Triacylglycerol is a key intermediate in membrane lipid breakdown and fatty acid β-oxidation, and blocking triacylglycerol hydrolysis reduces oxidative stress and enhances plant survival under extended darkness.
Abstract
Neutral lipid metabolism is a key aspect of intracellular homeostasis and energy balance and plays a vital role in cell survival under adverse conditions, including nutrient deprivation in yeast and mammals, but the role of triacylglycerol (TAG) metabolism in plant stress response remains largely unknown. By thoroughly characterizing mutants defective in SUGAR-DEPENDENT1 (SDP1) triacylglycerol lipase or PEROXISOMAL ABC TRANSPORTER 1 (PXA1), here we show that TAG is a key intermediate in the mobilization of fatty acids from membrane lipids for peroxisomal β-oxidation under prolonged dark treatment. Disruption of SDP1 increased TAG accumulation in cytosolic lipid droplets and markedly enhanced plant tolerance to extended darkness. We demonstrate that blocking TAG hydrolysis enhances plant tolerance to dark treatment via two distinct mechanisms. In pxa1 mutants, in which free fatty acids accumulated rapidly under extended darkness, SDP1 disruption resulted in a marked decrease in levels of cytotoxic lipid intermediates such as free fatty acids and phosphatidic acid, suggesting a buffer function of TAG accumulation against lipotoxicity under fatty acid overload. In the wild type, in which free fatty acids remained low and unchanged under dark treatment, disruption of SDP1 caused a decrease in reactive oxygen species production and hence the level of lipid peroxidation, indicating a role of TAG in protection against oxidative damage. Overall, our findings reveal a crucial role for TAG metabolism in membrane lipid breakdown, fatty acid turnover, and plant survival under extended darkness.
Photosynthesis provides the energy and reduced carbon for metabolism, growth, storage, and maintenance throughout the daily cycle. During the day, light energy is used to fuel photosynthetic carbon assimilation to produce organic compounds. In many plants including Arabidopsis (Arabidopsis thaliana), the majority of the immediate stable products of photosynthesis (up to 80%) are used for the synthesis of sugars and starch (Smith and Stitt, 2007; Stitt and Zeeman, 2012). At night when photosynthesis is not possible, starch accumulated during the day is hydrolyzed to provide a steady sugar and energy supply. A small fraction (approximately 10%) of photosynthetic carbon is used for the synthesis of fatty acids in the chloroplast in the light (Murphy and Leech, 1981). The end products of fatty acid synthesis can be used to acylate glycerol-3-phosphate (G3P) by acyltransferases to produce phosphatidic acid (PA) in the chloroplast, or in the endoplasmic reticulum (ER) following their export from the chloroplast (Bates et al., 2013). Dephosphorylation of PA yields diacylglycerol (DAG) in the ER and the chloroplast. Because the substrate specificity of enzymes responsible for PA assembly in the two compartments differs, DAG formed in the chloroplast or the ER is characterized by the presence of 16- or 18-carbon fatty acids at the sn-2 position of glycerol backbone, respectively (Heinz and Roughan, 1983; Frentzen, 1998).
PA and DAG are key intermediates in cellular glycerolipid metabolism. While PA and DAG generated in the chloroplast serve almost exclusively as a precursor for the synthesis of thylakoid membrane lipids at the chloroplast envelope, ER-derived DAG can be used for the assembly of both membrane lipids and triacylglycerol (TAG) in the ER (Bates and Browse, 2012; Chapman and Ohlrogge, 2012; Bates et al., 2013). In the model plant Arabidopsis (Arabidopsis thaliana), two enzymes, namely DAG acyltransferase1 (DGAT1) and phospholipid:DAG acyltransferase1 (PDAT1), play an overlapping role in TAG assembly in seed (Zhang et al., 2009) and nonseed (Fan et al., 2013) tissues. Due to lack of polar head groups, TAG formed in the ER is first sequestered in the hydrophobic region between the two leaflets of the ER membrane, leading to swelling of the membrane bilayer and eventually the budding of small TAG-containing lipid droplets (LDs) from ER into the cytosol (Murphy and Vance, 1999; Chapman et al., 2012). Cytosolic LDs grow by expansion or coalescence and are actively involved in many aspects of cellular metabolism and homeostasis (Chapman et al., 2012; Wilfling et al., 2014).
During vegetative growth, most of PA and DAG are used for membrane lipid assembly to support cellular membrane biogenesis, expansion, and maintenance (Bates and Browse, 2012). As a consequence, TAG does not accumulate to significant amounts (Xu and Shanklin, 2016), despite the occurrence of high TAG synthesis activities (Dahlqvist et al., 2000) and high transcript levels of genes encoding key enzymes for TAG assembly (Li et al., 2010; Hernández et al., 2012) in vegetative tissues such as leaves and roots. Recent studies showed that one reason for limited TAG accumulation in vegetative tissues is rapid TAG turnover, because disruption of SUGAR-DEPENDENT1 (SDP1), a cytosolic lipase responsible for hydrolyzing TAG in LDs into free fatty acids (FFAs) and DAG, significantly enhances TAG accumulation in roots and leaves (Kelly et al., 2013). In plants, fatty acids are broken down via β-oxidation in the peroxisome into acetyl-CoA, a key metabolite for energy production via mitochondrial respiration and for the synthesis of carbohydrates via the glyoxylate cycle and gluconeogenesis during oilseed germination (Graham, 2008). In Arabidopsis, a peroxisomal membrane protein named PEROXISOMAL ABC TRANSPORTER 1 (PXA1) is responsible for importing fatty acids as CoA esters into peroxisomes to enter the β-oxidation pathway (De Marcos Lousa et al., 2013). There is evidence that, unlike the situation in germinating oilseeds, fatty acid β-oxidation in vegetative tissues of Arabidopsis produces energy but not carbohydrates (Kunz et al., 2009).
The presence of high biochemical activities for TAG synthesis (Dahlqvist et al., 2000) and breakdown (Tjellström et al., 2015) raises an intriguing question of the functional role for TAG metabolism in plant vegetative tissues. Early studies in yeast and mammals showed that TAG accumulation plays a pivotal role in sequestering FFAs (Listenberger et al., 2003) and DAG (Zhang et al., 2003) into lipid droplets and thereby protecting against lipotoxic cell death under cellular conditions of fatty acid overload. In plants, deficiency in TAG synthesis results in premature cell death when fatty acids are produced in excess of cellular demands for membrane lipid synthesis (Fan et al., 2013). Blocking TAG hydrolysis by disrupting SDP1 compromises fatty acid β-oxidation and alters membrane lipid homeostasis in Arabidopsis under normal growth conditions, supporting a key role for TAG metabolism in fatty acid turnover in plants (Fan et al., 2014). Interestingly, recent studies in mammals showed that TAG accumulation in lipid droplets protects cell oxidative stress by limiting reactive oxygen species (ROS) generation and inhibiting lipid peroxidation of polyunsaturated fatty acids (Kuramoto et al., 2012; Bailey et al., 2015). Oxidative stress has been closely linked to stress tolerance, aging, and cell death in organisms ranging from yeast to plants to humans (Van Breusegem and Dat, 2006; Mullineaux and Baker, 2010; Gaschler and Stockwell, 2017). In plants, many abiotic stress treatments including prolonged darkness are known to induce ROS overproduction (Rosenwasser et al., 2011; Noctor et al., 2014) and TAG accumulation (Kunz et al., 2009; Moellering et al., 2010; Gasulla et al., 2013), but the physiological role of TAG in oxidative stress has to date not been studied in plants.
Cells suffer carbon starvation when starch reserve is exhausted, while photosynthetic carbon assimilation remains inactive under environmental constraints such as extended darkness. Metabolic and transcriptional profiling showed that many genes involved in the breakdown of cellular structural components such as proteins and lipids are induced. Notably, the transcripts of many genes assigned to fatty acid peroxisomal β-oxidation are markedly elevated in response to extended darkness (Thimm et al., 2004; Bläsing et al., 2005; Usadel et al., 2008). Blocking fatty acid (Kunz et al., 2009) and amino acid (Araújo et al., 2010) catabolism compromises the ability of plants to tolerate dark-induced starvation. These results suggest that plants use alternative substrates for respiration to minimize harmful effects of temporal carbon starvation and to aid cell survival during prolonged darkness. However, it remains unknown whether TAG turnover is required for membrane lipid breakdown and fatty acid peroxisomal β-oxidation and what the physiological function of TAG metabolism is during dark-induced carbon starvation in plants. Here, we demonstrate that TAG metabolism is an important aspect of membrane lipid breakdown during dark-induced carbon starvation. We also provide physiological, biochemical, and genetic evidence that, in addition to acting as a safe depot of cytotoxic lipid intermediates, TAG accumulation plays a vital role in protecting against oxidative damage under extended darkness in plants.
