Abstract
While substantial advances have been made in the treatment of chronic heart failure (CHF) in the past decade, the prevalence of CHF is increasing. CHF represents a growing financial burden on healthcare systems and, despite therapeutic advances, mortality remains high. There is a need for new therapeutic targets and treatment strategies. Beta-blockers remain the drugs of choice for reducing heart rate (HR) in CHF with reduced ejection fraction (EF), but evidence suggests that their use is suboptimal; a substantial proportion of patients with heart failure do not tolerate the doses of beta-blockers used in the large clinical trials and more than half of patients have inadequately controlled HR. For these patients, clinical evidence supports the addition of ivabradine to beta-blocker therapy. Ivabradine reduces HR via a different mechanism to beta-blockers and has been recommended in European Society of Cardiology guidelines to reduce the risk of CHF hospitalisation and cardiovascular death in symptomatic patients with EF ≤35 % who are in sinus rhythm and have a resting HR ≥70 beats per minute despite treatment with an evidence-based therapy. In addition to HR-lowering, ivabradine exerts other effects on the myocardium that are synergic and complementary to beta-blockers, and may be beneficial in CHF syndrome. In this review we summarise current findings on ivabradine therapy in CHF and advance the hypothesis, with related rationale, for combining ivabradine and beta-blocker therapy from the early stages of CHF in patients with reduced EF as an alternative strategy to up-titration of beta-blockers to an optimal dose.
Keywords: Ivabradine, beta-blockers, chronic heart failure
Chronic heart failure (CHF) is a progressive disorder characterised by elevated cardiac filling pressures, reduced cardiac output and decreased oxygen delivery to the tissues.[1] Activation of the sympathetic nervous system (SNS), along with activation of the renin–angiotensin–aldosterone system (RAAS), plays a fundamental role in the pathophysiology of CHF syndrome.[2–5] Early in the course of heart failure (HF) development, the neuro-endocrine system is activated and maintains haemodynamic stability and cardiac output, but over time these compensating mechanisms lead to deterioration of cardiovascular function through several pathways.[6] Thus, inhibition of SNS by beta-blockers and RAAS by angiotensin converting enzyme inhibitors, angiotensin receptor blockers and mineralocorticoid receptor antagonists has become the current standard pharmacological treatment for CHF. Despite the widespread use of these drugs, CHF patients still remain at high risk of death and worsening HF, possibly because of suboptimal drug therapy management.
There is growing clinical evidence that more than half of patients with CHF who are on beta-blockers have inadequately controlled heart rate (HR)[7–11] and a substantial proportion of patients do not tolerate the target doses of beta-blockers used in the large clinical trials.[8]
Moreover, further up-titration of beta-blockers is not achievable in many patients.[12] This is of concern since elevated HR is associated with an increased incidence of cardiovascular events in patients with CHF.[13–16] High resting HR has been found to be a predictor for clinical outcomes[17] and total and cardiovascular mortality independent of other risk factors in patients with coronary artery disease[18] and in the general population, as well as in CHF patients.[19] There is therefore a need for further strategies to reduce HR in CHF patients. Within this framework, clinical data support the addition of ivabradine to beta-blocker therapy. This brief review aims to summarise clinical evidence supporting the combined use of beta-blockers and ivabradine in patients suffering from systolic CHF.
SNS Activation and Beta-blocker Therapy in CHF
The left ventricle ‘remodelling’ process, resulting in a progressive enlargement of the left ventricle and decline in contractility – one measure of which is a reduced ejection fraction (EF) – characterises CHF with systolic dysfunction.[20,21] Continued SNS activation over time results in myocardial injury and in systemic effects that are detrimental for the blood vessels, kidneys and muscles. Together with RAAS activation, this creates a pathophysiological cycle responsible for worsening CHF syndrome and death.[20,21] The altered haemodynamic homeostasis of CHF patients is associated with an increased HR, which carries a negative prognosis;[22–24] whereas the beneficial effect of beta-blockers has been linked to their HR-lowering effect.[14–25] However, as previously mentioned, beta-blockers are often underused in clinical practice, are seldom prescribed at the doses proven to reduce events,[26–29] and their up-titration in response to persistently elevated HR can be associated with an increased risk of adverse reactions.[12] A non beta-blockade approach to HR reduction has recently become available[30,31] following discovery of the If current that modulates the slope of spontaneous diastolic depolarisation of the sino-atrial node: namely, ivabradine.
