CaMYB31, an R2R3-MYB transcription factor, regulates the capsaicinoid biosynthetic pathway in chili pepper fruits and is regulated by plant hormones, wounding, temperature, and light.
Abstract
Capsaicinoids are responsible for the hot taste of chili peppers. They are restricted to the genus Capsicum and are synthesized by the acylation of the aromatic compound vanillylamine (derived from the phenylpropanoid pathway) with a branched-chain fatty acid by the catalysis of the putative enzyme capsaicinoid synthase. R2R3-MYB transcription factors have been reported in different species of plants as regulators of structural genes of the phenylpropanoid pathway; therefore, we hypothesized that MYB genes might be involved in the regulation of the biosynthesis of pungent compounds. In this study, an R2R3-MYB transcription factor gene, designated CaMYB31, was isolated and characterized in Capsicum annuum ‘Tampiqueño 74’. Bioinformatic analysis suggested that CaMYB31 could be involved in secondary metabolism, stress and plant hormone responses, and development. CaMYB31 expression analysis from placental tissue of pungent and nonpungent chili pepper fruits showed a positive correlation with the structural genes Ca4H, Comt, Kas, pAmt, and AT3 expression and also with the content of capsaicin and dihydrocapsacin during fruit development. However, CaMYB31 also was expressed in vegetative tissues (leaves, roots, and stems). Moreover, CaMYB31 silencing significantly reduced the expression of capsaicinoid biosynthetic genes and the capsaicinoid content. Additionally, CaMYB31 expression was affected by the plant hormones indoleacetic acid, jasmonic acid, salicylic acid, and gibberellic acid or by wounding, temperature, and light, factors known to affect the production of capsaicinoids. These findings indicate that CaMYB31 is indeed involved in the regulation of structural genes of the capsaicinoid biosynthetic pathway.
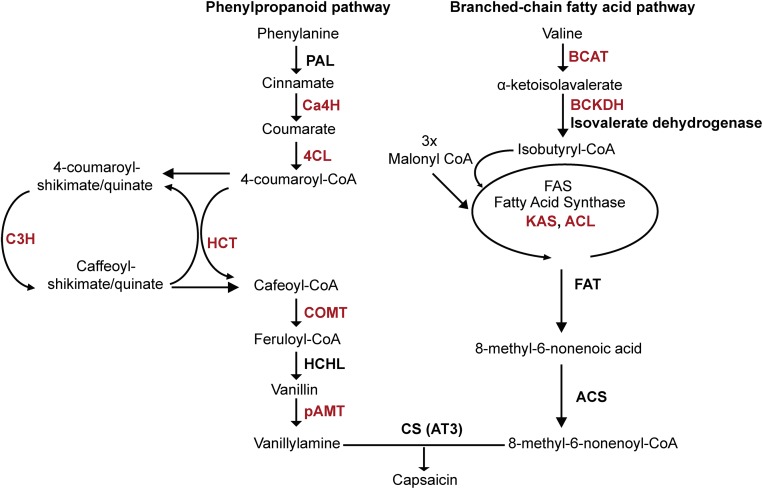
Chili peppers are members of the Capsicum genus, which belongs to the Solanaceae family. Capsicum annuum is the most cultivated species in the world. The importance of this crop is based on a great variety of industrial applications, such as food, pharmaceutical, cosmetic, and agronomic uses (Ochoa-Alejo and Ramírez-Malagón, 2001). One of the most valued compounds of chili pepper fruits are the capsaicinoids, which are synthesized and accumulated in the placental tissue through the convergence of phenylpropanoid and the branched-chain fatty acid pathways (Aza-González et al., 2011; Fig. 1). Capsaicin and dihydrocapsaicin are responsible for 90% of the pungency in the fruit, and their accumulation depends on the genotype, stage of fruit development, and environmental conditions (Lindsey and Bosland, 1996; Kim et al., 2009; Arce-Rodríguez and Ochoa-Alejo, 2015).
Figure 1.
Capsaicinoid biosynthetic pathway. PAL, Phe ammonia lyase; Ca4H, cinnamate 4-hydroxylase; 4CL, 4-coumaroyl-CoA ligase; HCT, hydroxycinnamoyl transferase; C3H, coumaroyl shikimate/quinate 3-hydroxylase; COMT, caffeic acid O-methyltransferase; pAMT, aminotransferase; BCAT, branched-chain amino acid transferase; KAS, ketoacyl-ACP synthase; ACL, acyl-CoA synthetase; FAT, acyl-ACP thioesterase; CS, capsaicinoid synthase; AT3, acyltransferase. The potential target genes of the CaMYB31 transcription factor are marked in red.
Research on the capsaicinoid biosynthesis pathway has uncovered key aspects of its biochemistry and molecular biology (Aza-González et al., 2011). The generation of chili pepper cDNA libraries and comparative gene expression analysis of pungent and nonpungent fruits has allowed the identification of several structural genes of the capsaicinoid biosynthesis pathway, such as Phe ammonia lyase (Pal), cinnamic acid 4-hydroxylase (Ca4H), 4-coumarate-CoA ligase (4CL), hydroxycinnamoyl transferase (HCT), caffeic acid O-methyltransferase (Comt), hydroxycinnamoyl-CoA hydratase/ligase, and a putative acyltransferase (AT3) proposed to be the possible capsaicinoid synthase (Curry et al., 1999; Kim et al., 2001; Stewart et al., 2005; Mazourek et al., 2009; Liu et al., 2013).
The role of transcription factors in the regulation of capsaicinoid biosynthesis is underexplored. Stewart et al. (2005) reported a differential expression analysis of two basic Leu zipper transcription factors in pungent and nonpungent fruits at different developmental stages, but their expression pattern did not correlate positively with the expression of the structural genes Pal, Ca4H, Comt, pAMT, BCAT, Kas, Acl, FatA, and AT3. Recently, Keyhaninejad et al. (2014) reported two ethylene response factor transcription factors whose expression correlated positively with the pungency level in nine chili pepper cultivars, and they were proposed as possible regulators of capsaicinoid biosynthesis.
Previous studies have shown that the phenylpropanoid pathway is regulated by R2R3-MYB transcription factors in different plant species, such as Arabidopsis (Arabidopsis thaliana), Antirrhinum majus, and Pinus taeda (Tamagnone et al., 1998; Patzlaff et al., 2003; Zhou et al., 2009). R2R3-MYB transcription factors represent one of the largest families in plants and regulate different biological processes, such as primary and secondary metabolism, responses to biotic and abiotic stresses, developmental processes, and hormonal responses (Dubos et al., 2010; Ambawat et al., 2013; Liu et al., 2015). Since the phenylpropanoid pathway is involved in the biosynthesis of precursors of the capsaicinoid biosynthetic pathway, we hypothesized that the R2R3-MYB family transcription factors participate in the biosynthesis of capsaicinoids. Here, we report on the isolation of a CaMYB31 cDNA, which encodes a putative R2R3-MYB protein, and its role in the regulation of capsaicinoid biosynthetic genes and in the accumulation of capsaicinoids in chili pepper fruits. Furthermore, we characterized CaMYB31 with regard to its gene organization, phylogeny, function, and responses to hormones, light, temperature, and wounding.
