Abstract
Background
Endeavor zotarolimus-eluting stents (E-ZES) and everolimus-eluting stents (EES) as second-generation stents were approved for use in percutaneous coronary interventions (PCIs) in 2008. We aimed to evaluate the long-term outcomes of E-ZES vs EES using New York State (NYS) cardiac registries and to compare long-term effectiveness of E-ZES vs EES in six “off-label” and two “high-risk” subgroups.
Methods
We created a longitudinal database by linking the NYS cardiac registries, the statewide hospital discharge data, the National Death Index, and the U.S. Census file (2010) for patients receiving either E-ZES or EES from July 2008 through December 2010. We examined outcome measures of all-cause mortality, acute myocardial infarction (AMI), target lesion PCI (TLPCI), and target vessel coronary artery bypass graft (TVCABG) surgery for 13,663 propensity score matched pairs in the 6-year follow-up period. We applied Kaplan-Meier methods and Cox proportional hazards regression for further adjustment of propensity-matched pairs.
Results
Compared with patients receiving EES, patients receiving E-ZES had a significantly higher rate of 6-year all-cause mortality (adjusted hazard ratio <AHR>: 1.10, 95% confidence interval <CI>: 1.04–1.17, P=0.003), AMI (AHR: 1.12, 95% CI: 1.02–1.23, P=0.01), TLPCI (AHR: 1.28, 95% CI: 1.18–1.39, P<0.001), and TVCABG (AHR: 1.47, 95% CI: 1.26–1.71, P<0.001). EES had better or similar long-term outcomes than E-ZES for the subgroups that were examined.
Conclusion
At 6 years, patients receiving EES generally had better or comparable mortality, AMI, TLPCI, and TVCABG outcomes compared with patients receiving E-ZES.
Keywords: Endeavor zotarolimus-eluting stents (E-ZES), everolimus-eluting stents (EES), long-term outcomes, mortality
Introduction
Drug-eluting stents (DES) have been commonly employed to treat coronary artery disease (CAD) for several years. Endeavor zotarolimus-eluting stents (E-ZES) and everolimus-eluting stents (EES) are second-generation DES that were approved by the U.S. Food and Drug Administration (FDA) in February 2008 and July 2008, respectively.[1] E-ZES is a thin strut cobalt-based alloy stent with a PC polymer and the anti-proliferative drug zotarolimus that is eluted within days after implantation into the coronary artery. Compared with first-generation DES, E-ZES use is associated with better or similar early- and late-term safety and efficacy based on the randomized clinical trials.[2–5] EES is designed to use everolimus as the anti-proliferative drug and biocompatible fluoropolymer to enhance the safety and efficacy of DES. Literature suggests that patients treated with EES have superior or similar outcomes compared with patients treated with first-generation DES.[6–8] Not surprisingly, both E-ZES and EES have been adopted into everyday percutaneous coronary intervention (PCI) practice rapidly after FDA’s approval. Recent randomized clinical trials have reported that use of EES leads to better or similar safety and efficacy relative to use of E-ZES over a 3-year follow-up period.[9–11]
However, it remains unknown whether patients receiving EES have better long-term outcomes compared with patients receiving E-ZES in real-world settings and whether such comparative advantages exist for “off-label” and “high risk” patient subgroups. To fill these knowledge gaps, high-quality cardiac registries can be used because they provide real-world clinical data for a much broader patient population that includes all providers and hospitals in a specific geographical region.[12–14] Therefore, we aimed to evaluate the long-term comparative effectiveness of E-ZES vs. EES in all patients as well as in a wide range of “off-label” and “high-risk” subgroups in New York State using the audited population-based PCI registry in New York State, the PCI Reporting System (PCIRS).[15]
Methods
Study Population
Patients treated with either E-ZES or EES (i.e., XIENCE V stents) from July 1, 2008 through December 31, 2010 were enrolled in the present study. Patients who received at least one E-ZES at the first recorded stent placement in the study period were placed into the E-ZES group, regardless of whether they had undergone coronary artery bypass graft (CABG) surgery or previous PCIs in an earlier admission prior to the study period. Patients who received at least one EES at the first recorded stent placement were placed into the EES group. Furthermore, six “off-label” subgroups (ST segment elevation myocardial infarction <STEMI>, totally occluded lesions, left main disease, multiple vessels diseased, bypassed graft lesions, and ejection fraction <35%), and two “high-risk” subgroups (age >75 years, and diabetes) were pre-defined for subgroup analysis.
Data
An aggregated dataset was generated by linking the New York State’s PCIRS registry, the New York State’s Cardiac Surgery Reporting System (CSRS) registry, the Statewide Planning and Research Cooperative System (SPARCS) database, and the National Death Index (NDI) file with the goal of comparing long-term effectiveness for the E-ZES and EES groups. A comprehensive set of clinical outcome measures was examined following E-ZES/EES insertion over a 6-year follow-up period.
