Abstract
Background
Recent failures of drugs that raised high-density lipoprotein (HDL) cholesterol levels to reduce cardiovascular events in clinical trials have led to increased interest in alternative indices of HDL quality, such as cholesterol efflux capacity, and HDL quantity, such as HDL particle number. However, no studies have directly compared these metrics in a contemporary population that includes potent statin therapy and low LDL cholesterol.
Methods
HDL cholesterol levels, apolipoprotein A-I (apoA-I), cholesterol efflux capacity, and HDL particle number were assessed at baseline and 12 months in a nested case-control study of the JUPITER trial, a randomized primary prevention trial that compared rosuvastatin treatment to placebo in individuals with normal LDL cholesterol but increased C-reactive protein levels. 314 cases of incident cardiovascular disease (myocardial infarction, unstable angina, arterial revascularization, stroke, or cardiovascular death) were compared to age- and gender-matched controls. Conditional logistic regression models adjusting for risk factors evaluated associations between HDL-related biomarkers and incident CVD.
Results
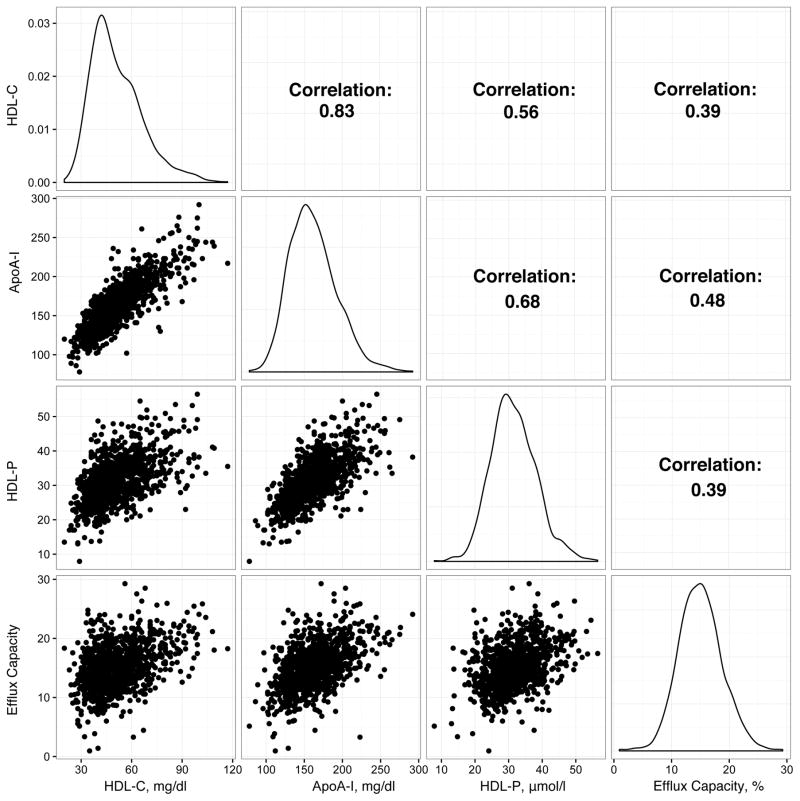
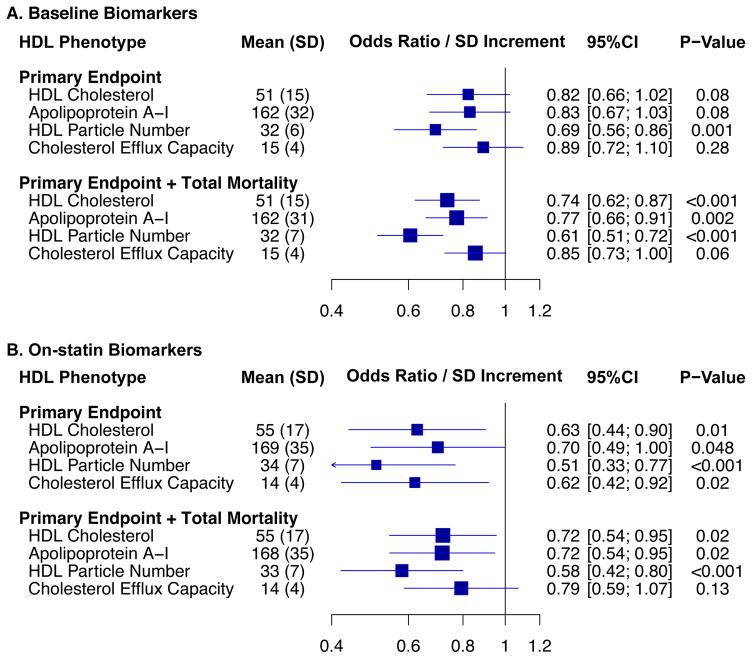
Cholesterol efflux capacity was moderately correlated with HDL cholesterol, apoA-I, and HDL particle number (Spearman r= 0.39, 0.48, and 0.39 respectively; P<0.001). Baseline HDL particle number was inversely associated with incident CVD (adjusted odds ratio per SD increment [OR/SD] 0.69; 95%CI 0.56 – 0.86; p< 0.001), while no significant association was found for baseline cholesterol efflux capacity (OR/SD 0.89; 95%CI 0.72–1.10; p=0.28), HDL cholesterol (OR/SD 0.82; 95%CI 0.66–1.02; P = 0.08), or apoA-I (OR/SD 0.83; 95%CI 0.67–1.03; p=0.08). 12 months of rosuvastatin (20mg/day) did not change cholesterol efflux capacity (average percent change −1.5%, 95%CI −13.3 to +10.2; p=0.80), but increased HDL cholesterol (+7.7%), apoA-I (+4.3%), and HDL particle number (+5.2%). On-statin cholesterol efflux capacity was inversely associated with incident CVD (OR/SD 0.62; 95%CI 0.42–0.92; p=0.02), although HDL particle number again emerged as the strongest predictor (OR/SD 0.51; 95%CI 0.33–0.77; p< 0.001).