RESULTS
Identification of CaMYB31 by Differential Gene Expression in Placental Tissue from Pungent and Nonpungent Fruits
The accumulation of capsaicin and dihydrocapsaicin in placental tissue of chili pepper fruits of C. annuum ‘Tampiqueño 74’ (pungent) and ‘California Wonder’ (nonpungent) during fruit development is presented in Table I. Capsaicin content was twice as high as dihydrocapsaicin in cv Tampiqueño 74 fruits, but these two capsaicinoids were not detected in cv California Wonder. In cv Tampiqueño 74, capsaicinoids were not recorded at 10 DPA, began accumulating at 20 DPA, peaked at 30 to 40 DPA, and decreased at 50 to 60 DPA.
Table I. Accumulation of capsaicin and dihydrocapsaicin in fruits of cv Tampiqueño 74 and cv California Wonder at different developmental stages.
Separation and determination of capsaicin and dihydrocapsaicin was performed by HPLC. Each value is the mean of six biological replicates ± sd. Lpt, Lyophilized placental tissue; N.D., not detected.
| Cultivar | Stages of Fruit Development | Capsaicin | Dihydrocapsaicin |
|---|---|---|---|
| DPA | mg g−1 Lpt | ||
| Tampiqueño 74 | 10 | N.D. | N.D. |
| 20 | 6.73 ± 0.55 | 3.43 ± 0.31 | |
| 30 | 9.91 ± 1.42 | 5.44 ± 1.59 | |
| 40 | 11.21 ± 1.19 | 5.85 ± 0.91 | |
| 50 | 7.94 ± 0.61 | 4.98 ± 0.87 | |
| 60 | 5.44 ± 0.46 | 3.93 ± 0.49 | |
| California Wonder | 10 | N.D. | N.D. |
| 20 | N.D. | N.D. | |
| 30 | N.D. | N.D. | |
| 40 | N.D. | N.D. | |
| 50 | N.D. | N.D. | |
| 60 | N.D. | N.D. |
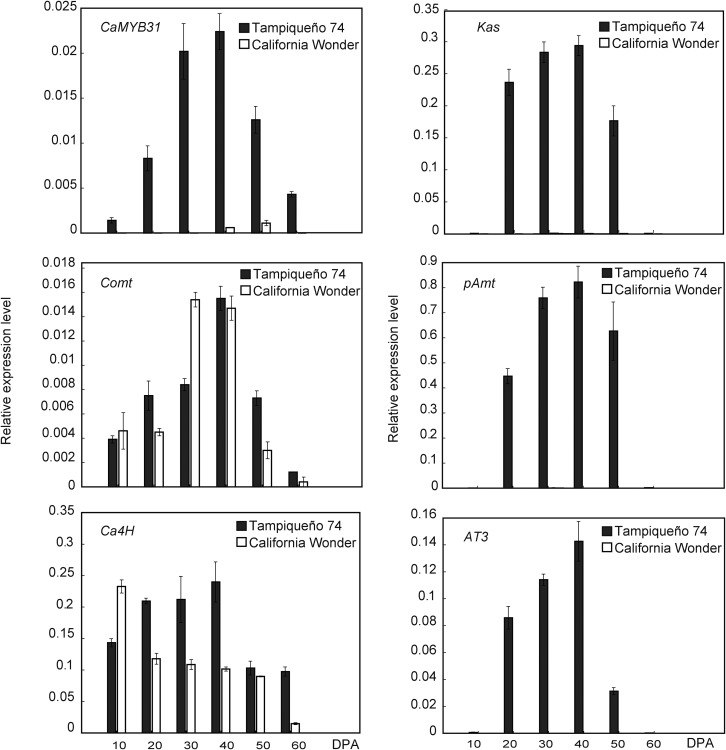
The expression of Ca4H, Comt, pAmt, AT3, and Kas genes was positively correlated with capsaicinoid accumulation in cv Tampiqueño 74 placental tissue. The expression of pAmt, Kas, AT3, and Comt was very low or even undetectable at 10 DPA, increased to a maximum between 30 and 40 DPA, decreased at 50 DPA, and was very low or undetectable at 60 DPA. Ca4H was expressed moderately at 10 DPA, increased to a maximum between 20 and 40 DPA, and then diminished slightly between 50 and 60 DPA. In cv California Wonder, the expression of AT3 was undetectable at all stages of fruit development, while that of Kas and pAmt was very low at 30 to 50 DPA. However, Comt and Ca4H were expressed at all stages of the nonpungent fruits (Fig. 2).
Figure 2.
Quantitative reverse transcription (qRT)-PCR expression assays of the capsaicinoid biosynthetic genes Kas, pAmt, AT3, Comt, Ca4H, and CaMYB31 in placental tissue from fruits of cv Tampiqueño 74 and cv California Wonder at different developmental stages. The data points represent means of three biological replicates ± sd.
Partial MYB sequences were identified from a cDNA library of cv Tampiqueño 74 (Supplemental Table S1). Only sequences that showed greater than 40% homology to MYB transcription factors and that were expressed in pungent placental tissue were selected for qRT-PCR analysis (Supplemental Fig. S1). CaMYB31 showed a clear expression pattern that correlated positively with both the expression levels of the capsaicinoid biosynthetic structural genes and capsaicinoid content during fruit development (Fig. 2).
CaMYB31 Structure and Phylogenetic Sequence Analyses
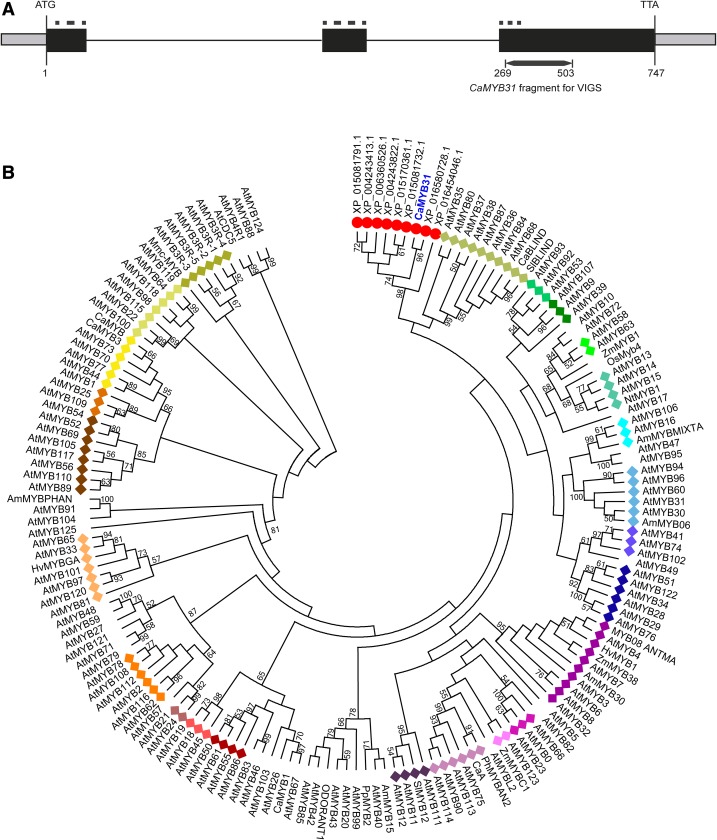
CaMYB31 contains a 747-bp reading frame encoding a putative R2R3-MYB protein of 249 amino acids that is preceded by a 5′ untranslated region (UTR) of 148 bp and followed by a 3′ UTR of 189 bp. The CaMYB31 cDNA sequence was compared with the chili pepper genome database (Qin et al., 2014), and three exons, of 133, 130, and 484 bp, and two introns were identified. The first intron of 724 bp was localized between amino acids 44 and 45 of the R2 domain, and the second one of 425 bp was between amino acids 88 and 89 of the R3 domain (Fig. 3A).
