Abstract
Osteoarthritis (OA), characterized by progressive destruction of articular cartilage, is the most common form of human arthritis. Here, we evaluated the potential chondroprotective and anti-inflammatory effects of Wogonin, a naturally occurring flavonoid, in IL-1β-stimulated human OA chondrocytes and cartilage explants. Wogonin completely suppressed the expression and production of inflammatory mediators including IL-6, COX-2, PGE2, iNOS and NO in IL-1β-stimulated OA chondrocytes. Further, Wogonin exhibits potent chondroprotective potential by switching the signaling axis of matrix degradation from catabolic towards anabolic ends and inhibited the expression, production and activities of matrix degrading proteases including MMP-13, MMP-3, MMP-9, and ADAMTS-4 in OA chondrocytes, and blocked the release of s-GAG and COL2A1 in IL-1β-stimulated OA cartilage explants. Wogonin also elevated the expression of cartilage anabolic factors COL2A1 and ACAN in chondrocytes and inhibited the IL-1β-mediated depletion of COL2A1 and proteoglycan content in the matrix of cartilage explants. The suppressive effect of Wogonin was not mediated through the inhibition of MAPKs or NF-κB activation. Instead, Wogonin induced mild oxidative stress through the generation of ROS and depletion of cellular GSH, thereby modulating the cellular redox leading to the induction of Nrf2/ARE pathways through activation of ROS/ERK/Nrf2/HO-1-SOD2-NQO1-GCLC signaling axis in OA chondrocytes. Molecular docking studies revealed that Wogonin can disrupt KEAP-1/Nrf-2 interaction by directly blocking the binding site of Nrf-2 in the KEAP-1 protein. Genetic ablation of Nrf2 using specific siRNA, significantly abrogated the anti-inflammatory and chondroprotective potential of Wogonin in IL-1β-stimulated OA chondrocytes. Our data indicates that Wogonin exerts chondroprotective effects through the suppression of molecular events involved in oxidative stress, inflammation and matrix degradation in OA chondrocytes and cartilage explants. The study provides novel insights into the development of Nrf2 as a promising candidate and Wogonin as a therapeutic agent for the management of OA.
Keywords: Osteoarthritis, Nrf2, Wogonin, ERK1/2, Redox
Introduction
Osteoarthritis (OA), the most prevalent form of arthritis, is a multifactorial disease and the major contributory factors are aging, age related mitochondrial dysfunction, oxidative stress and associated changes in inflammatory and catabolic gene expression in articular chondrocytes [1, 2]. Accumulating evidence indicates that inflammation plays a critical role in the pathogenesis of OA [3]. Interleukin-1β (IL-1β), a pro-inflammatory cytokine, plays a pivotal role in OA pathogenesis and triggers the production and secretion of inflammatory and catabolic factors associated with OA pathogenesis [4]. The cartilage degradation in OA results from disruption of metabolic homeostasis due to excess production of matrix degrading proteases including matrix metalloproteinases (MMPs) and aggrecanases [5]. Management of OA is difficult as current treatment options for preventing or slowing the development of OA are mostly ineffective and patients undergo the costly surgical intervention for joint arthroplasty. Thus, efforts to elucidate new molecules and mechanism that can be utilized in the management of OA are of utmost importance. The phytochemicals isolated from medicinal plants rich in flavonoids and polyphenols have been suggested as alternative and complementary therapies because of their minimal side effects, cost effective and local availability [6].
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), is a redox sensitive transcription factor that regulates the antioxidant defense system through a battery of cytoprotective genes in response to electrophilic and oxidative stresses [7]. Nrf2 regulate the expression of antioxidant, phase 2 detoxifying genes (such as superoxide dismutase (SOD-2) and NAD(P)H: quinone oxidoreductase (NQO1)) and stress response genes (for example, heme oxygenase-1(HO-1)) through consensus cis-elements called antioxidant-response elements (AREs). Activation of Nrf2/ARE has been shown to confer protection against various inflammatory stimuli, and oxidative stress associated diseases [8]. A recent study using histone deacetylase inhibition showed that activation of Nrf2 protect against OA [9]. Further, in an experimental model of OA, Nrf2-knockout mice displayed more severe cartilage damage than wild-type mice suggesting that Nrf2 activation has chondroprotective potential [9]. Some phyotchemicals rich in polyphenols and flavonoids have been reported to protect against cellular and tissue injury through activation of Nrf2 and induction of HO-1 and other cytoprotective enzymes [10–12]. Based on these reports, we hypothesized that Nrf2 activation using natural flavonoid might protect against OA related inflammatory and catabolic changes in human OA chondrocytes and cartilage explants under pathological condition in vitro.
