Abstract
Exercise training (ET) is a Guidelines Class 1A level of evidence adjunct therapy for heart failure (HF) with reduced ejection fraction treatment. As yet less certain is the role of ET for HF with preserved ejection fraction. Different ET types (endurance and resistance) and levels of intensity or domains (light, light-to-moderate and high-to-moderate) are used for ET programmes in patients with cardiac failure. Assessment of ET prescription can be performed through indirect (heart rate reserve) or direct metabolic measures (VO2 reserve, ventilatory threshold) with the most precise methodology based on the analysis of VO2 kinetics during constant work rate protocols of different workloads. The goals of assessing the effects of exercise prescription on functional capacity are traditionally represented by changes in VO2 during peak exercise by cardiopulmonary exercise testing (CPET). Nonetheless, the specific evaluation of how ET may favourably affect the abnormal patterns of VO2 linearity for work rate increase and the effects on ventilation seem important adjunctive parameters to be evaluated and monitored. Although a minority, some HF patients may not respond to ET programmes. This specific phenotype, once appropriately identified, needs a different approach and – intriguingly – should be switched to a higher ET intensity domain to yield the most comprehensive benefits from a personalised ET intervention.
Keywords: Exercise training, heart failure, oxygen uptake, exercise ventilation, oxygen kinetics
One of the most challenging nonpharmacological interventions to face heart failure (HF) and its consequent hallmark exercise intolerance is exercise training (ET), which is an approach used since early 1990s in HF with reduced ejection fraction (HFrEF) to mitigate the abnormal pathophysiology of cardiac failure and its influence on clinical outcomes.[1,2] Its practice has been more recently extended to HF with preserved ejection fraction (HFpEF) as this population[3] is similarly limited by fatigue and dyspnoea. ET benefits involve multiple organ systems, but the extent and targets of ET vary according to the protocol used.[4] ET can be planned according to different modalities (bike or treadmill); types (endurance versus resistance or their combination); intensity (continuous low or moderate intensity, or high intensity interval); frequency (weekly volume) and session dose or duration. Frequency and dose of ET are intuitively and casually dependent on the intensity, i.e. for the lower intensity the most frequent and longer session.
The purpose of this review is to briefly describe: how to plan a training session and determine the correct ET intensity level or domain; how to assess benefits of exercise prescription by monitoring functional capacity and its related phenotypes; and how to identify subjects who – despite adherence to the programme – are non- or poor-responders to this multilevel intervention.
How to Plan an ET Programme
Exercise intensity for an ET programme may be planned by indirect exercise intensity assessment or through a direct identification of exercise intensity domain by gas exchange analysis through cardiopulmonary exercise testing (CPET). Even when exercise intensity is indirectly estimated, measurement of VO2 by CPET should be considered.[5–7]
The most common and widely applied indirect method for ET prescription is based on heart rate (HR), which is used as a reference variable assuming that the relationship between HR and work rate (WR) is linear.[8,9] Thus, with the peak exercise corresponding to peak HR, the intensity is indirectly determined by regression equations or tables as the percentage of the peak HR value at a given percentage of peak VO2 – generally ranging between 70 and 85 % of maximum predicted VO2.[7]
An important concept when using HR for exercise prescription is the concept of HR reserve (HRR), defined as the difference between HR at rest and peak exercise. An HRR percentage equal to 60 % has been identified as corresponding at the first ventilatory threshold (1st VT) in both normal and HF patients (see Figure 1).[10] Practically, ET sessions for cardiac patients are proposed at a range of 40–70 % HRR.[11] Another indirect parameter proposed by the most recent joint position statement [7] for planning ET programmes, is the VO2 reserve calculated as: (peak VO2-rest VO2) x (% intensity desired) + rest VO2. VO2 reserve provides an indicator of exercise intensity by reflecting the true amount of energy for maximal exercise attainment, taking into account baseline levels.[7]
Figure 1: Non-invasive Identification of the 1st VT and 2nd VT by CPET Evaluation.