RESULTS
Rate of Fatty Acid Turnover Is Increased under Extended Darkness
Largely based on transcriptional profiling in Arabidopsis, it has been suggested that fatty acid catabolism is activated during carbon starvation such as extended darkness (Thimm et al., 2004; Bläsing et al., 2005; Usadel et al., 2008), but direct experimental evidence supporting this assumption is still lacking. Since fatty acid synthesis is negligible in the dark (Ohlrogge and Jaworski, 1997; Bao et al., 2000) and total leaf fatty acid levels were similar between wild-type and pxa1 plants at the end of the 16-h-light period (Fig. 1A), the difference in fatty acid content between light- and dark-treated plants should provide a simple estimate for net fatty acid turnover in the wild type and mutants. We initially focused our analysis on plants dark treated for 24 h as pxa1 mutant plants were reported to undergo severe necrotic cell death when dark treatment was extended a few hours beyond 24 h (Kunz et al., 2009). We also extended the analysis to sdp1 mutants (Eastmond, 2006), reasoning that if TAG metabolism is vital for fatty acid breakdown, a similar net fatty acid turnover in either the sdp1 or pxa1 background would be expected, because SDP1 is the major lipase controlling TAG breakdown in leaves (Kelly et al., 2013; Fan et al., 2014). Indeed, the total leaf fatty acid content was significantly higher in sdp1 and pxa1 mutants compared with wild type, but similar between sdp1 and pxa1 after 24 h of darkness (Fig. 1B). Because a direct comparison of lipid levels between light- and dark-treated samples is complicated by the difference in levels of starch (Maatta et al., 2012), which accumulates up to 10% by dry weight during the light period but is almost completely depleted at the end of normal night (Stitt and Zeeman, 2012), we sought to estimate the net lipid turnover by comparing the total fatty acid content of 8 h versus 24 h dark-treated plants. In the wild type, the amount of total leaf fatty acids per dry weight was 11.3% lower in 24-h dark-treated compared with 8-h dark-treated plants (Fig. 1C). This value is three times higher than the net lipid turnover of 2% to 4% of total fatty acids per day estimated in intact Arabidopsis plants growth under normal day/night cycles using radioisotope labeling (Bao et al., 2000; Bonaventure et al., 2004). As expected, disruption of SDP1 or PXA1 resulted in a drastic decrease in net fatty acid turnover during dark treatment (Fig. 1C). These results provide the first direct experimental evidence that under extended darkness lipid turnover is indeed enhanced, and TAG hydrolysis is a key step in the pathway of fatty acid degradation.
Figure 1.
Fatty acid turnover is enhanced during extended darkness. A, Total leaf fatty acid content at the end of the light period. B, Total leaf fatty acid content after dark treatment (D) for 8 and 24 h. C, Total leaf fatty acid loss between 8 and 24 h of darkness. Data represent mean ± se for three independent samplings of 3-week-old wild-type (WT) and mutant plants. Asterisks indicate statistically significant differences from the dark-treated wild type (B) or the wild type (C) based on Student’s t test (P < 0.05).
TAG Is a Key Intermediate in Fatty Acid Turnover during Extended Darkness
To further analyze the role of TAG metabolism in fatty acid breakdown in leaves, we monitored the changes in TAG levels in sdp1 and pxa1 mutants exposed to extended darkness. We reasoned that if TAG synthesis and hydrolysis are essential steps in fatty acid breakdown, we would expect to see similar levels of dark-induced TAG accumulation in sdp1 and pxa1 mutants, since TAG hydrolysis is subjected to feedback inhibition in mutants defective in fatty acid β-oxidation (Graham, 2008). As shown in Figure 2, TAG levels on a per dry weight basis were very low in both sdp1 and pxa1 mutants under normal growth conditions and remained largely unaltered during the initial 8 h of dark treatment. After 24 h of darkness, the average leaf TAG levels increased by 14.5-fold and 9.6-fold in sdp1 and pxa1, respectively, relative to the wild type. Compared with sdp1, pxa1 mutants accumulated a similar amount of TAG under normal growth conditions, but substantially less TAG after 24 h of dark treatment. TAG content in wild-type leaves was very low prior to dark treatment and remained largely unchanged during dark treatment for 24 h.
Figure 2.
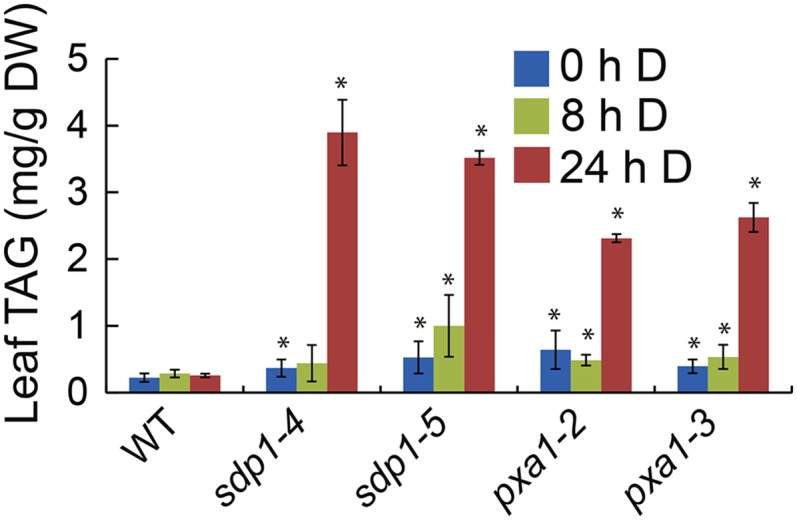
Changes in total leaf TAG content in wild-type (WT) and mutant plants during dark treatment. Three-week-old wild-type and mutant plants were exposed to darkness (D) for 24 h. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the untreated wild type based on Student’s t test (P < 0.05).
TAG Accumulation Is Due to Decreased Fatty Acid Turnover in pxa1 and sdp1
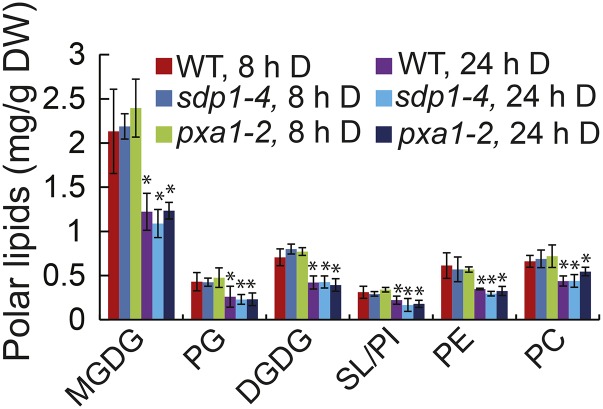
To test whether dark-induced TAG accumulation in pxa1 and sdp1 was due to increased conversion of membrane lipids to TAG or due to decreased TAG turnover, we quantified the changes in major membrane lipid levels in wild type, pxa1-2, and sdp1-4 after 8 and 24 h of darkness. On a per dry weight basis, the levels of major leaf lipids in pxa1-2 and sdp1-4 were identical to those in the wild type after 8 h of dark treatment (Fig. 3). After darkness for 24 h, levels of major membrane lipids, particularly those of monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), were decreased in the wild type, sdp1-4, and pxa1-2. Again, there were no differences in levels of major membrane lipids among the wild type, sdp1-4, and pxa1-2 after 24 h of darkness, except that PC content was significantly higher in pxa1-2 compared with the wild type (P > 0.05 based on Student’s t test). Together, these results suggest that the increased TAG accumulation in sdp1 and pxa1 is due to decreased TAG turnover rather than to increased membrane lipid conversion during dark treatment, and that TAG is mostly derived from MGDG and DGDG.
Figure 3.
Changes in polar lipid levels in wild-type and mutant plants during dark treatment. Three-week-old wild-type (WT) and mutant plants were exposed to darkness (D) for 24 h. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the 8-h dark-treated wild type based on Student’s t test (P < 0.05).
To test whether fatty acid breakdown was completely blocked in the mutants, we compared the accumulation of fatty acids in TAG (Fig. 2) with the reduction of fatty acids in polar lipids (Fig. 3) between 8 and 24 h of darkness. We found that the amounts of fatty acids accumulated in TAG (2.52 ± 0.46 mg/g dry weight [DW]) were quantitatively similar to total fatty acid loss in membrane lipids (2.30 ± 0.39 mg/g DW) in sdp1 mutants, suggesting again a crucial role of TAG hydrolysis in fatty acid turnover under dark treatment. In pxa1 mutants, the loss of fatty acids in membrane lipids (2.39 ± 0.28 mg/g DW) was greater than the accumulation of fatty acids in TAG (1.96 ± 0.19 mg/g DW) between 8 and 24 h of darkness, implying that TAG is continually hydrolyzed by SDP1 during darkness in pxa1. SDP1-mediated TAG hydrolysis may explain in part why FFA levels increased in pxa1 under extended darkness as reported by Kunz et al. (2009). However, at least part of the released acyl chains from TAG may be recycled into membrane lipid synthesis as indicated by an increase in PC levels in pxa1 compared with the wild type (Fig. 3).
Acyl Groups Derived from MGDG Are Used for the Synthesis of TAG, PC, and PE
In Arabidopsis, MGDG is characterized by a high level of 16:3 at the sn-2 position. Thus, analysis of fatty acid composition of individual lipid species should provide clues as to whether and how MGDG is converted to TAG. As shown in Figure 4, the fatty acid composition of TAG isolated from leaves of sdp1-4 was quite similar to that of TAG from pxa1-2 leaves before and after 24 h of darkness. During dark treatment, there were significant increases in polyunsaturated fatty acids including 18:2, 18:3, and 16:3 (Fig. 4A) at the expense of saturated and monosaturated fatty acids in TAGs isolated from leaves of sdp1-4 and pxa1-2 mutants. A marked increase in accumulation of polyunsaturated fatty acids including 16:3 in TAG was also observed in leaves of wild-type plants after 24 h of dark treatment.