Conclusions
In JUPITER, cholesterol efflux capacity was associated with incident CVD in individuals on potent statin therapy but not at baseline. For both baseline and on-statin analyses, HDL particle number was the strongest of four HDL-related biomarkers as an inverse predictor of incident events and biomarker of residual risk.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00239681.
Keywords: cardiovascular disease risk factors, high-density lipoprotein cholesterol
Introduction
High-density lipoprotein (HDL) cholesterol is a well-integrated biomarker of cardiometabolic health and remains a key component of the cardiovascular disease (CVD) risk prediction algorithms used to guide therapy.1 However, HDL cholesterol-raising therapeutics have failed to demonstrate efficacy in multiple recent clinical trials.2–4 These results have called into question both the causality of the observed relationship between HDL and CVD and the hypothesis that modulation of HDL cholesterol levels can improve clinical outcomes. At a minimum, it has become clear that changes in HDL cholesterol levels are an inadequate surrogate biomarker for therapeutic efficacy.
The static measurement of HDL cholesterol levels, as is performed in current clinical practice, may not adequately capture the anti-atherogenic properties of highly heterogeneous HDL particles. Beyond apolipoprotein A-I (apoA-I), proteomics analyses have identified more than 80 distinct proteins associated with circulating HDL particles.5 Previous studies have suggested that the number of circulating HDL particles may serve as a better predictor of cardiovascular risk than HDL cholesterol levels.6–13 A putative atheroprotective mechanism of HDL involves the reverse cholesterol transport pathway, the process by which excess cholesterol is removed from the periphery and returned to the liver for biliary excretion.14 Cholesterol efflux capacity is one metric of HDL functionality that quantifies the ability for an individual’s HDL to extract cholesterol from macrophages, the rate-limiting step of reverse cholesterol transport. Efflux capacity was demonstrated to be a better predictor of atherosclerotic burden than HDL cholesterol levels in several previous studies.15–19 This inverse relationship was extended to incident cardiovascular events in some,16–18 but not all,19 previous analyses. However, owing to challenges of standardizing a cell-based assay across laboratories, it is unlikely that assessment of efflux capacity will enter into routine clinical use.
Few previous studies have determined the relationships between these various HDL-related biomarkers, and none to-date have directly compared their associations with incident cardiovascular events, particularly in a contemporary cohort with low or normal levels of low-density lipoprotein (LDL) cholesterol levels that includes randomized treatment with potent statin therapy. Here, we examined prospective associations of HDL cholesterol, apoA-I, HDL particle number, and cholesterol efflux capacity in a nested case-control cohort derived from the Justification for the Use of Statins in Prevention: An Intervention Evaluating Rosuvastatin (JUPITER) clinical trial.20
Methods
Study Population
The study population was derived from the JUPITER trial, a previously reported primary prevention, randomized, double-blind, placebo-controlled trial investigating whether rosuvastatin 20 mg daily would decrease incident CVD in 17,802 asymptomatic individuals with LDL cholesterol <130 mg/dL and a high-sensitivity C-reactive protein (hsCRP) level ≥2.0 mg/L.20 Exclusion criteria for the JUPITER trial included diabetes mellitus, previous or current use of lipid-lowering therapy, or triglycerides >500 mg/dL. In addition to blood samples required for the trial protocol assays, 11,953 (67%) of trial participants voluntarily provided samples for additional biomarker phenotyping. Endpoints within the JUPITER trial were adjudicated by an independent committee blinded to treatment assignment.20 All participants provided written informed consent as part of the JUPITER trial enrollment. Institutional review board approval for the present study was obtained from Partners HealthCare (Boston, MA).
A nested case-control cohort was derived from individuals with available baseline blood samples. The primary analysis involved 314 cases who experienced the JUPITER trial primary endpoint (myocardial infarction, hospitalization for unstable angina, arterial revascularization, stroke, or cardiovascular death); an expanded secondary endpoint (N = 525 cases) was prespecified to additionally include death from any cause as implemented in previous JUPITER biomarker analyses (Supplementary Table 1)..21–23 Each case definition was compared to controls who remained free of events at the time of case event and matched in a 1:1 ratio based on age (within two years) and sex.
Laboratory Measurements
Fasting lipids, apolipoproteins, hsCRP, and glucose levels were measured in a core laboratory as previously described.20,23,24 HDL particle number was measured using nuclear magnetic resonance (NMR) spectroscopy LipoProfile III, by LipoScience, Inc (now LabCorp, Raleigh, NC). Total particle number was calculated to be the sum of levels across HDL subclasses, identified based on lipid methyl group NMR signals as previously described.12
Cholesterol efflux capacity was quantified in plasma samples thawed from liquid nitrogen storage using a previously validated cell-based ex vivo assay.15 In brief, J7774 macrophage cells were incubated with 3H-radiolabeled cholesterol. Expression of the ATP-binding cassette transporter A1 (ABCA1) transporter was upregulated using cyclic AMP. Diluted apolipoprotein B-depleted plasma was then added and liquid scintillation counting used to quantify the percent of radioactive cholesterol from the cells effluxed into the media. In order to minimize variation related to batch effects, paired baseline and 12-month samples from cases and matched controls were included on the same plate for efflux capacity assessment. Secondly, a pooled plasma control was used to correct for interassay variation across assays. In a pilot analysis involving blinded duplicate assessments of 20 patient samples divided into two aliquots, the mean intra- and interassay coefficients of variation were 3.7% and 4.7% respectively. Lipoprotein-associated phospholipase A2 activity levels were measured using an automated enzyme assay system.25 HDL-related phenotypes, including cholesterol efflux capacity, were measured both at baseline and after twelve months of therapy in individuals with samples available.