Figure 3.
Structural organization of the CaMYB31 gene and phylogenetic relationship of CaMYB31 with other plant MYB transcription factors. A, Schematic diagram of CaMYB31 showing the coding regions (black boxes), 5′ and 3′ UTRs (gray boxes), introns (solid lines), R2R3-MYB domains (dotted lines), and the fragment sequence of CaMYB31 used for virus-induced gene silencing (VIGS). B, Phylogenetic tree constructed using the neighbor-joining method based on the MYB domain alignment using MEGA6 software. The bootstrap values are shown as percentages (1,000 replicates) when greater than 50%. The clades were grouped as reported previously for Arabidopsis (Stracke et al., 2001; highlighted with colored diamonds for each subgroup), while CaMYB31 (highlighted with blue letters) grouped with solanaceous sequences (marked with red circles). Accession numbers for all protein sequences are listed in Supplemental Table S2.
A phylogenetic tree of 167 plant MYB proteins was constructed using the neighbor-joining method, based on alignment of the MYB domain, with a bootstrap test (n = 1,000; Fig. 3B). The full-length amino acid sequence of CaMYB31 was used to look for MYB proteins from RefSeq and the Swiss-Prot National Center for Biotechnology Information protein database, and the MYB Arabidopsis proteins also were included (Supplemental Table S2). The phylogenetic analysis was congruent with the subgroups defined for Arabidopsis MYB proteins (Kranz et al., 1998; Stracke et al., 2001).
According to our phylogenetic tree, CaMYB31 belongs to a cluster of MYB Solanaceae proteins with no assigned biological functions at this time (XP_016580728.1 with 98% identity to XP_015081732.1 with 67% identity; Fig. 3B). The nearest neighbor clade to CaMYB31 was subgroup 14 of Arabidopsis proteins (AtMYB36 with 63% identity to AtMYB68 with 46% identity), which are related mainly to development, while some of them are implicated in hormone response, stress response, and the regulation of lignin biosynthesis (Fig. 3B). We also constructed the phylogenetic tree using the IT3F software online (Bailey et al., 2008; Supplemental Fig. S2), and the results were consistent with our phylogenetic analysis.
CaMYB31 alignment with other MYB plant regulatory proteins showed a highly conserved R2R3 MYB domain in the N-terminal region and a highly variable C-terminal region (Supplemental Fig. S3A). As a complement to the phylogenetic analysis, we analyzed the C-terminal region of CaMYB31 by comparison with the nearest neighbor clade to search for conserved motifs using MEME motif-detection software, thinking that such a motif might be important for the function of CaMYB31. The C-terminal region of CaMYB31 shares one motif with these proteins related to development (Supplemental Fig. S3B). Moreover, PlantPAN 2.0 (Chow et al., 2016) was used as a database for signal scanning of MYB-binding sites in the putative promoter sequences of the structural capsaicinoid genes, and we found MYB cis-elements on all predictive promoters of the capsaicinoid structural genes (Supplemental Fig. S4).
Differential CaMYB31 Expression Analyses in Different Organ Tissues of Capsicum spp.
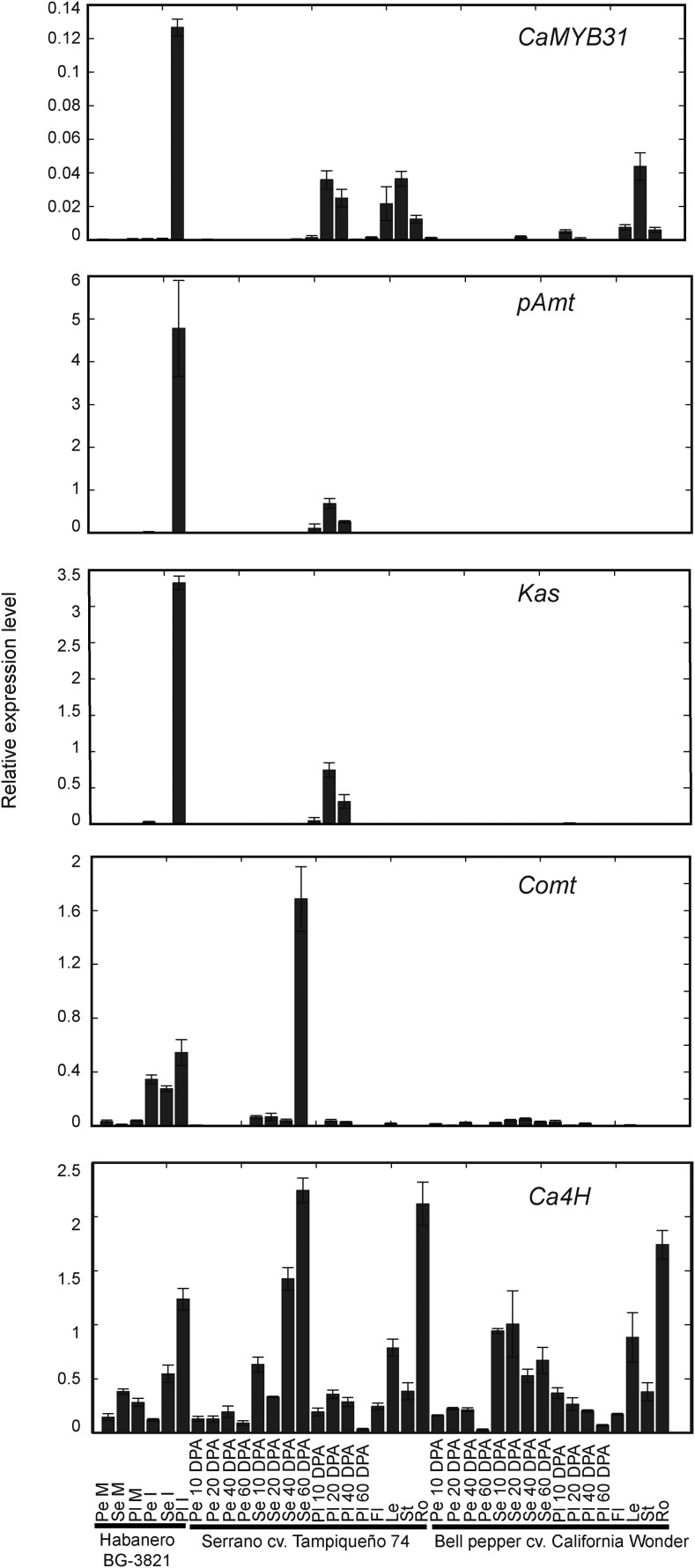
The expression of CaMYB31 and that of the structural genes Kas, pAmt, Comt, and Ca4H were analyzed by qRT-PCR in different tissues of the fruit (seeds, pericarp, and placenta) and plant (roots, stems, flowers, and leaves) from pungent and nonpungent cultivars. Kas and pAmt gene expression was detected exclusively in placental tissue (Fig. 4), although transcript levels were much higher in placental tissue from the immature fruits of Capsicum chinense ‘Habanero’ than from cv Tampiqueño 74, which correlated with the pungency level, whereas Comt and Ca4H also were expressed in seeds, pericarp, and vegetative tissues (Fig. 4).
