Abstract
Chronic hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. The introduction of direct acting antiviral agents (DAAs) for its treatment represents a major advance in terms of sustained virologic response (SVR) rates and adverse effect profiles. Mechanistically, DAAs inhibit specific HCV non-structural proteins (NS) that are vital for its replication. Boceprevir, telaprevir, simeprevir, asunaprevir, grazoprevir and paritaprevir are NS3/4A inhibitors. Ombitasvir, ledipasvir, daclatasvir, elbasvir and velpatasvir are NS5A inhibitors. Sofosbuvir and dasabuvir are NS5B inhibitors. Currently, a combination of two or more DAAs is the corner stone for the treatment of HCV infection. However, the success of DAA therapy is facing several challenges, including the potential of drug-drug interactions and resistant variance. Moreover, the shortage of relevant clinical pharmacological data and drug interaction regarding DAA is a clinical concern. The present review discusses the clinical pharmacology of DAAs with special emphasis on drug-drug interaction.
Key words: hepatitis C virus, direct acting antiviral, drug interactions, sofosbuvir, daclatasvir, ledipasvir
Introduction
Globally, the chronic hepatitis C virus (HCV) is a leading cause of liver diseases such as liver cirrhosis and hepatocellular carcinoma. It affects approximately 180 million people worldwide; about 3% of the world population.[1] Three to four million persons are newly infected each year, with the predominant prevalence being infection with genotype 1, followed by genotypes 2 and 3. The other genotypes, 4, 5, and 6, have specific geographical distribution. The infection with HCV occurs as the result of percutaneous transmission via infectious blood (blood-to-blood). Less than 20% of those acutely infected clear the virus; the rest (70-80%) become chronically infected for decades, a significant fraction of whom die of HCV-related illnesses.[2]
Since 1991, the interferon based therapy [interferon (INF) plus ribavirin (RBV)] has been the main component of treatment options with low virological response not exceeding 50%. In 2011, new medications named direct acting antivirals (DAA) were developed, which represents a major advancement in the treatment of HCV that achieved a virological response exceeding 90% in most genotypes. There is a very rapid progress in the development of DAAs that limits the ability to overwhelm the pharmacological details of each DAA. This review is a trial to give an overview of the pharmacology of most DAAs.
Timeline for DAA approval
The following Table 1 summarizes the timeline for the approval of DAA.
Table 1.
Timeline for DAA approval
| Date of approval | Drug |
|---|---|
| On May 13th, 2011 | Boceprevir was approved by the FDA for the treatment of chronic HCV to be used, in combination with peginterferon alfa and ribavirin, in adult patients. |
| On May 23th, 2011 | Telaprevir was approved by the FDA to be used in combination with peginterferon alfa and ribavirin for the treatment of HCV infection in adults. |
| In November, 2013 | Simeprevir was approved by the FDA to be used in combination with peginterferon alfa and ribavirin or in combination with sofosbuvir. |
| In December, 2013 | Sofosbuvir was approved to be used in combination with ribavirin or with pegylated interferon and ribavirin. |
| In October, 2014 | (Ledipasvir/Sofosbuvir) were approved by the FDA in one tablet. |
| In December, 2014 | FDA approved a combination (ombitasvir/paritaprevir/ritonavir and dasabuvir) for the treatment of patients with genotype 1. |
| In July, 2015 | Daclatasvir was approved to be used with sofosbuvir. |
| A combination (ombitasvir, paritaprevir and ritonavir) in one tablet to be used in combination with ribavirin for the treatment of HCV genotype 4 infections. | |
| In January, 2016 | On January 28th FDA approved a combination of elbasvir and grazoprevir, with or without ribavirin for treating patients with genotype 4. |
| In July, 2016 | A combination of sofosbuvir plus velpatasvir were approved with or without ribavirin for treating adult patients in all genotypes. |
DDA: direct acting antiviral agent; HCV: hepatitis C virus.
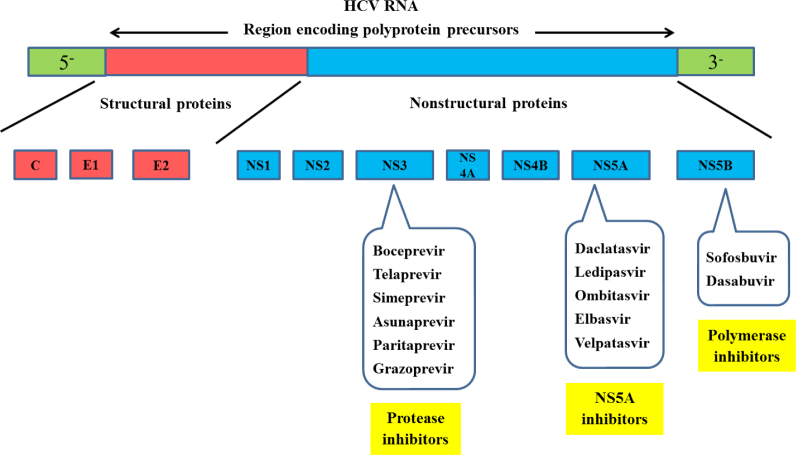
The genome HCV encodes 10 polyproteins: three structural proteins at the N-terminus and seven nonstructural (NS) proteins at the C-terminus (Figure 1). The three nonstructural proteins (NS3/4A, NS5A, and NS5B) are important for HCV replication, and therefore, represent important targets for inhibition by DAA.[3]
Figure 1.
Proteins encoded by the hepatitis C virus genome as targets for direct acting antiviral agents.