Wogonin (5, 7-dihydroxy-8-methoxyflavone), a naturally occurring flavonoid derived from the root extract of Scutellaria baicalensis, has been traditionally used in the treatment of inflammatory diseases [13]. The anti-inflammatory effect of Wogonin is known to be mediated through suppression of iNOS and COX-2 expression [13, 14]. Additionally, Wogonin has been shown to inhibit the inflammation associated colorectal carcinogenesis through suppression of NF-κB and activation of Nrf2 signaling pathways in HCT116 cells and THP-1 cells [15]. Further, a recent study showed that Wogonin inhibited the expression of MMP-3 in rabbit articular chondrocytes [16]. However, the chondroprotective effects of Wogonin have not been explored in detail and the data concerning the mechanisms underlying the potential chondroprotective actions of Wogonin is lacking. Therefore, the present study was undertaken to examine the anti-inflammatory and chondroprotective effect of Wogonin using OA chondrocytes and investigate its mechanisms. Our results demonstrate that Wogonin inhibited the IL-1β induced inflammatory and catabolic responses through the induction of Nrf2/ARE pathways via activation of ROS/ERK/Nrf2/HO-1-SOD2-NQO1 signaling axis in human OA chondrocytes.
Material and Methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and other reagents for cell culture were purchased from HyClone Laboratories (Logan, UT, USA). Ham’s F12 Medium was purchased from Lonza (Walkersville, MD, USA). Pronase and Collagenase were from Roche Diagnostics (Indianapolis, IN, USA). Wogonin was purchased from Extrasynthese (Genay Cedex, France). Recombinant human IL-1β, anti-COX-2 antibody and PGE2 and IL-6 ELISA kit were from R&D Systems (St Paul, MN, USA). Dichlorodihydrofluorescein diacetate (H2DCF-DA), fluorescein diacetate (FDA), dihydro-rhodamine (DHR123), propidium iodide (PI) were procured from Sigma Aldrich Chemicals Inc. (St. Louis, MO, USA). Antibodies specific for p-ERK1/2, ERK1/2, p-JNK, JNK, p-P38, P38, Nrf2, p-PI3K, p-AKT, p-GSK3β and Iκβα were purchased from Cell Signaling Technology (Beverly, MA). Antibodies specific for β-Actin, MMP-13, MMP-3, MMP-9, IL-6, iNOS, and COL2A1 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Appropriate horseradish peroxidase conjugated secondary antibodies were from Cell Signaling Technology (#7074) or Santa Cruz Biotechnology (sc-2020).
Preparation of primary human OA chondrocytes
Prior to the initiation of the studies the study protocol was reviewed and approved by the Institutional Review Board (IRB) of Northeast Ohio Medical University, Rootstown, Ohio as a “non-human subject study under 45 CFR” and that no informed consent was needed. All the methods used in this study were carried out in accordance with the approved guidelines and all experimental protocols were approved by the IRB of Northeast Ohio Medical University, Rootstown, Ohio. Discarded and de-identified knee or hip joint cartilage samples were collected through the NIH supported National Disease Research Interchange (NDRI) per the IRB approved protocol.
The unaffected areas of the cartilage were used to prepare OA chondrocytes by enzymatic digestion as described previously [17, 18]. OA cartilage was examined for histopathology grading according to Mankin scoring system [19]. In this system the scores are defined as follows: 0=Normal, 1=Superficial fibrillation, 2=Pannus and superficial fibrillation, 3=Fissures to the middle zone, 4=Fissures to the deep zone and 5=Fissures to the calcified zone. We have used OA cartilage with Mankin score of 1 for the preparation chondrocytes in the present study. To maintain the chondrogenic phenotype, chondrocytes were seeded at high density (1×106/well of 6-well plate). Further to confirm the phenotype at the time of analysis, the mRNA expression of chondrocytes phenotype markers COL2A1, ACAN, and COL10A1 was analyzed by real time PCR.
Treatment of primary human OA chondrocytes with Wogonin and IL-1β
Primary human OA chondrocytes (1×106/well of 6-well plate) were seeded in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (DMEM/F-12) supplemented with 10% fetal calf serum (FCS), 100 units/ml penicillin, and 100 mg/ml streptomycin for 2–3 days after plating. At about 80% confluence, OA chondrocytes were serum starved overnight and were then treated with different concentration of Wogonin for 2 h followed by stimulation with IL-1β (1 ng/ml). Chondrocytes treated with 0.1% DMSO served as control.
Cell viability assay
Primary human OA chondrocytes (20,000 cells/well of 96-well plate), were serum starved overnight, and then treated with Wogonin (10–50 μM) for 24 h in serum free medium and viability was determined using MTT assay using method described earlier [17].