Figure 4.
Fatty acid composition and positional distribution of TAG in dark-treated plants. A, Fatty acid composition of TAG. B, Fatty acids at the sn-2 position. C, Fatty acids at the sn-1 + 3 positions. Three-week-old wild-type (WT) and mutant plants were exposed to darkness (D) for 24 h. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the dark-treated wild type based on Student’s t test (P < 0.05).
At least two possible routes exist that enable a flow of lipid precursors derived from MGDG to the TAG assembly pathways at the ER: (1) MGDG is converted to DAG, which is directly exported from the chloroplast to serve as a backbone for TAG synthesis; (2) MGDG or DAG derived from MGDG are hydrolyzed by lipases to release FFAs and the exported FFAs are used for de novo TAG synthesis. To test these two possibilities, we carried out the stereo-specific analysis of fatty acid distribution in TAG isolated from leaves of 24-h dark-treated sdp1-4 and pxa1-2 plants. We found that about 80% of acyl chains at the sn-2 position of TAG are 18-carbon fatty acids, suggesting the majority of DAG for TAG synthesis is derived from the ER pathway. In addition, 16:3 was present at a similar level at sn-2 (Fig. 4B) and sn-1 and sn-3 positions (Fig. 4C) of TAG isolated from both sdp1-4 and pxa1-2 mutants. Since there is no known pathway for DAG export from the chloroplast, the observed even distribution of 16:3 across all three positions of TAG may suggest that only FFAs leave the chloroplast and are used for TAG assembly through the stepwise acylation of G3P in the ER.
To gain more information about the interconversion between different lipid classes, changes in fatty acid composition of major membrane lipids during 24 h of darkness were analyzed. No major differences in fatty acid composition of PC and PE were found among the wild type, sdp1-4 and pxa1-2 before dark treatment (Supplemental Fig. S1, A and B). After 24 h of darkness, there were decreases in relative amounts of 18:1 and 18:2 with a concomitant increase in 18:3 in PC in wild-type plants and both mutants, reflecting a continuation of fatty acid desaturation and/or the movement of 18:3 from MGDG to PC and PE in the dark (Maatta et al., 2012). Fatty acid composition of MGDG did not show major changes during 24 h of dark incubation in both wild type and mutants (Supplemental Fig. S1C). It is noteworthy that 16:3, which was mainly in MGDG before dark treatment, accumulated in both PC and PE after 24 h of darkness in the wild type, sdp1-4, and pxa1-2 (Supplemental Fig. S1, A and B). Together, these suggest that MGDG is converted to TAG and phospholipids during dark incubation, and there is no major difference with respect to mechanisms of conversion among the wild type and sdp1 and pxa1 mutants.
SFR2 Plays Limited Role in MGDG Breakdown under Extended Darkness
In Arabidopsis, one potential route for TAG synthesis from MGDG is initiated by FREEZING SENSITIVE 2 (SFR2), which converts MGDG to oligogalactolipids by transglycosylation with a concomitant production of DAG (Moellering et al., 2010). To test the role of SFR2 in MGDG breakdown in pxa1 mutants, we generated a double mutant between sfr2-4 and pxa1-2. Analysis of leaf lipid extracts from dark-treated plants revealed no major differences in TAG (Supplemental Fig. S2A) and MGDG (Supplemental Fig. S2B) levels between pxa1-2 and sfr2-4 pxa1-2. The fatty acid composition of leaf TAG was also similar between the 24-h dark-treated single and double mutants (Supplemental Fig. S3). Together these results suggest that SFR2 plays a limited role in mediating MGDG to TAG and PC conversion during extended dark treatment, likely reflecting the fact that SFR2 is localized in the outer envelope of chloroplasts (Xu et al., 2003), whereas its substrate MGDG is mostly present in thylakoid membranes (Douce and Joyard, 1990).
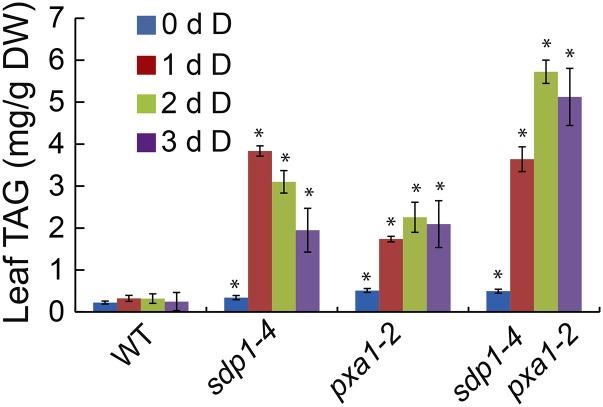
Enhancing Dark-Induced TAG Accumulation by Disruption of SDP1 in pxa1
TAG levels in pxa1 were significantly lower than that in sdp1 after 24 h of dark treatment (Fig. 2). To test whether the decreased TAG accumulation in pxa1 is due to decreased synthesis or increased hydrolysis, we constructed an sdp1-4 pxa1-2 double mutant and analyzed the time course of TAG accumulation during dark treatment for 3 d. Under normal growth conditions, TAG content in sdp1-4 pxa1-2 was comparable with either single mutant (Fig. 5). Under dark treatment, TAG accumulation in sdp1-4 leveled off after 1 d and started to decline thereafter. The amounts of TAG increased slightly after 1 d of darkness in pxa1-2 but almost linearly through the first 2 d of dark treatment in sdp1-4 pxa1-2. During the initial 1 d of darkness, TAG levels in the double mutant were similar to sdp1-4 but substantially higher than in pxa1-2. After 2 d of treatment, the double mutant accumulated approximately twice as much TAG as the single mutants. Together these results suggest that the decreased TAG accumulation in pxa1 mutants under extended darkness is due to increased TAG hydrolysis mediated by SDP1.
Figure 5.
Enhancing TAG accumulation by disruption of SDP1 in pxa1 during dark treatment. Three-week-old wild-type (WT) and mutant plants were exposed to darkness (D) for 3 d. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the untreated wild type based on Student’s t test (P < 0.05).
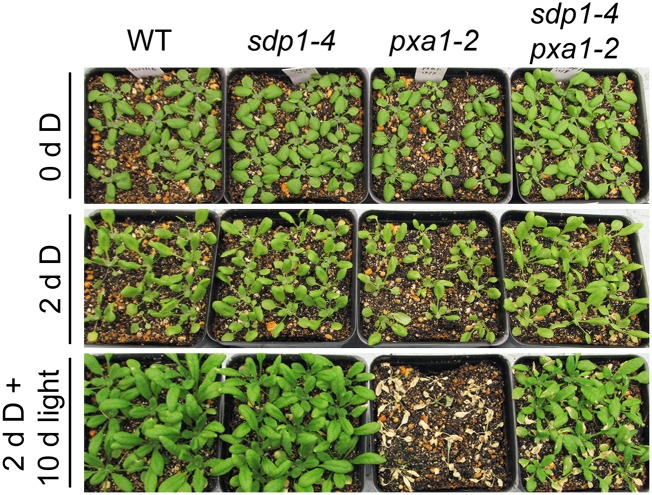
Disruption of SDP1 Enhances the Survival of pxa1 Plants under Extended Darkness
Mutants defective in fatty acid β-oxidation have been shown to be hypersensitive to extended darkness, likely due to the accumulation of FFAs (Kunz et al., 2009). Under our experimental conditions, both single pxa1-2 and double sdp1-4 pxa1-2 mutant plants were able to fully recover when transferred back to light growth conditions following 24 h of dark incubation (Supplemental Fig. S4). However, when dark treatment was extended to 48 h, pxa1-2 plants were severely wilted and unable to resume growth when reexposed to light for 24 h (Fig. 6). In contrast to the single pxa1-2 mutant, sdp1-4 pxa1-2 double mutant plants were much less affected after 48 h of dark treatment, and all of them were able to fully recover in the continuous light, similar to wild-type and sdp1-4 plants subjected to the same treatment, although the overall growth during the recovery was markedly reduced in the double mutant compared with the sdp1-4 and wild type.
Figure 6.
Increasing tolerance of pxa1 plants to extended darkness by disruption of SDP1. Three-week-old wild-type (WT) and mutant plants were exposed to darkness (D) for 2 d and then reexposed to light for 10 d before the photograph was taken.
