conditions (r = 0.45, P = 0.064). MYB44 codes for a transcription factor previously associated with ABA signaling, stomatal movement, and transpiration processes (Jaradat et al., 2013). By contrast, transpiration rate and the response of ANN2 expression were significantly correlated under both MWD (r = 0.63, P = 0.005) and SWD (r = 0.50, P = 0.034) conditions (Fig. 9B). The water content response was positively correlated with the response ratio of NCED3 expression under MWD (r = 0.54, P = 0.02) and SWD (r = 0.64, P = 0.0046) conditions (Fig. 9C), providing a link between the synthesis of ABA and leaf water status. The Ga-0 water content displayed an extremely high response to SWD; exclusion of this accession improved the relationship (r = 0.74, P < 0.001). The correlation between the leaf dry matter content response and the response ratio of NCED3 expression showed a similar relationship (Fig. 7). These combined results indicate that morphophysiological changes in response to WD are correlated with gene expression changes. However, it is not clear whether the morphophysiological response is driven directly by changes in the expression levels of these genes or whether the opposite is true. We cannot exclude either of these possibilities at the present time.
Figure 9.
Scatterplots demonstrating the relationships between the response ratios of gene expression and transpiration rate (A and B) and water content (C) under MWD (red) and SWD (black) conditions. Each point corresponds to one accession. Col-0 is indicated with open triangles. Solid lines display statistically significant correlations (Pearson’s test).
Relationships between Morphophysiological Responses to WD and Gene Expression Levels under Different Watering Regimes
In order to identify molecular markers for morphophysiological responses, we evaluated the relationships between the response ratios of morphophysiological traits and gene expression levels under WD and WW conditions. When the relative gene expression levels from WD conditions were analyzed, we observed strong relationships between morphophysiological responses (water content and transpiration rate) and gene expression levels under WD conditions (Fig. 10A). Under MWD conditions, the transpiration rate response was negatively correlated with HSP70 expression (r = −0.70, P = 0.0012) and positively correlated with NDL1 expression (r = 0.72, P < 0.001). Under SWD conditions, the transpiration rate response ratio was positively correlated with NDL1 expression (r = 0.63, P = 0.0048). Under MWD and SWD conditions, the water content response was positively correlated with NCED3 expression (r = 0.55, P = 0.019 and r = 0.57, P = 0.0128, respectively). Under SWD conditions, the correlation strength increased after Ga-0 was excluded from the analysis (r = 0.66, P = 0.0038). These results suggest that all three transcripts can be used as biomarkers of responses to WD.
Figure 10.
Scatterplots demonstrating relationships between the relative gene expression levels from WD (A) or WW (B) conditions and response ratios of morphophysiological traits under MWD (red) and SWD (black) conditions. Each point corresponds to one accession. Col-0 is indicated with open triangles. Solid lines display statistically significant correlations (Pearson’s test). Values of gene expression were ln transformed to improve linearity.
In order to investigate whether there is any relationship between initial (WW) gene expression and morphophysiological responses to WD, we repeated the correlation analysis using gene expression levels measured under WW conditions. The analysis revealed strong relationships between NDL1 and CHS expression levels and the response ratio of projected rosette area (Fig. 10B). NDL1 expression was positively correlated with projected rosette area under SWD conditions (r = 0.59, P = 0.0093). CHS expression was negatively correlated with projected rosette area under MWD (r = −0.51, P = 0.03) and SWD (r = −0.63, P = 0.0051) conditions. The projected rosette area response was slightly correlated with GA2OX6 expression under MWD conditions (r = 0.55, P = 0.02). The transpiration rate response ratio under MWD (r = −0.65, P = 0.0035) and SWD (r = −0.56, P = 0.015) conditions was negatively correlated with MYB44 expression under WW conditions. The expression levels of several genes also were associated with flowering time and plant size (rosette dry weight) under WW conditions. These results suggest that morphophysiological responses to WD may be driven not only by changes in the expression of the specific genes but also by transcript abundance in the period before the application of WD. The results of all bivariate correlations are presented in Supplemental Figures S3 and S4.
DISCUSSION
Food security under global climate change will depend on the availability of crop cultivars that tolerate WD (Tuberosa, 2012). Thus, it is essential to expand our understanding of plant responses to water stress conditions, including stress acclimation. Moderate or severe reductions in soil water content, such as the conditions during prolonged drought, markedly affect plant growth, morphology, physiology, and gene expression. Here, we identified accessions with different responses to MWD and/or SWD and elucidated the effects of different WD regimes on multiple plant traits. These analyses identified several relationships linking gene expression with morphophysiological acclimation to WD in Arabidopsis.
Morphophysiological and Molecular Responses to WD Vary among Accessions
The 18 accessions used in this study were selected according to their different ANN1 transcript levels. The accessions also differed greatly in size. Previous studies described allometric scaling in vascular plants, which is the dependence of morphophysiological traits on organism size (Reich et al., 2006; Vasseur et al., 2012; Vile et al., 2012). Here, we observed that multiple morphophysiological traits were correlated across accessions (Supplemental Fig. S2). Mutual correlations between size-related morphological traits and flowering time do not require explanation; however, allometric scaling also applied to transpiration rate, rosette water content, and leaf dry matter content. These effects may be due to relationships between allometric changes, tissue construction constraints, and leaf functioning. Allometric effects also were observed on gene expression levels in response to the different watering regimes, which might arise from differences in gene expression levels between tissues (Supplemental Figs. S3 and S4).