There are 3 main classes of DAAs, as per their target sites of action. The first group comprises NS3/4A inhibitors that include boceprevir, telaprevir, simeprevir, asunaprevir, grazoprevir and paritaprevir – boosted by ritonavir. Second group comprises NS5A inhibitors that include daclatasvir (DCV), ledipasvir, ombitasvir, elbasvir and velpatasvir. Third group includes NS5B nucleotide inhibitor (e.g., sofosbuvir) and non-nucleoside polymerase inhibitors (e.g., dasabuvir).[4,5]
Table 2.
Role of DAA in HCV guidelines
| Drug regimen | HCV genotype |
|---|---|
| Sofosbuvir/Ledipasvir +/-Ribavirin | Genotypes 1, 4, 5, and 6 |
| Paritaprevir/Ritonavir/Ombitasvir + Dasabuvir +/- Ribavirin: | Genotype 1 |
| Sofosbuvir + Simeprevir +/- Ribavirin: | Genotypes 1 and 4 |
| Sofosbuvir + Daclatasvir +/- Ribavirin: | All genotypes |
| Paritaprevir/Ritonavir/ombitasvir +/- Ribavirin: | Genotype 4 |
| Sofosbuvir + Ribavirin | Genotypes 2 and 3 |
HCV: hepatitis C virus; DDA: direct acting antiviral agent.
Role of DAA in the current guidelines for treating HCV
The following regimens are included in the new guidelines (EASL recommendation 2015),[6] along with the genotypes for which they are indicated:
N.B.: The duration of therapy of DAA ranges from 12 to 24 weeks. It depends on several factors including the presence of cirrhosis, genotype and previous failure of therapy.
Clinical pharmacology of newer DAA
The direct acting antiviral therapy was started when the first-wave, first-generation HCV NS3-4A protease inhibitors boceprevir and telaprevir were approved in combination with PegIFN-alpha and ribavirin for the treatment of chronic HCV genotype 1 infection in 2011.[7]However, these drugs have been reported to have several drug-drug interactions, and are largely replaced with newer DAA including Simeprevir, Paritaprevir, Daclatasvir, Ledipasvir, Ombitasvir, Sofosbuvir and Dasabuvir. Table 3 summarizes the pharmacokinetics of all approved DAA with special reference to the sites of drug-drug interaction. Clinical pharmacology of the newer DAA will be discussed in the next section.
Table 3.
Pharmacokinetics of Boceprevir, Telaprevir, Simeprevir, Asunaprevir, Paritaprevir, Grazoprevir, Daclatasvir, Ledipasvir, Ombitasvir, Sofosbuvir and Dasabuvir, which predispose them to drug-drug interactions
| Class drug | Absorption | Distribution | Metabolism | Elimination | |
|---|---|---|---|---|---|
| mainly | |||||
| NS3/4A Protease | substrate for | inhibitor or inducer | |||
| Inhibitors | |||||
| First-Wave | ↑ by food | PPB 75% | CYP3A4/5 | Moderate CYP3A | Hepatic |
| Boceprevir | P-gp inhibitor | AKR | inhibitor | ||
| Telaprevir | ↑ by fatty | PPB 60-76% | CYP3A4/5 | Strong CYP3A | Hepatic |
| Moderate P-gp inhibitor | inhibitor | ||||
| Second-Wave | ↑ by food | PPB 99% | CYP3A4 | mildly nhibits | Hepatic |
| Simeprevir | inhibitor of P-gp, OATP1B1 and | intestinal CYP3A4 and | |||
| MRP2 | CYP1A2 | ||||
| Asunaprevir | ↑ by fatty meals | PPB 99% | CYP3A | Moderate inhibitor | Hepatic |
| inhibitor of P-gp and OATP1B1 | of CYP2D6; weak | ||||
| inducer of CYP3A4 | |||||
| Paritaprevir | ↑ by food | PPB 97-98-% | CYP3A4/5 | ND | Hepatic |
| substrate for P-gp and BCRP; | |||||
| inhibitor of OATP1B1 | |||||
| Grazoprevir | PPB 98.8% | CYP3A | Weak inhibitor of | Hepatic | |
| ↑ by fatty meals | Transported by OATP1B1, P-gp. | CYP3A | |||
| Inhibits BCRP | |||||
| NS5A Inhibitors | |||||
| Daclatasvir | ↓ by fatty meals | PPB 99% | CYP3A | ND | Hepatic |
| inhibitor of P-gp | |||||
| and OATP1B1 | |||||
| Ledipasvir | ↓ by increasing gastric | PPB 99% | Minimally | ND | as parent drug |
| pH | substrate and an inhibitor of P-gp | metabolized | |||
| Ombitasvir | ↑ by food | PPB 99% | Amide hydrolysis | ND | Hepatic |
| substrate of P-gp and BCRP | |||||
| Elbasvir | ↓ by fatty meals | PPB 99.9% | CYP3A | ND | Hepatic |
| Transported by, P-gp. Inhibits | |||||
| BCRP, P-gp | |||||
| Velpatasvir | ↓ by fatty meals | PPB 99.5% | CYP2B6, | Hepatic | |
| Transported by and inhibits | CYP2C8, | ||||
| P-gp, BCRP, OATP1B1, | CYP3A4 | ||||
| OATP1B3 | |||||
| NS5B Polymerase Inhibitors | |||||
| Nucleoside Inhibitors | |||||
| Sofosbuvir | Food ↔ | PPB 61-65% | Prodrug is | ND | Renal |
| Substrate of P-gp and BCRP | phosphorylated | ||||
| to active-form | |||||
| Non-Nucleoside Inhibitors | |||||
| Dasabuvir | ↑ by food | PPB 99% | CYP2C8 | ND | Hepatic |
| substrate of P-gp and BCRP | CYP3A, CYP2D6 |
AKR: aldo keto reductase; BCRP: breast cancer resistance protein; CYP: cytochrome P450; DAA: direct-acting antiviral agent; MRP: multidrug resistant protein; ND: no data; OATP: organic anion transporting polypeptide; P-gp: P-glycoprotein; PPB: plasma protein binding. ↑ increase; ↓ decrease; ↔ nil
Sofosbuvir
Sofosbuvir (SOF) is a pyrimidine nucleotide analog inhibitor of NS5B, indicated in the treatment for HCV genotypes 1a, 1b, 2, 3, and 4 as a component of a combination antiviral treatment regimen.[8-10]
Sofosbuvir is phosphorylated within the cell, incorporates itself into the growing viral RNA strand, and terminates HCV RNA strand synthesis prematurely.[9] SOF confers an excellent genetic barrier to resistance; among several clinical trials, resistance has been reported in just one patient, when it was utilized as a monotherapy.[11]