Statistical Analysis
Medians, 25th, and 75th percentiles were calculated for continuous variables. The significance of variation in cholesterol efflux capacity across categorical patient characteristics was assessed using the nonparametric Wilcoxon rank sum or Chi-square tests. Spearman coefficients were calculated to determine the magnitude of correlation between biomarkers. The impact of treatment on HDL-related phenotypes was determined by calculating the percent change from baseline to 12-months in individuals stratified by treatment group. Statistical comparisons with baseline and placebo values determined via one-sample and two-sample t tests respectively.
Odds ratios were calculated across tertiles as well as per standard deviation increment in each biomarker. Conditional logistic regression models accounting for the matched study design were adjusted for age, race, randomized treatment group, smoking status, systolic blood pressure, body-mass index, fasting glucose, low-density lipoprotein cholesterol level, log-transformed triglyceride level, and family history of premature coronary artery disease. P values for trend were obtained by including tertile number as a variable in the regression model. Models analyzing on-statin biomarkers incorporated on-treatment values for LDL and log-transformed triglycerides. In order to preserve power while maintaining the matched case-control design, subgroup analyses were conducted by adding appropriate interaction terms to the models. Subgroup-specific linear regression parameters and odds ratios were calculated using combinations of main effect and interaction terms (Supplementary Methods).
All P values were two-tailed with a value < 0.05 used to indicate statistical significance. Analyses were performed using R version 3.1 software (The R Project for Statistical Computing, Vienna, Austria).
Results
The study population included 1050 trial participants—525 controls, 314 cases who experienced the primary JUPITER trial endpoint, and 525 cases who experienced the expanded secondary endpoint inclusive of all-cause mortality (Table 1). Those who experienced the primary JUPITER trial endpoint were more likely to have been allocated to placebo, had slightly lower body-mass index, were more likely to report current smoking, and had higher baseline triglycerides. HDL cholesterol, apolipoprotein A-I, and HDL particle number were each significantly lower in cases compared with controls. By contrast, no significant difference was noted in baseline cholesterol efflux capacity.
Table 1.
Baseline Characteristics
| Primary Endpoint Controls (N=314) | Primary Endpoint Cases (N=314) | Primary Endpoint + Total Mortality Controls (N = 525) | Primary Endpoint + Total Mortality Cases (N=525) | |
|---|---|---|---|---|
| Age, years | 69(64–74.8) | 69(63.2–75) | 69(64–75) | 70(64–75) |
| Female Sex | 86(27.4%) | 86(27.4%) | 149(28.4%) | 149(28.4%) |
| Rosuvastatin group | 140(44.6%) | 109(34.7%)* | 247(47%) | 203(38.7%)* |
| White Race | 290(92.4%) | 274(87.3%)* | 475(90.5%) | 436(83%)* |
| Body-Mass Index, kg/m2 | 28.5(25.5–31.5) | 27.7(24.9–30.7) | 28.5(25.7–31.6) | 27(24–30.5) * |
| Systolic Blood Pressure, mm Hg | 135(124–145) | 136(128–147) | 135(124–144) | 134(126–145) |
| Current smoker | 29(9.2%) | 67(21.3%)* | 55(10.5%) | 122(23.3%)* |
| FH of Premature CHD† | 44(14%) | 50(15.9%) | 77(14.7%) | 72(13.7%) |
| Metabolic syndrome | 120(38.5%) | 132(42.3%) | 201(38.5%) | 191(36.7%) |
| hsCRP, mg/l | 4.2(2.8–7.1) | 4.5(2.9–7.7) | 4.2(2.8–7) | 4.8(3–8.7)* |
| Lp-PLA2 Activity, nmol/min/mL | 197.9(167.4–224.1) | 202.8(177–238.3) | 197.4(167.3–229.5) | 203.1(172.7–236.7) |
| Fasting Glucose, mg/dl | 95(89–101) | 95(89–103) | 95(89–101) | 95(89–103) |
| Lipids, mg/dL | ||||
| LDL Cholesterol | 111(97–120) | 110.5(94–120) | 109(95–119) | 106(92–119) |
| HDL Cholesterol | 49(40–62) | 47(40–58)* | 49(40–62) | 47(40–59)* |
| Triglycerides | 111.5(82.2–159.5) | 121.5(93–171)* | 116(84–166) | 116(87–166) |
| Apolipoproteins, mg/dl | ||||
| Apolipoprotein B | 107(96–120) | 111(98–123.2) | 107(95–120) | 109(95–120) |
| Apolipoprotein A-I | 161(140–186) | 155(137.8–175)* | 162(141–183) | 156(139.5–177)* |
| HDL Particle Number, μmol/l | 32.8(27.9–37.6) | 30.4(26.6–35.2)* | 32.5(27.9–36.9) | 29.8(26–34.6)* |
| Cholesterol Efflux Capacity, % | 15.4(12.8–18) | 15.1(12.8–17.6) | 15(12.5–17.6) | 14.8(12.4–17.1) |
Values represent n(%) or median (25–75%). FH – Family History; CHD – Coronary Heart Disease; hsCRP – high-sensitivity C-Reactive Protein; Lp-PLA2 – lipoprotein-associated phospholipase A2; LDL – low-density lipoprotein; HDL – high-density lipoprotein.
indicates Chi-square or Wilcoxon rank-sum test p-value < 0.05
Family history of premature coronary disease was defined as diagnosis of the disease in a male first-degree relative before the age of 55 y or in a female first-degree relative before the age of 65 y.