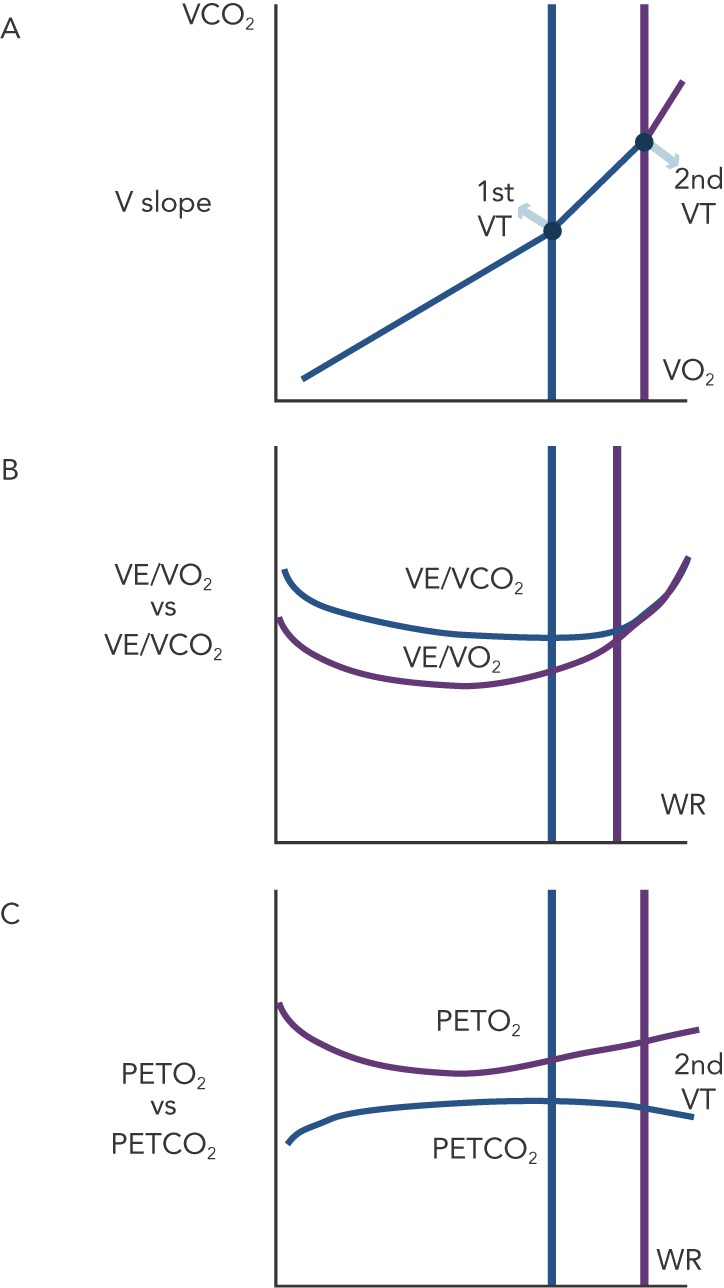
Three methods are vailable for identification of VTs by gas exchange analysis: the V-slope (a), the VE/VCO2 vs VE/VCO2 ratios behaviour; (b) and the pattern of PETCO2 vs PETO2 behaviour (c).
In order to optimise ET prescription, a comprehensive use of CPET-derived information is highly suggested for a pathophysiologically and clinically precise determination of ET intensity level. A range of aerobic exercise testing, from light- to moderate- to high- to severe-intensity aerobic training, have been used with HF patients with variable effectiveness, according to patients’ characteristics and predefined target of intervention.[12,13] The simpler and more effective way to plan ET programmes of mild to moderate intensity is the assessment of VO2 at the first ventilatory threshold, that is the metabolic condition above which blood lactic acid and pH starts to increase and decrease, respectively, generating a HCO3 buffering of the incoming acidosis. The 1st VT can be determined by three CPET-derived methods. The first is the V-slope, the point at which the incremental VCO2 production becomes higher than VO2 due to the additional CO2 produced by lactic acid buffering (see Figure 1a) with a slope of less than one to greater than one. There are two other gas exhange derived methods. One relies on the pattern of changes in ventilation (VE) to carbon dioxide (VCO2) production and VE to VO2 ratios, identifying the point of continuous increase in VE/VO2 and stable VE/VCO2 kinetics (see Figure 1b). The other is based on the definition of the point of divergent kinetics of the end-tidal partial pressure (PET) of CO2 vs the PETO2 (see Figure 1C).[5]
With increasing exercise intensity and lactic acid production above the 1st VT a second point is reached when bicarbonates no longer adequately compensate for metabolic acidosis. This is the second ventilatory threshold (2nd VT) (see Figure 1a) and is when hyperventilation occurs and ventilatory alkalosis develops. At this stage the VE/VCO2 ratio increases and inverts its trend and PETCO2 decreases (see Figure 1 b,c).