Sofosbuvir is activated in the liver by its phosphorylation to its triphosphate nucleoside analog, which is then dephosphorylated to the inactive GS-331007. Peak plasma concentrations (Cmax) of SOF is attained in 0.5–2 h and 2–4 h, while its terminal half-life is 0.5 h and that of GS-331007 is 27 h. SOF can be administrated with/without food; no gender or race difference has been reported with its use. Following a single 400 mg oral dose, 80% of the drug was eliminated in the urine (only 3.5% as the parent drug and 78% as GS-331007), while 12% was eliminated in the feces. No dosage adjustments are recommended for hepatic or mild-to-moderate renal impairment.[9,11]
Sofosbuvir has proven an efficacy in patients with genotypes 1–6 as a part of a regimen including peg-IFN and RBV.[12]SOF has been proven to be an excellent alterative in patients who are contraindicated to IFN therapy, or have stopped the IFN because of adverse effects.[13]
The most common side effects encountered for SOF was reported, when it was used in combination with IFN, and/or with longer (24 vs. 12 weeks) treatment. In phase 3 trials, when it was combined with RBV, the adverse effects were in combination with RBV. The adverse effects were fatigue, headache, nausea, insomnia, pruritus, irritability, anemia, asthenia, and diarrhea. When it was combined with RBV and IFN the adverse effects were decreased appetite, influenza like illness, pyrexia, chills, neutropenia and myalgia.[9]
Ledipasvir
Ledipasvir (LDV) is an NS5A inhibitor and is currently available only as ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1a, 1b hepatitis C.[14] LDV has shown efficacy to treat HCV patients without PEG-interferon or ribavirin.
Natural resistance can emerge quickly against HCV NS5A, with suboptimal therapy.[15] Patients with mutation in Y93H and Q30K are typically more susceptible to develop such resistance, a condition that may be minimized by the use of a combination treatment.[16]
Ledipasvir undergoes oxidative metabolism by unknown mechanism, and is excreted mainly through the biliary tract (86%), while less than 1% in the urine. The parent drug accounts for about 70% of the excreted dose. The terminal half-life of LDV is 47 h.[14]
Ledipasvir has demonstrated a high efficacy as a combination with SOF in treating HCV 1 patients, with or without RBV in phase 2 trials. Moreover, it provides better sustained virological response in relapsing cases from RBV/SOF combination.[17] While it is only currently available as a combination with Sof. Ledipasvir several studies have shown its value as a combination therapy with other DAA therapies, other than SOF.[18] The current recommendation for LDV use suggests there is no need to combine LDV/ SOF with RBV or to prolong the treatment duration for more than 12 weeks.[19] Moreover, a more recent study recommends that a patient with HCV RNA level of < 6 million IU/mL may need LDV/SOF for only an 8-weeks treatment schedule.[14,20]
The most common side effects reported for LDV (> 5% of patients) by phase 3 clinical trial are diarrhea, nausea, fatigue, headache, insomnia and elevations in both bilirubin and lipase elevations. These side effects are more common with a longer duration of treatment (24 weeks) or concomitant RBV therapy.[14]
Simeprevir
Simeprevir (SIM) is an NS3/4A protease inhibitor, its efficacy has been reported against HCV genotypes 1a and 1b.[21] SIM has demonstrated a higher genetic barrier to resistance compared to older generations of protease inhibitors, telaprevir and boceprevir.[22] SIM has proven efficacy in non-responders from IFN-RBV treatment when it is combined with SOF in a daily dose of 150 mg with/without RBV for only 12 weeks schedule therapy.[21]
Upon oral administration, SIM reaches Cmax after 4–6 h. It is advisable to take SIM with food, since the latter can increase its AUC by 60–70%. It is metabolized primarily by way of oxidative metabolism through the CYP3A family. Because of the intestinal CYP3A4 and p-glycoprotein inhibition, a careful monitoring for drug interactions is a mandatory precaution. The drug interactions that are of clinical significance are anticonvulsants, statins, macrolide antibiotics, triazole antifungals, digoxin, antiarrhythmics, and others. 91% of the drug is recovered in the feces, while less than 1% of the parent drug is recovered in urine. The terminal half-life of SIM reaches up to 41 hours in HCV infected patients. No dose adjustment is required in the case of renal and mild hepatic impairment, but moderate and severe hepatic impairments may increase the plasma concentration of SIM to 2 folds, and 5 folds receptively.[21]
Simeprevir is a well-tolerated drug. Most of the reported side effects are known to result from the concomitantly taken medication. The side effects reported when it is combined with RBV/IFN are nausea, myalgia, rash, photosensitivity, pruritus and dyspnea. In other studies, when IFN is not included, the side effects were nausea and headache. In the absence of IFN and RBV, the reported adverse effects for SIM/SOF were rash, photosensitivity and pruritus.[21]
Daclatasvir
Daclatasvir inhibits both viral RNA replication and virion assembly by binding to the N-terminus of NS5A causing structural distortions that interfere with NS5A functions. Absorption and bioavailability: daclatasvir is given once daily in oral dose (60 mg/day). It is well absorbed after oral administration with peak plasma concentrations occurring within 2 hours post dose; a high-fat, diet decreases daclatasvir bioavailability when compared with fasted conditions. There is no effect on daclatasvir bioavailability with normal diet. It binds to plasma protein by approximately 99%, with volume of distribution about 47 L.[23,24]