Use of Ivabradine in Heart Failure
Ivabradine (Procolaran® , Servier) selectively and specifically inhibits the If current in the sino-atrial node, reducing HR without affecting the autonomic nervous system (see Figure 1).[32–34] The effectiveness of ivabradine in CHF has been tested in the Systolic Heart Failure Treatment with the If inhibitor Ivabradine Trial (SHIFT)[35] in which 6,558 patients with CHF on stable background therapy, including beta-blockers, and a HR ≥70 beats per minute (bpm) with sinus rhythm were randomised to ivabradine (up to 7.5 mg twice daily) or placebo. At the median follow-up of 22.9 months, the results indicated improved clinical outcomes: 18 % reduction in the primary composite endpoint of cardiovascular death or hospitalisation for worsening HF, 26 % reduction in hospitalisation for worsening HF and 26 % reduction in pump failure death in the ivabradine group. Since the majority of patients in SHIFT were taking beta-blockers, it was hypothesised that the combination of beta-blockers plus ivabradine rather than the dose of beta-blocker was relevant to these findings. A subanalysis of SHIFT appeared to confirm this hypothesis, by showing that the combination of drugs rather than the dose of beta-blockers was important in improving the primary endpoints of cardiovascular death and hospitalisation.[36] A further study concluded that the combination of beta-blockers plus ivabradine resulted in improved outcomes regardless of the individual beta-blocker prescribed.[37]
Figure 1: Mechanism of Action of Ivabradine.
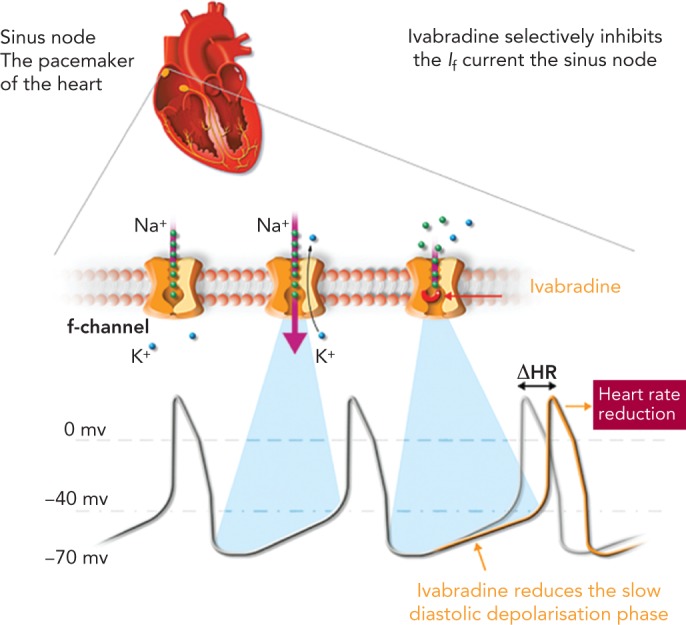
Source: http://www.shift-study.com/ivrabradine/mode-of-action/ Reproduced with the permission of Servier © 2016.
A number of clinical studies have evaluated the use of ivabradine in combination with beta-blockers (Table 1). The first large randomised controlled study of ivabradine was the MorBidity-mortality EvAlUaTion of the If Inhibitor Ivabradine in Patients with Coronary Artery Disease and Left Ventricular Dysfunction (BEAUTIFUL) trial[38] in which patients (n=10,917) with stable coronary artery disease and an EF <40 % were randomised to ivabradine 7.5 mg twice daily or placebo. Most patients (87 %) were receiving beta-blockers in addition to the study drug. After a median of 19 months, no significant difference was found between ivabradine and placebo in terms of the primary composite endpoints (cardiovascular death, hospitalisation for myocardial infarction and worsening HF). However, ivabradine reduced hospitalisation for myocardial infarction and coronary revascularisation in patients with a HR >70 bpm by 36 % (p=0.001), suggesting that the lowering of raised HR may be associated with improved outcomes. Importantly, the study also showed that the combination of a beta-blocker and ivabradine was well tolerated.
Table 1: Clinical Studies Investigating the Combination of Beta-blockers and Ivabradine.
| Study Name | Description | Efficacy Outcomes | Safety/Tolerability |
|---|---|---|---|
| CARVedilol, IVAbradine or their Combination on Exercise Capacity in Patients with Heart Failure (CARVIVA HF)[37] | HF patients, three groups: carvedilol <25 mg twice daily (n=38); ivabradine <7.5 mg twice daily (n=41); and carvedilol/ivabradine <12.5/7.5 mg twice daily (n=42). | Heart rate reduced in all three groups, but to a greater extent by the combination. Six-minute walk test myocardial venous oxygen consumption results significantly improved in the ivabradine and combination groups (both p<0.01), as did peak venous oxygen and ventilatory anaerobic threshold (p<0.01 for ivabradine and p<0.03 for combination versus carvedilol). No changes in those with carvedilol. Ivabradine and combination groups had better quality of life (p<0.01 versus baseline for ivabradine and p<0.02 for combination) versus no change with carvedilol. | Maximal dose of study treatment was more frequently tolerated in patients receiving ivabradine (36/41) than in those receiving carvedilol (18/38) or combination therapy (32/42; p<0.01 ivabradine versus carvedilol). |