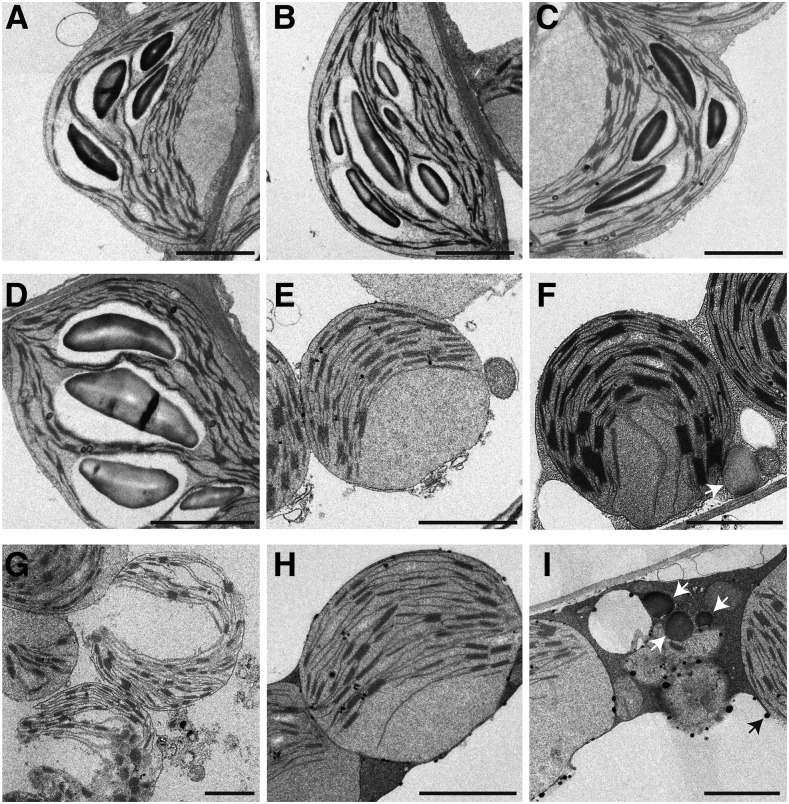
At the ultrastructural level, cellular organelles were indistinguishable among the wild type, sdp1-4, pxa1-2, and sdp1-4 pxa1-2 before darkness (Fig. 7, A–D). Chloroplasts were typically lens-shaped in all genotypes, containing extensive thylakoid membrane systems and large starch granules. After 2 d of darkness, the shape of most chloroplasts became spherical in wild-type and single and double mutant plants (Fig. 7, E–I). LD, which was absent in leaves of plants before dark treatment, accumulated in the cytosol in both sdp1-4 single and sdp1-4 pxa1-2 double mutants after dark treatment for 2 d (Fig. 7, F and I), consistent with increases in TAG levels during dark treatment as shown in Figure 5. While cellular organelles appeared to remain largely undamaged in the wild type (Fig. 7E), sdp1-4 (Fig. 7F), and sdp1-4 pxa1-2 (Fig. 7H), cell compartmentation was lost, chloroplasts were swollen, and the envelope was ruptured in pxa1-2 after 2 d of darkness (Fig. 7G).
Figure 7.
Ultrastructural changes in plants exposed to extended darkness. A to D, Transmission electron micrographs of leaf cells of the wild type (A), sdp1-4 (B), pxa1-2 (C), and sdp1-4 pxa1-2 (D) before dark treatment. E to G, Transmission electron micrographs of leaf cells of the wild type (E), sdp1-4 (F), and pxa1-2 (G) after 2 d of darkness. H and I, Transmission electron micrographs of leaf cells of the sdp1-4 pxa1-2 double mutant after 2 d of darkness. Plants were 3 weeks old prior to dark treatment. Arrows indicate LDs. Bars = 2 µm.
Disruption of SDP1 in pxa1 Increases the Sequestration of FFA and PA in TAG
Studies in yeast and mammals have shown that TAG accumulation protects against cell death through at least two mechanisms. One involves sequestration of toxic lipid intermediates such as FFAs (Listenberger et al., 2003); another is associated with the protection by TAG accumulation against oxidative stress (Kuramoto et al., 2012; Bailey et al., 2015). To test whether the increased TAG by disrupting SDP1 affects FFA levels, total lipids were extracted from dark-treated plants and neutral lipids were separated by thin-layer chromatography and FFA content was quantified by gas chromatography. FFA levels increased only slightly at 1 d, but markedly at 2 d in pxa1-2 during dark treatment (Fig. 8A). Notably, disruption of SDP1 in pxa1-2 led to a marked reduction in FFA accumulation after 2 d of darkness.
Figure 8.
Changes in FFA (A) and PA (B) levels in wild-type and mutant plants under extended darkness. Three-week-old wild-type (WT) and mutant plants were exposed to darkness (D) for 2 d. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the untreated wild type based on Student’s t test (P < 0.05).
In addition to FFA, PA, a key intermediate in glycerolipid metabolism, also markedly increased after 2 d, but not 1 d of darkness in pxa1-2 (Fig. 8B). Blocking TAG hydrolysis by SDP1 disruption resulted in a 61% reduction in the PA level in pxa1-2 mutants at 2 d of darkness. PA levels remained low and unchanged during dark treatment for 2 d in wild-type and sdp1-4 mutant plants. Together, these results suggest that the increased FFA and PA levels in pxa1 are partially due to TAG hydrolysis mediated by SDP1.
Increased Tolerance to Prolonged Darkness in sdp1 Single Mutants Is Not Related to Decreases in FFA Levels
When dark treatment was extended to 10 d, most wild-type plants collapsed and showed severe signs of cell death, whereas sdp1 mutants were much less affected (Fig. 9A). When transferred back to normal growth conditions, only <10% of wild-type plants survived, whereas up to 47% of sdp1 mutants recovered (Fig. 9B). No discernable morphological and developmental differences between the wild type and sdp1 mutants were found under normal growth conditions (Fig. 9A).
Figure 9.
Increased tolerance to extended darkness in sdp1 single mutants. A, Three-week-old wild-type (WT) and mutant plants were exposed to darkness (D) for 10 d and then reexposed to light for 10 d before the photograph was taken. Control (CK) plants were grown for 3 weeks under normal growth conditions. B, Increased survival rates of sdp1 single mutants under extended darkness. Three-week-old wild-type and mutant plants were exposed to darkness for 10 d. Surviving plants were scored after reexposure to light for 10 d. C, Changes in leaf FFA levels during dark treatment. D, Changes in TAG content during dark treatment. Data in B to D are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the untreated wild type based on Student’s t test (P < 0.05).
Unlike the situation in pxa1 mutants, FFA levels remained low and showed no significant change during dark treatment for up to 10 d in both the wild type and sdp1-4 (Fig. 9C), despite large increases in TAG accumulation in sdp1 compared with the wild type during dark treatment (Fig. 9D). These results suggest that the improved survival rate in sdp1 is due to mechanisms other than sequestering toxic lipid intermediates such as FFA via TAG accumulation.
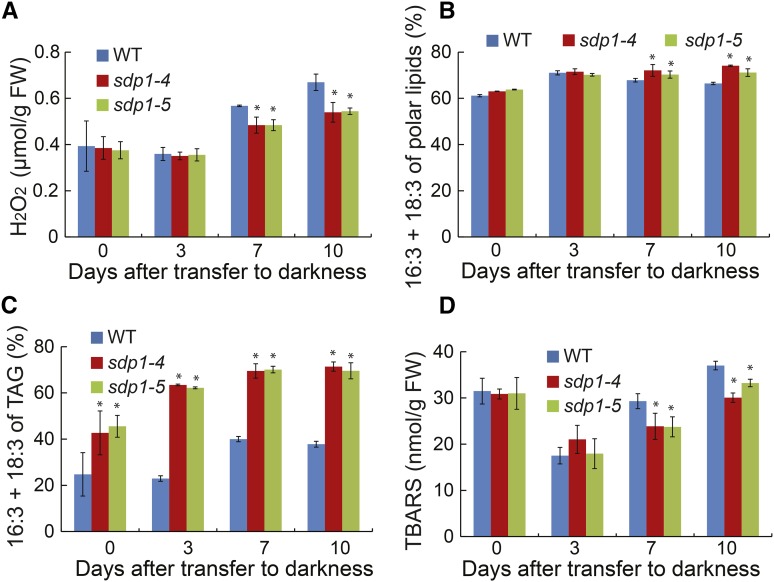
TAG Accumulation Protect against Membrane Lipid Peroxidation under Extended Darkness
Oxidative damage has long been known to play an important role in dark-induced cell death in plants, and peroxisomes are the major sites of ROS production, particularly under extended darkness (Rosenwasser et al., 2011). Because ROS such as H2O2 are the byproducts of fatty acid oxidation, the increased TAG accumulation in sdp1-4 under dark treatment might lead to a decrease in ROS production and hence an alleviation of oxidative stress-induced cell death during dark treatment. To test this possibility, changes in H2O2 levels were compared between the wild type and sdp1 mutants during dark treatment for 10 d. Leaf H2O2 content remained at the basal level during the initial 3 d of darkness, but slowly increased thereafter in both wild-type and mutant plants (Fig. 10A). There were no differences in H2O2 levels between sdp1 and the wild type during the initial 3 d of dark treatment. However, at 7 and 10 d posttreatment, sdp1 mutants showed significantly lower levels of H2O2 compared with the wild type.
Figure 10.
TAG accumulation protects against dark-induced oxidative stress. A to D, Changes in levels of H2O2 (A), trienes of polar lipids (B), trienes of TAG (C), and TBARS during dark treatment. Three-week-old wild-type (WT) and mutant plants were exposed to darkness for 10 d. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the wild type based on Student’s t test (P < 0.05).