Daclatasvir is metabolized by the liver with CYP3A4 being the primary CYP isoform responsible for metabolism. The majority of the drug is eliminated in the feces (53% of the dose as unchanged form and about 7% of the dose was excreted in the urine (primarily as unchanged daclatasvir). Its elimination half-life is about 13 hours and its clearance equals 4.2L/h. Generally, daclatasvir is well tolerated and the most common reported adverse reaction are headache 21 (14%), fatigue 21 (14%), nausea 12 (8%) and diarrhea 7 (5%). Sever bradycardia was reported when it was coadministered with amiodarone and sofosbuvir.[25]
Daclatasvir is contraindicated with strong CYP 3A inducers like phenytoin, carbamazepine and rifampicin. In animal studies, there was no evidence of fetal toxicity with oral administration of daclatasvir at doses up to 22 times normal human dose. However, fetal toxicity was observed in rats and rabbits at maternally toxic doses that reached 33 and 98 times the human dose, respectively. Consider the benefits and risks when prescribing daclatasvir to a pregnant woman. No data is available regarding the presence of daclatasvir in human milk. However, daclatasvir is secreted in the milk of lactating rats. Benefit/risk patients should be considered in administrating daclatasvir to lactating mothers.
No dosage adjustment of daclatasvir is required for patients with any degree of renal impairment. In patients with mild (Child-Pugh A), moderate (Child-Pugh B), or severe (Child-Pugh C) hepatic impairment, no dosage adjustment of daclatasvir is required. However, in patients with decompensated cirrhosis, the safety and efficacy of daclatasvir have not been established.[23]
Paritaprevir/Ritonavir/Ombitasvir plus Dasabuvir
The all-oral regimen is available as a fixed-combination of paritaprevir 75 mg, ritonavir 50 mg, and ombitasvir 25 mg in one tablet, to be taken once daily. The latter product is copackaged with dasabuvir 250 mg, which is to be taken twice daily. The all-oral medications have distinct mechanisms of action, targeting multiple steps in the hepatitis C life cycle and may have additive effect.
Paritaprevir is an inhibitor of the HCV NS3/4A protease, has activities against genotypes 1a and 1b with good activities against genotypes 4a and 6a, whereas it is less active against genotype 2A and 3a in terms of EC50. Ritonavir is an HIV-1 protease inhibitor that acts as a pharmacokinetic booster of paritaprevir.
Ombitasvir is an inhibitor of HCV NS5A with pangenotypic efficacy against 1a, 1b, 2a, 2b, 3a, 4a and 5a with an EC50 ranging from 0.68 pM to 366 pM.
Dasabuvir is a non-nucleoside inhibitor of NS5B RNA-dependent RNA polymerase acting as an allosteric inhibitor of the palm domain of NS5B. Dasabuvir is active against replicons of genotypes 1a and 1b, whereas it has a reduced activity in the genotypes 2a, 2b, 3a, and 4a.[26]
All-oral Paritaprevir/Ritonavir/Ombitasvir plus Dasabuvir regimen is given with meals and is recommended for the treatment of genotypes 1a, 1b, and 4 with or without ribavirin. The All-oral regimen has high rates of SVR in treatment of both naïve and experienced patients with best outcome in non-cirrhotic patients with genotype 1b infections.[27,28]
The all-oral members are highly bound to plasma proteins, and apart from ombitasvir, which is metabolized by amide hydrolysis, they are metabolized through hepatic CYP450 enzymes. The latter pharmacokinetic profile subjects them to multiple drug-drug interactions via CYP3A system, particularly paritaprevir and ritonavir. [29] Because non-renal mechanisms accounted for the elimination of all-oral regimen, renal impairment may not require specific recommendation. On the other hand, due to dependence on hepatic elimination, all-oral regimen is not recommended for patients with moderate hepatic impairment and is contraindicated in those with severe hepatic impairment. The regimen is well tolerated but some mild adverse effects may be associated, such as nausea, pruritus, insomnia and asthenia.[26]
Elbasvir/Grazoprevir
Elbasvir is an inhibitor of NS5A, whereas grazoprevir is an NS3/4A protease inhibitor. The combination of elbasvir and grazoprevir was approved with or without ribavirin for treating adult patients with chronic HCV genotype 1 or 4. A fixed-dose combination of elbasvir 50 mg and grazoprevir 100 mg is to be taken orally once daily without regard to meals. Genetic assessment for patients of genotype 1a is recommended to determine if the patient is NS5A resistant before the initial treatment, in order to determine the combination with ribavirin in regimen and the duration of therapy. Both elbasvir and grazoprevir undergo metabolism, primarily via the CYP3A pathway, and more than 90% of a dose is eliminated in the feces. Subjects receiving Elbasvir/Grazoprevir for 12 weeks, most complained of fatigue, headache, and nausea as the common adverse reactions. In subjects receiving Elbasvir/ Grazoprevir with ribavirin for 16 weeks, the most often reported adverse reactions were anemia and headache.[30]
The safety and effectiveness of elbasvir/grazoprevir has not been established in pregnant women or patients younger than 18 years of age. No dosage adjustment of Elbasvir/Grazoprevir is recommended in patients with renal impairment, including patients on dialysis. No dosage adjustment of Elbasvir/Grazoprevir is recommended in patients with mild hepatic impairment. However, Elbasvir/ Grazoprevir is contraindicated in patients with moderate or severe hepatic impairment.