Baseline cholesterol efflux capacity was higher in female participants (Table 2), but did not appreciably vary according to treatment group, smoking status, family history of premature coronary artery disease, or the presence of the metabolic syndrome. Moderate correlations were noted between efflux capacity and other HDL-related phenotypes (Figure 1; Supplementary Table 2). LDL-related biomarkers demonstrated modest correlations with cholesterol efflux capacity, with Spearman correlation coefficients of 0.19, 0.15, and 0.18 for non-HDL cholesterol, LDL cholesterol, and apolipoprotein B levels, respectively (p < 0.0001 for each). HsCRP and lipoprotein-associated phospholipase A2 activity levels, biomarkers of inflammation, both demonstrated inverse correlations with efflux capacity (Spearman correlation coefficients −0.09 and −0.21 respectively; p = 0.003 and p < 0.0001).
Table 2.
Baseline Cholesterol Efflux Capacity According to Clinical Subgroups
| N | Median (25th – 75th%) | P Value | |
|---|---|---|---|
| Sex | |||
| Male | 752 | 14.5(12.2–16.9) | <.0001 |
| Female | 298 | 15.7(13.4–18.2) | |
| Treatment Group | |||
| Placebo | 600 | 14.8(12.4–17) | 0.11 |
| Rosuvastatin | 450 | 15.2(12.4–17.8) | |
| Current Smoker | |||
| No | 872 | 14.9(12.5–17.4) | 0.75 |
| Yes | 177 | 14.9(12.4–17.1) | |
| FH of Premature CHD* | |||
| No | 900 | 14.9(12.4–17.3) | 0.42 |
| Yes | 149 | 15.3(12.8–17.4) | |
| Metabolic Syndrome | |||
| No | 650 | 14.9(12.6–17.4) | 0.28 |
| Yes | 392 | 14.8(12.3–17) |
P values derived from Wilcoxon rank-sum test.
Family history of premature coronary disease was defined as diagnosis of the disease in a male first-degree relative before the age of 55 y or in a female first-degree relative before the age of 65 y.
Figure 1.
Spearman correlations between HDL-related biomarkers.
Distributions for each biomarker and pairwise Spearman correlation coefficients and scatterplots are displayed. HDL-C – high-density lipoprotein cholesterol; ApoAI – apolipoprotein A-I; HDLP – high-density lipoprotein particle number; Efflux capacity – cholesterol efflux capacity.
Overall, no significant association was found between baseline cholesterol efflux capacity and incident cardiovascular events, with adjusted odds ratios for tertiles two and three compared to tertile one of 1.08 (95%CI 0.70 – 167) and 0.75 (95%CI 0.47 – 1.21) respectively. Similarly, the adjusted OR/SD increment in efflux capacity was not statistically significant − 0.89 (95%CI 0.72 – 1.10; p = 0.28) (Table 3 and Figure 2). No significant heterogeneity of effect was noted according to sex, randomization to rosuvastatin versus placebo, or in participants stratified according to median hsCRP levels, or other clinical subgroups (Supplementary Table 3). The efficacy of rosuvastatin was also similar across tertiles of baseline cholesterol efflux capacity (Supplementary Table 4).
Table 3.
Association between Baseline Cholesterol Efflux Capacity and Incident Cardiovascular Events
| Tertile One | Tertile Two | Tertile Three | P-Trend | OR/SD Increment | |
|---|---|---|---|---|---|
|
| |||||
| Primary Endpoint | |||||
|
| |||||
| Range, % | 1.0 – 13.6 | 13.6 – 16.8 | 16.9 – 28.5 | ||
|
| |||||
| N (N Cases/N Controls) | 210 (105/105) | 209 (111/98) | 209 (98/111) | 628 (314/314) | |
|
| |||||
| Model One | |||||
| Odds Ratio (95%CI) | Reference | 1.12 (0.75 – 1.67) | 0.84 (0.55 – 1.27) | 0.43 | 0.94 (0.79 – 1.13) |
| P-value | 0.59 | 0.41 | 0.52 | ||
|
| |||||
| Model Two | |||||
| Odds Ratio (95%CI) | Reference | 1.08 (0.70 – 1.67) | 0.75 (0.47 – 1.21) | 0.26 | 0.89 (0.72 – 1.1) |
| P-value | 0.73 | 0.24 | 0.28 | ||
|
| |||||
| Primary Endpoint Plus Total Mortality | |||||
|
| |||||
| Range, % | 1.0 – 13.4 | 13.4 – 16.5 | 16.5 – 29.3 | ||
|
| |||||
| N (N Cases/N Controls) | 350 (182/168) | 350 (180/170) | 350 (163/187) | 1050 (525/525) | |
|
| |||||
| Model One | |||||
| Odds Ratio (95%CI) | Reference | 0.95 (0.69 – 1.30) | 0.77 (0.55 – 1.06) | 0.11 | 0.90 (0.78 – 1.03) |
| P-value | 0.75 | 0.11 | 0.12 | ||
|
| |||||
| Model Two | |||||
| Odds Ratio (95%CI) | Reference | 0.98 (0.7 – 1.39) | 0.69 (0.48 – 1.01) | 0.06 | 0.85 (0.73 – 1.00) |
| P-value | 0.92 | 0.05 | 0.06 | ||
Model One: Conditional logistic regression odds ratio adjusted for age and treatment group. Model Two: Odds ratio adjusted for age, treatment group, race, smoking status, systolic blood pressure, body-mass index, fasting glucose, baseline low-density lipoprotein cholesterol level, baseline log-transformed triglyceride level, and family history of premature coronary artery disease. OR – Odds Ratio. P-trend – P-value for linear trend across tertiles.