| MorBidity-mortality EvAlUaTion of the If Inhibitor Ivabradine in Patients with Coronary Artery Disease and Left Ventricular Dysfunction (BEAUTIFUL)[39] | Phase III coronary artery disease and left ventricular ejection fraction <40 % (n=10,917): 5,479 patients received 5 mg ivabradine, (increased to target dose of 7.5 mg twice a day), and 5,438 received matched placebo in addition to appropriate cardiovascular medication; 87 % of patients were taking beta-blockers. Median follow-up 19 months. | Ivabradine did not affect the primary composite endpoint (HR 1.00; 95 % CI [0.91–1.1]; p=0.94). In a subgroup of patients with a heart rate of ≥70 bpm, ivabradine did not affect the primary composite outcome (HR 0.91; 95 % CI [0.81–1.04]; p=0.17), cardiovascular death or admission to hospital for new-onset or worsening HF. It did reduce hospitalisation for fatal and non-fatal MI (HR 0.64; 95 % CI [0.49–0.84]; p=0.001) and coronary revascularisation (HR 0.70; 95 % CI [0.52–0.93]; p=0.016). | Serious adverse events were similar: 22.5 % patients in the ivabradine group verus 22.8 % of controls (p=0.70). |
| Systolic Heart Failure Treatment with the If inhibitor Ivabradine Trial (SHIFT)[40] | Phase III HF (n=6,558) 90 % taking beta-blockers, randomised to ivabradine (n=3,268) <7.5 mg twice daily or placebo (n=3,290). Data available for 3,241 patients in the ivabradine group and 3,264 patients allocated to placebo. Median follow-up 22.9 months (interquartile range: 18–28 months). | Primary endpoint event (composite of cardiovascular death or hospital admission for worsening HF (HR 0.82; 95 % CI [0.75–0.90]; p<0.0001): 24 % ivabradine versus 29 % placebo group. Events driven mainly by hospital admissions for worsening HF (21 % placebo versus 16 % ivabradine; HR 0.74; 95 % CI [0.66–0.83]; p<0.0001) and deaths due to HF (3 % ivabradine versus 5 % placebo; HR 0.74; 95% CI [0.58–0.94]; p=0.014). | Serious adverse events: 3,388 ivabradine patients versus 3847 placebo patients (p=0.025). Symptomatic bradycardia: 150 (5 %) ivabradine patients versus 32 (1 %) placebo patients (p<0.0001). Visual side-effects (phosphenes): 89 (3 %) ivabradine patients versus 17 (1 %) placebo patients (p<0.0001) |
| PractIcal Daily EffectiveNess and TolEraNce of Procoralan® in Chronic SystolIc Heart Failure in GermanY (INTENSIFY)[48] | HF (n=1,956) prospective, open-label multicentre study. Initial mean European quality of life-5 dimensions (EQ-5D) index score: 0.64±0.28. | After 4 months of treatment with ivabradine, HR was reduced to 67±8.9 bpm; patients presenting with signs of decompensation decreased to 5.4 %; brain natriuretic peptide levels >400 pg/mL dropped to 26.7 %; NYHA classification shifted towards lower grading (24.0 % and 60.5 % in NYHA I and II, respectively). EQ-5D index improved to 0.79±0.21. | |
| Bagryi et al.[56] | Systolic HF (n=69) prospective, open-label, single-centre study, 5-month follow-up. | Patients receiving ivabradine had lower resting heart rate at 5 months (61.6±3.1 versus 70.2±4.4 bpm; p<0.05). Adding ivabradine to carvedilol was associated with increases in 6-minute walk test and ejection fraction (all p<0.05). Patients receiving ivabradine and carvedilol had lower heart rates and better exercise capacity than those on carvedilol alone. | Treatment tolerability was satisfactory. |
| Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalized with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF)[57] | Systolic HF (n=71): beta-blockers + ivabradine (33) versus beta-blockers alone (38), starting 24 hours after hospital admission for acute HF; 4-month follow-up. | Heart rate 28 days (64.3±7.5 versus 70.3±9.3 bpm; p=0.01) and 4 months (60.6±7.5 versus 67.8±8 bpm; p=0.004) after discharge were significantly lower in the combination therapy group. Ejection fraction, brain natriuretic peptide levels and severity of symptoms significantly improved in the combination therapy group. No differences were found in morbidity and mortality. | No severe side effects attributable to early administration of ivabradine (asymptomatic bradycardia <60 bpm in seven patients in the ivabradine + beta-blocker group versus six patients in the beta-blocker alone group). |
bmp = beats per minute; HF = heart failure; HR = hazard ratio; NYHA = New York Heart Association.