The 1st VT signals the limit between mild-to-moderate and the moderate-to-high intensity domains corresponding to around 50–60 % of peak VO2 and 60–70 % of peak HR. Admittedly, in advanced HF and in a rate of approximately 20 %, VO2 at 1st VT cannot be determined. When identifiable, the 2nd VT is usually attained at around 70–80 % peak VO2 and 80–90 % peak HR reached during incremental exercise.
It is unknown if these methods can be interchangeably applied to ET programme prescriptions in the HF setting. It has also to be considered that VO2 at 1st VT may be affected by the type of exercise; use of a treadmill leads to a 10 % higher peak VO2 compared to bike. Thus, ET programmes should be promoted and performed with the same modality with which they are planned.[14] Despite these caveats, there is evidence that ET programmes based on VO2 at 1st VT compared to HRR method provide significantly higher improvements in peak VO2 and cardiac output as assessed by O2 pulse.[15]
The recent joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation provides directions on the identification of ET intensity domains by using constant WR exercise tests, and looking at the different VO2 on kinetics response,[7] which – though in most cases is impractical – remains the most accurate physiology-based approach. Briefly, VO2 kinetics during constant WR exercise reflects three phases of adaptation of the organ systems and factors involved in alveolar-to-cell O2 coupling: phase I, or cardiodynamic, during which the increase in VO2 is mediated by the immediate increase in cardiac output and pulmonary blood flow at the start of exercise; phase II, or cell respiration, reflecting decreased O2 content (muscle extraction) and increased CO2 content in venous blood secondary to increased cell respiration as well as a further increase in cardiac output; phase III, or the steady state, during which an equilibrium is reached between O2 extraction and CO2 production rates. If WR is above the subject’s 1st VT, the rate of increase during phase III is not steady and correlates strongly with the increase of lactate (see Figure 2a). This allows for the precise identification of four ET domains, whose intensity is based on the physiology of O2 uptake kinetics: light to moderate, moderate to high, high to severe and severe to extreme (see Figure 2b).
Figure 2: Study of VO2 kinetics.
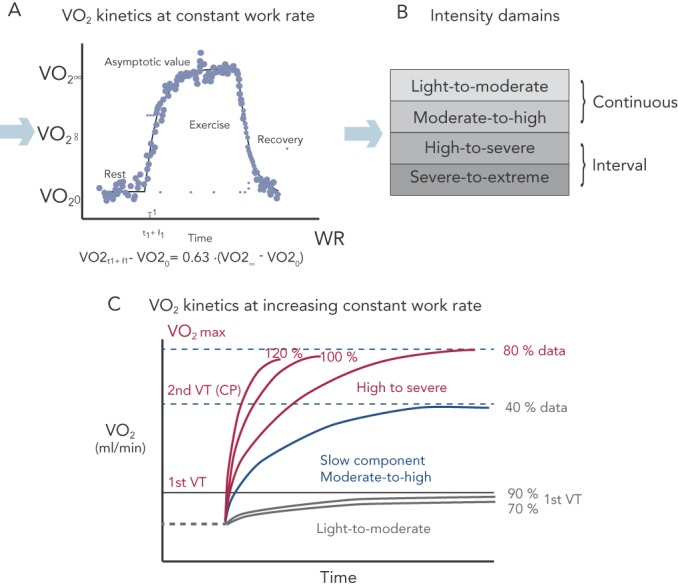
(A) Constant work rate exercise helps define the physiological background leading to O2 uptake and is the gold standard for the precise identification of four ET intensity: light-to-moderate, moderate-to-high, high-to-severe and severe-to-extreme. (B) Intensity domains are identified in light-to-moderate when VO2 steady state is reached rapidly and no lactate production occurs (steady state below 1st VT); moderate to severe that corresponds to workloads between the 1st and snd VTs. This is the maximal attained aerobic effort and a slow component is detectable due to a progressive loss of muscle efficiency. The high-to-severe domain comprises all work rate above the 2nd VT with the severe to extreme domain that is the condition that can not be sustainable unless for few minutes.