New York State’s PCIRS and CSRS registries are comprehensive high-quality registries developed by the New York State Department of Health. Since their establishment approximately 25 years ago, these registries have been routinely used to publish statewide public reports on quality and outcomes for PCI procedures and adult cardiac surgeries.[15] The cardiac registries include important information on patient demographics, pre-procedural risk factors, hemodynamic status, left ventricular function, coronary vessels diseased and attempted (vessel attempted information only available in PCIRS registry), coronary lesion information (only available in PCIRS registry), procedure details, provider identifiers, post-procedure complications and discharge status. An evident strength of New York State’s cardiac registries is that the completeness, quality, reliability, and timeliness of clinically relevant variables are ensured through rigorous systematic efforts.[15] Moreover, New York State’s cardiac registries have benefitted remarkably from the guidance and supervision of the New York State Cardiac Advisory Committee, which is comprised of reputable cardiologists, cardiac surgeons, and experts in cardiovascular quality and outcomes, access, and ethics. As this study’s goal was to assess 6-year comparative effectiveness between E-ZES and EES, the PCIRS and CSRS registries (through 2014) were used to identify target lesion PCI (TLPCI) and a proxy for target vessel CABG (TVCABG) using PCIRS and CSRS data from subsequent years.
The SPARCS dataset contains all-inclusive patient discharges from nonfederal acute care hospitals in New York State. It contains information on patient demographics, diagnoses, procedures, admission date, discharge date, and discharge disposition. SPARCS data (2008 – 2014) were matched to the July 2008–December 20010 PCIRS registry data using patient identifiers to identify subsequent acute myocardial infarctions (AMIs) during 6-year follow-up.
The multi-year NDI files (2008 – 2014) contain information on death and date of death as well as personal identifiers for all residents in the United States. The NDI files were linked to the patients treated with E-ZES and EES using unique patient identifiers to identify the 6-year post-procedure vital status of these patients.
In addition, the residence zip code level median household income from the most recent U.S. Census File (2010) was matched to the merged data to enable us to account for differences in socioeconomic status of patients treated with E-ZES and EES.
Outcomes
The primary outcome measure was 6-year all-cause mortality after the initial E-ZES/EES placement. The secondary outcome measures were post-E-ZES/EES AMI, TLPCI, and TVCABG during the 6-year follow-up period. All-cause mortality was defined using aggregated NDI files (2008–2014). Subsequent AMI was defined using longitudinal SPARCS data (2008 – 2014) to identify hospital admissions with a principal diagnosis code in the range of ICD-9-CM 410.X1 where X denotes the region of the AMI and the fifth digit of “1” denotes a new AMI episode. Subsequent TLPCI was identified as a repeat PCI procedure performed in a coronary artery lesion that had been treated during the index E-ZES/EES placement. Subsequent TVCABG was defined as a patient who underwent CABG surgery during the 6-year follow-up period with significant (≥70% stenosis) target vessel disease in the same vessel where the index E-ZES/EES stent was placed. This is a proxy for TVCABG because the CSRS registry contains the coronary vessels diseased but not the vessels attempted. Therefore, the 6-year TVCABG indicates that at least there is subsequent CABG in the presence of target vessel disease, which suggests failure of the index E-ZES/EES implantation.
Statistical Analysis
We applied propensity score matching methods to reduce the selection bias, which is inherent in any observational study.[16, 17] We summarized the distributions of patient level risk factors for the E-ZES and EES groups (see Table 1). These variables included demographics (age, gender, race/ethnicity, payer, residence zip-code level median household income), baseline characteristics (body mass index <BMI> and left ventricular ejection fraction <LVEF>), co-morbidities (myocardial infarction, cerebrovascular disease, peripheral vascular disease, hemodynamically instability, congestive heart failure, malignant ventricular arrhythmia, chronic obstructive pulmonary disease, diabetes, previous CABG surgery, previous PCI, renal failure), and vessel/lesion-related risk factors (number of vessels diseased, left main disease, lesion characteristics, number of stents implanted). In addition, hospital level characteristics (whether the hospital’s risk-adjusted in-hospital/30-day PCI mortality during 2005–2007 was above the median value of NYS hospitals’ PCI mortality, whether the hospital’s PCI volume during 2005–2007 was above the median value of NYS hospitals’ PCI volume in that time period) and physician level characteristics (whether the physician’s risk-adjusted in-hospital/30-day PCI mortality during 2005–2007 was above the median value of NYS physicians’ PCI mortality in that time period, whether the physician’s PCI volume during 2005–2007 was above the median value of NYS physicians’ PCI volume between 2005 and 2007) were compared for E-ZES and EES patients. Next, we ran a multivariable logistic regression model to predict the probability of receiving E-ZES (vs EES) based on variables noted above. We used calipers of width 0.2 of the standard deviation of the logit of the propensity score. Then, each patient treated with E-ZES was matched to a patient treated with EES based on the closest predicted propensity score generated from the multivariable logistic regression model. We re-examined the distributions of the variables after the propensity matching, and almost all had a standardized difference < 10% (see Table 1).