In SHIFT,[35] patients with symptomatic CHF had a higher baseline HR and a greater HR reduction due to ivabradine than in the BEAUTIFUL trial. In this study, patients (n=6,558) with CHF on stable background therapy were randomised to ivabradine (up to 7.5 mg twice daily) or placebo. At the median follow-up of 22.9 months, data were available for 3,241 patients in the ivabradine group and 3,264 patients in the placebo group. Use of ivabradine was associated with an 18 % reduction in the primary composite endpoint of cardiovascular death or hospitalisation for worsening HF: 24 % of patients in the ivabradine group and 29 % of those taking placebo had a primary endpoint event (hazard ratio (HR) 0.82; 95 % CI [0.75–0.90]; p<0.0001; Figure 2). The effects were driven mainly by hospital admissions for worsening HF (21 % placebo versus 16 % ivabradine; HR 0.74, 95% CI [0.66–0.83]; p<0.0001) and deaths due to HF (5 % versus 3 %; HR 0.74; 95 % CI [0.58–0.94]; p=0.014). The risk of cardiovascular outcomes increased with HR, and every 5-bpm increase in baseline HR was associated with a 16 % increase in the risk of primary outcome in the placebo arm.[35] This is in agreement with a meta-analysis by McAlister et al. indicating that, in CHF patients, a reduction of 5 bpm with beta-blocker treatment was associated with an 18 % reduction in the risk of death.[39] An analysis of the SHIFT data found that in the ivabradine group there was a direct association between HR achieved at 28 days and subsequent cardiac outcomes. Patients receiving treatment who reached a target HR below 60 bpm at 28 days had the lowest event rate compared with patients with higher HRs (event rate 17.4 %; 95 % CI [15.3–19.6]), suggesting that resting HR is a powerful predictor of outcomes in CHF.[13]
Figure 2: Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT) Primary Composite Endpoint of Death or Hospitalisation for Worsening Heart Failure[36].

CI = confidence interval; HR = hazard ratio. Source: Swedberg et al.[36] Reproduced with the permission of Elsevier © 2010.
Since the majority of patients in SHIFT (90 %) were taking beta-blockers, researchers hypothesised that the combination of beta-blockers plus ivabradine was important. A substudy of SHIFT to assess the impact of background beta-blocker dose on response to ivabradine found that the combination rather than the dose was important, and that the primary endpoint and HF hospitalisations were significantly reduced by ivabradine in all subgroups with <50 % of target beta-blocker dose, including patients not taking beta-blockers (p=0.012).[36] A further study concluded that the combination of beta-blockers plus ivabradine resulted in improved outcomes regardless of the individual beta-blocker prescribed.[37]
Pathophysiological Mechanisms Underlying the Combined Use of Beta-blockers and Ivabradine
The rationale for combining beta-blockers and ivabradine is that their actions at heart level are synergic and not limited to sinus node rate; whereas beta-blockers have several other target points that are beneficial in CHF syndrome. The randomised CARVedilol, IVAbradine or their Combination on Exercise Capacity in Patients with Heart Failure (CARVIVA-HF) study[40] found that ivabradine alone or in combination with carvedilol was more effective than carvedilol alone in improving exercise tolerance and quality of life (QoL) in CHF patients. A subanalysis of SHIFT[41] also found that HR reduction with ivabradine was associated with improved QoL. This finding was consolidated by data from the prospective, open-label multicentre PractIcal Daily EffectiveNess and TolEraNce of Procoralan® in Chronic SystolIc Heart Failure in GermanY (INTENSIFY) study.[42]
The beneficial effects of beta-blockers may only in part be related to HR reduction; their protective action against the deleterious effects of excessive sympathetic activity on the heart and other organs and their humoral mechanisms may significantly contribute to the benefits of this class of drugs. Similarly, it has been suggested that HR-independent mechanisms could contribute to the additional beneficial effects associated with ivabradine treatment.[43]
Haemodynamic Mechanisms
HR reduction can increase the duration of diastole[44,45] and therefore improve myocardial perfusion. Beta-blockers reduce HR and prolong diastolic duration, but they also impair isovolumic ventricular relaxation, offsetting part of this benefit in terms of the diastolic pressure–time integral.[46] Beta-blockers also increase alpha-adrenergic coronary vasoconstriction. Ivabradine protects isovolumic ventricular relaxation and does not offset the benefit in terms of coronary blood flow[46] because ivabradine does not increase alpha-adrenergic coronary vasoconstriction, as is typically seen with beta-blockers.[47] This explains why, for the same level of HR reduction, the increase in diastolic time and the perfusion duration and volume are greater with ivabradine than with beta-blockers.[4] Treatment with beta-blockers plus ivabradine therefore improves myocardial perfusion by these mechanisms, both at rest and during exercise.[45]
In addition to this, ivabradine administration has been shown to significantly increase stroke volume in patients with severe CHF.[48] The increase in stroke volume caused by ivabradine is of clinical relevance as beta-blockers reduce stroke volume during initiation, the first months of treatment and up-titration. This effect of beta-blockade could be compensated for by prescribing ivabradine with lower initial doses of beta-blockers. Ivabradine also reduces left ventricular (LV) end-diastolic pressure, unlike beta-blockers, with this effect being still present when ivabradine is co-prescribed with a beta-blocker, resulting in increased stroke volume and maintenance of cardiac output.[48,49]
Left Ventricular Structure and Function
In addition to the haemodynamic mechanisms reported above, ivabradine slows the progressive modification of LV structure in the 2–3 months after the initiation of therapy, which contributes to the improvement in cardiac function.[49–51] Ivabradine increases vascular compliance, thus reducing LV load, and this effect is related to beneficial outcomes in ivabradine-treated patients. Like beta-blockers, treatment with ivabradine significantly lowers RAAS activation compared with placebo. Lower RAAS activation results in improved renal and vascular pressures, and in a decrease in cardiac wall stress, thereby preventing worsening cardiac fibrosis and cardiac remodelling.