Light-to-moderate and moderate-to-high intensity programmes comprise continuous exercise, a condition that is not sustainable for high-to-severe and severe-to-extreme protocols requiring an interval exercise approach. Light-to-moderate intensity domains encompass the corresponding WR that engender a VO2 steady-state value below the corresponding 1st VT (see Figure 2c). During this WR and in this domain, a VO2 steady state is attained relatively rapidly following the commencement of exercise and there is no lactate production. For this reason, exercise can be well-tolerated and is generally sustainable for longer periods of time (30–40 min) with only a mild sense of fatigue and breathlessness.
The moderate-to-high intensity domain corresponds to those workloads between the 1st and 2nd VTs. The 2nd VT represents the maximal WR sustainable in conditions of both VO2 and lactate steady state and are the highest limit of sustainable prolonged aerobic exercise in HF patients. This work intensity determines a slow component (see Figure 2c) increase of VO2 after 2–3 minutes of constant WR, a component that is not detectable during incremental exercise. Interestingly, the VO2 slow component elevates the VO2 above the level expected for a given WR, yielding a delayed attainment of the VO2 steady-state by 10–15 minutes or more. The VO2 steady-state is attained at a level higher then expected for a below 1st VT VO2/WR relationship. The slow component represents an additional cost due to progressive loss of muscle efficiency.
The high-to-severe intensity domain comprises all the work rates above the 2nd VT that determine a peak VO2 attainment with no steady state. In this intensity domain, no slow component is evident and VO2 rises close to a monoexponential pattern that is terminated at maximal VO2 (see Figure 2c). The severe-to-extreme intensity is a domain characterised by a very short tolerable duration; fatigue occurs before peak VO2 can be achieved.
How to Assess Benefits on Functional Capacity and Exercise Performance
An improvement in functional capacity is the most immediate and objective result of an effective ET programme. Despite the fact that functional capacity is governed by an integrated response of multiple organ systems, ET intervention outcomes are generally quantified as changes in VO2. The wide physiological background behind exercise capacity response and the progressive familiarisation of the cardiac rehab personel with gas exchange analysis technique point represent, however, the solid bases for a more indepth analysis and comprehensive interpretation of results of exercise intervention trial. Further understanding is required of the determinants of exercise improvement that may add to peak VO2 and result in even more remarkable efficacy.
The question of how much ET may improve VO2 kinetics – rather than peak VO2 – is intriguing. Specifically, during maximal symptom-limited exercise, the VO2 increase is linearly related to the increase in WR and accurately reflects the extent of aerobically regenerated adenosine triphosphate (ATP). In physiological conditions, the VO2/WR linearity corresponds to a 10 ml/min increase per watt, irrespective of the load imposed and slightly changing according to exercise duration (see Figure 3a). This assumption, however, is not true in cardiovascular disorders such as HF and the pattern of VO2 increase may change in a shallow downward shift (see Figure 3b) or in a VO2 flattening at a given WR (see Figure 3c). Consequently, there are infrequent concerning conditions with a VO2 decrease associated with acute drop in blood pressure and cardiac output (see Figure 3d). Aside from this extreme condition, the other abnormal phenotypes may represent a very likely target of ET interventions worth of consideration even when changes in peak VO2 per se are not remarkable.
Figure 3: Different Patterns in VO2 vs WR Linear Increase During Maximal Exerciset Test.