Figure 2.
Associations for baseline or on-statin HDL-related biomarkers and incident events.
Odds ratios for baseline (A) and on-statin (B) HDL-related biomarkers are reported per standard deviation (SD) increment based on a conditional logistic regression analysis adjusted for matched design as well as for the following risk factors and biomarkers: age, race, treatment group, smoking status, systolic blood pressure, body-mass index, fasting glucose, LDL cholesterol level, log-transformed triglycerides, and family history of premature coronary artery disease. Models analyzing on-statin biomarkers incorporated on-treatment values for LDL cholesterol and log-transformed triglycerides.
Twelve-month levels of cholesterol efflux capacity were available in 617 of 1,050 (59%) study participants. Rosuvastatin treatment compared with placebo did not lead to a significant change in cholesterol efflux capacity (Table 4). By contrast, a modest increase in HDL cholesterol, apoA-I, and HDL particle number levels was noted in individuals randomized to rosuvastatin treatment, with average percent change values of 7.7, 4.3, and 5.2% respectively (Table 4). Moderate correlations were noted between change in cholesterol efflux capacity and change in HDL cholesterol, apoA-I, and HDL particle number (Spearman r = 0.26, 0.36, 0.20 respectively; p < 0.001 for each; Supplementary Table 5). Change in efflux capacity was not related to incident cardiovascular events (adjusted odds ratio per 10% change 0.96; 95%CI 0.88 – 1.04; p = 0.33; Supplementary Table 6).
Table 4.
Impact of Randomized Rosuvastatin Therapy Versus Placebo on HDL-Related Biomarkers Levels
| HDL Phenotype | Placebo | Rosuvastatin | P Value vs. Placebo |
|---|---|---|---|
| HDL Cholesterol | |||
| N | 481 | 369 | |
| Mean at baseline, mg/dl | 51.0 | 51.6 | |
| Mean at 12mo, mg/dl | 51.8 | 54.8 | |
| Percent Change (95%CI) | 2.6 (1.2 – 2.4) | 7.7 (5.8 – 9.5) | <0.0001 |
| P Value vs. Baseline | 0.0003 | <0.0001 | |
| Apolipoprotein A-I | |||
| N | 476 | 364 | |
| Mean at baseline, mg/dl | 161.5 | 162.6 | |
| Mean at 12mo, mg/dl | 163.4 | 168.2 | |
| Percent Change (95%CI) | 1.9 (0.7 – 3.1) | 4.3 (2.7 – 6.0) | 0.02 |
| P Value vs. Baseline | 0.002 | <0.0001 | |
| HDL Particle Number | |||
| N | 428 | 312 | |
| Mean at baseline, μmol/l | 31.5 | 31.4 | |
| Mean at 12mo, μmol/l | 31.4 | 33.2 | |
| Percent Change (95%CI) | −1.6 (−3.1 – 0.1) | 5.2 (3.1–7.3) | <.0001 |
| P Value vs. Baseline | 0.04 | <0.0001 | |
| Cholesterol Efflux Capacity | |||
| N | 369 | 248 | |
| Mean at baseline, % | 14.9 | 15.2 | |
| Mean at 12mo, % | 14.1 | 13.8 | |
| Percent Change (95%CI) | −3.3 (−7.9 – 1.2) | −1.5 (−13.3 – 10.2) | 0.78 |
| P Value vs. Baseline | 0.15 | 0.80 | |
Mean percent changes from baseline to twelve-months of therapy calculated. P values versus baseline and placebo determined via one-sample and two-sample t tests respectively.
On-statin levels of cholesterol efflux capacity were available for 248 of 450 (55%) of rosuvastatin-treated study participants (Supplementary Table 7). On-statin cholesterol efflux capacity was significantly associated with risk of incident cardiovascular events for the primary endpoint (OR/SD increment 0.62; p = 0.02) but not for the expanded endpoint inclusive of total mortality (OR/SD increment 0.79; p = 0.13) – Table 5 and Figure 2. No significant heterogeneity of effect was noted across clinical or biomarker parameters (Supplementary Table 8).
Table 5.