Table 1.
Characteristics of 1:1 Propensity Matched EES vs E-ZES Patients (N=13,663 Pairs)
| EES | E-ZES | Standardized Difference (%) | |||
|---|---|---|---|---|---|
| Variable | Frequency | Percent | Frequency | Percent | |
| Demographics | |||||
| Age | |||||
| <55 | 2,713 | 19.9 | 2,565 | 18.8 | 2.7 |
| 55–64 | 2,746 | 27.4 | 3,767 | 27.6 | 0.3 |
| 65–74 | 4,064 | 29.7 | 3,843 | 28.1 | 3.6 |
| 75–84 | 2,618 | 19.2 | 2,852 | 20.9 | 4.3 |
| 85+ | 522 | 3.8 | 636 | 4.7 | 4.1 |
| Female | 4,358 | 31.9 | 4,213 | 30.8 | 2.3 |
| Ethnicity | |||||
| Hispanic | 1,112 | 8.1 | 965 | 7.1 | 4.1 |
| Race | |||||
| White | 10,073 | 73.7 | 10,490 | 76.8 | 8.9 |
| Black | 1,223 | 9.0 | 1,169 | 8.6 | 1.4 |
| Asian | 733 | 5.4 | 743 | 5.4 | 0.3 |
| Other | 1,634 | 12.0 | 1,261 | 9.2 | 7.1 |
| Median Household Income | |||||
| <$40,000 | 1,502 | 11.0 | 1,099 | 8.0 | 10 |
| $40,000–$69,999 | 5,989 | 43.8 | 5,189 | 38.0 | 11.9 |
| $70,000+ | 6,172 | 45.2 | 7,375 | 54.0 | 17.7 |
| Primary payer | |||||
| Medicare | 4,812 | 35.2 | 4,995 | 36.6 | 2.7 |
| Medicaid | 498 | 3.6 | 450 | 3.3 | 1.9 |
| Private | 7,942 | 58.1 | 7,852 | 57.5 | 1.3 |
| Other | 411 | 3.0 | 366 | 2.7 | 2.0 |
| Risk Factors | |||||
| LVEF (%) | |||||
| <20 | 981 | 7.2 | 809 | 5.9 | 5.1 |
| 20–29 | 424 | 3.1 | 387 | 2.8 | 1.6 |
| 30–49 | 2,818 | 20.6 | 2,939 | 21.5 | 2.2 |
| 50+ | 9,440 | 69.1 | 9,528 | 69.7 | 1.4 |
| Myocardial infarction (from symptom onset to PCI) | |||||
| No MI within 14 days | 9,994 | 73.2 | 10,053 | 73.6 | 1.0 |
| STEMI <6 hrs | 983 | 7.2 | 1,078 | 7.9 | 2.6 |
| STEMI 6–11 hrs | 197 | 1.4 | 210 | 1.5 | 0.8 |
| STEMI 12–23 hrs | 116 | 0.9 | 127 | 0.9 | 0.9 |
| Non-STEMI <6 hrs | 97 | 0.7 | 80 | 0.6 | 1.6 |
| Non-STEMI 6–11 hrs | 142 | 1.0 | 126 | 0.9 | 1.2 |
| Non-STEMI 12–23 hrs | 282 | 2.1 | 253 | 1.9 | 1.5 |
| MI 1–14 days | 1,852 | 13.6 | 1,736 | 12.7 | 2.5 |
| Cerebrovascular disease | 1,141 | 8.4 | 1,088 | 8.0 | 1.4 |
| Peripheral vascular disease | 1,140 | 8.3 | 1,037 | 7.6 | 2.8 |
| Hemodynamical status (unstable) | 40 | 0.3 | 38 | 0.3 | 0.3 |
| CHF(this admission) | 706 | 5.2 | 733 | 5.4 | 0.9 |
| Malignant ventricular arrhythmia | 72 | 0.5 | 81 | 0.6 | 0.9 |
| COPD | 942 | 6.9 | 958 | 7.0 | 0.5 |
| Diabetes | 4,708 | 34.5 | 4,258 | 31.2 | 7.0 |
| Previous CABG | 2,191 | 16.0 | 2,010 | 14.7 | 3.7 |
| Previous PCI | 4,151 | 30.4 | 3,854 | 28.2 | 4.8 |
| Renal failure | |||||
| No renal failure | 12,330 | 90.2 | 12,438 | 91.0 | 2.7 |
| Renal failure, creatinine 1.6–2.0 | |||||
| mg/dl | 737 | 5.4 | 711 | 5.2 | 0.8 |
| Renal failure, creatinine > 2.0 | |||||
| mg/dl | 312 | 2.3 | 269 | 2.0 | 2.2 |
| Renal failure, requiring dialysis | 284 | 2.1 | 245 | 1.8 | 2.1 |
| BMI | |||||
| <25 | 2,814 | 20.6 | 3,012 | 22.0 | 3.6 |
| 25–29 | 5,401 | 39.5 | 5,152 | 37.7 | 3.7 |
| 30+ | 5,448 | 40.0 | 5,499 | 40.3 | 0.8 |
| Number of vessels diseased | |||||
| One or two | 11,456 | 83.9 | 11,861 | 86.8 | 8.4 |
| Three | 2,056 | 15.1 | 1,681 | 12.3 | 8.0 |
| Left main disease | 530 | 3.9 | 403 | 3.0 | 5.1 |
| Number of stents | |||||
| One | 9,299 | 68.1 | 9,655 | 70.7 | 4.1 |
| Two | 3,363 | 24.6 | 3,148 | 23.0 | 3.7 |