PCA of absolute values of morphophysiological traits revealed a general pattern of more pronounced response to SWD than MWD (Fig. 4A). However, the first two principal component axes remained uncorrelated despite mutual correlations between traits. The lack of parallelism between trends observed for groups with different watering regimes arose from the high correlation between transpiration rate response and plant size (Supplemental Fig. S5). Negative correlations between the changes in transpiration rate in response to SWD and MWD and plant size under WW conditions suggested that larger-sized accessions need to reduce water loss per unit area to a greater extent than smaller-sized accessions to maintain an acceptable leaf water potential during WD acclimation (Supplemental Fig. S5). Contrary to the observations of Vile et al. (2012), we did not observe strong correlations between rosette dry weight under WW conditions and its response ratio under MWD conditions; however, under SWD conditions, the correlation was close to statistical significance (ρ = −0.46, P = 0.054). Our analysis of response ratios instead of absolute trait values was motivated by two considerations: allometric effects on plant traits and a strong accession effect on many traits, which often exceeded the effect of soil water content (Table II). Unsurprisingly, PCA of response ratios confirmed that plant responses were more pronounced under SWD than MWD conditions, and rosette size and water status were the main parameters differentiating plants with respect to MWD and SWD (Fig. 4B). PCA also revealed the high degree of variability in plant responses to WD among accession groups. Ludlow (1989) proposed that plant strategies to cope with WD effects include avoidance (maintenance of high rosette water content), tolerance (ability to grow under reduced rosette water content), and escape (adjustment of life cycle length to minimize exposure to WD). Our observed accession clustering pattern, especially on the PC1:PC2 plane, suggested that accessions from the first cluster prevented excessive water loss, which is indicative of the avoidance strategy. Accessions from the second cluster continued growing despite reduced water content, thereby displaying moderate tolerance to WD. Accessions from the third cluster, including An-1 and Est-1, displayed extreme tolerance to WD conditions. This result is consistent with previous studies describing An-1 and Est-1 as tolerant to WD (Granier et al., 2006; Vile et al., 2012). Furthermore, we also observed that the accession Ga-0 did not display severe size reduction despite considerable reductions in rosette water content and leaf dry matter content under SWD conditions. Thus, we consider that Ga-0 also responds as tolerant to WD, and this accession should be included in future studies.
Gene expression analyses of the 18 accessions indicated that few transcripts responded to WD in a systematic manner, and often the one-directional response trend was clear only for one of the WD regimes (Fig. 5). This may suggest that acclimation to different levels of WD severity requires the activation of different molecular mechanisms. This hypothesis should be tested in future experiments using high-throughput methods such as transcriptome profiling. Most of the observed transcripts responded to WD with down- or up-regulation depending on the accession, which was reported previously by other researchers. Wang et al. (2013) subjected Sha, Ler-1, and Col-0 accessions to salt treatment and identified a set of differentially expressed genes. Cold treatment also induced differential gene expression among accessions, and variations in expression levels were ascribed to polymorphic regions in gene promoters (Barah et al., 2013). Mild progressive WD also induced similar effects (Des Marais et al., 2012). Lasky et al. (2014) suggested that the presence/absence of ABA response element motifs in gene promoters could be responsible for fine-tuning gene expression responses to abiotic stress and is a major factor influencing phenotypic plasticity. However, it was recently reported that the impact of gene × environment interaction on gene expression levels is mostly regulated in trans, while expression changes independent of the environment are regulated in cis (Clauw et al., 2016). Harb et al. (2010) suggested that acclimation to constant WD results from the stabilization of cellular metabolism as well as from new hormonal homeostasis settings. Therefore, it is possible that the final status quo of biologically active molecules varies among accessions, which may be reflected in their transcriptional responses.
Morphophysiological and Molecular Responses to WD Are Closely Related
Relationships between morphophysiological and gene expression responses to WD in Arabidopsis accessions were investigated previously by Des Marais et al. (2012). They performed transcriptome profiling of 17 accessions that were subjected to mild progressive soil drying for 7 d and then conducted PCA to evaluate the results. They reported statistically significant correlations between principal components for gene expression responses and several morphophysiological traits, including specific leaf area and leaf carbon isotope composition. Clauw et al. (2015, 2016) performed similar studies using young developing leaves. Although these studies generated massive amounts of transcriptomic data, they utilized relatively short WD acclimation periods and did not report specific relationships between gene expression and morphophysiological responses. Here, we implemented a long WD acclimation period before the measurements and focused on the relationships between a large set of morphophysiological traits and a selection of genes.