Association between On-Statin Cholesterol Efflux Capacity and Incident Cardiovascular Events
| Tertile One | Tertile Two | Tertile Three | P-Trend | OR/SD Increment | |
|---|---|---|---|---|---|
|
| |||||
| Primary Endpoint | |||||
| Range, % | 5.85–11.87 | 11.88–15.54 | 15.55–25.31 | ||
|
| |||||
| N (N Cases/N Controls) | 60 (31/29) | 59 (29/30) | 59 (20/39) | ||
|
| |||||
| Model One | |||||
| Odds Ratio (95%CI) | Reference | 0.92 (0.45 – 1.90) | 0.36 (0.16 – 0.84) | 0.02 | 0.66 (0.47 – 0.94) |
| P-value | 0.82 | 0.02 | 0.02 | ||
|
| |||||
| Model Two | |||||
| Odds Ratio (95%CI) | Reference | 0.79 (0.35 – 1.77) | 0.31 (0.12 – 0.75) | 0.01 | 0.62 (0.42 – 0.92) |
| P-value | 0.57 | 0.01 | 0.02 | ||
|
| |||||
| Primary Endpoint + Total Mortality | |||||
|
| |||||
| Range, % | 5.17–11.5 | 11.6–15.4 | 15.5–25.3 | ||
|
| |||||
| N (N Cases/N Controls) | 83 (39/44) | 82 (39/43) | 83 (34/49) | ||
|
| |||||
| Model One | |||||
| Odds Ratio (95%CI) | Reference | 1.04 (0.56 – 1.92) | 0.64 (0.33 – 1.23) | 0.20 | 0.86 (0.66 – 1.13) |
| P-value | 0.90 | 0.18 | 0.27 | ||
|
| |||||
| Model Two | |||||
| Odds Ratio (95%CI) | Reference | 0.99 (0.5 – 1.94) | 0.51 (0.25 – 1.04) | 0.07 | 0.79 (0.59 – 1.07) |
| P-value | 0.98 | 0.07 | 0.13 | ||
Model One: Odds ratio adjusted for age and treatment group. Model Two: Odds ratio adjusted for age, treatment group, race, smoking status, systolic blood pressure, body-mass index, fasting glucose, on-statin low-density lipoprotein cholesterol level, on-statin log-transformed triglyceride level, and family history of premature coronary artery disease. OR – Odds Ratio. P-trend – P-value for linear trend across tertiles.
Among HDL-related biomarkers assessed at baseline, HDL particle number had the strongest inverse association with incident cardiovascular events, followed by HDL cholesterol and apoA-I levels (Figure 2 and Supplementary Table 9). A similar pattern emerged in the on-statin analysis; although point estimates for all four HDL-related biomarkers suggested an inverse association with incident events, HDL particle number emerged as the strongest inverse predictors of incident events (Figure 2, Table 6, Supplementary Table 10). Effect estimates for baseline and on-treatment associations were similar in a sensitivity analysis that was restricted to cases (N = 182) with an incident ‘hard’ cardiovascular event of myocardial infarction, stroke, or cardiovascular death. Importantly, the magnitude of association for these biomarkers compares favorably to that observed for Non-HDL cholesterol, a frequently used surrogate for atherogenic apolipoprotein-B containing lipoproteins. For example, in a model adjusting for age, treatment, race, systolic blood pressure, smoking, BMI, and fasting glucose, the OR/SD lower non-HDL cholesterol of 0.73 (95% CI 0.53 – 1.00; p = 0.05) was observed.
Table 6.
Baseline and On-statin Biomarkers and Incident Cardiovascular Events After Additional Adjustment for HDL-related Biomarkers
| N*(N Cases/N Controls) | OR/SD Increment95%CI | P-Value | N*(N Cases/N Controls) | OR/SD Increment95%CI | P-Value | |
|---|---|---|---|---|---|---|
| HDL Cholesterol | ||||||
| Adjusted Odds Ratio | 622(310/312) | 0.82 (0.66 – 1.02) | 0.08 | 217(83/134) | 0.63 (0.44 – 0.9) | 0.01 |
| + Apolipoprotein A-I | 619(308/311) | 1.01 (0.68 – 1.48) | 0.97 | 214(81/133) | 0.76 (0.40 – 1.42) | 0.39 |
| + HDL Particle Number | 585(299/286) | 1.25 (0.92 – 1.71) | 0.16 | 187(77/110) | 0.91 (0.57 – 1.45) | 0.69 |
| + Cholesterol Efflux Capacity | 622(310/312) | 0.83 (0.65 – 1.07) | 0.16 | 174(77/97) | 0.66 (0.44 – 1.00) | 0.05 |
| Apolipoprotein A-I | ||||||
| Adjusted Odds Ratio | 619(308/311) | 0.83 (0.67 – 1.03) | 0.08 | 214(81/133) | 0.7 (0.49 – 1.00) | 0.05 |
| + HDL Cholesterol | 619(308/311) | 0.83 (0.57 – 1.19) | 0.31 | 214(81/133) | 0.89 (0.48 – 1.64) | 0.71 |
| + HDL Particle Number | 582(297/285) | 1.23 (0.89 – 1.71) | 0.21 | 185(76/109) | 0.99 (0.61 – 1.6) | 0.95 |
| + Cholesterol Efflux Capacity | 619(308/311) | 0.81 (0.63 – 1.05) | 0.11 | 172(76/96) | 0.73 (0.49 – 1.09) | 0.13 |
| HDL Particle Number | ||||||
| Adjusted Odds Ratio | 585(299/286) | 0.69 (0.56 – 0.86) | 0.001 | 187(77/110) | 0.51 (0.33 – 0.77) | 0.001 |
| + HDL Cholesterol | 585(299/286) | 0.61 (0.46 – 0.81) | 0.001 | 187(77/110) | 0.52 (0.32 – 0.85) | 0.008 |
| + Apolipoprotein A-I | 582(297/285) | 0.62 (0.46 – 0.84) | 0.002 | 185(76/109) | 0.53 (0.32 – 0.88) | 0.01 |
| + Cholesterol Efflux Capacity | 585(299/286) | 0.65 (0.51 – 0.83) | <0.0001 | 155(74/81) | 0.53 (0.34 – 0.82) | 0.005 |
| Cholesterol Efflux Capacity | ||||||
| Adjusted Odds Ratio | 622(310/312) | 0.89 (0.72 – 1.10) | 0.28 | 174(77/97) | 0.62 (0.42 – 0.92) | 0.02 |
| + HDL Cholesterol | 622(310/312) | 0.98 (0.76 – 1.25) | 0.86 | 174(77/97) | 0.79 (0.5 – 1.22) | 0.29 |
| + Apolipoprotein A-I | 619(308/311) | 1.04 (0.80 – 1.35) | 0.76 | 172(76/96) | 0.79 (0.5 – 1.26) | 0.33 |
| + HDL Particle Number | 585(299/286) | 1.16 (0.90 – 1.50) | 0.25 | 155(74/81) | 0.86 (0.54 – 1.36) | 0.51 |
Odds ratio for the JUPITER trial primary endpoint for baseline phenotypes adjusted for age, treatment group, race, smoking status, systolic blood pressure, body-mass index, fasting glucose, low-density lipoprotein cholesterol level, log-transformed triglyceride level, and family history of premature coronary artery disease. Additional adjustment was subsequently performed for baseline values of alternate HDL-related biomarker levels. Odds ratio for the JUPITER trial primary endpoint for on-statin phenotypes adjusted for age, race, smoking status, systolic blood pressure, body-mass index, fasting glucose, on-statin low-density lipoprotein cholesterol level, on-statin log-transformed triglyceride level, and family history of premature coronary artery disease. Additional adjustment was subsequently performed for on-statin values of alternate HDL-related biomarker levels. OR – Odds Ratio. SD – standard deviation.