| Three or more | 1,001 | 7.3 | 860 | 6.3 | 4.0 |
| Vessel<2.5mm | 363 | 2.7 | 238 | 1.7 | 6.2 |
| Lesion | |||||
| Length>33mm | 768 | 5.6 | 575 | 4.2 | 6.5 |
| Hospital and physician level characteristics | |||||
| Hospital risk-adjusted in -hospital/30-day mortality (2005–2007) above median (0.901) | |||||
| 2,942 | 21.5 | 2,525 | 18.5 | 7.6 | |
| Hospital PCI volume (2005–2007) above median (2,582 patients) | |||||
| 10,731 | 78.5 | 10,881 | 79.6 | 2.7 | |
| Physician risk-adjusted in -hospital/30-day mortality in 2005 - 2007 above median (0.854) | |||||
| 5,692 | 41.7 | 5,504 | 40.3 | 4.2 | |
| Physician PCI volume in 2005 –2007 above median (385 patients) | |||||
| 9,674 | 70.8 | 10,295 | 75.4 | 3.5 | |
We developed Kaplan-Meier survival curves to examine each outcome measure for the E-ZES and EES groups. We also conducted proportional hazards regression analyses to further adjust for differences in the distribution of patient level factors among matched E-ZES/EES pairs of patients. The robust sandwich variance method was used to adjust for the matched-pairs design. Finally, we repeated the above analyses to evaluate 6-year comparative effectiveness of E-ZES vs EES for each of six “off-label” and two “high-risk” subgroups.
We used SAS version 9.3 software (SAS Institute, Cary, North Carolina) for all descriptive and regression analyses, and 2-sided tests with a 0.05 significance level were used for hypothesis testing.
Results
We obtained 13,663 1:1 propensity matched pairs of patients receiving E-ZES or EES from July 1, 2008 through December 31, 2010 in the final cohort for analysis. This cohort was selected from the initial pool of 13,665 patients treated with E-ZES and 33,006 patients treated with EES. The demographics, socioeconomic status, pre-procedure risk factors, vessel/lesion information, and hospital characteristics and physician characteristics of the matched pairs were compared. The distributions of almost all variables were very similar between E-ZES and EES groups, which was suggested by the standardized difference < 10% in Table 1[18]. The mean and the standard deviation of the difference in propensity scores between matched pairs were 0.03 and 0.006, respectively. Approximately three fourths of patients were from 55 to 84 years old. About 31% of patients were female and over 73% were whites. More than 38% of patients had public insurance (Medicare, 35%; Medicaid, 3%) and around 58% had private insurance. Over 73% of patients did not have a myocardial infarction within 14 days prior to undergoing PCI. About one third of patients had diabetes, 30% had a previous PCI, and 15% had a previous CABG surgery. Nearly 70% of patients had a left ventricular ejection fraction (LVEF) greater than 50%. Over 83% of patients had one or two vessels diseased, 3% had left main disease, and about 30% received multiple stents during the index E-ZES/EES placement. Kaplan-Meier survival curve for each 6-year outcome measure was drawn (see Figures 1A–1D).
Figure 1A.
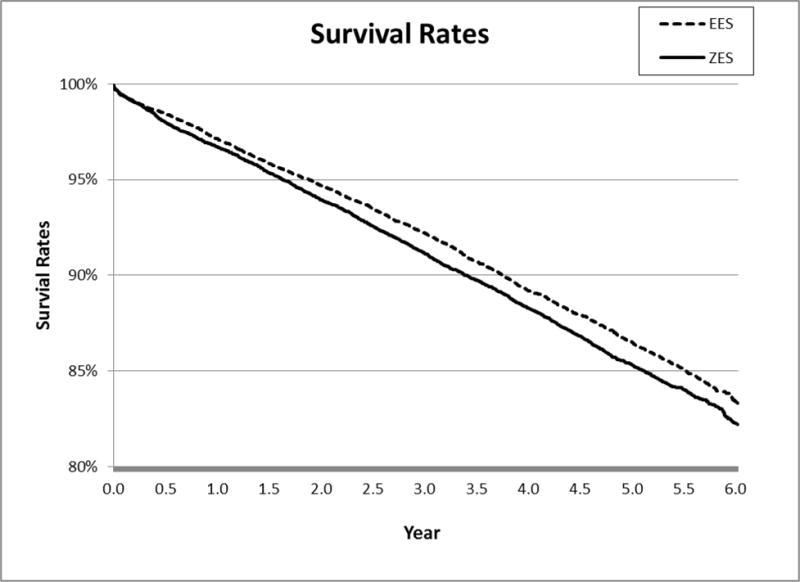
6-Year All-Cause Mortality
Figure 1D.