Velpatasvir/Sofosbuvir
A combination velpatasvir (an inhibitor of viral NS5A) and sofosbuvir (a nucleotide inhibitor of NS5B polymerase) was approved for the treatment of adult patients with chronic HCV genotype 1, 2, 3, 4, 5 or 6 infection. A fixed-dose combination of velpatasvir 100 mg and sofosbuvir 400 mg is to be taken orally once daily with or without food. The combination of velpatasvir/sofosbuvir being pan-genotypic has clinical importance as it cover HCV patients will all 6 genotypes and it may not be necessary to perform genotype testing. Velpatasvir/Sofosbuvir combination is indicated for chronic HCV patients without cirrhosis or with compensated cirrhosis. For indicated patients with decompensated cirrhosis, the regimen should include ribavirin.
Sofosbuvir is rapidly converted to the predominant circulating metabolite, GS-331007. As mentioned above, majority of sofosbuvir and its metabolite is excreted in the urine. On the other hand, velpatasvir is subjected to metabolism via the CYP2B6, CYP2C8, and CYP3A4 pathways, and most of the dose is excreted in feces, primarily via biliary excretion of the parent drug.
The commonly reported adverse events during the use of velpatasvir/sofosbuvir in patients without cirrhosis or with compensated cirrhosis were headache, fatigue and nausea. As with other sofosbuvir-containing regimens, symptomatic bradycardia has been reported in patients taking amiodarone concurrently, and some have required pacemaker intervention.[31] Concurrent therapy is not recommended; however, closed cardiac monitoring is needed for the first 48 hours during co-administration.
For velpatasvir/sofosbuvir per se, there is no need for dosage adjustment in patients with hepatic impairment, but clinical and laboratory monitoring is recommended for patients with decompensated cirrhosis who are co-treated with ribavirin. Dosage adjustment is not necessary in patients with mild to moderate renal impairment. However, sofosbuvir cannot be recommended for patients with GFR <30 mL/min or end-stage renal disease.
Overview of direct acting antiviral drug interactions
The introduction of direct acting antiviral agents (DAAs) for treatment of hepatitis C viral infections has achieved high virological cure rates (>90%) encouraging more patients to start the treatment. However, the wide use of combined DAAs regimens offers a possibility of exposure to drug interactions especially in patients under treatment for other co-morbidities.[32] It is not only important to monitor for drug-drug interaction during HCV therapy with DAAs, but also to evaluate the interaction potential even before the start of treatment course in order to limit the drug interaction. Indeed, this is a complex issue given the large number of drug classes that may be prescribed or non-prescribed and the combined DAAs regimens particularly those including protease inhibitors. Moreover, the disease stage of liver that itself is the target for DAAs therapy and is the common site of drug-drug interactions. Direct acting antiviral could be a victim, as they are substrates and/or a culprit and they may act as enzyme inhibitors or inducers in drug-drug interactions. Because DAAs are recently approved, most of the available data comes from in vitro research and there is a limitation in available clinical data regarding these drugs. With regard to this complex issue, there are online websites that provide help tools for the clinicians in exploring potential drug-drug interactions (e.g., www.hep-druginteractions.org and www.aasld.org).
Pharmacologic basis of direct acting antiviral drug interaction
Drug-drug interaction is the modification of the action of one drug by another and may be pharmacodynamic or pharmacokinetic in nature. Pharmacodynamic interactions don’t result due to a change in drug concentrations but lead to alteration of the response of body to another drug. Pharmacodynamic interactions may occur between drugs with opposite actions leading to antagonism or may occur between drugs with similar actions leading to potentiation, addition or synergism. Pharmacodynamic interactions are not easy to be quantified, and subsequently, are difficult to manage. For example, during the interferon-based therapy for chronic HCV, Peg-INF enhances the ribavirin-induced anemia (sometimes severe) to discontinue the therapy. On the other hand, pharmacokinetic interactions do result in a change in drug concentrations; and occur at the level of drug absorption, distribution, metabolism and elimination. Pharmacokinetic interactions are partially predictable based on the available data and can be limited or prevented by a dosage adjustment.[33,34]
Direct acting antiviral-related pharmacodynamic drug interactions
The co-administration of protease inhibitors with other drugs such as clarithromycin or moxifloxacin may alter the QT interval result in critical arrhythmia due to the prolongation of QT interval. So, the precautions for QT interval prolongation should be considered in patients who are initiating protease inhibitor therapy and are under medications with proarrhythmic potential.[35]Another observation reported extreme bradycardia after first doses of sofosbuvir and daclatasvir and indicated that patients treated with amiodarone should be continuously monitored within the first 48 hours following the initiation of therapy with these DAAs.[36]
Direct acting antiviral-related pharmacokinetic drug interactions
Food increases the absorption of the all-oral paritaprevir/ ritonavir/ombitasvir plus dasabuvir, boceprevir and simeprevir, and so, it is recommended to take these medications with food. Fatty meals increase the absorption of telaprevir and asunaprevir. Sofosbuvir and daclatasvir are taken with or without regard to food. Ledipasvir solubility can be reduced at higher gastric pH, so the concomitant administration of medications such as H2 receptor blockers or proton pump inhibitors may decrease its concentration. [37-39] Similarly, concurrent administration of Velpatasvir/ Sofosbuvir with the proton pump inhibitor omeprazole is not recommended, or taken 4 hours before it is necessary, as the concentration and activity of velpatasvir is reduces with increase in gastric pH value.