Numbers reflective of individuals with biomarkers values available and nonmissing covariates.
Inclusion of alternate HDL-related biomarkers in the same regression model was used to isolate independent predictors of residual risk for incident cardiovascular events (Table 6). In this analysis, the inverse association between HDL particle number levels incident CVD was the most robust and maintained significance in each of the mutually adjusted models.
Discussion
In a nested case-control study in the JUPITER trial of participants with elevated hsCRP but normal or low LDL cholesterol, we determined the relationship between four key markers of HDL metabolism – HDL cholesterol, apoA-I, HDL particle number, and cholesterol efflux capacity – and risk of incident CVD. An inverse relationship was noted for on-statin, but not baseline, cholesterol efflux capacity and incident cardiovascular events. Among all four HDL related phenotypes analyzed, HDL particle number emerged as the strongest inverse predictor of incident events, both at baseline and during potent statin therapy.
Our findings of moderate correlations between cholesterol efflux capacity and HDL cholesterol levels, apoA-I, and HDL particle number are in keeping with multiple previous reports.15–17 We extend these observations to note an inverse association between cholesterol efflux capacity and the inflammatory biomarkers hsCRP and lipoprotein-associated phospholipase A2 activity. This finding is consistent with previous mouse and human data suggesting that remodeling of the HDL proteome or site-specific oxidation of apolipoprotein A-I by myeloperoxidase in an inflammatory setting inhibits cholesterol efflux and the reverse cholesterol transport pathway.26–28
Rosuvastatin was associated with an increase in HDL cholesterol, apoA-I, and HDL particle number as previously reported,12 but had no impact on cholesterol efflux capacity, consistent with a similar result noted in a randomized trial involving pravastatin or atorvastatin.15
In contrast with most previous studies,15–18 we did not observe a significant inverse relationship between baseline cholesterol efflux capacity and incident cardiovascular events in JUPITER. One potential explanation for this directionally consistent but less striking relationship may relate to the elevated hsCRP for all JUPITER participants. The drivers of atherosclerotic events in this inflammatory context may be different than those in previous population-based studies.15–19 Interestingly, on-statin cholesterol efflux capacity was inversely associated with incident cardiovascular events. This finding could reflect the increased importance of HDL functionality in the context of a very low-achieved LDL cholesterol or during statin therapy. Although not conclusive, this association supports ongoing efforts to incorporate therapies that promote reverse cholesterol transport into statin-based regimens to improve patient outcomes.
The current study findings add to a growing body of evidence supporting HDL particle number as a stronger predictor of incident cardiovascular events than HDL cholesterol or apolipoprotein A-I levels. Similar observations were noted from less contemporary population cohorts6–8 and clinical trial-based analyses.9–12 In the Women’s Health Study, a significant inverse relationship was noted with incident coronary events,29 but not for overall cardiovascular outcomes.30 The present study extends this previous evidence base, noting that HDL particle number was the strongest HDL-related biomarker of residual risk in participants treated with potent statin therapy. Secondly, we note that HDL particle number was a better risk predictor than the best-validated metric of HDL functionality, cholesterol efflux capacity. If confirmed in additional studies, HDL particle number assessment may have value as a surrogate for clinical efficacy in the assessment of interventions targeting HDL metabolism. This NMR-based assessment affords considerable logistical advantages over cholesterol efflux capacity, a cell-based assay that requires significant resources and is difficult to standardize across studies or research groups.
Strengths of the study include detailed assessment of four HDL-related phenotypes in a contemporary clinical trial population, prospective adjudication of all trial endpoints, and the randomized allocation of potent statin therapy to achieve very low levels of circulating LDL cholesterol. A limitation of this study is that the JUPITER trial was terminated after a median follow-up of 1.9 years on the basis of demonstrated efficacy of rosuvastatin therapy in cardiovascular event reduction.20 The relatively small number of CVD events may have limited power to detect associations between HDL related phenotypes and outcomes, particularly in analyses restricted to those receiving rosuvastatin therapy. Furthermore, the generalizability of our findings may be limited beyond the population studied in the JUPITER trial.