We identified several new correlations between molecular and morphophysiological responses, which may broaden our current understanding of plant acclimation to WD. The response ratio of projected rosette area was most highly correlated with the response ratios of NDL1, GA2OX6, and CHS expression (Fig. 8). These three genes are indirectly related to plant growth. NDL1 physically interacts with REGULATOR OF G-PROTEIN SIGNALING1 and the GTP-BINDING PROTEIN BETA1 subunit of the heterotrimeric G-protein and regulates auxin transport in roots and shoots (Mudgil et al., 2009, 2013). NDL1 gene expression is regulated by sugar and light (Mudgil et al., 2009; Hummel et al., 2010). Low sugar content was related to low biomass in Arabidopsis, and sugars were postulated to be involved in growth regulation (Sulpice et al., 2009). GA2OX6 encodes a GA 2-oxidase that catalyzes the reduction of GA levels and leads to DELLA protein stabilization (Skirycz and Inzé, 2010; Colebrook et al., 2014). DELLA proteins modulate gene transcription patterns and reduce cell proliferation and cell expansion in response to suboptimal environmental conditions (Skirycz and Inzé, 2010; Davière and Achard, 2013). Low GA2OX6 levels indirectly lead to reduced inhibition of growth under WD conditions. CHS encodes a key enzyme in flavonoid biosynthesis. Flavonoids act as regulators of auxin transport and, thereby, affect plant growth (Brown et al., 2001; Besseau et al., 2007). The correlations between the responses of NDL1, CHS, and GA2OX6 expression and the response of projected rosette area support the central role of auxin and GA signaling in growth regulation upon WD. These transcripts may have potential as biomarkers to determine the rate of growth retardation upon WD.
The response of the transpiration rate was correlated with the response ratios of ANN2 and MYB44 expression (Fig. 9). Two annexins (ANN1 and ANN4) were reported previously to be associated with transpiration (Huh et al., 2010). Water loss from cut rosettes was reduced in ann1ann4 double knockout mutants due to enhanced stomatal responses to ABA. To the best of our knowledge, the response of ann2 knockout mutants to WD has not been studied. Wang et al. (2015) described functional redundancy between ANN1 and ANN2 in heat-mediated calcium signaling. ANN2 also may be involved in regulating stomatal movements. We observed that the response ratio of the transpiration rate also was correlated with the response ratio of MYB44 expression. MYB44 is a member of the MYB transcription factor family and is expressed in leaf vasculature and guard cells. Plants overexpressing MYB44 were reported to display rapid ABA-induced stomatal closure and reduced transpiration compared with wild-type plants (Jung et al., 2008). Our results suggest that, under constant WD conditions, changes in ANN2 and MYB44 expression levels are proportional to the reduction in water loss through stomata and may hold potential as biomarkers for this component of acclimation to WD.
One of the most interesting correlations identified in this study was the relationship between the response ratios of rosette water content and NCED3 expression (Fig. 9C). These responses were positively correlated under both MWD and SWD. In this study, NCED3 was coexpressed with the ABA-induced genes RAB18 (under MWD) and RD29A (under MWD and SWD conditions; Fig. 6A). These combined results provide further confirmation of the links between all three transcripts with ABA-mediated responses to WD.
Gene Expression as a Marker and Predictor of Morphophysiological Responses to WD
The discovery of easily measurable traits for predicting plant behavior is one of the main goals of ecophysiology (Vile et al., 2006, 2012; Violle et al., 2007). Several studies searched for biomarkers (transcripts) that can be used to assess the physiological state of a plant using high-throughput and candidate-gene approaches (Yang et al., 2011; Rengel et al., 2012; Marchand et al., 2013). We observed a strong relationship between relative NCED3 expression levels during MWD and SWD and the response ratios of water content and leaf dry matter content (Fig. 10A; Supplemental Fig. S3). This suggests that the accumulation of NCED3 transcripts can be used as a marker for the water status response when studying natural variation in Arabidopsis. NCED3 expression also was correlated with water content under WW conditions (r = 0.69, P = 0.0017). The question of whether higher NCED3 expression levels directly prevent leaves from losing water requires further study. However, silencing of NCED3 expression increased the transpiration rate and reduced plant tolerance to severe dehydration, whereas ectopic NCED3 expression enhanced plant tolerance to severe dehydration (Iuchi et al., 2001). We also observed correlations between NDL1 and HSP70 expression levels and the response ratio of transpiration rate under WD conditions (Fig. 10A). However, this observation might be biased by the allometric scaling effects on these three parameters (Fig. 6B; Supplemental Fig. S3).
We identified a number of relationships with differing strengths between gene expression levels under WW conditions and the morphophysiological responses (Fig. 10B; Supplemental Fig. S4). The response of projected rosette area correlated with the expression levels of CHS, NDL1, and (to some extent) GA2OX6. The response ratio of the transpiration rate correlated with HSP70 and MYB44 expression. The observation that the response ratios of CHS, GA2OX6, NDL1, and MYB44 expression levels correlated with the response ratios of respective morphophysiological traits raised a question about the effect of initial gene expression levels on changes in gene expression during WD acclimation. We observed that WD-induced changes in the expression levels of several genes were negatively correlated with their expression levels under WW conditions (Supplemental Fig. S6). The highest correlations observed under SWD conditions were with NDL1 (ρ = −0.84, P < 0.001) and PIP1;4 (ρ = −0.798, P < 0.001) expression, suggesting that the response at the transcript level was partially dependent on the quantity of the specific mRNA molecule under control conditions. It is known that the expression levels of certain genes in the early stages of development can be used to predict the growth parameters of fully grown maize (Zea mays) plants (Baute et al., 2015). We propose that the abundance of a specific transcript before the plant encounters WD may shape the plasticity of the morphophysiological responses. A similar observation was reported in studies on plant stress memory, which showed the central role for ABA signaling in this process (for review, see Fleta-Soriano and Munné-Bosch, 2016 and Viirlouvet and Fromm, 2005). In this regard, it is worth noting that MYB44 was characterized previously as a WD memory gene (Ding et al., 2013).