H2O2 can discompose into highly reactive ROS such as hydroxyl radicals, which is capable of directly abstracting hydrogen from polyunsaturated fatty acids leading to the generation of cytotoxic lipid peroxides via a self-propagating chain reaction (Farmer and Mueller, 2013). In Arabidopsis leaves, as much as 75% of malondialdehyde, one of the major end products of lipid peroxidation (Hodges et al., 1999; Weber et al., 2004; Zoeller et al., 2012), is derived from polyunsaturated fatty acids with three double bonds (Weber et al., 2004; Mène-Saffrane et al., 2007). To test whether lipid peroxidation contributes to dark-induced cell death, we first analyzed the changes in the fatty acid composition of leaf membrane lipids and TAG during the prolonged dark treatment. The relative levels of polyunsaturated fatty acids with three double bonds (16:3 + 18:3, trienes) in total membrane lipids increased during the initial 3 d of darkness then started to decrease thereafter in the wild type, but remained largely unchanged in sdp1 (Fig. 10B). By day 10, the relative amounts of trienes were significantly higher in membrane lipids of sdp1 compared with those of wild type. The relative levels of trienes in TAG increased during the first 7 d of darkness and then stayed largely unaltered in both the wild type and sdp1 (Fig. 10C). By day 10, more than 70% of TAG acyl chains were fatty acids containing three double bonds in sdp1. In the wild type, the relative amounts of trienes in TAG increased from 25% to 40% of total TAG acyl chains during the initial 7 d of dark treatment and then decreased thereafter.
In many biological systems, malondialdehyde content can be measured as thiobarbituric acid reactive substances (TBARS), and levels of TBARS are widely used as an indicator of lipid peroxidation under oxidative stress (Havaux et al., 2003; Li et al., 2012). In both wild-type and sdp1 plants, TBARS levels decreased during the initial 3 d of treatment but slowly increased thereafter (Fig. 10D). Compared with the wild type, sdp1 mutants had similar levels of TBARS during the initial 3 d of darkness, but significantly decreased TBARS levels at 7 and 10 d posttreatment, mirroring changes in H2O2 levels in wild-type and sdp1 mutant plants in response to prolonged darkness (Fig. 10A).
The H2O2 (Supplemental Fig. S5A) and TBARS (Supplemental Fig. S5B) levels were very similar among the wild type, sdp1-4, pxa1-2, and sdp1-4 pxa1-2 after 2 d of darkness, suggesting that oxidative damage arising from H2O2 accumulation does not contribute significantly to dark-induced cell death in pxa1 mutants and that the increased survival rate of sdp1-4 pxa1-2 double mutant compared with pxa1-2 single mutant during dark treatment is not due to the protective effects of TAG accumulation against ROS-mediated lipid peroxidation as observed in sdp1 single mutants.
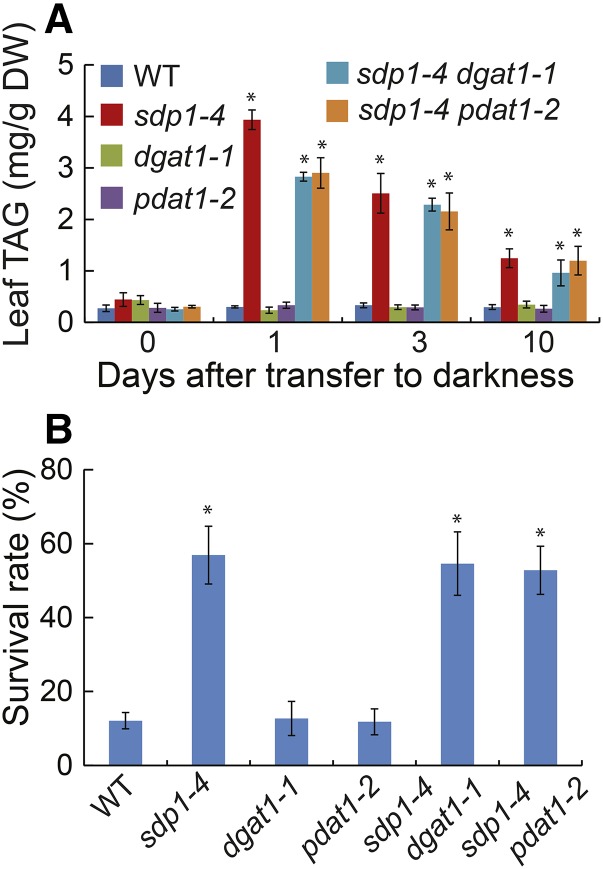
Disruption of DGAT1 or PDAT1 Has No Major Impact, Whereas Overexpression of PDAT1 Decreases Plant Survival Rates under Extended Darkness
The final step of TAG synthesis is catalyzed by PDAT1 and DGAT1 in seed (Zhang et al., 2009) and leaf (Fan et al., 2013) tissues of Arabidopsis. To test the relative contributions of DGAT1 and PDAT1 to TAG synthesis under extended darkness and their role in dark-induced cell death, we generated double mutants of sdp1-4 and dgat1-1 or pdat1-2 (Fan et al., 2013). During the initial 24 h of dark treatment, TAG levels were 28% and 26% lower in sdp1-4 dgat1-1 and sdp1-4 pdat1-2, respectively, compared with sdp1-4 (Fig. 11A). At day 3 and beyond, however, there were no significant differences in TAG levels among sdp1-4, sdp1-4 dgat1-1, and sdp1-4 pdat1-2, suggesting that either DGAT1 or PDAT1 activity is sufficient to mediate the last step of TAG synthesis in sdp1-4 or other DAG acyltransferases were activated during the dark treatment. In addition, no significant differences in plant survival rates were found among the wild type, dgat1-1, and pdat1-2 or among sdp1-4, sdp1-4 dgat1-1, and sdp1-4 pdat1-2 after 10 d of darkness (Fig. 11B).
Figure 11.
Disruption of DGAT1 or PDAT1 has limited impact on TAG accumulation and plant survival under extended darkness. A, TAG levels in the wild type (WT) and single and double mutants. B, Plant survival rates after 10 d of darkness followed by 10 d of recovery in the light. Three-week-old wild-type and mutant plants were exposed to darkness (D) for 10 d. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the wild type based on Student’s t test (P < 0.05).
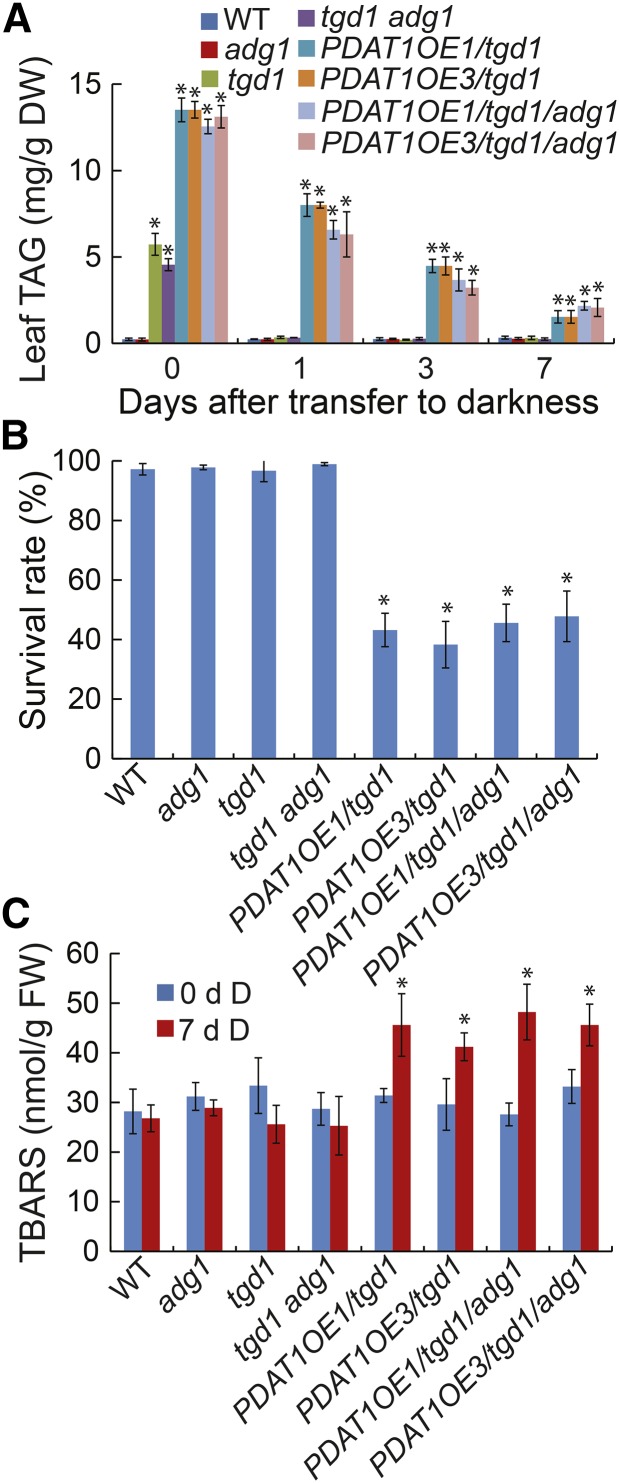
To test the potential interaction between starch and TAG metabolism in plant survival during extended darkness, we took advantage of PDAT1 overexpression lines in the tgd1 background (PDAT1OE/tgd1; Fan et al., 2013) and the adg1 mutant defective in starch synthesis due to a mutation in ADP-Glc pyrophospharylase (Lin et al., 1988). By genetic crossings, we obtained two independent PDAT1OE/tgd1 lines in the adg1 homozygous mutant background (PDAT1OE/tgd1/adg1). Under normal growth conditions, both PDAT1OE/tgd1 and PDAT1OE/tgd1/adg1 lines accumulated over 50-fold more TAG in leaves at the end of the light period compared with the wild type (Fig. 12A). During dark treatment, TAG levels in PDAT1OE/tgd1 and PDAT1OE/tgd1/adg1 lines steadily declined to <20% of the original levels by day 7. Surprisingly, both PDAT1OE/tgd1 and PDAT1OE/tgd1/adg1 lines died much faster than the wild type, adg1, tgd1, and tgd1 adg1 under extended darkness. After 7 d of darkness, more than 50% of PDAT1OE/tgd1 and PDAT1OE/tgd1/adg1 died, whereas all the wild-type, adg1, tgd1, and tgd1 adg1 plants survived (Fig. 12B). Analysis of lipid peroxidation showed that both PDAT1OE/tgd1 and PDAT1OE/tgd1/adg1 lines accumulated significantly higher levels of TBARS compared with the wild type after 7 d of darkness (Fig. 12C), supporting the idea that peroxisomal β-oxidation of fatty acids released from TAG enhances oxidative stress and hence cell death under extended darkness.