Figure 4.
Differential expression assays of CaMYB31 and of the capsaicinoid biosynthetic genes pAmt, Kas, Comt, and Ca4H in different tissues of cv Tampiqueño 74 and cv California Wonder and in fruits of cv Habanero BG-3821. M, Mature; I, immature; Pe, pericarp; Se, seed; Pl, placenta; Fl, flower; Le, leaf; St, stem; Ro, root. The data points are means of three biological replicates ± sd.
CaMYB31 showed the highest expression in placental tissue of cv Habanero immature fruits, which are considered highly hot (Fig. 4). Moreover, the characteristic expression pattern of CaMYB31 during the development of cv Tampiqueño 74 fruits was confirmed. The CaMYB31 expression observed in placental tissue of cv California Wonder fruits was much lower. CaMYB31 presented very low expression in the seeds and pericarp.
In the case of vegetative tissues, CaMYB31 was expressed at very low levels in flower tissues of cv Tampiqueño 74 and was undetectable in flowers of cv California Wonder (Fig. 4). However, CaMYB31 was expressed moderately in roots and highly in leaf and stem tissues when compared with the placenta of cv Tampiqueño 74 chili pepper fruits.
Effects of CaMYB31 Silencing on the Expression of Capsaicinoid Biosynthetic Genes and on Capsaicinoid Content
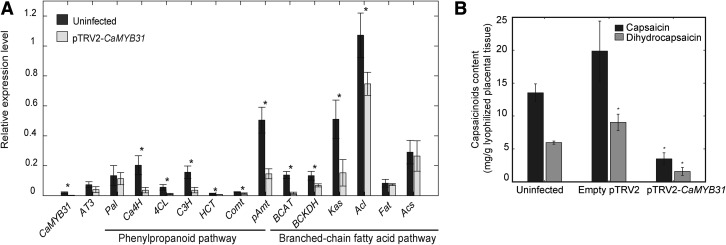
CaMYB31-silencing experiments were carried out using a tobacco rattle virus (TRV)-derived vector to generate the construct pTRV2-CaMYB31, which contained a 234-bp region from 90 amino acids of the R3 domain toward the C-terminal region (Fig. 3A). Fruits of 40 DPA were collected to investigate the effect of CaMYB31 silencing.
Fruits from plants infected with the pTRV2-CaMYB31 construct exhibited a significant diminution of CaMYB31 gene expression (82%) compared with uninfected plants (Fig. 5A). CaMYB31 silencing caused a significant reduction in the expression of the capsaicinoid biosynthetic genes Ca4H (81.8%), 4CL (80.6%), C3H (76.4%), HCT (49%), Comt (42.7%), pAmt (71%), BCAT (88.6%), BCKDH (48.5%), Kas (70%), and Acl (30.3%) compared with fruits from uninfected plants (Fig. 5A). It has been shown previously that agroinfection with the empty pTRV2 vector did not cause statistically significant changes in the expression patterns of tested structural genes in chili pepper fruits (Arce-Rodríguez and Ochoa-Alejo, 2015).
Figure 5.
Effects of CaMYB31 gene silencing on biosynthetic structural gene expression and on capsaicinoid content in fruits of chili pepper. A, qRT-PCR analysis of capsaicinoid biosynthetic genes and CaMYB31 in fruits of chili pepper plants infected with the viral construct pTRV2-CaMYB31 compared with uninfected control plants. B, HPLC analysis of capsaicin and dihydrocapsaicin in fruits of uninfected plants and pTRV2- and pTRV2-CaMYB31-infected plants. The data points are means of three biological replicates ± sd. Asterisks indicate significant differences between the control (uninfected) and infected plants (P ≤ 0.05; Tukey’s test).
Capsaicin and dihydrocapsaicin contents in fruits of plants agroinfected with pTRV2-CaMYB31 showed significant reductions of 74.2% and 73%, respectively, compared with fruits from uninfected plants (Fig. 5B). Fruits from plants agroinfected with the empty pTRV2 vector exhibited capsaicin content statistically similar to that of uninfected plants; however, dihydrocapsaicin content was significantly higher. Therefore, fruits from plants agroinfected with pTRV2-CaMYB31 had an 82.4% decrease in capsaicin and dihydrocapsaicin compared with fruits from plants infected with the empty pTRV2 vector (Fig. 5B).
Effects of Plant Hormones, Light, Temperature, and Wounding on the Expression of CaMYB31 and Capsaicinoid Biosynthesis Structural Marker Genes
Environmental stimuli and some plant hormones affect capsaicinoid accumulation in chili pepper fruits (Kim et al., 2009; Gutiérrez-Carbajal et al., 2010). In order to test whether these factors regulate CaMYB31 expression, several physical stimuli and plant hormones were applied to 30-DPA fruits of cv Tampiqueño 74. The expression of CaMYB31 and the capsaicinoid biosynthetic gene markers Kas and pAmt were analyzed in placental tissue by qRT-PCR.
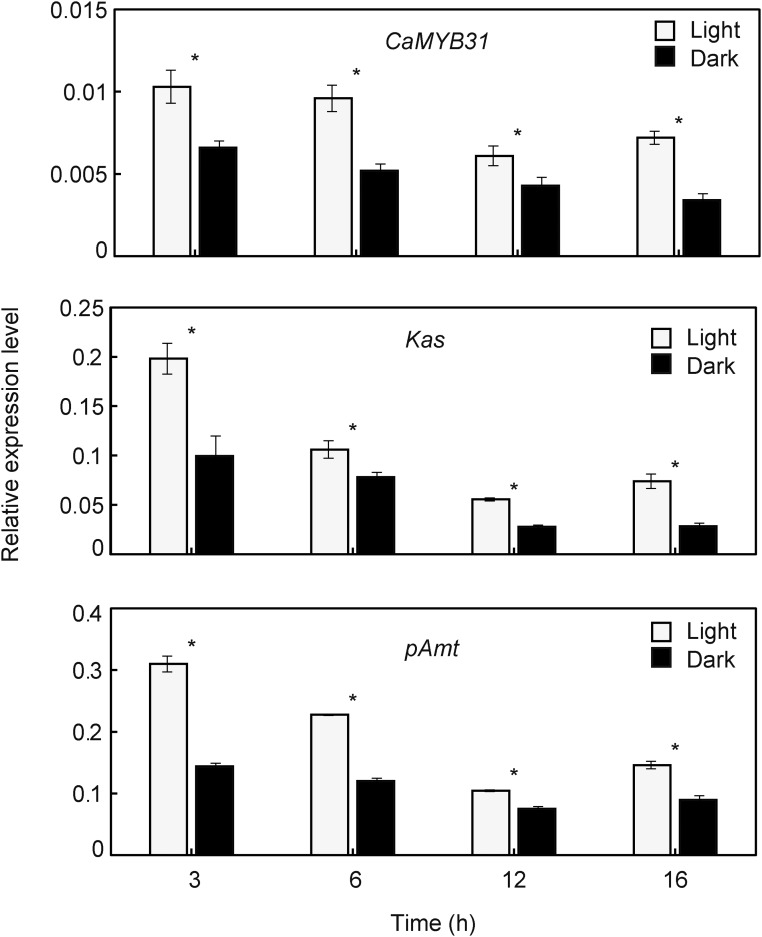
Fruits were treated with light or dark conditions with varying exposure times, and the transcript levels were examined. Dark exposure caused a significant diminution on CaMYB31 expression similar to the expression pattern for Kas and pAmt (Fig. 6). Temperature was another tested stimulus; we monitored gene expression at high temperature (37°C), low temperature (4°C), and standard growth temperature (25°C) at different exposure times. CaMYB31, Kas, and pAmt expression at 37°C was significantly lower, while that in fruits at 4°C was higher, compared with standard growth conditions (Fig. 7). The expression of CaMYB31, Kas, and pAmt in wounded fruits had a significant decrease when compared with nonwounded fruits (Fig. 8).