We could not exclude that the expression of some stress-responsive genes also was triggered under WW conditions. Plant growth and physiology are influenced by a plethora of factors. The An-1 accession is usually described in the literature as a small plant that does not respond to WD (Granier et al., 2006; Bouchabke et al., 2008). However, Clauw et al. (2015) reported that An-1 displayed a significant response to the WD conditions applied in their study. Consecutive expression of stress-responsive genes leads to reduced growth rate and final biomass (Todesco et al., 2010; Miller et al., 2015). We observed a negative correlation between NDL1 (ρ = −0.61, P = 0.0087) and P5CS1 (ρ = −0.61, P = 0.0079) expression levels and plant size under WW conditions (Supplemental Fig. S4). We cannot exclude the effects of allometric scaling on gene expression; however, it is possible that activation of these and other genes might have led to reductions in plant growth under WW conditions.
CONCLUSION
We analyzed the morphophysiological and gene expression responses of 18 Arabidopsis accessions to two severities of constant soil WD. Multivariate analyses grouped accessions into clusters showing different strategies for coping with WD conditions. Three accessions (An-1, Est-1, and Ga-0) had superior tolerance to WD. We also obtained a simplified profile of stress-response gene expression and identified correlations between morphophysiological and gene expression responses. Strong relationships between gene expression under WW conditions and morphophysiological responses to WD also were identified. From a basic science point of view, this study has identified previously unknown correlations between gene expression levels and morphophysiological acclimation to prolonged WD and demonstrated a putative link between initial gene expression levels and WD responsiveness. The results point to the potential of using gene expression levels as a predictor of morphophysiological WD responses, which would be particularly useful for breeders because it would not be necessary to apply WD during the process of cultivar selection. Marchand et al. (2013) created linear models for assessing water status on the basis of gene expression in sunflower (Helianthus annuus) plants. We applied a simplified version of their pipeline to select transcripts (see Supplemental Methods S1), and obtained sets of expression-based models to predict the response ratios of all morphophysiological traits (Supplemental Fig. S7). For example, predicting the response of final biomass to WD based on the expression levels of five genes under WW conditions showed a very good fit (Supplemental Fig. S8); however, larger sets of observations will be required to demonstrate its generality or specificity with regard to the conditions used in this study. The identification and development of expression-based predictors for use in crop breeding programs, however, is a potentially promising strategy that will be elucidated further in future studies.
MATERIALS AND METHODS
Plant Material
Eighteen Arabidopsis (Arabidopsis thaliana) accessions (Na-1, Sha, Kas-1, Ak-1, Col-0, Ga-0, Ler-1, Bay-0, Est-1, Mt-0, Cvi-0, Ct-1, Or-0, Mr-0, Nok-1, Bla-5, Agu-1, and An-1) were used for this study. The main selection criterion was the expression level of the drought-response marker ANN1, which was analyzed and quantified during a preliminary experiment (Konopka-Postupolska et al., 2009; Lu et al., 2015). Seedlings of 94 accessions derived from three different collections (McKhann, Weigel, and Nordborg) were screened during that preliminary experiment and provided data on the extremely high and low levels of ANN1 mRNA. The results were used to select six accessions with high ANN1 expression levels and six accessions with low ANN1 expression levels (Supplemental Table S1; Supplemental Fig. S1). The set was completed by six accessions with moderate ANN1 expression levels, which displayed contrasting behaviors in previous studies of plant responses to WD (Granier et al., 2006; Vile et al., 2012).
Growth Conditions
Seeds were kept in the dark at 4°C for at least 1 week before sowing. Four to six seeds were sown at the soil surface in 225-mL pots filled with a 1:1 (v/v) mixture of loamy soil and organic compost (Neuhaus N2). The soil surface was moistened with one-tenth-strength Hoagland solution, and pots were kept in the dark for 48 h under controlled environmental conditions (20.5°C and 70% air relative humidity). Then, pots were placed in the PHENOPSIS growth chamber with a 12-h-light photoperiod, 190 µmol m−2 s−1 photosynthetic photon flux density, 70% air relative humidity, 0.7 kPa water vapor pressure deficit, and air temperature set to 20.8°C. Pots were sprayed with deionized water three times per day until germination. Soil water content was adjusted to 0.35 g water g−1 dry soil in every pot after germination. After emergence of the first two true leaves (stage 1.02 [Boyes et al., 2001]), soil water content was automatically maintained at 0.35, 0.2, and 0.15 g water g−1 dry soil depending on the WD treatment, namely, WW, MWD, and SWD, by daily adjustment with one-tenth-strength Hoagland nutrient solution (Fig. 1). Field water capacity of the substrate was determined to be 0.78 g water g−1 dry soil (Granier et al., 2006); thus, WW, MWD, and SWD conditions represented 45%, 26%, and 19% of the field water capacity, respectively. After emergence of the fourth leaf, only one plant was left in each pot, giving eight biological replicates per accession per treatment.