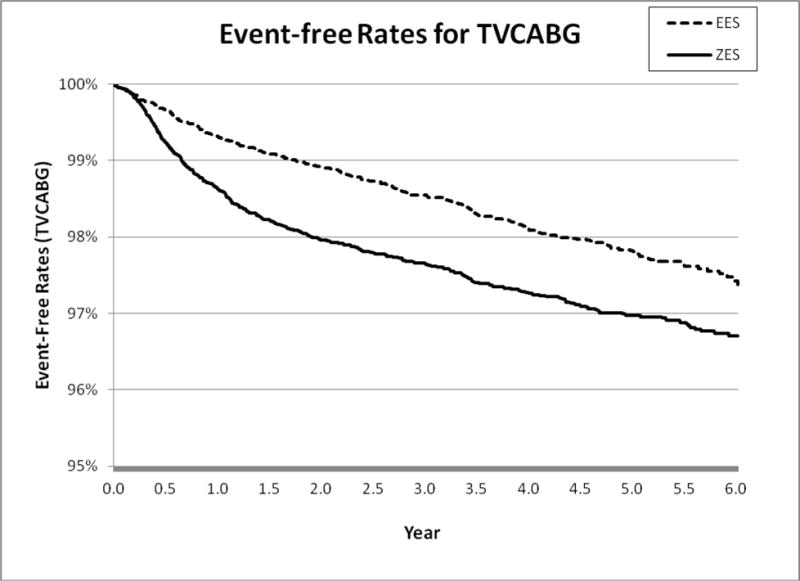
6-Year Target Vessel CABG (TVCABG)
After further adjusting matched patients using patient, physician, and hospital level factors, we found that patients receiving E-ZES were associated with a significantly higher rate of 6-year all-cause mortality (adjusted hazard ratio <AHR> = 1.10, 95% confidence interval <CI>, 1.04–1.17, P=0.003), AMI (AHR = 1.12, 95% CI, 1.02–1.23, P=0.01), TLPCI (AHR = 1.28, 95% CI, 1.18–1.39, P<0.001), and TVCABG (AHR = 1.47, 95% CI, 1.26–1.71, P<0.001) (see Table 2). The findings from Cox regression analysis suggest that there were significant differences in all outcomes measures between E-ZES and EES although Kaplan-Meier survival curve showed that there was no significant difference in AMI. These findings indicate that at 6 years, patients treated with EES had better primary outcome and secondary outcomes relative to patients treated with E-ZES. The findings from the “off-label” and “high-risk” subgroup analyses indicate that patients receiving E-ZES had a significantly higher rate of TLPCI and TVCABG for subgroups with STEMI, totally occluded lesions, multiple vessels diseased, and diabetes. There were no significant differences in all-cause mortality and AMI outcomes between E-ZES and EES groups except that compared with EES use, E-ZES use was associated with significantly higher all-cause mortality rate (AHR = 1.29, 95% CI, 1.13–1.48, P<0.001) in patients with diabetes.
Table 2.
6-Year Primary and Secondary Outcomes (Cox Regression)
| Outcome | Hazard Ratio (ZES vs EES) | 95% Confidence Interval | P-value |
|---|---|---|---|
| All-Cause Mortality | 1.10 | 1.03 – 1.17 | 0.003 |
| AMI | 1.12 | 1.02 – 1.23 | 0.01 |
| TLPCI | 1.28 | 1.18 – 1.39 | <0.001 |
| TVCABG | 1.47 | 1.26 – 1.71 | <0.001 |
Discussion
Our study represents the largest observational study used to compare long-term effectiveness between E-ZES and EES in everyday PCI practice. We used population-based high-quality NYS cardiac registries linked to other national/statewide datasets to capture a comprehensive set of outcomes with the longest follow-up to date (i.e., 6 years). This study enabled us to compare differences in long-term outcomes between E-ZES and EES in real-world setting and compare differences in a variety of “off-label” and “high-risk” subgroups. The strength of the study is that we included all patients treated with E-ZES/EES from all non-federal hospitals in New York State. To minimize the selection bias inherent in any observational study, we applied a propensity score matching method to adjust for all available risk factors in the PCIRS registries.