Figure 6.
Effects of light on CaMYB31, Kas, and pAmt expression in placental tissue from chili pepper fruits at 30 DPA. Gene expression was measured at different incubation times (3, 6, 12, and 16 h). The data points are means of three biological replicates ± sd. Asterisks show significant differences between fruits incubated under dark and light conditions (P ≤ 0.05; Tukey’s test).
Figure 7.
Effects of temperature on CaMYB31, Kas, and pAmt expression in placental tissue from chili pepper fruits at 30 DPA. Gene expression was quantified at different incubation times (3, 6, 12, and 16 h) and different temperatures (4°C, 25°C, and 37°C). The data points are means of three biological replicates ± sd. Asterisks show significant differences between fruits incubated at standard temperature (25°C) and high temperature (37°C) or low temperature (4°C; P ≤ 0.05; Tukey’s test).
Figure 8.
Effects of wounding on CaMYB31, Kas, and pAmt expression in placental tissue from chili pepper fruits at 30 DPA. Gene expression was quantified at different incubation times (3, 6, 12, and 16 h). The data points are means of three biological replicates ± sd. Asterisks show significant differences between control (no injuries) and wounded fruits (P ≤ 0.05; Tukey’s test).
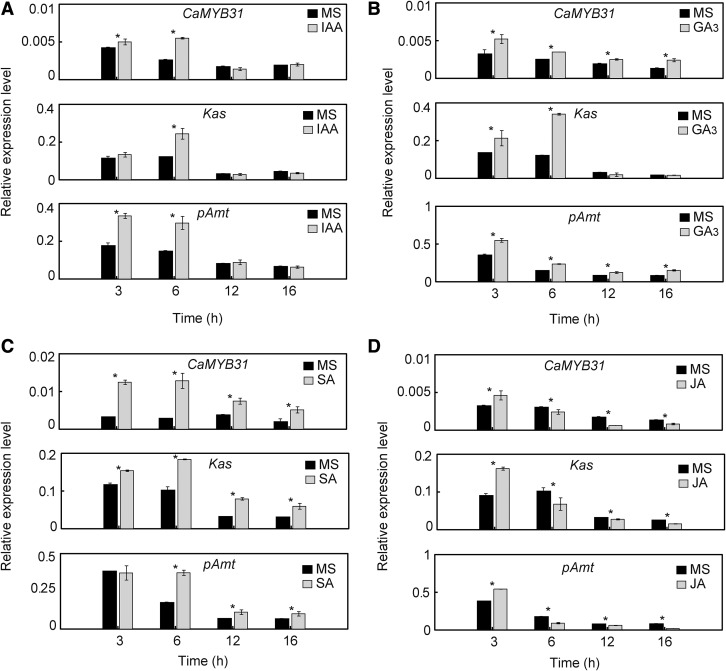
In addition, the transcript levels of CaMYB31 in fruits treated with 100 µm jasmonic acid (JA), salicylic acid (SA), gibberellic acid GA3, or indoleacetic acid (IAA) compared with control fruits (treated with Murashige and Skoog [MS] medium) were determined. CaMYB31, Kas, and pAmt expression increased significantly by IAA, SA, and GA3 treatment, whereas a significant diminution by JA treatment was detected, except at 3 h of exposure (Fig. 9). The effect of these treatments was dependent on the time of exposure. In general, CaMYB31 expression correlated with the expression of Kas and pAmt under exposure to different environmental stimuli.
Figure 9.
Effects of plant hormones on CaMYB31, Kas, and pAmt expression in placental tissue from chili pepper fruits at 30 DPA. Gene expression was quantified at different incubation times (3, 6, 12, and 16 h) in fruits treated with 100 µm IAA (A), GA3 (B), SA (C), or JA (D). The data points are means of three biological replicates ± sd. Asterisks show significant differences between control (MS medium) and treated fruits (P ≤ 0.05; Tukey’s test).
DISCUSSION
The generation of chili pepper cDNA libraries and comparative gene expression analysis of pungent (cv Tampiqueño 74) and nonpungent (cv California Wonder) fruits allowed us to identify CaMYB31, an R2R3-MYB transcription factor gene, as a strong candidate to regulate the capsaicinoid pathway.
In previous studies, the positive correlation between the expression pattern of structural genes (Pal, Ca4H, Comt, Acl, Fat, Kas, and AT3) of the capsaicinoid biosynthetic pathway and the level of pungency of chili pepper fruits has been reported (Curry et al., 1999; Aluru et al., 2003; Arce-Rodríguez and Ochoa-Alejo, 2015). It has been shown that capsaicinoid accumulation is dependent on the developmental stage of chili pepper fruits, and this synthesis and accumulation are highly likely to be regulated and coordinated at the transcriptional level.
Capsaicinoid Content and Expression of Genes Involved in the Capsaicinoid Pathway in Chili Pepper Fruits
A positive correlation was observed between the expression pattern of the structural genes Kas, pAmt, and AT3 and the pungency level during the development of Serrano and bell pepper fruits, indicating that these are specific genes of the capsaicinoid pathway. On the other hand, Comt and Ca4H genes also were expressed in nonpungent fruit and in vegetative tissues, suggesting that they participate in other biological processes, such as lignin biosynthesis (Humphreys and Chapple, 2002). CaMYB31 showed an expression pattern similar to that of the Kas, pAmt, and AT3 genes in the placental tissue of fruits that correlated with pungency, which strongly suggested it as a candidate to regulate the structural genes of the capsaicinoid pathway. However, CaMYB31 also was expressed in vegetative tissues such as roots, leaves, and stems, indicating a possible role in more than one biological process, such as lignin biosynthesis, or other metabolites derived from the phenylpropanoid pathway.