Measurement of Plant Traits
Projected Rosette Area and Growth Rate
Visible light images of each pot were automatically taken once per day during the whole period of the experiment. Images were processed using ImageJ software (Schneider et al., 2012) to extract projected rosette areas during the course of the experiment. Growth rate was calculated by fitting a sigmoidal curve (Gompertz function) after extraction of the maximal slope value using the gcFitModel function from the R/grofit package (Kahm et al., 2010).
Infrared Imaging
Leaf temperature was measured using an infrared camera (FLIR SC645; FLIR Systems) fitted on the PHENOPSIS robotized arm during 3 consecutive days at bolting (stage 5.10). A thin sheet of cigarette paper was suspended under the camera lens to account for ambient temperature. The leaf temperature value for each plant was calculated as a mean of six regions of interest on the rosette, which was corrected with respect to the ambient temperature in close proximity to the rosette.
Gravimetric Transpiration Measurement
To evaluate the whole-plant transpiration rate, six to eight pots per accession per treatment were sealed at bolting (stage 5.10) with four layers of polyethylene film placed between the rosette and the soil surface to eliminate evaporation from soil. Then, the pots were automatically weighed twice during the night and twice during the day for 3 consecutive days. The transpiration rate was calculated as averaged water loss normalized by averaged projected rosette area during the period of measurement.
Stomatal Density Measurement
The second largest leaf of each rosette was collected from three to four biological replicates after the appearance of the first flower (stage 6.00). For each accession and in each treatment, this leaf had reached its final area. Epidermal imprints were taken from the leaf abaxial side using nail polish. Approximately 1,000 stomata per leaf were counted with the microscope (Eclipse E-800; Nikon) and normalized by the unit of area. Leaves from the late-flowering accessions (Mr-0, Bla-5, Kas-1, Agu-1, and Nok-1) were collected approximately 2 months after sowing.
RNA Extraction and Gene Expression Analyses
After the appearance of the first flower (stage 6.00), the largest leaf was harvested from three to four biological replicates during the last dark hour before the light came on in the growth chamber. The leaf was frozen rapidly in liquid nitrogen. Leaves from the late-flowering accessions were collected approximately 2 months after sowing. Total RNA was extracted using a modified guanidinium-phenol-chloroform protocol (Chomczynski and Sacchi, 1987), treated with DNase1 (ThermoFisher Scientific), and subjected to reverse transcription using random hexamer primer and the RevertAid First-Strand cDNA Synthesis Kit (ThermoFisher Scientific). Gene expression analysis via reverse transcription-qPCR was performed using the LightCycler480 instrument and the LightCycler480 SYBR Green I Master Kit (Roche). Relative gene expression levels were determined using a standard curve method, and the value for each target gene was then normalized against the mean of expression values of four reference genes. Genes were chosen using publicly available data from microarray experiments and the literature. The expression of all selected genes responded to the treatments in a large portion of available microarray experiments associated with dehydration, salinity, and hormonal treatments. Primers were designed in the coding sequence regions with the lowest possible number of single-nucleotide polymorphisms. Primers for qPCR used in the study are listed in Supplemental Table S3
Other Destructive Analyses
Whole rosettes of four to five biological replicates were harvested after the appearance of the first siliques, and rosette fresh weights were determined. Leaves from late-flowering accessions were collected approximately 2 months after sowing. Detached rosettes were kept in deionized water for 24 h at 4°C, and their water-saturated weights were determined. Individual leaves were then attached to a sheet of paper and scanned for subsequent determination of the final leaf number and calculation of the total leaf area using ImageJ software. Dry weights of laminas and petioles were obtained after drying for 72 h at 80°C. Rosette dry weight was expressed as the sum of lamina and petiole dry weights. Rosette water content was calculated as the ratio of the absolute rosette water content and the rosette fresh weight. Relative water content was calculated as the ratio of the absolute rosette water content and maximal (water-saturated) rosette water content. The leaf dry matter content was calculated as the ratio of the dry and water-saturated weights of laminas. All physiological traits used in the experiment are listed in Supplemental Table S4.