Cardiac remodelling plays a crucial role in the pathophysiology of CHF and also affects the prognosis of this patient population.[52] Ivabradine has been reported to induce reverse remodelling in patients with New York Heart Association Functional class II and IV HF, including modifications of LV structure (i.e. decreased in LV end-systolic and end-diastolic volumes), that have been observed after just 3 months of therapy,[50,51] being accompanied by a 2.7 % increase in LVEF. A reduction in cardiac collagen[53,54] and fibrosis have been also reported in animal models of HF. Another SHIFT substudy found that ivabradine reverses cardiac remodelling in patients with CHF and LV systolic dysfunction, and this effect was independent of beta-blocker use.[51] Taken together, these results support the role of ivabradine in protecting LV structure and function.[34]
Safety Issues Associated with Combined Ivabradine and Beta-blocker Therapy
Studies to date indicate that ivabradine is well tolerated in combination with beta-blockers. In the SHIFT[35] there was a lower incidence of serious adverse events in the ivabradine versus placebo group.
In total, 21 % of patients on ivabradine discontinued treatment compared to 19 % of patients on placebo (p=0.017). Bradycardia was more frequent with ivabradine than with placebo (5 % versus 1 %, p<0.0001 respectively). Visual luminous phenomena (phosphenes) were reported in 3 % of patients in the ivabradine group.[35] Adverse events were not influenced by beta-blocker dosage.[37]
In the BEAUTIFUL trial[38] no additional safety concerns were identified in patients taking beta-blockers plus ivabradine. The incidence of serious adverse events in the ivabradine and placebo groups was similar (22.5 % versus 22.8 %; p=0.70). There was, however, a higher incidence of bradycardia (including asymptomatic bradycardia) in the ivabradine group than in the placebo group (13 % versus 2 %).
Current Ivabradine Use
Currently, ivabradine can be given early in hospitalisation, and can be initiated at the same time as beta-blockers.[55,56] It is recommended that treatment commence with the administration of 5 mg ivabradine twice daily. After 2 weeks, the resting HR should be checked. If it exceeds 60 bpm, the dose should be raised to 7.5 mg twice daily. At a resting HR of 50–60 bpm, the dose can be maintained at 5 mg twice daily, and if HR is below 50 bpm it should be reduced to 2.5 mg twice daily.[6] HR should be regularly checked throughout treatment and the dose adjusted accordingly. If HR remains below 50 bpm despite dose reduction, treatment must be discontinued. In patients aged 75 years or more, a lower starting dose should be considered (2.5 mg twice daily, i.e. half a 5 mg tablet twice daily) before up-titration if necessary. Importantly, no dose adjustment is needed in patients with hepatic or renal impairment.[57]
Practical guidance on the use of ivabradine in CHF is provided in an addendum to the 2016 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure (see Table 2).[58]
Table 2: European Society of Cardiology Practical Guidance on the Use of Ivabradine in Patients with Heart Failure with Reduced Ejection Fraction[58].
| WHY? |
| To reduce the risk of HF hospitalization and cardiovascular death. |
| IN WHOM AND WHEN? |
| Indications: |
|
| Contra-indications: |
|
| Cautions/seek specialist advice: |
|
| WHAT DOSE? |
| Ivabradine: starting dose 5 mg b.i.d., target dose 7.5 mg b.i.d. |
| WHERE? |
|
| HOW TO USE? |
|
| PROBLEM SOLVING |
|
| ADVICE TO PATIENT |
|
ACE = angiotensin-converting enzyme; AF = atrial fibrillation; ARB = angiotensin receptor blocker; b.i.d. = twice daily; bpm = beats per minute; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HIV = human immunodeficiency virus; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; TIA = transient ischemic attack. Source: Reproduced from Ponikowski et al.[58] with the permission of Oxford University Press (UK) © 2016 European Society of Cardiology, www.escardio.org
Discussion
Current ESC guidelines[59] on CHF recommend the use of ivabradine in symptomatic patients with LVEF ≤35 % who are in sinus rhythm and have a resting heart rate ≥70 bpm despite treatment with an evidence-based dose of beta-blocker (or maximum tolerated dose below that or those who are unable to tolerate or have contraindications to a beta-blocker), angiotensin converting enzyme inhibitor, angiotensin receptor blocker and mineralocorticoid receptor antagonist. The US Food and Drug Administration has recommended similar indications for ivabradine.[60]
It should be recalled that in SHIFT only around a quarter of patients achieved the recommended ESC target dose, and around half achieved at least 50 % of the target dose.[35] This reflects current clinical practice.[35,61] SHIFT has provided evidence for additional HR lowering with ivabradine for patients in sinus rhythm who are receiving beta-blockers. Ivabradine is easier to use than beta-blockers and is better tolerated. The benefit provided by ivabradine was similar in the small subgroup of SHIFT that did not receive a beta-blocker to that observed in the overall population,[36] raising the possibility that combining ivabradine with suboptimal doses of beta-blockers may be a better strategy than uptitrating beta-blockers to an optimal dose.