We found that EES use led to better long-term outcomes relative to E-ZES use. Our results are generally consistent with results from a meta-analysis summarizing prior observational studies on comparative effectiveness of ZES vs. EES.[11] The meta-analysis reported that EES significantly reduced the rate of target vessel revascularization (RR = 0.61, 95% CI: 0.47–0.79, P<0.001) based on 8 previous observational studies with the total sample size of 12,299. The meta-analysis also found that target lesion revascularization (RR = 0.57, 95% CI: 0.38–0.83, P=0.004) relative to ZES based on 13 prior observational studies with a total sample size of 14,589. However, the meta-analysis study did not find a significant difference in long-term mortality and did not include subsequent AMI as an outcome measure. It is important to note that compared with our study, the above 13 observational studies cited in the meta-analysis had much shorter follow-up (i.e., 1–3 years vs. 6 years in our study) and much smaller sample size (i.e., total 6,550 matched pairs from all 13 prior studies vs. 13,663 matched pairs in our study. Also, the ZES group used in the meta-analysis included both E-ZES and RESOLUTE zotarolimus-eluting stents (R-ZES), without any clear distinction between the two ZES, whereas only E-ZES was compared to EES in our study. It is very likely that such differences in study designs could explain why we found both survival benefits and a long-term reduced AMI rate in EES group relative to E-ZES group. In addition, our findings echo the results from a recent registry study of patients with ST-segment elevation myocardial infarction (STEMI) that compared 3-year outcomes between E-ZES and EES. In that study, Velders et al demonstrated that compared with E-ZES (n=519), EES (n=412) was associated with a better 3-year composite endpoint (which is comprised of cardiac death, target vessel-related myocardial infarction, and target lesion revascularization) (AHR = 0.64, 95% CI: 0.42–0.99). Their main findings align well with the results from the subgroup analysis for “STEMI” patients in our study. However, they did not detect any significant difference in mortality between E-ZES and EES, possibly because of the very small sample size of the study.
When we evaluated the long-term comparative effectiveness of E-ZES vs EES in six “off-label” and two “high-risk” subgroups, we found that EES was associated with a significantly lower rate of TLPCI and TVCABG in subgroups of STEMI, totally occluded lesions, multiple vessels diseased, and diabetes. Patients treated with EES had better or at least similar outcomes compared with patients treated with E-ZES for almost all “off-label” or “high-risk” subgroups. To the best of our knowledge, our study represents the largest observational study and the longest follow-up period using a high-quality cardiac registry to examine the relative effectiveness of E-ZES and EES for important “off-label” and “high-risk” subgroups. Overall, our results suggest that EES is a better option than E-ZES for “off-label” and “high-risk” patient subgroups. Although further research is needed to confirm our findings of the comparative advantages of EES vs. E-ZES for those particular subgroups, our findings highlight the need and urgency of providing timely patient-centered evidence for improving quality and outcomes in PCI practice.
This study has several limitations. First and foremost, this was a nonrandomized observational study and we could not rule out the possibility of selection bias and residual confounders even after applying propensity score matching to minimize the selection bias and further adjusting for all available risk factors in multivariable regression analyses. For example, there is the possibility that risk factors such as differences in the use of post-stent placement antiplatelet therapy could have had an impact on relative long-term outcomes. Unfortunately, such information was not available in our databases and thus we were unable to control for such practically important factors. Second, we focused on E-ZES only in this long-term comparative effectiveness study. R-ZES is a more recent type of ZES that received FDA’s approval in February 2012. It has been found to have superior short-term outcomes relative to E-ZES.[19] However, although R-ZES is more commonly employed in current practice, R-ZES has not been in practice long enough to examine its longer-term (more than 4 years) outcomes. When more recent data become available, a long-term comparative effectiveness study between R-ZES and EES would be helpful to provide updated, valuable, and more relevant evidence to improve medical decision making on DES selection. Third, we relied on the diseased coronary artery information in the CSRS registry to examine the TVCABG outcome because the vessels attempted information was not available in the CSRS registry. Instead, we had to assume that if the target coronary vessel was diseased and any subsequent CABG operation was performed that there was a TVCABG in the target vessel. Fourth, we could not capture a small portion of patients who were hospitalized for AMI or repeat revascularization outside New York State after the index E-ZES/EES implantation. We decided to include only New York residents in analysis in order to minimize any systematic bias between E-ZES and EES groups with respect to hospital readmissions occurring outside New York State. Fifth, we were unable to study stent thrombosis and restenosis rates after E-ZES/EES placement in the present study because such clinical information was not available in the PCI and CABG registries. Sixth, dual antiplatelet therapy (DAPT) and peri-operative management might affect the long-term outcomes of E-ZES/EES outcomes. Unfortunately, we were unable to account for these factors due to unavailability of such information. Finally, our study focused on 6-year all-cause mortality for E-ZES vs. EES. It would be ideal to examine cardiac mortality as well as all-cause mortality, but we were unable to obtain such detailed information.