Data Analysis
Differences in average trait values between watering conditions were analyzed with ANOVAs, with accession and soil water content as the interactive factors, and with Dunnett’s posthoc test, where appropriate. Response ratios were used to illustrate the behaviors of all studied accessions. Response ratios between WW and WD conditions were calculated as follows:
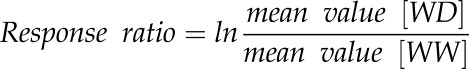 |
where WD can be MWD or SWD. For consistency, the response ratios of gene expression also were ln transformed instead of the canonical log2 transformation. For their calculation, two of the closest replicates were used out of three, for better precision. PCAs were performed on the averaged absolute trait values and their response ratios to determine relationships between the traits and effects of the environmental conditions. The statistical significance of multivariate differences between treatments was tested using the Monte-Carlo permutation test (randtest.between function in the R/ade4 package [n = 999]; Dray and Dufour, 2007). k-means clustering was performed on PCA coordinates to identify groups of accessions that responded to WDs in a similar way. Bivariate relationships between the responses to WD in terms of gene expression and morphophysiological traits were investigated using both Spearman’s (ρ) and Pearson’s (r) correlation tests depending on the distribution and linearity assumptions. All statistical analyses were performed in R version 3.2.3 (R Core Team, 2016). Calculated means of trait values and their response ratios are available in Supplemental Data Set S1.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers RD29A, AT5G52310; RAB18, AT5G66400; APX1, AT1G07890; GPX3, AT2G43350; HSP70, AT3G12580; P5CS1, AT2G39800; NCED3, AT3G14440; CHS, AT5G13930; NDL1, AT5G56750; P450, AT3G03470; GA2OX6, AT1G02400; MYB44, AT5G67300; NFYA5, AT1G54160; PIP1;4, AT4G00430; ANN1, AT1G35720; ANN2, AT5G65020; PP2A, AT1G13320; PEX4, AT5G25760; UPL7, AT3G53090; YLS8, AT5G08290.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Relative ANN1 expression levels in 7-d-old seedlings of 94 Arabidopsis accessions normalized against PEX4.
Supplemental Figure S2. Bivariate correlations between the values of morphophysiological traits under WW, MWD, and SWD conditions.
Supplemental Figure S3. Bivariate correlations between the relative gene expression levels from WD conditions and response ratios of morphophysiological traits under MWD and SWD conditions.
Supplemental Figure S4. Bivariate correlations between the relative gene expression levels from WW conditions and response ratios of morphophysiological traits under MWD and SWD conditions.
Supplemental Figure S5. Bivariate correlations between the absolute values from WW conditions and response ratios of morphophysiological traits under MWD and SWD conditions.
Supplemental Figure S6. Bivariate correlations between gene expression levels under WW conditions and their response ratios under MWD and SWD conditions.
Supplemental Figure S7. Modeling of the morphophysiological responses to MWD and SWD conditions based on gene expression under WW conditions.
Supplemental Figure S8. Scatterplot demonstrating relationships between the measured and predicted response ratios of rosette dry weight under MWD and SWD conditions.
Supplemental Table S1. Geographic origins of the selected Arabidopsis accessions.
Supplemental Table S2. Morphophysiological trait loadings in PCA.
Supplemental Table S3. Transcripts analyzed in the study.
Supplemental Table S4. Morphophysiological traits used in the study.
Supplemental Data Set S1. Raw, averaged, and response ratio values of all measured variables.
Supplemental Methods S1. Linear modeling methodology.
Acknowledgments
We thank our coworkers, in particular, Dr. Magdalena Krzymowska for critical comments on the article and Dr. Dorota Konopka-Postupolska and Maciej Lirski for discussions and help in the preliminary phase of the project, as well as Katarzyna Gawarecka and Katarzyna Krzyczmonik for providing the seeds for accession selection and the technical staff for their support.
Glossary
- WD
water deficit
- ABA
abscisic acid
- WW
well-watered
- MWD
moderate water deficit
- SWD
severe water deficit
- PCA
principal component analysis
- PC1
principal component 1
- PC2
principal component 2
- PC3
principal component 3
- qPCR
quantitative PCR
Footnotes
This work was supported by the European Plant Phenotyping Network (grant no. 284443), funded by the FP7 Research Infrastructures Programme of the European Union, and by the National Science Centre, Poland (PRELUDIUM grant no. 2012/05/N/NZ9/01396 to W.R.).
Articles can be viewed without a subscription.
References
- Alexandersson E, Danielson JA, Råde J, Moparthi VK, Fontes M, Kjellbom P, Johanson U (2010) Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J 61: 650–660 [DOI] [PubMed] [Google Scholar]
- Bac-Molenaar JA, Granier C, Keurentjes JJ, Vreugdenhil D (2016) Genome-wide association mapping of time-dependent growth responses to moderate drought stress in Arabidopsis. Plant Cell Environ 39: 88–102 [DOI] [PubMed] [Google Scholar]