Figure 12.
Overexpression of PDAT1 increases the plant sensitivity to extended darkness. A, TAG levels in the wild type (WT) and single and double mutants. B, Plant survival rates after 7 d of darkness followed by 10 d of recovery in the light. C, Levels of TBARS after 7 d of darkness (D). Three-week-old wild type, mutants, and PDAT1 overexpression line 1 and 3 in the tgd1 (PDAT1OE/tgd1) or tgd1 adg1 double mutant background (PDAT1OE/tgd1/adg1) were exposed to darkness for 7 d. Data are the means of three biological replicates with SE. Asterisks indicate statistically significant differences from the wild type based on Student’s t test (P < 0.05).
There were no apparent differences in plant survival rates between PDAT1OE/tgd1 and PDAT1OE/tgd1/adg1 following 7 d of dark treatment (Fig. 12B). The lack of effect of starch accumulation on the survival of PDAT1OE/tgd1 following the prolonged dark treatment is perhaps not surprising, since starch accumulated during the day is the major carbon and energy source during the normal night but not under extended darkness (Stitt and Zeeman, 2012).
DISCUSSION
Under carbon starvation conditions, fatty acids released from membrane lipids are used as one of alternative substrates for respiration. In this study, the use of mutants defective in TAG hydrolysis or fatty acid β-oxidation enabled us to carry out in-depth biochemical and genetic analysis of TAG metabolism and function in Arabidopsis plants under dark-induced carbon starvation conditions. Our results reveal crucial roles of TAG metabolism in membrane lipid breakdown and fatty acid β-oxidation and uncover the evolutionarily conserved function of TAG in protection against lipotoxicity and ROS-induced oxidative damage in plant model systems. TAG accumulation has also been linked to lifespan extension in yeast (Handee et al., 2016) and Caenorhabditis elegans (Narbonne and Roy, 2009). The finding that disruption of SDP1 increases plant survival rates under dark-induced carbon starvation suggests that the role of intracellular TAG in preserving cell viability is likely to be conserved in plants as well.
TAG as a Key Intermediate in Fatty Acid Respiration
Our studies show that rather than directly being used for respiration, fatty acids released from membrane lipids are first incorporated into TAG and acyl groups derived from TAG hydrolysis are used for fatty acid peroxisomal oxidation. In wild-type plants, TAG remained at very low levels during prolonged darkness. Since TAG accumulation is dependent upon the balance between rates of synthesis and degradation, the low levels of TAG most likely reflect a rapid turnover of TAG in dark-treated leaves. Indeed, disruption of SDP1 or PXA1 resulted in an up to 15-fold increase in leaf TAG content during the initial 24 h of darkness (Fig. 2). We verified that the major membrane lipid content and their fatty acid composition were almost identical among the wild type, sdp1, and pxa1, suggesting that disruption of either SDP1 or PXA1 does not affect the process of membrane lipid breakdown per se. However, the total fatty acid levels were >10% higher in both sdp1 and pxa1 mutants compared with the wild type after 24 h of darkness (Fig. 1B). These results suggest that (1) net fatty acid turnover is similar among the wild type, sdp1, and pxa1; (2) fatty acid degradation is almost completely blocked in sdp1 during the initial 24 h of dark treatment; and (3) net fatty acid turnover is enhanced under extended darkness.
The finding that dark-induced TAG accumulation was significantly lower in pxa1 compared with sdp1 suggests that TAG was hydrolyzed by SDP1 in pxa1, although at a slower rate compared with the rate in the wild type. This was confirmed by comparatively analyzing TAG content in sdp1-4 single and sdp1-4 pxa1-2 double mutants. During the initial 24 h of dark treatment, TAG content in the sdp1-4 pxa1-2 double mutant was identical to that in sdp1-4, but higher than that in pxa1-2 (Fig. 5), suggesting that SDP1 and PXA1 function in a linear metabolic pathway, with SDP1 genetically epistatic to PXA1 in TAG accumulation in leaves. However, when dark treatment was extended to 2 d, the sdp1-4 pxa1-2 double mutant accumulated double the amount of TAG compared with either single mutant. Because the rate of TAG synthesis appeared to remain constant as evidenced by a steady increase in TAG content in sdp1-4 pxa1-2 during the initial 2 d of dark treatment (Fig. 5), such an additive effect of SDP1 and PXA1 on TAG accumulation might reflect increases in rates of TAG hydrolysis mediated by SDP1 following prolonged dark treatment in pxa1 but not in sdp1.
Pathways of Membrane Lipid Conversion to TAG
Earlier structural studies have shown that chloroplasts are the first organelle of mesophyll cells to be affected during prolonged dark incubation in leaves (Peoples et al., 1980; Thompson et al., 1998). At biochemical levels, the breakdown of chloroplast lipids, particularly MGDG, the major lipid of chloroplast membranes precedes the degradation of lipids in other cellular compartments, and the decline in thylakoid lipids is accompanied by a rise in TAG content (Wanner et al., 1991; Kaup et al., 2002). On the basis of the observations that TAG contains large amounts of fatty acids characteristic of MGDG and that there is a large increase in size and number of plastoglobules, chloroplasts have been suggested as the site of TAG synthesis and storage under extended darkness (Kaup et al., 2002; Kunz et al., 2009). Using a regiospecific lipase, we found that the majority of TAG contains 18-carbon fatty acids at the sn-2 position of the glycerol backbone. In addition, lipid droplets, the TAG storage structures, were accumulated in the cytosol. These results indicate that the ER rather than the chloroplast is the site of TAG synthesis, and that MGDG and other thylakoid lipids are first hydrolyzed and the exported fatty acids are used for de novo TAG assembly by ER-resident acyltransferases.
Role of TAG in Protecting against Cell Death
Our results reveal two related mechanisms by which TAG accumulation protects against cell death in plants. The first mechanism is sequestration of toxic lipid intermediates such as FFA and PA into TAG. Such a cytoprotective role of TAG is clearly illustrated in pxa1 mutants. During the initial 1 d of dark treatment, neither FFA nor PA levels showed major increases due to rapid TAG synthesis and limited TAG hydrolysis. Mutant plants suffered no visible damage following 1 d of darkness and recovered well when transferred back to light. Between day 1 and 2, TAG accumulation leveled off, and FFA and PA levels increased dramatically, likely due to increased TAG hydrolysis and massive membrane lipid breakdown associated with cell disintegration. All pxa1 plants died after 2 d of dark treatment. Blocking TAG hydrolysis by disruption of SDP1 in pxa1 resulted in a marked increase in TAG accumulation with concomitant decreases in levels of FFA and PA and a dramatic increase in the survival rate of pxa1 plants following prolonged dark treatment.
An interesting question arising from this work relates to the metabolic origin of PA accumulated in pxa1 under extended darkness. Although PA is not a direct intermediate of TAG degradation, the finding that disruption of SDP1 caused a marked decrease in PA accumulation (Fig. 8B) suggests that TAG is an important source of PA in dark-treated pxa1. In this scenario, PA may be assembled de novo through G3P acylation by ER-resident acyltransferases (Frentzen, 1998) using fatty acids released from TAG hydrolysis. Alternatively, DAG derived from SDP1-mediated TAG breakdown may be converted into PA by the action of DAG kinase (Katagiri et al., 1996). In addition to TAG as a PA source, structural phospholipids such as PC and PE may be converted into PA by the action of phospholipase D or by the combined action of phospholipase C and DAG kinase. Further studies are needed to distinguish between these and other possibilities. Nevertheless, it is interesting to note that rapid PA accumulation in Arabidopsis seedlings in response to cold stress has been shown to be catalyzed by DAG kinase (Arisz et al., 2013).
The second mechanism that enhances plant survival under extended darkness by SDP1 disruption involves protection by TAG accumulation against ROS-induced oxidative stress. One of the primary targets of ROS is polyunsaturated fatty acids (Weber et al., 2004). We found that during prolonged darkness, increases in TBARS levels were accompanied by decreases in levels of polyunsaturated fatty acids, particularly 18:3, supporting the notion that oxidative stress is enhanced under extended darkness. Oxidative stress reflects an imbalance between the generation of ROS and the cellular capacity to detoxify their harmful effects (Mullineaux and Baker, 2010; Noctor et al., 2014; Gaschler and Stockwell, 2017). Since H2O2 is a by-product of peroxisomal fatty acid oxidation (Graham, 2008; Theodoulou and Eastmond, 2012), the increased H2O2 is likely due to a combined effect of the increased rate of fatty acid turnover (Fig. 1B) and a decrease in ROS scavenging activities (del Rio et al., 1998; Jimenez et al., 1998) during prolonged dark treatment. In sdp1, the decreased TAG hydrolysis is associated with a decrease in TBARS and H2O2 levels and an increase in levels of polyunsaturated fatty acids in membrane lipids (Fig. 10). Conversely, increased TAG accumulation prior to the dark treatment by overexpression of PDAT1 enhances oxidative stress and dark-induced cell death (Fig. 12). Together, these results, along with the data from a previous study (Kunz et al., 2009), support the view that peroxisomal β-oxidation of fatty acids remobilized from membrane lipids and TAG is a double-edged sword for plants, in a sense that it not only generates useful energy for metabolism, growth, and maintenance but also produces highly toxic ROS, thereby causing oxidative stress and contributing to cell death under extended darkness.