Bioinformatic Analysis of CaMYB31
Bioinformatic analysis of R2R3-MYB proteins showed that the putative CaMYB31 protein was closer to unknown function proteins of chili pepper, tomato (Solanum lycopersicum), potato (Solanum tuberosum), and tobacco (Nicotiana tabacum). Moreover, subgroup 14 of MYB Arabidopsis proteins was the nearest clade to CaMYB31, which shared one motif in the C-terminal region and, therefore, might have some similar functions (lignin biosynthesis, stress and plant hormone response, or development). The lignin and capsaicinoid biosynthetic pathways have common steps at early stages of the phenylpropanoid biosynthesis, suggesting that CaMYB31 is probably implicated in the early regulation of the capsaicinoid pathway. Additionally, we found putative MYB-binding sites on all the predicted promoters of the structural genes of the capsaicinoid pathway, suggesting that they are potential target genes of CaMYB31.
Effects of CaMYB31 Silencing on the Expression of Capsaicinoid Biosynthesis-Related Genes and Capsaicinoid Content
Virus-induced gene silencing was used to investigate the function of CaMYB31 in the capsaicinoid pathway. CaMYB31 silencing caused a significant decrease in capsaicinoid accumulation and on the expression of all structural genes of the phenylpropanoid pathway, except that of Pal, and also on the early genes of the branched-chain fatty acid pathway (BCAT, BCKDH, Kas, and Acl) of capsaicinoid biosynthesis, showing strong evidence of its participation in the regulation of this metabolic process. Based on these results, we propose that the structural genes that showed a significant decrease in expression when CaMYB31 was silenced are potential target genes of CaMYB31. Future studies of the CaMYB31 interaction with promoters of the structural genes whose expression was affected should provide more evidence of the direct transcriptional effect.
Effects of Hormones, Light, Temperature, and Wounding on CaMYB31 Expression
The biosynthesis and accumulation of capsaicinoids in chili pepper fruits is sensitive to environmental factors such as temperature and light, to stress conditions (wounding and drought), and also to plant hormones (Lindsey and Bosland, 1996; Suresh and Ravishankar, 2005; Kim et al., 2009; Gutiérrez-Carbajal et al., 2010). For this reason, we analyzed the effects of plant hormones and stress on CaMYB31 expression and two of the possible target genes: Kas and pAmt.
In previous studies, it was reported that SA induced the production of capsaicinoids and intermediary compounds (Gutiérrez-Carbajal et al., 2010; Altúzar-Molina et al., 2011; Rodas-Junco et al., 2013) while JA induced or repressed the accumulation of capsaicinoids and intermediary metabolites depending on the concentration and exposure time (Suresh and Ravishankar, 2005; Gutiérrez-Carbajal et al., 2010; Altúzar-Molina et al., 2011). CaMYB31, Kas, and pAmt expression was increased significantly with SA treatment, while JA treatment caused a significant decrease in its expression, except at 3 h of exposure. In general, a typical antagonistic effect between SA and JA treatment was revealed, similar to that observed in genes related to defense against pathogens.
Wounding stress has been reported to promote the formation of JA while repressing SA production (Lee et al., 2004). A significant decrease in CaMYB31, Kas, and pAmt expression in wounded chili pepper fruits was observed consistently with JA treatment, except at 3 h.
Other hormones, such as GA3 and auxin, interact with the SA and JA pathways, increasing the complexity of the signaling pathway in the mechanism of defense in plants (Robert-Seilaniantz et al., 2011; Pieterse et al., 2012). CaMYB31, Kas, and pAmt expression was incremented significantly under IAA and GA3 treatment. It has been reported that GAs suppress JA signaling and induce SA signaling (Pieterse et al., 2012), consistent with our results. Conversely, it has been reported that the auxin response was antagonistic to the SA response (Pieterse et al., 2012); however, we did not observe an antagonistic effect on the expression of CaMYB31, Kas, and pAmt. The concentration of the plant hormone and exposure time highly influence gene expression.
Temperature is another factor that positively or negatively affects the level of pungency in chili pepper fruits and the expression of capsaicinoid structural genes (Kim et al., 2009; Rahman and Inden, 2012; González-Zamora et al., 2013). The expression of CaMYB31, Kas, and pAmt was increased significantly at 4°C and was reduced at 37°C compared with 25°C; thus, cv Tampiqueño 74 perhaps is a variety in which the accumulation of capsaicinoids is negatively affected by high temperature.
Light intensity has been found to exert a significant effect on capsaicinoid content in chili pepper fruits (Iwai et al., 1979; Gangadhar et al., 2012). Kim et al. (2009) reported that the enzymatic activity of AT3 was induced by exposure to light and repressed in the dark. Similarly, the expression of CaMYB31, Kas, and pAmt was significantly higher under light compared with dark conditions in our investigation.
CONCLUSION
Our work showed strong evidence of the involvement of a MYB transcription factor in the regulation of capsaicinoid biosynthesis and its stress response in chili pepper fruits. Future studies are necessary to investigate whether CaMYB31 acts directly on the promoter of structural genes of the capsaicinoid pathway or through the formation of a protein complex with other transcription factors.
MATERIALS AND METHODS
Plant Growth Conditions
Chili pepper (Capsicum annuum) ‘Tampiqueño 74’ (Serrano type) and ‘California Wonder’ (bell pepper type) plants were grown in greenhouse conditions and fertilized every 2 weeks with FerviaFol (Agroquímicos Rivas) solution (N:P:K, 30:20:10). Chili pepper fruits were harvested at 10, 20, 30, 40, 50, and 60 DPA from at least six plants. Only placental tissue was collected and was immediately frozen in liquid nitrogen, stored at −80°C, and used for qRT-PCR expression analysis to select MYB gene candidates.
The cv Tampiqueño 74 and cv California Wonder and Capsicum chinense ‘Habanero’ BG-3821 plants were grown for differential expression analysis in different organs. Chili pepper fruits were harvested at 10, 20, 40, and 60 DPA from C. annuum, and immature and mature stages from C. chinense were dissected to separate the seeds, placenta, and pericarp, which were stored separately. Roots, leaves, stems, and flowers of adult plants from the different genotypes of C. annuum were collected. All tissues were frozen and stored at −80°C.
Seeds of cv Tampiqueño 74 were germinated in a growth chamber at a temperature of 28°C under a 16-h photoperiod, a photon flux of 70 µmol m−2 s−1 (T8W/Starcoat GE fluorescent lamps), and a relative humidity of 66% for silencing experiments. Three-week-old chili pepper seedlings were agroinfected and, after 6 weeks, were transferred to greenhouse conditions until 40-DPA chili pepper fruits were collected for each treatment.
Seeds of cv Tampiqueño 74 were germinated and grown in a greenhouse for the studies of environmental stimuli in entire fruits. Placental tissue was collected from 30-DPA chili pepper fruits for each treatment.
Identification and Isolation of the CaMYB31 Gene
We selected 24 MYB partial sequences from a cDNA library of cv Tampiqueño 74 with more than 40% identity to MYB proteins (Supplemental Table S1). The expression of these MYB genes was analyzed by qRT-PCR, comparatively with the expression of the capsaicinoid biosynthetic genes AT3, Kas, pAmt, Ca4H, and Comt, in placental tissue of fruits at different developmental stages (10, 20, 30, 40, 50, and 60 DPA) of cv Tampiqueño 74 (pungent) and cv California Wonder (nonpungent). Moreover, the levels of capsaicin and dihydrocapsaicin were quantified at the same stages of development in pungent and nonpungent fruits. The CaMYB31 gene was selected as the candidate based on the positive correlation of its expression pattern with capsaicinoid content and the expression levels of the structural genes.