Estimation of apoptosis by PI staining
Chondrocytes (1×106/well of 6-well plate) were treated with Wogonin (50 μM) for 24 h and Wogonin induced chondrocytes apoptosis were estimated by PI staining as described previously [20]. A total of 20,000 cells were acquired in BDAccuri C6 Flowcytometer and percent apoptotic cells were determined by analyzing a sub-G1 population (<2 n DNA content) using FlowJo software.
Reactive oxygen species (ROS) measurement
Basal and induced ROS levels were measured by using cell-permeable fluorogenic probes DHR123 and (H2DCF-DA) as described previously [21, 22]. To detect intracellular ROS, OA chondrocytes (0.5×106/well of 12-well plate) were stained with 20 μM oxidation-sensitive (DCFH2-DA) for 0.5 h at 37 °C and were further treated with Wogonin (10–50 μM) or vehicle for 1 h. To ascertain the contribution of H2O2 and O2− to Wogonin-mediated increase in ROS, chondrocytes were treated with catalase (1000 units/ml) or SOD (200 unit/ml) for 2 h before the addition of Wogonin (50 μM).
For the measurement of IL-1β induced ROS, chondrocytes were pretreated with Wogonin (50 μM) for 2h and then labeled with DHR123 (5μM) or H2DCF-DA (20 μM) for 0.5 h followed by stimulation with IL-1β (1ng/ml) for 5 minutes and ROS levels were estimated by measuring the fluorescence emission due to oxidation of DHR123 to Rhodamine123 at 500 nm excitation and 536 nm emission or oxidation of H2DCF to DCF at an excitation and emission wavelengths of 485 and 525 nm using the Synergy H1 multi-mode plate reader.
Estimation of H2O2 in supernatant using Amplex red assay
We measured H2O2 generation in the supernatants, not in the cells to rule out the possibility of H2O2 production that may occur during lysates preparation due to cell destruction. Interestingly, H2O2 can diffuse across cell membranes probably through the aquaporins [23], therefore level of H2O2 is predicted to be similar within the cytoplasm and in the culture medium. For the measurement H2O2 generation, OA chondrocytes were treated with Wogonin (25, 50 μM) for 1 h and supernatant were collected and assay was performed as per the manufacturer’s protocol. Amplex® Red reagent reacts with H2O2 in the presence of peroxidase (HRP) and produces the red-fluorescent oxidation product, resorufin. The release of H2O2 in the supernatant of Wogonin treated chondrocytes was estimated by measuring fluorescence of resorufin at an excitation and emission wavelengths of 571 and 585 nm using the Synergy H1 multi-mode plate reader.
Estimation of mitochondrial O2− using MitoSox
For measurement of mitochondrial O2−, chondrocytes were stained with MitoSOX Red (5 μM) for 10 minutes at 37°C followed by treatment with Wogonin (25, 50 μM) for 1 h and O2− generation were estimated by measuring fluorescence using 510 nm excitation and 580 nm emission using Synergy H1 multi-mode plate reader.
Estimation of nitric oxide (NO)
Chondrocytes (1×106/well of 6-well plate) were pretreated with Wogonin (50 μg/ml) for 2h followed by treatment with IL-1β (1 ng/ml) for 24 h and NO was estimated in the culture supernatant using Greiss assay as described previously [22]. Culture supernatant was incubated with Greiss reagent and NO levels were estimated by measuring the absorbance at 550 nm using the Synergy H1 multi-mode plate reader. The amount of nitric oxide in each sample was calculated using standard curve generated with known dilutions of sodium nitrite.
Intracellular GSH assay
Monocholorbimane (mBCl) was used to measure intracellular GSH as described previously [20]. Briefly, OA Chondrocytes (0.5×106/well of 12-well plate) were treated with Wogonin (10–50μM, 1h) and mBCl (40μM, 0.5 h at 37°C) was loaded into the cells and fluorescence emission from cellular SH-reacted monochlorobimane was measured at 394 nm excitation and 490 nm emission using the Synergy H1 multi-mode plate reader. Monocholorbimane is also known to react with small molecular weight thiols other than GSH, but GSH forms the major monochlorobimane reactive thiol [20].
Total RNA isolation and real time PCR
Chondrocytes (1×106/well of 6-well plate) were pretreated with Wogonin (50 μM) for 2h followed by treatment with IL-1β (1 ng/ml) for 24h. Total RNA from isolated chondrocytes was prepared essentially as previously described [17]. For mRNA expression analysis, cDNA was synthesized from 1μg of total RNA using high-capacity cDNA reverse transcription kit (Life Technologies) and mRNA expression of MMP-13, MMP-3, MMP-9, IL-6, iNOS, COX-2, ADAMTS-4, COL2A1, ACAN was quantified using TaqMan Gene Expression Assays as previously described [24]. Relative expression levels were calculated using the 2−ΔΔCT method [25].