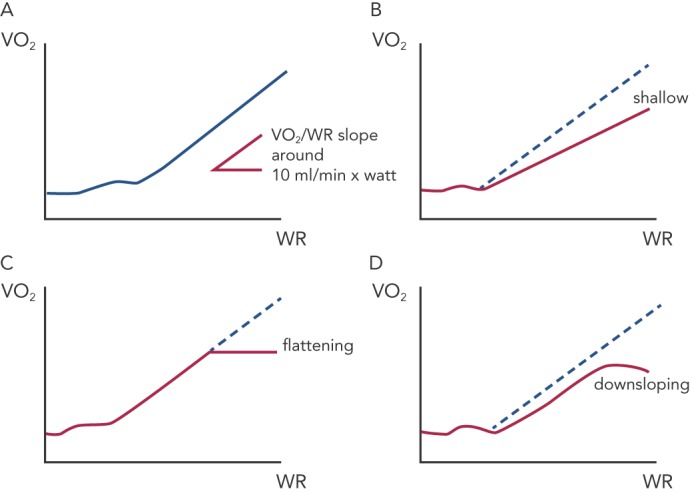
During a maximal symptom-limited exercise, VO2 increase lineraly to the increase in work rate (WR) and accurately reflects the extent of aerobically regenerated ATP. In physiological conditions, the VO2/WR linearity corrisponds to 10 ml/min increase (a). In HF, the pattern of VO2 increase may change in a shallow downward shift (b) or in a VO2 flattening at a given WR c). There are then unfrequent worrysome conditions with a VO2 downsloping associated with acute drop in blood pressure and cardiac output (d).
The other set of abnormalities that are typical of HF and may considerably benefit from ET interventions are those involving the inefficient ventilation (VE), which is an integral part of the abnormal response to exercise and represents a mainstay target of therapeutic interventions. Ventilation inefficiency is described by the slope of the rate of increase of VE vs carbon dioxide production (VCO2).[16]
VE/VCO2 slope is a clear, but underestimated, endpoint of ET. Studies that have addressed how exercise interventions may modulate exercise hyperpnoea have not been numerous, however, they have been consistent in showing a positive effect of ET (see Table 1).[1,17–22] These studies have been performed with aerobic continuous ET of variable intensity and, on average, a reduction in the VE/VCO2 slope of around 10 % has been observed. Mechanisms implicated in this beneficial effects may be multifactorial, including a modulatory activity on chemoreflex sensistivity and an improved perfusion of lung microvessels.
Table 1: Studies Assessing Changes in VE/VCO2 Slope in HF Patients Undergoing ET Programmes.
| Study | Type of HF / N of pts | Type of ET | Changes in VE VCO2 Slope |
|---|---|---|---|
| Coats et al.[1] | HFrEF/17 | 8 weeks/low intensity | From 38.0–34.0 |
| Davey et al.[17] | HFrEF/22 | 8 weeks/ low intensity | From 39.0–35.0 |
| Myers et al.[18] | HFrEF/25 | 8 weeks/ high intensity | From 33.0–27.0 |
| Guazzi et al.[19] | HFrEF/16 | 8 weeks/ moderate intensity | From 33.0–28.0 |
| Anaya et al.[20] | HFrEF/401 | 8 weeks/mild to moderate intensity | 1.7 unit reduction in high risk women |
| Zurek et al.[21] | HFrEF/52 | 8 weeks/ moderate intensity | From 35.0–32.0 |
| Smart et al.[22] | HFpEF/30 | 16 weeks/ moderate intensity | From 33.9–29.6 |
Another important target of ET programmes is exercise oscillatory ventilation (EOV), a phenomenon charcaterised by a cyclic fluctuation of ventilation and expired gas kinetics, occurring in approximately 20–30 % of heart failure patients. The proposed aetiology includes a prolonged circulatory time with failure of cardiac output to adequately increase, causing delay in circulatory time and a demodulated chemoreflex sensitivity to blood gas tension.[16]
ET seems to be the most comprehensive intervention that is able to modulate at a multisystem level the pathogenetic mechanisms involved in this relevant ventilatory abnormality, by ‘resetting’ the central and peripheral control of VE[23] and preventing the haemodynamic perturbations responsible for an increased circulatory time.[24] In a trial by Zurek et al.[21] the hypothesis was tested and confirmed: ET programmes may effectively impact EOV. Patients were optimally treated, 100 % were receiving renin–angiotensin system inhibitors and 90 % β-blockers, suggesting that EOV is a phenomenon that may not be responsive to standard HF therapy, requiring a targeted ad hoc approach, such as ET.