The full-length CaMYB31 gene was predicted using the GeneScan Web server from the pepper genome (Qin et al., 2014), and primers were designed (forward, 5′-TACACGTGATGGTGAGAACAACACCTTGCT-3′, and reverse, 5′-CGCACGTGTTAATAATTAAAATGATCAAA-3′) for amplification by reverse transcription-PCR from placental tissue of 40-DPA chili pepper fruits of cv Tampiqueño 74. The PCR conditions for amplification were 94°C for 3 min, followed by 30 cycles (94°C for 30 s, 57.5°C for 30 s, and 72°C for 1 min), and 72°C for 7 min. The PCR product was diluted (1 µL of PCR product with 19 µL of sterile water) and used as a template for a second PCR under the same conditions. The PCR product was cloned into the pCR 8 vector (Invitrogen) according to the manufacturer’s instructions and sequenced.
Phylogenetic and Sequence Analysis
BLASTp was used to search MYB protein sequences from the RefSeq_protein and Swiss-Prot National Center for Biotechnology Information databases using the full-length amino acid sequence of CaMYB31. The characterized Solanaceae proteins (SlMYB12, SlBLIND, CaBLIND, CaMYB, CaMYB1, CaA, and CaMYB3) and all the Arabidopsis (Arabidopsis thaliana) MYB proteins reported by Stracke et al. (2001) and at the IT3F Web site (Bailey et al., 2008) also were included (Supplemental Table S2). The sequences were aligned with ClustalW using default parameters, and it was manually adjusted. Based on the MYB domain alignment, a phylogenetic tree was constructed with the neighbor-joining method, model JTT+G, and a bootstrap test (1,000 replicates) using MEGA6 software. In addition, we constructed a phylogenetic tree using the IT3F Web site with CaMYB31 and the MYB proteins of Arabidopsis. The clades were grouped as reported previously (Kranz et al., 1998; Stracke et al., 2001). MEME version 4.11.3 (http://meme-suite.org/tools/meme) was used to discover putative motifs in the C-terminal region of CaMYB31. PlantPAN 2.0 (Chow et al., 2016) was used to search MYB-binding sites in the predictive promoters of the structural genes of the capsaicinoid biosynthesis pathway.
Virus-Induced Gene Silencing of the CaMYB31 Gene
A 234-bp fragment of CaMYB31 was amplified (bases 269–503) using the primers forward (5′-AGTCTAGATCCTTTGGTGACCATTCTGA-3′) and reverse (5′-AGTCTAGAGGCGATTGCTGCTCACTT-3′). Amplification conditions were 94°C for 5 min, followed by 30 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 1 min), and extension at 72°C for 7 min. CaMYB31 viral silencing plasmid construction and agroinfiltration were carried out as reported for tomato (Solanum lycopersicum; Liu et al., 2002), with some modifications (Arce-Rodríguez and Ochoa Alejo, 2015).
Quantification of Capsaicinoids
Capsaicin and dihydrocapsaicin were quantified in placental tissue from cv Tampiqueño 74 (pungent) and cv California Wonder (nonpungent) chili pepper fruits in three biological samples composed of 10 placentas each in the case of 10-DPA fruits and three placentas for the 20-, 30-, 40-, 50-, and 60-DPA fruits. The extraction and quantification of capsaicin and dihydrocapsaicin were carried out as reported by Arce-Rodríguez and Ochoa-Alejo (2015).
Plant Hormones, Light, Temperature, and Wounding Chili Pepper Fruit Treatments
Chili pepper fruits of cv Tampiqueño 74 at 30 DPA were collected to evaluate the effect of different factors on the expression of CaMYB31 and the capsaicinoid biosynthetic genes Kas and pAmt for 3, 6, 12, and 16 h. For light/dark response assays, complete fruits were first wrapped with plastic film and foil for 24 h and then were uncovered and exposed to fluorescent light conditions (50 µmol m−2 s−1). For temperature treatments, entire fruits were wrapped with plastic film and foil and incubated at 4°C, 25°C, and 37°C. For the wounding responses experiment, a scalpel blade was introduced approximately 6 mm deep several times into whole fruits, which were then covered with plastic film and incubated at 25°C under standard light conditions. To apply plant hormone treatments, placental tissue was separated from 30-DPA fruits and submerged into MS (Murashige and Skoog, 1962) liquid medium containing 0 or 100 µm JA, SA, GA3, or IAA (Sigma) and incubated at 25°C under standard light conditions. After incubation, the placental tissues from all treatments were immediately frozen in liquid nitrogen and stored at −80°C for gene expression analysis.
Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from placenta, pericarp, seeds, flowers, stems, leaves, and roots with Trizol (Invitrogen) according to the protocol provided by the manufacturer. The extraction was performed in triplicate for each sample. RNA was purified with the PureLink Micro-To-Midi kit (Invitrogen) according to the instructions. cDNA was synthesized from purified RNA with SuperScript II or SuperScript III reverse transcriptase (Invitrogen) following the protocol provided by the manufacturer.
Real-Time Quantitative PCR Assays
Total RNA was extracted, purified, and treated with DNase I (Invitrogen) following the manufacturer’s instructions. cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen) and adjusted to 100 ng mL−1. qRT-PCR was performed as reported by Arce-Rodríguez and Ochoa-Alejo (2015) using the primer pairs described in Supplemental Table S3.
Statistical Analysis
The data generated in silencing experiments and the effect of different factors on the expression of CaMYB31 were subjected to ANOVA with Tukey’s test (P ≤ 0.05) to find statistically significant differences between the mean values.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. qRT-PCR expression assays of the putative MYB transcription factor genes in placental tissue from chili pepper fruits of cv Tampiqueño 74 and cv California Wonder at different developmental stages.
Supplemental Figure S2. Phylogenetic relationship of CaMYB31 with MYB transcription factors of Arabidopsis.
Supplemental Figure S3. Alignment of amino acid sequences of CaMYB31 with others plant R2R3-MYB proteins.
Supplemental Figure S4. Schematic diagram of the putative MYB-binding site localizations in the predicted promoter of capsaicinoid structural genes.
Supplemental Table S1. Sequences from the cDNA library of cv Tampiqueño 74 (Serrano type) for qRT-PCR analysis.
Supplemental Table S2. Accession numbers of MYB proteins listed in Figure 3.
Supplemental Table S3. Primers used for qRT-PCR analysis.
Acknowledgments
We thank Yolanda Rodríguez for technical assistance in the HPLC analysis; Dr. Cesar Aza-González, Dr. María del Rocío Gomez-García, and José Luis Pablo-Rodríguez for technical assistance in the agroinfiltration experiments; and Dr. Luis Delaye, Dr. Octavio Martínez de la Vega, and Magdalena Rivera for their important insights in bioinformatic analysis.
Glossary
- qRT
quantitative reverse transcription
- UTR
untranslated region
- JA
jasmonic acid
- SA
salicylic acid
- IAA
indoleacetic acid
Footnotes
This work was supported by the National Council of Science and Technology (grant no. 177063 and a scholarship to M.L.A.-R.).