Most of DAAs have a high rate of plasma protein binding average 99%. Telaprevir and boceprevir binding rate to plasma proteins is around 75 %. Approximately 61% to 65% of sofosbuvir is bound to plasma proteins, but plasma protein binding of its active metabolite is negligible. Significant drug-drug interactions due to the displacement from Protein-binding are more likely to occur when drugs demonstrating very high rate of protein binding are co-administered; this in turn increases the free drug fraction. The increased free drug concentration could potentially cause increased therapeutic and/or toxic effects of the drug and the dose regimen is recommended to be adjusted.[40]
A large number of membrane transporters have been identified, however, only few of them have been implicated in clinically relevant drug–drug interactions. Examples include P-glycoprotein (P-gp), organic anion transporting polypeptide 1B1 (OATP1B1), and breast cancer resistance protein (BCRP). Almost all the DAA agents have a relation with P-gp and/or other transporters that can be induced or inhibited and have the potential to affect drug bioavailability by either increasing elimination or decreasing cellular absorption. Drug interactions with transporters have the potential to alter drug concentrations by interfering with elimination or cellular absorption. For example, it has been reported that co-administration of telaprevir or boceprevir plus digoxin altered the bioavailability of digoxin due to interaction via P-gp, suggesting the need to cut digoxin dose to avoid an overdose. Also, Boceprevirmay increase the toxicity of statins via inhibition of OATP1B1 decreasing their hepatic uptake and Simeprevir concentrations may be increased when co-administered with the immunosuppressant cyclosporine due to interaction via transporters.[33,41-43] Because grazoprevir is a substrate of OATP1B1/3, its plasma concentration may be significantly increased by OATP1B1/3 inhibitors (e.g., cyclosporine, lopinavir, saquinavir), and its concurrent use is contraindicated.
Drug-drug interactions at the level of metabolism and elimination by cytochrome P450 (CYP450) enzyme system can occur, but commonly mistaken as the only site of interactions. Most of DAA agents are mainly metabolized via by CYP450 enzyme system, in particular the 3A4 isozyme. Boceprevir is metabolized by the aldoketo reductase enzymes mainly and by CYP3A, whereas ombitasvir is metabolized by amide hydrolysis followed by oxidative metabolism. Ledipasvir is minimally metabolized and excreted as a parent drug, whereas sofosbuvir is a prodrug activated by phosphorylation enzymes within host cell. As most of the first-generation protease inhibitors for DAA agents are metabolized through the CYP3A system, they have numerous drug interactions. Boceprevir and telaprevir, the first wave members of NS3/4A inhibitors, were reported to interact with azole antifungal, immunosuppressants.[34,44] Second generation are not yet approved and data about their interactions are limited. The newer DAA agents such as sofosbuvir (an HCV NS5B polymerase inhibitor) and ledipasvir (an HCV NS5A inhibitor), which are not or only marginally affected by CYP450 enzymes, are relatively less vulnerable to significant hepatic pharmacokinetic interactions.
Coadministration of the 3D regimen of OBV, PTV/r, and DSV with ethinyl estradiol-containing contraceptives was reported to have increased risk of alanine aminotransferase elevations. The mechanism for this interaction is not clear. Estradiol is metabolized to estriol by CYP3A4 and CYP1A2, which is then glucuronidated. Coadministration could increase the estradiol concentrations due to inhibition of CYP3A4, but decrease the concentrations due to induction of CYP1A2. Stopping the Estradiol-containing medications for at least 1 week prior to starting 3D and using alternative methods of contraception (e.g., progestin only contraception or non-hormonal methods) are recommended. The Estradiol-containing medications can be restarted approximately 2 weeks following the completion of HCV treatment. It has been reported that coadministration of boceprevir with combined oral contraceptives containing ethinyl estradiol is unlikely to alter the contraceptive effectiveness, and patients should be clinically monitored. [27,45] Since both elbasvir and grazoprevir are metabolized via the CYP3A, their plasma concentrations and action of elbasvir/grazoprevir may be significantly reduced by strong CYP3A inducers (e.g., rifampin carbamazepine, phenytoin). Conversely, the action of elbasvir/grazoprevir may be increased by CYP3A inhibitors such as ketoconazole, as well as certain combination products for human immunodeficiency virus infection (e.g., Stribild), and concurrent use is not recommended.