Future studies performed in large cohort of patients should aim to clarify how much ventilatory abnormalities may benefit from ET programmes, extending clinical endpoints to variables that, although pathophysiologically relevant, are still poorly analysed and considered.
Identification of ET Nonresponders
Some patients performing supervised ET do not show any improvement in exercise capacity and peak VO2 and represent a subset worthy of attention. Few studies have, however, addressed the clinical relevance of poor response to ET, though predicting nonresponders and how to successfully intervene by selecting appropriate and personalised intensity domain defined ET programmes would be of use.
In 155 HF patients undergoing an ET programme (aerobic continuous training at 1st VT workload), Tabet et al.[25] identified a subgroup at higher risk that did not improve peak VO2 much and in need of a tight monitored. No mechanistic explanations were provided except for evidence at multivariate analysis of B-type natriuretic peptide level along peak VO2 as only independent predictive factors of outcome (p=0.01).
Interestingly, in a large group of HF patients Scmidt et al.[26] provided the only available characterisation of ET responders identified as patients who did not improved peak VO2 by more than 5 % and work load by more than 10 %, or reduced VE/VCO2 slope by more than 5 %. Subjects who did not fulfil at least one of the above criteria were classified as non-responders. The best predictors of positive ET were HR recovery at 1 min, and peak HR and optimal thresholds separating responders from non-responders were at less than 30 bpm for HR reserve, less than 6 bpm for HR recovery and less than 101 bpm for peak HR.
In summary, it is intriguing to prospect that once more attention is posed on the nonresponder phenotype, ET should conceivably be switched to a personalised ET intensity domain that would yield to the most efficient and of ET-derived benefits across the wide spectrum of HF syndrome.
Acknowledgments
The present investigation was supported by a grant provided by the Monzino Foundation, Milano, Italy.
References
- 1.Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 2.Belardinelli R, Georgiou D, Cianci G, et al. Exercise training improves left ventricular diastolic filling in patients with dilated cardiomyopathy. Clinical and prognostic implications. Circulation. 1995;91:2775–2784. doi: 10.1161/01.cir.91.11.2775. [DOI] [PubMed] [Google Scholar]
- 3.Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: Meta-analysis of randomized control trials. Circulation. Heart failure. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ismail H, McFarlane JR, Nojoumian AH, et al. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: A systematic review and meta-analysis. JACC Heart failure. 2013;1:514–522. doi: 10.1016/j.jchf.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Balady GJ, Arena R, Sietsema K, et al. American Heart Association Exercise CR, Prevention Committee of the Council on Clinical C, Council on E, Prevention, Council on Peripheral Vascular D, Interdisciplinary Council on Quality of C, Outcomes R. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the american heart association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 6.Guazzi M, Adams V, Conraads V, et al. European Association for Cardiovascular P, Rehabilitation, American Heart A. Eacpr/aha scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezzani A, Hamm LF, Jones AM, et al. Canadian Association of Cardiac R. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: A joint position statement of the european association for cardiovascular prevention and rehabilitation, the american association of cardiovascular and pulmonary rehabilitation and the canadian association of cardiac rehabilitation. Eur J Prev Cardiol. 2013;20:442–467. doi: 10.1177/2047487312460484. [DOI] [PubMed] [Google Scholar]