Alternative Routes for TAG Hydrolysis in the Absence of SDP1
The finding that TAG levels in sdp1 mutants increased during the initial 1 d of darkness and then declined (Fig. 5) suggests the existence of alternative routes for TAG hydrolysis that was activated as dark treatment progressed. In the Arabidopsis genome, there are many genes annotated as TAG lipases, most of which remain uncharacterized (Troncoso-Ponce et al., 2013). Thus, one simple explanation for decreased TAG content is that in the absence of SDP1, other TAG lipases are responsible for continued TAG hydrolysis. Studies in yeast and humans have shown that in addition to TAG hydrolysis by cytosolic lipases, an acid lipase plays an important role in storage lipid breakdown through the vacuolar/lysosomal degradative pathway of autophagy (Jaishy and Abel, 2016; Shatz et al., 2016; Wang, 2016). In this scenario, cytosolic LDs are engulfed by double membrane structures named autophagosomes and delivered to vacuoles/lysosomes for lipid catabolism by acidic lipases. The released fatty acids are oxidized in peroxisomes through β-oxidation to generate acetyl-CoA for energy production through the tricarboxylic acid cycle in mitochondria under conditions of nutrient scarcity. A homolog of mammalian acid lipase in Arabidopsis has been shown to exhibit bono fide TAG lipase activity (El-Kouhen et al., 2005), but its role in TAG hydrolysis in nonseed tissues remains unknown. Autophagy plays a critical role in nutrient recycling in plants, and autophagic activity is induced during carbon starvations conditions (Izumi et al., 2013; Avin-Wittenberg et al., 2015). In future studies, it will be interesting to examine whether the autophagy-vacuole pathway contributes to TAG breakdown and fatty acid oxidation in plants.
In summary, this study provides the direct experimental evidence that fatty acid turnover is enhanced under extended darkness. The mobilization of fatty acids from photosynthetic membrane lipids for peroxisomal β-oxidation requires TAG synthesis in the ER. Disruption of PDAT1, DGAT1, or starch synthesis has a limited impact on plant survival rates under dark treatment. On the other hand, blocking TAG hydrolysis and hence fatty acid β-oxidation reduces oxidative damage to membrane lipids and, consequently, enhances plant survival under extended darkness. Many abiotic stresses are known to induce lipid catabolism (Essigmann et al., 1998; Moellering et al., 2010) and ROS production (Van Breusegem and Dat, 2006; Noctor et al., 2014; Huang et al., 2016). Therefore, the observed protective role of TAG accumulation against lipid peroxidation may apply to other stress conditions. Further experiments are under way to test this hypothesis and to better understand the role of TAG metabolism in plant tolerance to abiotic stress.
METHODS
Plant Materials and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) plants used in this study were of the Columbia ecotype. The sdp1-4 and sdp1-5 mutants were previously described by Eastmond (2006), pxa1-2 and pxa1-3 by Kunz et al. (2009), sfr2-3 by Moellering et al. (2010), adg1 by Lin et al. (1988), and tgd1, dgat1-1, pdat1-2, and transgenic plants overexpressing PDAT1 by Fan et al. (2013).
For growth on plates, surface-sterilized seeds of Arabidopsis were germinated on 0.6% (w/v) agar-solidified one-half-strength Murashige and Skoog medium (Murashige and Skoog, 1962) supplemented with 1% (w/v) Suc in an incubator with a photon flux density of 80 to 120 μmol m–2 s–1, a light period of 16 h (22°C), and a dark period of 8 h (18°C). For growth on soil, plants were first grown on MS medium for 10 d, transferred to soil, and grown under a photosynthetic photon flux density of 150 to 200 μmol m−2 s−1 at 22/18°C (day/night) with a 16-h-light/8-h-dark period. Dark treatment was conducted in growth incubators at 24°C.
Lipid and Fatty Acid Analyses
Lipids were extracted and analyzed as described previously (Fan et al., 2013). Separation and identification of the fatty acid methyl esters were performed on an HP5975 gas chromatograph-mass spectrometer (Hewlett-Packard) fitted with a 60-m × 250-μm SP-2340 capillary column (Supelco) with helium as the carrier gas. The TAG content was calculated according to Li et al. (2006). The fatty acid composition at the sn-2 position of the glycerol backbone was determined by Rhizopus arrhizus lipase digestion as described by Härtel et al. (2000).
Quantification of TBARS Level
TBARS were prepared by extraction with chilled solution consisting of 0.3% thiobarbituric acid in 10% trichloroacetic acid (TCA). After incubation at 90°C for 15 min, samples were cooled to room temperature and centrifuged at 12,000g for 5 min. TBARS concentrations in the clear supernatant were measured at 532 nm, with a correction of nonspecific A600, using a molar extinction coefficient of 155 mm−1cm−1 (Hodges et al., 1999).
Quantification of H2O2 Content
H2O2 levels in leaves were measured according to Velikova et al. (2000). Briefly, leaf tissues were frozen in liquid nitrogen and ground to a fine powder. The powder was then suspended with ice-cold 0.1% (w/v) TCA. After centrifugation at 12,000g for 10 min, the supernatant was used to determine H2O2 levels following reaction with potassium iodine for 1 h in the dark. The reaction mixture contained 0.5 mL leaf extract, 0.5 mL potassium phosphate buffer (100 mm, pH 7.8) and 1 mL potassium iodine (1 m). The absorbance was measured at 390 nm against a blank sample prepared with 0.1% TCA instead of leaf extracts.
Transmission Electron Microscopy
Leaf tissues were fixed with 2.5% (v/v) glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.4) for 2 h and then postfixed with 1% osmium tetroxide in the same buffer for 2 h at room temperature. After dehydration in a graded series of ethanol, the tissues were embedded in EPON812 resin (Electron Microscopy Sciences), sectioned, and stained with 2% uranyl acetate and lead citrate before viewing under a JEOL JEM-1400 LaB6 120-keV transmission electron microscope.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ADG1, At5g48300; DGAT1, At2g19450; PDAT1, At5g13640; PXA1, At4g39850; SDP1, At5g04040; SFR2, AT3g06510; TGD1, At1g19800.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Changes in fatty acid composition of PC, PE, and MGDG in wild-type and mutant plants during dark treatment.
Supplemental Figure S2. Changes in levels of TAG and MGDG in wild-type, sfr2-4, pxa1-2, and sfr2-4 pxa1-2 and mutant plants during dark treatment.
Supplemental Figure S3. Fatty acid composition of TAG in wild-type, sfr2-4, pxa1-2, and sfr2-4 pxa1-2 and mutant plants before and after dark treatment.
Supplemental Figure S4. Growth phenotype of sdp1-4, pxa1-2, and sdp1-4 pxa1-2 plants after 24 h of darkness.
Supplemental Figure S5. Changes in levels of H2O2 and TBARS in wild-type, sdp1-4, pxa1-2, and sdp1-4 pxa1-2 plants during dark treatment.
Acknowledgments
We thank John Shanklin for valuable discussions and advice.
Glossary
- DAG
diacylglycerol
- DW
dry weight
- ER
endoplasmic reticulum
- FFA
free fatty acid
- G3P
glycerol-3-phosphate
- LD
lipid droplet
- PA
phosphatidic acid
- ROS
reactive oxygen species
- TAG
triacylglycerol
- TBARS
thiobarbituric acid reactive substances
- TCA
trichloroacetic acid
Footnotes
This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract number DE-SC0012704, specifically through the Physical Biosciences program of the Chemical Sciences, Geosciences and Biosciences Division. Use of the transmission electron microscope and the confocal microscope at the Center of Functional Nanomaterials was supported by the Office of Basic Energy Sciences, U.S. Department of Energy, under contract DE-SC0012704.
Articles can be viewed without a subscription.