- Bac-Molenaar JA, Vreugdenhil D, Granier C, Keurentjes JJ (2015) Genome-wide association mapping of growth dynamics detects time-specific and general quantitative trait loci. J Exp Bot 66: 5567–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerenfaller K, Massonnet C, Walsh S, Baginsky S, Bühlmann P, Hennig L, Hirsch-Hoffmann M, Howell KA, Kahlau S, Radziejwoski A, et al. (2012) Systems-based analysis of Arabidopsis leaf growth reveals adaptation to water deficit. Mol Syst Biol 8: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barah P, Jayavelu ND, Rasmussen S, Nielsen HB, Mundy J, Bones AM (2013) Genome-scale cold stress response regulatory networks in ten Arabidopsis thaliana ecotypes. BMC Genomics 14: 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baute J, Herman D, Coppens F, De Block J, Slabbinck B, Dell’Acqua M, Pè ME, Maere S, Nelissen H, Inzé D (2015) Correlation analysis of the transcriptome of growing leaves with mature leaf parameters in a maize RIL population. Genome Biol 16: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19: 148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchabke O, Chang F, Simon M, Voisin R, Pelletier G, Durand-Tardif M (2008) Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. PLoS ONE 3: e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero A, Barthakur S, Bushart TJ, Chou S, Morgan RO, Fernandez MP, Clark GB, Roux SJ (2006) Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol Biochem 44: 13–24 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Clauw P, Coppens F, De Beuf K, Dhondt S, Van Daele T, Maleux K, Storme V, Clement L, Gonzalez N, Inzé D (2015) Leaf responses to mild drought stress in natural variants of Arabidopsis. Plant Physiol 167: 800–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw P, Coppens F, Korte A, Herman D, Slabbinck B, Dhondt S, Van Daele T, De Milde L, Vermeersch M, Maleux K, et al. (2016) Leaf growth response to mild drought: natural variation in Arabidopsis sheds light on trait architecture. Plant Cell 28: 2417–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217: 67–75 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davière JM, Achard P (2013) Gibberellin signaling in plants. Development 140: 1147–1151 [DOI] [PubMed] [Google Scholar]
- Des Marais DL, McKay JK, Richards JH, Sen S, Wayne T, Juenger TE (2012) Physiological genomics of response to soil drying in diverse Arabidopsis accessions. Plant Cell 24: 893–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Liu N, Virlouvet L, Riethoven JJ, Fromm M, Avramova Z (2013) Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol 13: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22: 1–20 [Google Scholar]
- El-Soda M, Kruijer W, Malosetti M, Koornneef M, Aarts MG (2015) Quantitative trait loci and candidate genes underlying genotype by environment interaction in the response of Arabidopsis thaliana to drought. Plant Cell Environ 38: 585–599 [DOI] [PubMed] [Google Scholar]
- Fleta-Soriano E, Munné-Bosch S (2016) Stress memory and the inevitable effects of drought: a physiological perspective. Front Plant Sci 7: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Thioux JJ, Rolland G, Bouchier-Combaud S, Lebaudy A, et al. (2006) PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol 169: 623–635 [DOI] [PubMed] [Google Scholar]
- Hansen BG, Halkier BA, Kliebenstein DJ (2008) Identifying the molecular basis of QTLs: eQTLs add a new dimension. Trends Plant Sci 13: 72–77 [DOI] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MM, Pereira A (2010) Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol 154: 1254–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SM, Noh EK, Kim HG, Jeon BW, Bae K, Hu HC, Kwak JM, Park OK (2010) Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant Cell Physiol 51: 1499–1514 [DOI] [PubMed] [Google Scholar]
- Hummel I, Pantin F, Sulpice R, Piques M, Rolland G, Dauzat M, Christophe A, Pervent M, Bouteillé M, Stitt M, et al. (2010) Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: an integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol 154: 357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Jaradat MR, Feurtado JA, Huang D, Lu Y, Cutler AJ (2013) Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol 13: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146: 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahm M, Hasenbrink G, Lichtenberg-Fraté H, Ludwig J, Kschischo M (2010) grofit: fitting biological growth curves with R. J Stat Softw 33: 1–2120808728 [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J (2009) The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55: 141–172 [DOI] [PubMed] [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R (2008) Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283: 34197–34203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky JR, Des Marais DL, Lowry DB, Povolotskaya I, McKay JK, Richards JH, Keitt TH, Juenger TE (2014) Natural variation in abiotic stress responsive gene expression and local adaptation to climate in Arabidopsis thaliana. Mol Biol Evol 31: 2283–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK (2008) The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20: 2238–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JT, Mullen JL, Lowry DB, Awole K, Richards JH, Sen S, Verslues PE, Juenger TE, McKay JK (2015) Exploiting differential gene expression and epistasis to discover candidate genes for drought-associated QTLs in Arabidopsis thaliana. Plant Cell 27: 969–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry DB, Logan TL, Santuari L, Hardtke CS, Richards JH, DeRose-Wilson LJ, McKay JK, Sen S, Juenger TE (2013) Expression quantitative trait locus mapping across water availability environments reveals contrasting associations with genomic features in Arabidopsis. Plant Cell 25: 3266–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Peng JJ, Yu ZB, Du JJ, Xu JN, Wang XY (2015) Thylakoid membrane oxidoreductase LTO1/AtVKOR is involved in ABA-mediated response to osmotic stress in Arabidopsis. Physiol Plant 154: 28–38 [DOI] [PubMed] [Google Scholar]
- Ludlow MM. (1989) Strategies in response to water stress. In Kreeb HK, Richter H, Hinkley TM, eds, Structural and Functional Response to Environmental Stresses: Water Shortage. SPB Academic Press, The Hague, The Netherlands, pp 269–281 [Google Scholar]