Beneficial drug-drug interaction
As mentioned above, most of the drug-drug interactions are harmful to patients, particularly when they are unpredictable during prescription. However, sometimes drug-drug interactions are employed to be beneficial to patients. With regard to DAA, paritaprevir is an NS3/4A protease inhibitor metabolized by cytochrome P450 (CYP) 3A and is given in the all-oral paritaprevir/ritonavir/ ombitasvir plus dasabuvir. Ritonavir is an HIV protease inhibitor and has no direct activity against HCV, but acts as a CYP3A inhibitor. Adding ritonavir in low dose to the regimen as a pharmacokinetic enhancer prolongs the half-life of paritaprevir and allowing once-daily dosing commonly referred to as ritonavir boosting.[24]
The newer DAA agents have a very high SVR, but the data about drug-drug interaction that may decrease their efficacy or increase their adverse effects is limited. For DAA, as all drugs, there exists a measure which relates their toxic dose to therapeutic dose to which is the therapeutic index. Thus, targeting a range of drug concentrations that is effective and safe in the same time is essential. However, pharmacokinetic drug interactions with CYP inhibitors may increase drug concentrations leading to an increase in dose-related toxicities. On the other hand, interactions with CYP inducers may decrease drug concentrations leading to failure of therapy. The impact of drug-drug interaction depends upon many factors including the patient’s age, sex, genomics, disease stage, comorbidities, and the pharmacologic character particularly, the extent of interaction and the safety margin of DAA and the co-administered drugs. A clinically significant drug-drug interaction occurs only for drugs with low therapeutic index especially with high degree of interaction. The risk of drug interaction must be considered during planning of the therapeutic regimen for HCV, with more potential with protease inhibitors and to a lesser extent with NS5A and NS5B inhibitors. In the above-mentioned section, we have tried to shed light on the principles of pharmacologic interaction with DAA-related examples to help the clinicians to limit such possibility and balance the therapeutic options keeping both good efficacy and safety profiles.
Challenges and future direction of DAA
Though DAA has provided much needed, safe and effective therapeutic option for chronic HCV patient, some challenges need further effort. Such challenges include the presence of resistant variance, low efficacy in cirrhotic patients, presence of drug-drug interactions, and the cost. The future direction should go through multiple directions, for example, continuous monitoring and developing DAA, use of combined groups of DAA with different mechanisms of action to minimize resistance, searching for other antiviral groups with different mechanisms of action, and finding a solution for improving cirrhosis by developing antifibrotic drugs. A recent study showed promising results in the possible incorporation of a new cyclophilin inhibitor, STG-175 in DAA-regimen.[46]
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Parfieniuk A, Jaroszewicz J, Flisiak R.. Specifically targeted antiviral therapy for hepatitis C virus. orld J Gastroenterol 2007. 21;13:5673–81. doi: 10.3748/wjg.v13.i43.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–2. doi: 10.1016/j.jhep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS. HCV NS5A inhibitors in development. Clin Liver Dis. 2013;17:111–21. doi: 10.1016/j.cld.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Asselah T, Marcellin P. Direct acting antivirals for the treatment of chronic hepatitis C: one pill a day for tomorrow. Liver Int. 2012;32(S1):88–102. doi: 10.1111/j.1478-3231.2011.02699.x. [DOI] [PubMed] [Google Scholar]
- 5.Herbst DA, Reddy KR. NS5A inhibitor, daclatasvir, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs. 2013;22:1337–46. doi: 10.1517/13543784.2013.826189. [DOI] [PubMed] [Google Scholar]
- 6.EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–44. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 9.SOVALDI. Foster City, CA: Gilead Sciences Inc; 2013. [Package Insert] Available from Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204671s007lbl.pdf Accessed on Feb 17 2017. [Google Scholar]
- 10.Koff RS. The efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5b polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014;39:478–87. doi: 10.1111/apt.12601. [DOI] [PubMed] [Google Scholar]
- 11.Keating GM, Vaidya A. Sofosbuvir: first global approval. Drugs. 2014;74:273–82. doi: 10.1007/s40265-014-0179-7. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Torres M, Lawitz E, Kowdley KV, Nelson DR, De Jesus E, McHutchison JG. et al. Sofosbuvir (Gs-7977) plus peginterferon/ribavirin in treatment-naive patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58:663–8. doi: 10.1016/j.jhep.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS. et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 14.HARVONI. Foster City, CA: Gilead Sciences Inc; 2014. [Package Insert] Available from Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205834s018lbl.pdf Accessed on Feb 17 2017. [Google Scholar]
- 15.Nakamoto S, Kanda T, Wu S, Shirasawa H, Yokosuka O. Hepatitis C virus NS5a inhibitors and drug resistance mutations. World J Gastroenterol. 2014;20:2902–12. doi: 10.3748/wjg.v20.i11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile I, Buonomo AR, Borgia F. Ledipasvir: a novel synthetic antiviral for the treatment of HCV infection. Expert Opin Investig Drugs. 2014;23:561–71. doi: 10.1517/13543784.2014.892581. [DOI] [PubMed] [Google Scholar]
- 17.Osinusi A, Kohli A, Marti MM, Nelson A, Zhang X, Meissner EG. et al. Re-treatment of chronic hepatitis C virus genotype 1 infection after relapse: an open-label pilot study. Ann Intern Med. 2014;161:634–8. doi: 10.7326/M14-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyles DL, Rodriguez-Torres M, Lawitz E, Shiffman ML, Pol S, Herring WR. et al. All-oral combination of ledipasvir, vedroprevir, tegobuvir, and ribavirin in treatment-naive patients with genotype 1 HCV infection. Hepatology. 2014;60:56–64. doi: 10.1002/hep.27053. [DOI] [PubMed] [Google Scholar]