One study found that the use of beta-blockers and resting HR were independent predictors of prognosis but beta-blocker dose was not.[62] Thus, it may be hypothesised that achieving a HR within the target range may be a more appropriate therapeutic goal than optimising beta-blocker dose in patients with CHF. In SHIFT, patients with the lowest risk reached a HR <60 bpm; therefore it might be reasonable, at present, to recommend this target in daily practice. There is, however, no direct evidence for this. Further trials are clearly needed before first-line use of ivabradine is recommended in patients other than those for whom beta-blockers are contraindicated.
Conclusion
In this review we have reported consistent data suggesting it is possible to safety extend the use of ivabradine plus beta-blocker therapy in patients with CHF, even in less advanced stages of the disease. This combined therapy could favour lower beta-blocker doses, facilitate up-titration for the achievement of target HR, and avoid the possible dose-dependent adverse events related to their use.
Acknowledgments
Medical Media Communications (Scientific) Ltd provided medical writing and editing support to the authors.
References
- 1.Schrier RW, Abdallah JG, Weinberger HH, Abraham WT. Therapy of heart failure. Kidney Int. 2000;57:1418–1425. doi: 10.1046/j.1523-1755.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin–angiotensin–aldosterone system to clinical state in congestive heart failure. Circulation. 1981;63:645–651. doi: 10.1161/01.cir.63.3.645. [DOI] [PubMed] [Google Scholar]
- 3.Kalidindi SR, Tang WH, Francis GS. Drug insight: aldosterone-receptor antagonists in heart failure – the journey continues. Nat Clin Pract Cardiovasc Med. 2007;4:368–378. doi: 10.1038/ncpcardio0914. [DOI] [PubMed] [Google Scholar]
- 4.Mizuno Y, Yoshimura M, Yasue H et al. Aldosterone production is activated in failing ventricle in humans. Circulation. 2001;103:72–77. doi: 10.1161/01.cir.103.1.72. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 6.Zannad F, Gattis Stough W, Rossignol P et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J. 2012;33:2782–2795. doi: 10.1093/eurheartj/ehs257. 10.1093/eurheartj/ehs257 [DOI] [PubMed] [Google Scholar]
- 7.Franke J, Wolter JS, Meme L et al. Optimization of pharmacotherapy in chronic heart failure: is heart rate adequately addressed? Clin Res Cardiol. 2013;102:23–31. doi: 10.1007/s00392-012-0489-2. 10.1007/s00392-012-0489-2. [DOI] [PubMed] [Google Scholar]
- 8.Russell SJ, Oliver M, Edmunds L et al. Optimized beta-blocker therapy in heart failure: is there space for additional heart rate control? Br J Cardiol. 2012;19:21–23. 10.5837/bjc.2012.001 [Google Scholar]
- 9.Maggioni AP, Anker SD, Dahlstrom U et al. Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry, Eur J Heart Fail. 2013;15:1173–1184. doi: 10.1093/eurjhf/hft134. 10.1093/eurjhf/hft134 [DOI] [PubMed] [Google Scholar]
- 10.Zugck C, Martinka P, Stöeckl G et al. Heart rate control in chronic systolic heart failure patients in Germany: results of a nationwide survey. Dtsch Med Wochenschr. 2015;140:e48–55. doi: 10.1055/s-0041-100608. 10.1055/s-0041-100608 [DOI] [PubMed] [Google Scholar]
- 11.Cowie MR, Davidson L. Clinical perspective: the importance of heart rate reduction in heart failure. Int J Clin Pract. 2012;66:728–730. doi: 10.1111/j.1742-1241.2012.02968.x. 10.1111/j.1742-1241.2012.02968.x [DOI] [PubMed] [Google Scholar]
- 12.Erdmann E. Safety and tolerability of beta-blockers: prejudices and reality. Eur Heart J. 2009;11:21–25. (Suppl):A. [PubMed] [Google Scholar]
- 13.Bohm M, Swedberg K, Komajda M et al. SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. 10.1016/S0140-6736(10)61259-7 [DOI] [PubMed] [Google Scholar]
- 14.Lechat P, Hulot JS, Escolano S et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428–1433. doi: 10.1161/01.cir.103.10.1428. 10.1161/01.CIR.103.10.142. [DOI] [PubMed] [Google Scholar]
- 15.Gullestad L, Wikstrand J, Deedwania P et al. MERIT-HF Study. What resting heart rate should one aim for when treating patients with heart failure with a beta-blocker? Experiences from the Metoprolol Controlled Release/Extended Release Randomized Intervention Trial in Chronic Heart Failure (MERIT-HF). J Am Coll Cardiol. 2005;45:252–259. doi: 10.1016/j.jacc.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Castagno D, Skali H, Takeuchi M et al. CHARM Investigators. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. J Am Coll Cardiol. 2012;59:1785–1795. doi: 10.1016/j.jacc.2011.12.044. 10.1016/j.jacc.2011.12.044 [DOI] [PubMed] [Google Scholar]