Articles can be viewed without a subscription.
References
- Altúzar-Molina AR, Muñoz-Sánchez JA, Vázquez-Flota F, Monforte-González M, Racagni-Di Palma G, Hernández-Sotomayor SMT (2011) Phospholipidic signaling and vanillin production in response to salicylic acid and methyl jasmonate in Capsicum chinense J. cells. Plant Physiol Biochem 49: 151–158 [DOI] [PubMed] [Google Scholar]
- Aluru MR, Mazourek M, Landry LG, Curry J, Jahn M, O’Connell MA (2003) Differential expression of fatty acid synthase genes, Acl, Fat and Kas, in Capsicum fruit. J Exp Bot 54: 1655–1664 [DOI] [PubMed] [Google Scholar]
- Ambawat S, Sharma P, Yadav NR, Yadav RC (2013) MYB transcription factor genes as regulators for plant responses: an overview. Physiol Mol Biol Plants 19: 307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce-Rodríguez ML, Ochoa-Alejo N (2015) Silencing AT3 gene reduces the expression of pAmt, BCAT, Kas, and Acl genes involved in capsaicinoid biosynthesis in chili pepper fruits. Biol Plant 59: 477–484 [Google Scholar]
- Aza-González C, Núñez-Palenius HG, Ochoa-Alejo N (2011) Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep 30: 695–706 [DOI] [PubMed] [Google Scholar]
- Bailey PC, Dicks J, Wang TL, Martin C (2008) IT3F: a web-based tool for functional analysis of transcription factors in plants. Phytochemistry 69: 2417–2425 [DOI] [PubMed] [Google Scholar]
- Chow CN, Zheng HQ, Wu NY, Chien CH, Huang HD, Lee TY, Chiang-Hsieh YF, Hou PF, Yang TY, Chang WC (2016) PlantPAN 2.0: an update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res 44: D1154–D1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry J, Aluru M, Mendoza M, Nevarez J, Melendrez M, O’Connell MA (1999) Transcripts for capsaicinoids biosynthetic genes are differentially accumulated in pungent and non-pungent Capsicum spp. Plant Sci 148: 47–57 [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 15: 573–581 [DOI] [PubMed] [Google Scholar]
- Gangadhar BH, Mishra RK, Pandian G, Park SW (2012) Comparative study of color, pungency, and biochemical composition in chili pepper (Capsicum annuum) under different light-emitting diode treatments. HortScience 47: 1729–1735 [Google Scholar]
- González-Zamora A, Sierra-Campos E, Luna-Ortega JG, Pérez-Morales R, Rodríguez Ortiz JC, García-Hernández JL (2013) Characterization of different Capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography, determination of pungency and effect of high temperature. Molecules 18: 13471–13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Carbajal MG, Monforte-González M, Miranda-Ham ML, Godoy-Hernández G, Vázquez-Flota F (2010) Induction of capsaicinoid synthesis in Capsicum chinense cell cultures by salicylic acid or methyl jasmonate. Biol Plant 54: 430–434 [Google Scholar]
- Humphreys JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5: 224–229 [DOI] [PubMed] [Google Scholar]
- Iwai K, Suzuki T, Fujiwake H (1979) Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues, in Capsicum annuum var. annuum cv. Karayatsubusa at different growth stages after flowering. Agric Biol Chem 43: 2493–2498 [Google Scholar]
- Keyhaninejad N, Curry J, Romero J, O’Connell MA (2014) Fruit specific variability in capsaicinoid accumulation and transcription of structural and regulatory genes in Capsicum fruit. Plant Sci 215-216: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim S, Kim S, Ki BD (2001) Isolation of cDNA clones differentially accumulated in the placenta of pungent pepper by suppression subtractive hybridization. Mol Cells 11: 213–219 [PubMed] [Google Scholar]
- Kim JS, Park M, Lee DJ, Kim BD (2009) Characterization of putative capsaicin synthase promoter activity. Mol Cells 28: 331–339 [DOI] [PubMed] [Google Scholar]
- Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C, et al. (1998) Towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J 16: 263–276 [DOI] [PubMed] [Google Scholar]
- Lee A, Cho K, Jang S, Rakwal R, Iwahashi H, Agrawal GK, Shim J, Han O (2004) Inverse correlation between jasmonic acid and salicylic acid during early wound response in rice. Biochem Biophys Res Commun 318: 734–738 [DOI] [PubMed] [Google Scholar]
- Lindsey K, Bosland PW (1996) A field study of environmental interaction on pungency. Capsicum Eggplant Newslett 14: 36–38 [Google Scholar]
- Liu J, Osbourn A, Ma P (2015) MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol Plant 8: 689–708 [DOI] [PubMed] [Google Scholar]
- Liu S, Li W, Wu Y, Chen C, Lei J (2013) De novo transcriptome assembly in chili pepper (Capsicum frutescens) to identify genes involved in the biosynthesis of capsaicinoids. PLoS ONE 8: e48156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Mazourek M, Pujar A, Borovsky Y, Paran I, Mueller L, Jahn MM (2009) A dynamic interface for capsaicinoid systems biology. Plant Physiol 150: 1806–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Ochoa-Alejo N, Ramírez-Malagón R (2001) In vitro chili pepper biotechnology. In Vitro Cell Dev Biol Plant 37: 730–741 [Google Scholar]
- Patzlaff A, McInnis S, Courtenay A, Surman C, Newman LJ, Smith C, Bevan MW, Mansfield S, Whetten RW, Sederoff RR, et al. (2003) Characterisation of a pine MYB that regulates lignification. Plant J 36: 743–754 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28: 489–521 [DOI] [PubMed] [Google Scholar]
- Qin C, Yu C, Shen Y, Fang X, Chen L, Min J, Cheng J, Zhao S, Xu M, Luo Y, et al. (2014) Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc Natl Acad Sci USA 111: 5135–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MJ, Inden H (2012) Effect of nutrient solution and temperature on capsaicin content and yield contributing characteristics in six sweet pepper (Capsicum annuum L.) cultivars. J Food Agric Environ 10: 524–529 [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JDG (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49: 317–343 [DOI] [PubMed] [Google Scholar]
- Rodas-Junco BA, Cab-Guillén Y, Muñoz-Sánchez JA, Vázquez-Flota F, Monforte-González M, Hernández-Sotomayor SMT (2013) Salicylic acid induces vanillin synthesis through the phospholipid signaling pathway in Capsicum chinense cell cultures. Plant Signal Behav 8: doi/10.4161/psb.26752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C Jr, Kang BC, Liu K, Mazourek M, Moore SL, Yoo EY, Kim BD, Paran I, Jahn MM (2005) The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J 42: 675–688 [DOI] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Suresh B, Ravishankar GA (2005) Methyl jasmonate modulated biotransformation of phenylpropanoids to vanillin related metabolites using Capsicum frutescens root cultures. Plant Physiol Biochem 43: 125–131 [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Merida A, Parr A, Mackay S, Culianez-Macia FA, Roberts K, Martin C (1998) The AmMYB308 and AmMYB330 transcription factors from Antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 10: 135–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lee C, Zhong R, Ye ZH (2009) MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 21: 248–266 [DOI] [PMC free article] [PubMed] [Google Scholar]