Conclusions
On-statin but not baseline cholesterol efflux capacity was inversely associated with incident cardiovascular events in JUPITER trial participants. Among four HDL-related biomarkers, HDL particle number was the strongest inverse predictor of risk in both baseline and on-statin analyses. Whether a therapy designed to enhance cholesterol efflux capacity or increase HDL particle number can complement high-potency statin therapy in reducing cardiovascular risk remains to be determined.
Supplementary Material
Clinical Perspective.
What is new?
Moderate correlations between HDL-related biomarkers – HDL-cholesterol, apolipoprotein A-I, HDL particle number, and cholesterol efflux capacity – were noted in JUPITER trial participants, reinforcing the complementary information provided by each assessment.
No significant relationship was observed between baseline or serial changes in cholesterol efflux capacity (a commonly used measure of HDL functionality) and incident cardiovascular events; however, cholesterol efflux capacity assessed on potent statin therapy was inversely associated with incident cardiovascular events.
Among HDL-related biomarkers, HDL particle number demonstrated the strongest inverse association with incident cardiovascular events when assessed both at baseline and on potent rosuvastatin therapy.
What are the clinical implications?
Cholesterol efflux capacity was a marker of residual risk when assessed on statin therapy, consistent with a potential role in driving residual risk in a low LDL-cholesterol environment.
HDL particle number was a stronger predictor of CVD than other HDL-related biomarkers and may serve as a biomarker of residual risk or response to therapy in the future.
Whether a therapy designed to enhance cholesterol efflux capacity or increase HDL particle number can reduce cardiovascular risk remains uncertain.
Acknowledgments
Sources of Funding
The research for this article was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers R01HL117861, HL117861-S1, and HL117861-S2 to Dr. Mora and 1K01HL135342-01 to Dr. Demler. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The JUPITER trial was financially supported by AstraZeneca (Wilmington, DE). Cholesterol efflux capacity was measured by VascularStrategies (Plymouth Meeting, PA). HDL particles number was measured by LipoScience, Inc (now LabCorp; Raleigh, NC) at no additional cost to the study.
Footnotes
Twitter: @amitvkhera
Disclosures
Dr. Khera reports funding support from an ACCF/Merck Cardiovascular Research Fellowship, a John S. LaDue Memorial Fellowship in Cardiology from Harvard Medical School, and a KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst funded by the National Institutes of Health (NIH) (TR001100). Drs. Adelman and Collins are employees of Vascular Strategies. Dr. Glynn has received research grant support from AstraZeneca. Dr. Ridker has received research grant support from AstraZeneca, Novartis, Amgen, Pfizer, Kowa, and NHLBI; he is listed as a co-inventor on patents held by the Brigham and Women’s Hospital related to the use of inflammatory biomarkers in CVD (licensed to AstraZeneca and Siemens). Dr. Rader has received consulting fees from Aegerion, Alnylam, Eli Lilly, Pfizer, and Novartis; he is a cofounder of VascularStrategies and Staten Biotechnology. Dr. Mora has received research grant support from Atherotech Diagnostics and NHLBI, and has received consulting fees from Amgen, Lilly, Pfizer, Cerenis Therapeutics, and Quest Diagnostics. Dr. Mora and Dr. Otvos have a patent application on the use of an NMR spectroscopy biomarker unrelated to HDL for predicting risk of colorectal cancer.
References
- 1.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 2.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 3.HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 5.Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high-density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Harchaoui K, Arsenault BJ, Franssen R. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra A, Neeland IJ, Das SR. Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas Heart Study) Am J Cardiol. 2015;115:890–894. doi: 10.1016/j.amjcard.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 10.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R Heart Protection Study Collaborative Group. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–2478. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 11.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R Multiple Risk Factor Intervention Trial Research Group. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monette JS, Hutchins PM, Ronsein GE, Wimberger J, Irwin AD, Tang C, Sara JD, Shao B, Vaisar T, Lerman A, Heinecke JW. Patients With Coronary Endothelial Dysfunction Have Impaired Cholesterol Efflux Capacity and Reduced HDL Particle Concentration. Circ Res. 2016;119:83–90. doi: 10.1161/CIRCRESAHA.116.308357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 15.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritsch A, Scharnagl H, März W. HDL cholesterol efflux capacity and cardiovascular events. N Engl J Med. 2015;372:1870–1871. doi: 10.1056/NEJMc1503139. [DOI] [PubMed] [Google Scholar]
- 19.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mora S, Glynn RJ, Boekholdt SM, Nordestgaard BG, Kastelein JJ, Ridker PM. On-treatment non-high-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and lipid ratios in relation to residual vascular risk after treatment with potent statin therapy: JUPITER (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin) J Am Coll Cardiol. 2012;59:1521–1528. doi: 10.1016/j.jacc.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, MacFadyen JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A2 mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin Chem. 2012;58:877–886. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]
- 26.Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, Heinecke JW. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res. 2015;56:1519–1530. doi: 10.1194/jlr.M059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high-density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewing B, Parathath S, Barrett T, Chung WK, Astudillo YM, Hamada T, Ramkhelawon B, Tallant TC, Yusufishaq MS, Didonato JA, Huang Y, Buffa J, Berisha SZ, Smith JD, Hazen SL, Fisher EA. Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:779–789. doi: 10.1161/ATVBAHA.113.303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinkuolie AO, Paynter NP, Padmanabhan L, Mora S. High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes. 2014;7:55–63. doi: 10.1161/CIRCOUTCOMES.113.000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.