- 17.Kolloch R, Legler UF, Champion A et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J. 2008;29:1327–1334. doi: 10.1093/eurheartj/ehn123. 10.1093/eurheartj/ehn123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz A, Bourassa MG, Guertin MC et al. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Kannel C, Paffenbarger RS Jr et al. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362:228–238. doi: 10.1056/NEJMcp0909392. 10.1056/NEJMcp0909392 [DOI] [PubMed] [Google Scholar]
- 21.Shah AM, Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378:704–712. doi: 10.1016/S0140-6736(11)60894-5. 10.1016/S0140-6736(11)60894-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox K, Borer JS, Camm AJ et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 23.Pocock SJ, Wang D, Pfeffer MA et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 24.Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J. 1999;1:H64–9. [Google Scholar]
- 25.Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57 doi: 10.1016/0002-9149(86)90888-x. 43F–49F. [DOI] [PubMed] [Google Scholar]
- 26.Vitale C, Iellamo F, Volterrani M et al. Heart rate control in an unselected consecutive population of outpatients with stable coronary artery disease: analysis of the CARDIf Study Cohort. Angiology. 2010;61:763–767. doi: 10.1177/0003319710369102. 10.1177/0003319710369102 [DOI] [PubMed] [Google Scholar]
- 27.Butler J, Arbogast PG, BeLue R et al. Outpatient adherence to beta-blocker therapy after acute myocardial infarction. J Am Coll Cardiol. 2002;40:1589–1595. doi: 10.1016/s0735-1097(02)02379-3. [DOI] [PubMed] [Google Scholar]
- 28.de Groote P, Isnard R, Assyag P et al. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the Impact Reco programme. Eur J Heart Fail. 2007;9:1205–1211. doi: 10.1016/j.ejheart.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Komajda M, Follath F, Swedberg K et al. Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure Survey programme-asurvey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J. 2003;24:464–474. doi: 10.1016/s0195-668x(02)00700-5. [DOI] [PubMed] [Google Scholar]
- 30.DiFrancesco D. Cardiac pacemaker I(f) current and its inhibition by heart rate-reducing agents. Curr Med Res Opin. 2005;21:1115–1122. doi: 10.1185/030079905x50543. [DOI] [PubMed] [Google Scholar]
- 31.Thollon C, Cambarrat C, Vian J et al. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deedwania P. Selective and specific inhibition of If with ivabradine for the treatment of coronary artery disease or heart failure. Drugs. 2013;73:1569–1586. doi: 10.1007/s40265-013-0117-0. 10.1007/s40265-013-0117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canet E, Lerebours G, Vilaine JP. Innovation in coronary artery disease and heart failure: clinical benefits of pure heart rate reduction with ivabradine. Ann N Y Acad Sci. 2011;1222:90–99. doi: 10.1111/j.1749-6632.2011.05960.x. 10.1111/j.1749-6632.2011.05960.x [DOI] [PubMed] [Google Scholar]
- 34.Pereira-Barretto AC. Cardiac and hemodynamic benefits: mode of action of ivabradine in heart failure. Adv Ther. 2015;32:906–919. doi: 10.1007/s12325-015-0257-6. 10.1007/s12325-015-0257-6 [DOI] [PubMed] [Google Scholar]
- 35.Swedberg K, Komajda M, Bohm M et al. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. 10.1016/S0140-6736(10)61198-1 [DOI] [PubMed] [Google Scholar]
- 36.Swedberg K, Komajda M, Bohm M et al. SHIFT Investigators. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. doi: 10.1016/j.jacc.2012.01.020. 10.1016/j.jacc.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 37.Bocchi EA, Bohm M, Borer JS et al. SHIFT Investigators. Effect of combining ivabradine and beta-blockers: focus on the use of carvedilol in the SHIFT population. Cardiology. 2015;131:218–224. doi: 10.1159/000380812. 10.1159/000380812 [DOI] [PubMed] [Google Scholar]
- 38.Fox K, Ford I, Steg PG et al. BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. 10.1016/S0140-6736(08)61170-8 [DOI] [PubMed] [Google Scholar]
- 39.McAlister FA, Wiebe N, Ezekowitz JA et al. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 40.Volterrani M, Cice G, Caminiti G et al. Effect of Carvedilol, Ivabradine or their combination on exercise capacity in patients with Heart Failure (the CARVIVA HF trial). Int J Cardiol. 2011;151:218–224. doi: 10.1016/j.ijcard.2011.06.098. 10.1016/j.ijcard.2011.06.098 [DOI] [PubMed] [Google Scholar]
- 41.Ekman I, Chassany O, Komajda M et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32:2395–2404. doi: 10.1093/eurheartj/ehr343. 10.1093/eurheartj/ehr343 [DOI] [PubMed] [Google Scholar]
- 42.Zugck C, Martinka P, Stockl G. Ivabradine treatment in a chronic heart failure patient cohort: symptom reduction and improvement in quality of life in clinical practice. Adv Ther. 2014;31:961–974. doi: 10.1007/s12325-014-0147-3. 10.1007/s12325-014-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becher PM, Lindner D, Miteva K et al. Role of heart rate reduction in the prevention of experimental heart failure: comparison between If-channel blockade and beta-receptor blockade. Hypertension. 2012;59:949–957. doi: 10.1161/HYPERTENSIONAHA.111.183913. 10.1161/HYPERTENSIONAHA.111.183913 [DOI] [PubMed] [Google Scholar]