- 19.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M. et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–198. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 20.Kowdley KV, An D, Pang PS, Wyles D. Analysis of subgroup differences in the ion-3 trial of ledipasvir-sofosbuvir in chronic hepatitis C infection. Open Forum Infect Dis. 2015;2:ofv056. doi: 10.1093/ofid/ofv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OLYSIO. Titusville, NJ: Janssen Therapeutics; 2013. [Package Insert] Available from Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205123s012lbl.pdf Accessed on Feb 17 2017. [Google Scholar]
- 22.Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34(S1):69–78. doi: 10.1111/liv.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DAKLINZA. Bristol-Myers Squibb Company; 2015. [package insert] Available from Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206843s006lbl.pdf Accessed on Feb 17 2017. [Google Scholar]
- 24.Sulkowski MS, Jacobson IM, Nelson DR. Daclatasvir plus sofosbuvir for HCV infection. N Engl J Med. 2014;370:1560–1. doi: 10.1056/NEJMc1401726. [DOI] [PubMed] [Google Scholar]
- 25.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N. et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–35. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VIEKIRA PAK. North Chicago, IL: AbbVie Inc.; 2014. [Package Insert] Available from Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206619s014lbl.pdf Accessed on Feb 17 2017. [Google Scholar]
- 27.Andreone P, Colombo MG, Enejosa JV, Bernstein B. ABT-450, ritonavir, ombitasvir, anddasabuvir achieves 97% and 100% sustained virologic response with or withour ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014147:359–365.e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 28.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C. et al. ABT-450-/r-ombi-tasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 29.Menon R, Badri P, Wang T, Polepally AR, Zha J, Khatri A. et al. Drugdrug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir and dasabuvir. J Hepatol. 2015;63:20–9. doi: 10.1016/j.jhep.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 30.ZEPATIER. Merck & Co., Inc.; Whitehouse Station, NJ: [package insert] Available from Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208261s002lbl.pdf Accessed on Feb 17 2017. [Google Scholar]
- 31.EPCLUSA. Gilead Sciences, Inc: 2016. [package insert] Available from Drugs@FDA http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208341s002lbl.pdf Accessed on Feb 17 2017. [Google Scholar]
- 32.Bunchorntavakul C, Tanwandee T. Treatment of Chronic Hepatitis C in Special Populations. Gastroenterol Clin N Am. 2015;44:883–900. doi: 10.1016/j.gtc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Talavera Pons S, Lamblin G, Boyer-Grand A, Sautou V, Abergel A. Drug interactions and protease inhibitors used in the treatment of hepatitis C: how to manage? Eur J Clin Pharmacol. 2014;70:775–89. doi: 10.1007/s00228-014-1679-9. [DOI] [PubMed] [Google Scholar]
- 34.Kiser JJ, Burton JR, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt K, Hughes CA, Hills-Nieminen C. Protease inhibitor-associated QT interval prolongation. Ann Pharmacother. 2011;45:1544–50. doi: 10.1345/aph.1Q422. [DOI] [PubMed] [Google Scholar]
- 36.Renet S, Chaumais MC, Antonini T, Zhao A, Thomas L, Savoure A. et al. Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology. 2015;149:1378–80. doi: 10.1053/j.gastro.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 37.Childs-Kean LM, Hand EO. Simeprevir and sofosbuvir for treatment of chronic hepatitis C infection. Clin Ther. 2015;37:243–67. doi: 10.1016/j.clinthera.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Smith MA, Chan J, Mohammad RA. Ledipasvir-sofosbuvir: interferon ribavirin-free regimen for chronic hepatitis C virus infection. Ann Pharmacother. 2015;49:343–50. doi: 10.1177/1060028014563952. [DOI] [PubMed] [Google Scholar]
- 39.McConachie SM, Wilhelm SM, Kale-Pradhan PB. New direct-acting antivirals in hepatitis C therapy: a review of sofosbuvir, ledipasvir, daclatasvir, simeprevir, paritaprevir, ombitasvir and dasabuvir. Expert Rev Clin Pharmacol. 2016;9:287–302. doi: 10.1586/17512433.2016.1129272. [DOI] [PubMed] [Google Scholar]
- 40.Benet L, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71:115–21. doi: 10.1067/mcp.2002.121829. [DOI] [PubMed] [Google Scholar]
- 41.Marquez B, Van Bambeke F. ABC multidrug transporters: target for modulation of drug pharmacokinetics and drug-drug interactions. Curr Drug Targets. 2011;12:600–20. doi: 10.2174/138945011795378504. [DOI] [PubMed] [Google Scholar]
- 42.Garg V, Chandorkar G, Farmer HF, Smith F, Alves K, van Heeswijk RPG. Effect of Telaprevir on the pharmacokinetics of midazolam and digoxin. J ClinPharmacol. 2012;52:1566–73. doi: 10.1177/0091270011419850. [DOI] [PubMed] [Google Scholar]
- 43.Hulskotte EG, Feng HP, Xuan F, Gupta S, van Zutven MG, O’Mara E. et al. Pharmacokinetic evaluation of the interaction between the HCV protease inhibitor Boceprevir and the HMG-CoA reductase inhibitors Atorvastatin and Pravastatin. Antimicrob Agents Chemother. 2013;57:2582–8. doi: 10.1128/AAC.02347-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tischer S, Fontana RJ. Drug-drug interactions with oral anti-HCV agents and idiosyncratic hepatotoxicity in the liver transplant setting. J Hepatol. 2014;60:872–84. doi: 10.1016/j.jhep.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin WH, Feng HP, Shadle CR, O’Reilly T, Wagner JA, Butterton JR. Pharmacokinetic and pharmacodynamic interactions between the hepatitis C virus protease inhibitor, boceprevir, and the oral contraceptive ethinyl estradiol/norethindrone. Eur J Clin Pharmacol. 2014;70:1107–13. doi: 10.1007/s00228-014-1711-0. [DOI] [PubMed] [Google Scholar]
- 46.Gallay PA, Chatterji U, Bobardt MD, Long Z, Zhang S, Su Z. Characterization of the Anti-HCV Activities of the New Cyclophilin Inhibitor STG-175. PLoS One. 2016;22(11):e0152036. doi: 10.1371/journal.pone.0152036. [DOI] [PMC free article] [PubMed] [Google Scholar]