- Marchand G, Mayjonade B, Varès D, Blanchet N, Boniface MC, Maury P, Nambinina Andrianasolo F, Burger P, Debaeke P, et al. (2013) A biomarker based on gene expression indicates plant water status in controlled and natural environments. Plant Cell Environ 36: 2175–2189 [DOI] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18: 2749–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Song Q, Shi X, Juenger TE, Chen ZJ (2015) Natural variation in timing of stress-responsive gene expression predicts heterosis in intraspecific hybrids of Arabidopsis. Nat Commun 6: 7453. [DOI] [PubMed] [Google Scholar]
- Mudgil Y, Ghawana S, Jones AM (2013) N-MYC down-regulated-like proteins regulate meristem initiation by modulating auxin transport and MAX2 expression. PLoS ONE 8: e77863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgil Y, Uhrig JF, Zhou J, Temple B, Jiang K, Jones AM (2009) Arabidopsis N-MYC DOWNREGULATED-LIKE1, a positive regulator of auxin transport in a G protein-mediated pathway. Plant Cell 21: 3591–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Osakabe K, Shinozaki K, Tran LS (2014) Response of plants to water stress. Front Plant Sci 5: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna [Google Scholar]
- Rasheed S, Bashir K, Matsui A, Tanaka M, Seki M (2016) Transcriptomic analysis of soil-grown Arabidopsis thaliana roots and shoots in response to a drought stress. Front Plant Sci 7: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Tjoelker MG, Machado JL, Oleksyn J (2006) Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439: 457–461 [DOI] [PubMed] [Google Scholar]
- Rengel D, Arribat S, Maury P, Martin-Magniette ML, Hourlier T, Laporte M, Varès D, Carrère S, Grieu P, Balzergue S, et al. (2012) A gene-phenotype network based on genetic variability for drought responses reveals key physiological processes in controlled and natural environments. PLoS ONE 7: e45249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21: 197–203 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Vandenbroucke K, Clauw P, Maleux K, De Meyer B, Dhondt S, Pucci A, Gonzalez N, Hoeberichts F, Tognetti VB, et al. (2011) Survival and growth of Arabidopsis plants given limited water are not equal. Nat Biotechnol 29: 212–214 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, et al. (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely G, Abrahám E, Cséplo A, Rigó G, Zsigmond L, Csiszár J, Ayaydin F, Strizhov N, Jásik J, Schmelzer E, et al. (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53: 11–28 [DOI] [PubMed] [Google Scholar]
- Tardieu F. (1996) Drought perception by plants: do cells of droughted plants experience water stress? Plant Growth Regul 20: 93–104 [Google Scholar]
- Tardieu F. (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. J Exp Bot 63: 25–31 [DOI] [PubMed] [Google Scholar]
- Tardieu F, Granier C, Muller B (2011) Water deficit and growth: co-ordinating processes without an orchestrator? Curr Opin Plant Biol 14: 283–289 [DOI] [PubMed] [Google Scholar]
- Tardieu F, Tuberosa R (2010) Dissection and modelling of abiotic stress tolerance in plants. Curr Opin Plant Biol 13: 206–212 [DOI] [PubMed] [Google Scholar]
- Thioulouse J, Chessel D, Dole’dec S, Olivier JM (1997) ADE-4: a multi-variate analysis and graphical display software. Stat Comput 7: 75–83 [Google Scholar]
- Tisné S, Serrand Y, Bach L, Gilbault E, Ben Ameur R, Balasse H, Voisin R, Bouchez D, Durand-Tardif M, Guerche P, et al. (2013) Phenoscope: an automated large-scale phenotyping platform offering high spatial homogeneity. Plant J 74: 534–544 [DOI] [PubMed] [Google Scholar]
- Todesco M, Balasubramanian S, Hu TT, Traw MB, Horton M, Epple P, Kuhns C, Sureshkumar S, Schwartz C, Lanz C, et al. (2010) Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature 465: 632–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuberosa R. (2012) Phenotyping for drought tolerance of crops in the genomics era. Front Physiol 3: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur F, Violle C, Enquist BJ, Granier C, Vile D (2012) A common genetic basis to the origin of the leaf economics spectrum and metabolic scaling allometry. Ecol Lett 15: 1149–1157 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Lasky JR, Juenger TE, Liu TW, Kumar MN (2014) Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol 164: 144–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile D, Pervent M, Belluau M, Vasseur F, Bresson J, Muller B, Granier C, Simonneau T (2012) Arabidopsis growth under prolonged high temperature and water deficit: independent or interactive effects? Plant Cell Environ 35: 702–718 [DOI] [PubMed] [Google Scholar]
- Vile D, Shipley B, Garnier E (2006) A structural equation model to integrate changes in functional strategies during old-field succession. Ecology 87: 504–517 [DOI] [PubMed] [Google Scholar]
- Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116: 882–892 [Google Scholar]
- Virlouvet L, Fromm M (2015) Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol 205: 596–607 [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9: 244–252 [DOI] [PubMed] [Google Scholar]
- Wang X, Ma X, Wang H, Li B, Clark G, Guo Y, Roux S, Sun D, Tang W (2015) Proteomic study of microsomal proteins reveals a key role for Arabidopsis annexin 1 in mediating heat stress-induced increase in intracellular calcium levels. Mol Cell Proteomics 14: 686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang L, Zheng Z, Grumet R, Loescher W, Zhu JK, Yang P, Hu Y, Chan Z (2013) Transcriptomic and physiological variations of three Arabidopsis ecotypes in response to salt stress. PLoS ONE 8: e69036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D. (2012) Natural variation in Arabidopsis: from molecular genetics to ecological genomics. Plant Physiol 158: 2–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XS, Wu J, Ziegler TE, Yang X, Zayed A, Rajani MS, Zhou D, Basra AS, Schachtman DP, Peng M, et al. (2011) Gene expression biomarkers provide sensitive indicators of in planta nitrogen status in maize. Plant Physiol 157: 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]