References
- Araújo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, Witt S, Obata T, Schauer N, Graham IA, et al. (2010) Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 22: 1549–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisz SA, van Wijk R, Roels W, Zhu JK, Haring MA, Munnik T (2013) Rapid phosphatidic acid accumulation in response to low temperature stress in Arabidopsis is generated through diacylglycerol kinase. Front Plant Sci 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avin-Wittenberg T, Bajdzienko K, Wittenberg G, Alseekh S, Tohge T, Bock R, Giavalisco P, Fernie AR (2015) Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in Arabidopsis seedlings under carbon starvation. Plant Cell 27: 306–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, Postle AD, Gould AP (2015) Antioxidant role for lipid droplets in a stem cell niche of Drosophila. Cell 163: 340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Focke M, Pollard M, Ohlrogge J (2000) Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J 22: 39–50 [DOI] [PubMed] [Google Scholar]
- Bates PD, Browse J (2012) The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front Plant Sci 3: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Stymne S, Ohlrogge J (2013) Biochemical pathways in seed oil synthesis. Curr Opin Plant Biol 16: 358–364 [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G, Bao X, Ohlrogge J, Pollard M (2004) Metabolic responses to the reduction in palmitate caused by disruption of the FATB gene in Arabidopsis. Plant Physiol 135: 1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD, Dyer JM, Mullen RT (2012) Biogenesis and functions of lipid droplets in plants. J Lipid Res 53: 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD, Ohlrogge JB (2012) Compartmentation of triacylglycerol accumulation in plants. J Biol Chem 287: 2288–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97: 6487–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jimenez A, Lopez-Huertas E, Hernandez JA, Hernandez JA (1998) The activated oxygen role of peroxisomes in senescence. Plant Physiol 116: 1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcos Lousa C, van Roermund CW, Postis VL, Dietrich D, Kerr ID, Wanders RJ, Baldwin SA, Baker A, Theodoulou FL (2013) Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc Natl Acad Sci USA 110: 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R, Joyard J (1990) Biochemistry and function of the plastid envelope. Annu Rev Cell Biol 6: 173–216 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ. (2006) SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kouhen K, Blangy S, Ortiz E, Gardies AM, Ferté N, Arondel V (2005) Identification and characterization of a triacylglycerol lipase in Arabidopsis homologous to mammalian acid lipases. FEBS Lett 579: 6067–6073 [DOI] [PubMed] [Google Scholar]
- Essigmann B, Güler S, Narang RA, Linke D, Benning C (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 95: 1950–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yan C, Roston R, Shanklin J, Xu C (2014) Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis. Plant Cell 26: 4119–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yan C, Xu C (2013) Phospholipid:diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis. Plant J 76: 930–942 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Mueller MJ (2013) ROS-mediated lipid peroxidation and RES-activated signaling. Annu Rev Plant Biol 64: 429–450 [DOI] [PubMed] [Google Scholar]
- Frentzen M. (1998) Acyltransferases from basic science to modified seed oils. Fett-Lipid 100: 161–166 [Google Scholar]
- Gaschler MM, Stockwell BR (2017) Lipid peroxidation in cell death. Biochem Biophys Res Commun 482: 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasulla F, Vom Dorp K, Dombrink I, Zähringer U, Gisch N, Dörmann P, Bartels D (2013) The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J 75: 726–741 [DOI] [PubMed] [Google Scholar]
- Graham IA. (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59: 115–142 [DOI] [PubMed] [Google Scholar]
- Handee W, Li X, Hall KW, Deng X, Li P, Benning C, Williams BL, Kuo MH (2016) An energy-independent pro-longevity function of triacylglycerol in yeast. PLoS Genet 12: e1005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Dormann P, Benning C (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA 97: 10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Lütz C, Grimm B (2003) Chloroplast membrane photostability in chlP transgenic tobacco plants deficient in tocopherols. Plant Physiol 132: 300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E, Roughan PG (1983) Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol 72: 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández ML, Whitehead L, He Z, Gazda V, Gilday A, Kozhevnikova E, Vaistij FE, Larson TR, Graham IA (2012) A cytosolic acyltransferase contributes to triacylglycerol synthesis in sucrose-rescued Arabidopsis seed oil catabolism mutants. Plant Physiol 160: 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611 [DOI] [PubMed] [Google Scholar]
- Huang S, Van Aken O, Schwarzländer M, Belt K, Millar AH (2016) The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol 171: 1551–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Hidema J, Makino A, Ishida H (2013) Autophagy contributes to nighttime energy availability for growth in Arabidopsis. Plant Physiol 161: 1682–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaishy B, Abel ED (2016) Lipids, lysosomes, and autophagy. J Lipid Res 57: 1619–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A, Hernandez JA, Pastori G, Sevilla F, del Rio LA, Sevilla F (1998) Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol 118: 1327–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Mizoguchi T, Shinozaki K (1996) Molecular cloning of a cDNA encoding diacylglycerol kinase (DGK) in Arabidopsis thaliana. Plant Mol Biol 30: 647–653 [DOI] [PubMed] [Google Scholar]
- Kaup MT, Froese CD, Thompson JE (2002) A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol 129: 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AA, van Erp H, Quettier AL, Shaw E, Menard G, Kurup S, Eastmond PJ (2013) The sugar-dependent1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol 162: 1282–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Scharnewski M, Feussner K, Feussner I, Flügge UI, Fulda M, Gierth M (2009) The ABC transporter PXA1 and peroxisomal β-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. Plant Cell 21: 2733–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, et al. (2012) Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem 287: 23852–23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yu K, Hildebrand DF (2010) DGAT1, DGAT2 and PDAT expression in seeds and other tissues of epoxy and hydroxy fatty acid accumulating plants. Lipids 45: 145–157 [DOI] [PubMed] [Google Scholar]
- Li X, Moellering ER, Liu B, Johnny C, Fedewa M, Sears BB, Kuo MH, Benning C (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24: 4670–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Beisson F, Pollard M, Ohlrogge J (2006) Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67: 904–915 [DOI] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J (1988) Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol 86: 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100: 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta S, Scheu B, Roth MR, Tamura P, Li M, Williams TD, Wang X, Welti R (2012) Levels of Arabidopsis thaliana leaf phosphatidic acids, phosphatidylserines, and most trienoate-containing polar lipid molecular species increase during the dark period of the diurnal cycle. Front Plant Sci 3: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mène-Saffrané L, Davoine C, Stolz S, Majcherczyk P, Farmer EE (2007) Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an Arabidopsis tocopherol-deficient mutant. Whole-body mapping of malondialdehyde pools in a complex eukaryote. J Biol Chem 282: 35749–35756 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Muthan B, Benning C (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330: 226–228 [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Baker NR (2010) Oxidative stress: antagonistic signaling for acclimation or cell death? Plant Physiol 154: 521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Murphy DJ, Leech RM (1981) Photosynthesis of lipids from 14CO2 in Spinacia oleracea. Plant Physiol 68: 762–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, Vance J (1999) Mechanisms of lipid-body formation. Trends Biochem Sci 24: 109–115 [DOI] [PubMed] [Google Scholar]
- Narbonne P, Roy R (2009) Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457: 210–214 [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164: 1636–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 109–136 [DOI] [PubMed] [Google Scholar]
- Peoples MB, Beilharz VC, Waters SP, Simpson RJ, Dalling MJ (1980) Nitrogen redistribution during grain growth in wheat (Triticum aestivum L.): II. Chloroplast senescence and the degradation of ribulose-1,5-bisphosphate carboxylase. Planta 149: 241–251 [DOI] [PubMed] [Google Scholar]
- Rosenwasser S, Rot I, Sollner E, Meyer AJ, Smith Y, Leviatan N, Fluhr R, Friedman H (2011) Organelles contribute differentially to reactive oxygen species-related events during extended darkness. Plant Physiol 156: 185–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz O, Holland P, Elazar Z, Simonsen A (2016) Complex relations between phospholipids, autophagy, and neutral lipids. Trends Biochem Sci 41: 907–923 [DOI] [PubMed] [Google Scholar]
- Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC (2012) Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol 15: 282–292 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Eastmond PJ (2012) Seed storage oil catabolism: a story of give and take. Curr Opin Plant Biol 15: 322–328 [DOI] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37: 914–939 [DOI] [PubMed] [Google Scholar]
- Thompson JE, Froese CD, Madey E, Smith MD, Hong Y (1998) Lipid metabolism during plant senescence. Prog Lipid Res 37: 119–141 [DOI] [PubMed] [Google Scholar]
- Tjellström H, Strawsine M, Ohlrogge JB (2015) Tracking synthesis and turnover of triacylglycerol in leaves. J Exp Bot 66: 1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso-Ponce MA, Cao X, Yang Z, Ohlrogge JB (2013) Lipid turnover during senescence. Plant Sci 205-206: 13–19 [DOI] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141: 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants - Protective role of exogenous polyamines. Plant Sci 151: 59–66 [Google Scholar]
- Wang CW. (2016) Lipid droplets, lipophagy, and beyond. Biochim Biophys Acta 1861(8 Pt B): 793–805 [DOI] [PubMed] [Google Scholar]
- Wanner L, Keller F, Matile P (1991) Metabolism of radiolabeled galactolipids in senescent barley leaves. Plant Sci 78: 199–206 [Google Scholar]
- Weber H, Chételat A, Reymond P, Farmer EE (2004) Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J 37: 877–888 [DOI] [PubMed] [Google Scholar]
- Wilfling F, Haas JT, Walther TC, Farese RV Jr (2014) Lipid droplet biogenesis. Curr Opin Cell Biol 29: 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fan J, Riekhof W, Froehlich JE, Benning C (2003) A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J 22: 2370–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Shanklin J (2016) Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu Rev Plant Biol 67: 179–206 [DOI] [PubMed] [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB (2009) DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21: 3885–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chieu HK, Low CP, Zhang S, Heng CK, Yang H (2003) Schizosaccharomyces pombe cells deficient in triacylglycerols synthesis undergo apoptosis upon entry into the stationary phase. J Biol Chem 278: 47145–47155 [DOI] [PubMed] [Google Scholar]
- Zoeller M, Stingl N, Krischke M, Fekete A, Waller F, Berger S, Mueller MJ (2012) Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of pimelic and azelaic acid. Plant Physiol 160: 365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]