- 44.Meiler SE, Boudoulas H, Unverferth DV, Leier CV. Diastolic time in congestive heart failure. Am Heart J. 1987;114:1192–1198. doi: 10.1016/0002-8703(87)90196-7. [DOI] [PubMed] [Google Scholar]
- 45.Colin P, Ghaleh B, Monnet X et al. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol. 2003;284:H676–82. doi: 10.1152/ajpheart.00564.2002. [DOI] [PubMed] [Google Scholar]
- 46.Colin P, Ghaleh B, Hittinger L et al. Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol. 2002;282:H672–9. doi: 10.1152/ajpheart.00547.2001. [DOI] [PubMed] [Google Scholar]
- 47.Custodis F, Schirmer SH, Baumhakel M et al. Vascular pathophysiology in response to increased heart rate . J Am Coll Cardiol. 2010;56:1973–1978. doi: 10.1016/j.jacc.2010.09.014. 10.1016/j.jacc.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 48.De Ferrari GM, Mazzuero A, Agnesina L et al. Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail. 2008;10:550–555. doi: 10.1016/j.ejheart.2008.04.005. 10.1016/j.ejheart.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 49.Reil JC, Tardif JC, Ford I et al. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. J Am Coll Cardiol. 2013;62:1977–1985. doi: 10.1016/j.jacc.2013.07.027. 10.1016/j.jacc.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 50.Sarullo FM, Fazio G, Puccio D et al. Impact of “off-label” use of ivabradine on exercise capacity, gas exchange, functional class, quality of life, and neurohormonal modulation in patients with ischemic chronic heart failure. J Cardiovasc Pharmacol Ther. 2010;15:349–355. doi: 10.1177/1074248410370326. 10.1177/1074248410370326 [DOI] [PubMed] [Google Scholar]
- 51.Tardif JC, O’Meara E, Komajda M et al. SHIFT Investigators. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–2515. doi: 10.1093/eurheartj/ehr311. 10.1093/eurheartj/ehr311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. On behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 53.Mulder P, Barbier S, Chagraoui A et al. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–1679. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- 54.Milliez P, Messaoudi S, Nehme J et al. Beneficial effects of delayed ivabradine treatment on cardiac anatomical and electrical remodeling in rat severe chronic heart failure. Am J Physiol Heart Circ Physiol. 2009;296:H435–41. doi: 10.1152/ajpheart.00591.2008. 10.1152/ajpheart.00591.2008 [DOI] [PubMed] [Google Scholar]
- 55.Bagriy AE, Schukina EV, Samoilova OV et al. Addition of ivabradine to beta-blocker improves exercise capacity in systolic heart failure patients in a prospective, open-label study. Adv Ther. 2015;32:108–119. doi: 10.1007/s12325-015-0185-5. 10.1007/s12325-015-0185-5 [DOI] [PubMed] [Google Scholar]
- 56.Hidalgo FJ, Anguita M, Castillo JC et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalized with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): A randomized study. Int J Cardiol. 2016;217:7–11. doi: 10.1016/j.ijcard.2016.04.136. 10.1016/j.ijcard.2016.04.136 [DOI] [PubMed] [Google Scholar]
- 57.European Medicines Agency. Procolaran: Summary of Product Characteristics. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000597/WC500043590.pdf. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000597/WC500043590.pdf (accessed 26 June 2016).
- 58.Ponikowski P, Voors AA, Anker SD ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure – Web Addenda. Eur Heart J. 2016. 2016. pp. 1–17.10.1093/eurheartj/ehw128http://www.escardio.org/static_file/Escardio/Guidelines/ehw128_Addenda.pdf (accessed 26 June 2016)
- 59.Ponikowski P, Voors AA, Anker SD ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016. 2016 pii: ehw128; [DOI] [PubMed]
- 60.US Food and Drug Administration. Corlanor. Highlights of Prescribing Information. 2015. http://www.accessdata.fda. gov/drugsatfda_docs/label/2015/206143Orig1s000lbl.pdf. http://www.accessdata.fda. gov/drugsatfda_docs/label/2015/206143Orig1s000lbl.pdf (accessed 26 June 2016)
- 61.Kalra PR, Morley C, Barnes S et al. Discontinuation of beta-blockers in cardiovascular disease: UK primary care cohort study. Int J Cardiol. 2013;167:2695–2699. doi: 10.1016/j.ijcard.2012.06.116. 10.1016/j.ijcard.2012.06.116 [DOI] [PubMed] [Google Scholar]
- 62.Cullington D, Goode KM, Clark AL, Clelend JG. Heart rate achieved or beta-blocker dose in patients with chronic heart failure: which is the better target? Eur J Heart Fail. 2012;14:737–747. doi: 10.1093/eurjhf/hfs060. 10.1093/eurjhf/hfs060 [DOI] [PubMed] [Google Scholar]