- 8.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 9.Kitzman DW, Brubaker PH, Morgan TM, et al. Exercise training in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. Circulation. Heart Failure. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brawner CA, Keteyian SJ, Ehrman JK. The relationship of heart rate reserve to vo2 reserve in patients with heart disease. Med Sci Sports Exerc. 2002;34:418–422. doi: 10.1097/00005768-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Durstine JL, Moore G, Painter P, Roberts S. American Colege of Sports Medicine. Champaign: Human Kinetics.; 2009. ACSM’s exercise management for persons with chronic diseases and disabilities. [Google Scholar]
- 12.Demopoulos L, Bijou R, Fergus I, et al. Exercise training in patients with severe congestive heart failure: Enhancing peak aerobic capacity while minimizing the increase in ventricular wall stress. J Am Coll Cardiol. 1997;29:597–603. doi: 10.1016/s0735-1097(96)00526-8. [DOI] [PubMed] [Google Scholar]
- 13.Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 14.Beckers PJ, Possemiers NM, Van Craenenbroeck EM, et al. Impact of exercise testing mode on exercise parameters in patients with chronic heart failure. Eur J Prev Cardiol. 2012;19:389–395. doi: 10.1177/1741826711400664. [DOI] [PubMed] [Google Scholar]
- 15.Vallet G, Ahmaidi S, Serres I, et al. Comparison of two training programmes in chronic airway limitation patients: Standardized versus individualized protocols. Eur Respir J. 1997;10:114–122. doi: 10.1183/09031936.97.10010114. [DOI] [PubMed] [Google Scholar]
- 16.Guazzi M. Abnormalities in cardiopulmonary exercise testing ventilatory parameters in heart failure: Pathophysiology and clinical usefulness. Curr Heart Fail Rep. 2014;11:80–87. doi: 10.1007/s11897-013-0183-3. [DOI] [PubMed] [Google Scholar]
- 17.Davey P, Meyer T, Coats A, et al. Ventilation in chronic heart failure: Effects of physical training. Br Heart J. 1992;68:473–477. doi: 10.1136/hrt.68.11.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers J, Dziekan G, Goebbels U, Dubach P. Influence of high-intensity exercise training on the ventilatory response to exercise in patients with reduced ventricular function. Med Sci Sports Exerc. 1999;31:929–937. doi: 10.1097/00005768-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Guazzi M, Reina G, Tumminello G, Guazzi MD. Improvement of alveolar-capillary membrane diffusing capacity with exercise training in chronic heart failure. J Appl Physiol. 2004;97:1866–1873. doi: 10.1152/japplphysiol.00365.2004. [DOI] [PubMed] [Google Scholar]
- 20.Anaya SA, Church TS, Blair SN, et al. Exercise dose-response of the v(e)/vco(2) slope in postmenopausal women in the drew study. Med Sci Sports Exerc. 2009;41:971–976. doi: 10.1249/MSS.0b013e3181930009. [DOI] [PubMed] [Google Scholar]
- 21.Zurek M, Corra U, Piepoli MF, et al. Exercise training reverses exertional oscillatory ventilation in heart failure patients. Eur Respir J. 2012;40:1238–1244. doi: 10.1183/09031936.00167011. [DOI] [PubMed] [Google Scholar]
- 22.Smart NA, Haluska B, Jeffriess L, Leung D. Exercise training in heart failure with preserved systolic function: A randomized controlled trial of the effects on cardiac function and functional capacity. J Card Fail. 2012;18:295–301. doi: 10.1111/j.1751-7133.2012.00295.x. [DOI] [PubMed] [Google Scholar]
- 23.Stickland MK, Miller JD. The best medicine: Exercise training normalizes chemosensitivity and sympathoexcitation in heart failure. J Appl Physiol. 2008;105:779–781. doi: 10.1152/japplphysiol.90889.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erbs S, Hollriegel R, Linke A, et al. Exercise training in patients with advanced chronic heart failure (nyha iiib) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circulation Heart Failure. 2010;3:486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 25.Tabet JY, Meurin P, Beauvais F, et al. Absence of exercise capacity improvement after exercise training program: A strong prognostic factor in patients with chronic heart failure. Circulation. Heart Failure. 2008;1:220–226. doi: 10.1161/CIRCHEARTFAILURE.108.775460. [DOI] [PubMed] [Google Scholar]
- 26.Schmid JP, Zurek M, Saner H. Chronotropic incompetence predicts impaired response to exercise training in heart failure patients with sinus rhythm. Eur J Prev Cardiol. 2013;20:585–592. doi: 10.1177/2047487312444368. [DOI] [PubMed] [Google Scholar]