In conclusion, our study provides the most comprehensive comparison to date between E-ZES and EES in everyday PCI practice over a 6-year follow-up using all-inclusive high-quality NYS cardiac registries. Patients receiving E-ZES were associated with a higher rate of 6-year all-cause mortality and subsequent AMI, TLPCI and TVCABG relative to patients receiving EES. The comparative advantages of EES were also observed for subgroups of patients with STEMI, totally occluded lesions, multiple vessels diseased, and for older patients. Our findings are generally consistent with prior observational studies, and results from our subgroup analysis are valuable and relevant for patient-centered DES selection. Future research is needed to confirm our findings and examine the long-term comparative effectiveness of the more recent type of ZES (i.e., R-ZES) vs. EES in contemporary PCI practice.
Supplementary Material
Figure 1B.
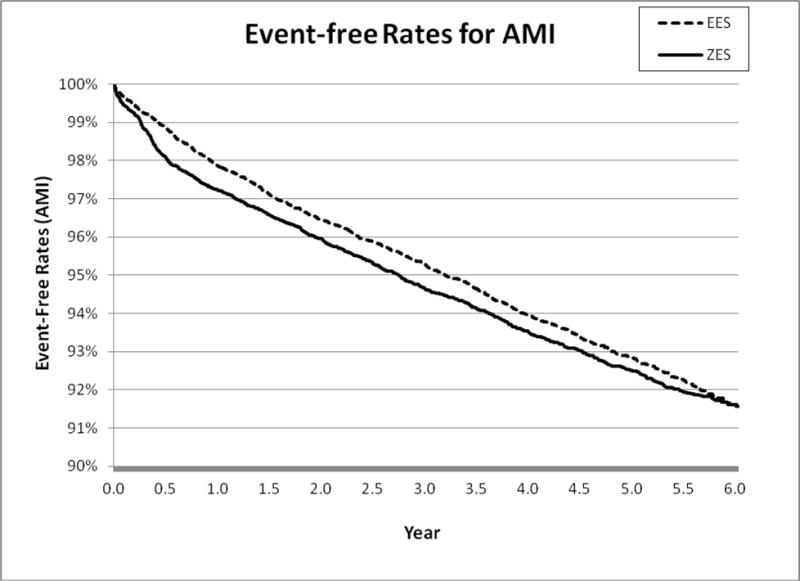
6-Year Acute Myocardial Infarction (AMI)
Figure 1C.
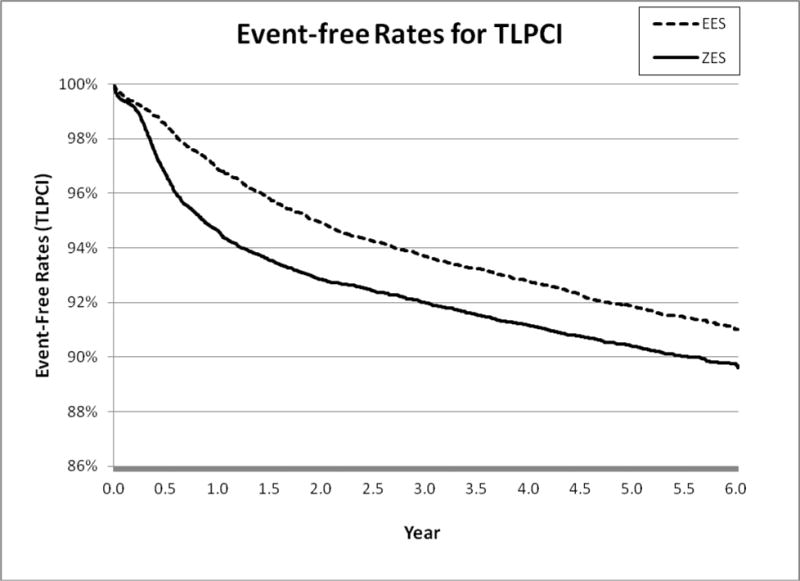
6-Year Target Lesion PCI (TLPCI)
Acknowledgments
This project was funded by a R01 grant (HS022289–01) from the Agency for Healthcare Research and Quality (AHRQ). We thank the New York State Cardiac Advisory Committee for their encouragement and support of this study; and thank Kimberly S. Cozzens, Cynthia L. Johnson, and the cardiac surgery departments and cardiac-catheterization laboratories of the participating hospitals for their tireless efforts to ensure the timeliness, completeness, and accuracy of the PCI and CSRS registries.
This project was funded by a R01 grant (HS022289-01) from the Agency for Healthcare Research and Quality (AHRQ).
Abbreviations List
- AMI
acute myocardial infarction
- CABG
coronary artery bypass graft
- CHD
coronary heart disease
- CSRS
Cardiac Surgery Reporting System
- DES
drug-eluting stents
- EES
everolimus-eluting stents
- E-ZES
Endeavor zotarolimus-eluting stents
- FDA
Food and Drug Administration
- LVEF
left ventricular ejection fraction
- NDI
National Death Index
- NSTEMI
non-ST-segment elevation myocardial infarction
- PCI
percutaneous coronary intervention
- PCIRS
Percutaneous Coronary Interventions Reporting System
- STEMI
ST-segment elevation myocardial infarction
- SPARCS
Statewide Planning and Research Cooperative System
- TLPCI
target lesion percutaneous coronary intervention
- TVCABG
target vessel coronary artery bypass graft
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Nothing to disclose
References
- 1.Garg S, Serruys PW. Coronary stents: current status. Journal of the American College of Cardiology. 2010;56:S1–42. doi: 10.1016/j.jacc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Kandzari DE, Leon MB, Meredith I, Fajadet J, Wijns W, Mauri L. Final 5-year outcomes from the Endeavor zotarolimus-eluting stent clinical trial program: comparison of safety and efficacy with first-generation drug-eluting and bare-metal stents. JACC Cardiovascular interventions. 2013;6:504–12. doi: 10.1016/j.jcin.2012.12.125. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Nikolsky E, Cutlip DE, Mauri L, Liberman H, Wilson H, et al. Improved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trial. JACC Cardiovascular interventions. 2010;3:1043–50. doi: 10.1016/j.jcin.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Fajadet J, Wijns W, Laarman GJ, Kuck KH, Ormiston J, Munzel T, et al. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114:798–806. doi: 10.1161/CIRCULATIONAHA.105.591206. [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Mauri L, Popma JJ, Cutlip DE, Nikolsky E, O’Shaughnessy C, et al. A randomized comparison of the Endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. Journal of the American College of Cardiology. 2010;55:543–54. doi: 10.1016/j.jacc.2009.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Kedhi E, Gomes ME, Lagerqvist B, Smith JG, Omerovic E, James S, et al. Clinical impact of second-generation everolimus-eluting stent compared with first-generation drug-eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC Cardiovascular interventions. 2012;5:1141–9. doi: 10.1016/j.jcin.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211–23. doi: 10.1161/CIRCULATIONAHA.113.001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smits PC, Vlachojannis GJ, McFadden EP, Royaards KJ, Wassing J, Joesoef KS, et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice: The COMPARE Trial (A Trial of Everolimus-Eluting Stents and Paclitaxel Stents for Coronary Revascularization in Daily Practice) JACC Cardiovascular interventions. 2015;8:1157–65. doi: 10.1016/j.jcin.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Velders MA, Boden H, van der Hoeven BL, Liem SS, Atary JZ, van der Wall EE, et al. Long-term outcome of second-generation everolimus-eluting stents and Endeavor zotarolimus-eluting stents in a prospective registry of ST-elevation myocardial infarction patients. Euro Intervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2013;8:1199–206. doi: 10.4244/EIJV8I10A184. [DOI] [PubMed] [Google Scholar]
- 10.Omar A, Torguson R, Kitabata H, Pendyala LK, Loh JP, Magalhaes MA, et al. Long-Term Safety and Efficacy of Second-Generation Everolimus-Eluting Stents Compared to Other Limus-Eluting Stents and Bare Metal Stents in Patients With Acute Coronary Syndrome Catheter. Cardio Inte. 2014;84:1053–60. doi: 10.1002/ccd.25469. [DOI] [PubMed] [Google Scholar]
- 11.Gu H, Hua K, Li W, Wang Y, Yang J. Safety and efficacy of everolimus-eluting stent versus zotarolimus-eluting stent: A meta-analysis of randomized controlled clinical trials and observational studies. International journal of cardiology. 2015;201:552–60. doi: 10.1016/j.ijcard.2015.02.097. [DOI] [PubMed] [Google Scholar]
- 12.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA: the journal of the American Medical Association. 2001;286:708–13. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 13.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Archives of internal medicine. 2002;162:1682–8. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 14.Masoudi FA, Havranek EP, Wolfe P, Gross CP, Rathore SS, Steiner JF, et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. American heart journal. 2003;146:250–7. doi: 10.1016/S0002-8703(03)00189-3. [DOI] [PubMed] [Google Scholar]
- 15.Hannan EL, Cozzens K, King SB, 3rd, Walford G, Shah NR. The New York State cardiac registries: history, contributions, limitations, and lessons for future efforts to assess and publicly report healthcare outcomes. Journal of the American College of Cardiology. 2012;59:2309–16. doi: 10.1016/j.jacc.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in medicine. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–3. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134:1128–35. doi: 10.1016/j.jtcvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Tada T, Byrne RA, Cassese S, King L, Schulz S, Mehilli J, et al. Comparative efficacy of 2 zotarolimus-eluting stent generations: resolute versus endeavor stents in patients with coronary artery disease. American heart journal. 2013;165:80–6. doi: 10.1016/j.ahj.2012.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