Western Immunoblotting
After treatments, OA chondrocytes were harvested, washed with cold PBS and lysed in ice-cold RIPA buffer and lysate were prepared as described previously [24]. Equivalent amounts of lysate protein (20 μg) were resolved by 10% SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, USA) and the blots were incubated with primary antibodies diluted in 2% blocking buffer for overnight at 4°C. Blots were then incubated with horse radish peroxidase-conjugated secondary antibody, followed by washing with TBST. Blot were developed using Luminata Western HRP substrate (EMS Millipore) and the antibody reactive proteins were visualized by chemiluminescence and imaged using the Pxigel imaging system (Syngene, Frederick, MD).
Visualization of Nrf2 nuclear translocation using confocal microscopy
OA Chondrocytes (0.1×106) were seeded in 8-well chamber slide and then treated with Wogonin (50 μM) for 6, 12 h at 37°C. After completion of treatment, cells were fixed with 4% paraformaldehyde and permiabilzed in 0.3% Triton X-100 in phosphate buffered saline. The cells were probed with anti-Nrf2 primary antibody followed by anti-mouse or ant-rabbit Alexa Fluor 488 secondary antibody (Life Technologies). Cells were mounted using antifade mounting medium containing DAPI stain (Vectashield, Burlingame, CA) and visualized by Olympus FV1000 confocal microscope using a 60xoil immersion lens.
siRNA mediated depletion of Nrf2 expression using nucleofection
Primary human OA chondrocytes were transfected with 100 nM Nrf2 siRNA (SMARTpool: ON-TARGETplus NFE2L2 siRNA Dharmacon, Lafayette, CO, USA) or MISSION® siRNA Universal Negative Controls (Sigma Aldrich, St. Louis, MO) using P3 Primary Cell 4D-Nucleofector™ X Kit on 4D-Nucleofector equipment (Lonza, Walkersville, MD) following the instructions provided by the manufacturers. Transfected cells were plated in 6-well plates. Forty-eight hours after the transfections, cells were serum starved overnight followed by treatment with Wogonin (10 μM) followed by stimulation with IL-1β (1 ng/ml) in serum free medium for 16 h. Gene expression levels were measured by quantitative PCR using the TaqMan assay system as described previously [18].
Molecular docking of Wogonin with Keap1 protein
The Crystal structure of Keap1 protein in complex with a small chemical compound K67 (PDB CODE: 4ZY3) was extracted from the Protein Data Bank and used as docking structure template. Docking studies were performed using Glide tool in Schrödinger Maestro suite [26]. An energy minimised three dimensional structure of Wogonin compatible for docking was used throughout the docking process. Keap1 protein was processed using protein preparation wizard to ensure chemical correctness and to optimize the protein structure for docking. A receptor grid has been generated around the ligand binding site of the Keap1 protein followed up for docking process. The Glide docking output contains multiple docking combinations ranked according to Glide gscore, docking score, binding energy, and other properties.
Estimation of IL-6, MMP-13 and PGE2 using ELISA
After the treatment of OA chondrocytes, culture supernatants were collected and levels of IL-6, MMP-13 and PGE2 in the supernatants were estimated using a commercially available ELISA kit (Boster Immunoleader, Pleasanton, CA or R&D System, St Paul, MN).
MMP-13 activity assay
MMP-13 activity was measured in the culture media from the Wogonin (10–50 μM) treated and IL-1 β-stimulated chondrocytes using SensoLyte Fluorimetric MMP-13 activity assay kit essentially as per manufacturer’s instruction (AnaSpec). The culture supernatant proteins were first activated with Aminophenyl mercuric acetate (APMA; 1 mM) at 37°C for 40 minutes. To initiate the enzymatic reaction, activated culture supernatant was incubated with MMP-13 FRET substrate for 1h at 37°C and fluorescence signal was recorded at 510 nm excitation and 580 nm emission using Synergy H1 multi-mode plate reader.
Collagenase activity assay
Collagenase/gelatinase activity was estimated in culture supernatant from Wogonin (10–50 μM) treated and IL-1β stimulated chondrocytes using Enzchek Gelatinase/Collagenase Assay Kit (Molecular Probes) according to the manufacturer’s instructions. Collagenase in the culture supernatants was activated by APMA (1 mM) at 37°C for 40 minutes. To analyze collagenase activity, DQ Collagen Fluorescein conjugate (100μg/mL) was added to culture medium and incubated for 4 h at 37°C and activity was determined by measuring fluorescence emission of fluorescence peptide generated by cleavage of DQ collagen at 480 nm excitation and 520 nm emission using Synergy H1 multi-mode plate reader.
Estimation of type II collagen (COL2A1) levels
Cartilage degradation occurs due to enzymatic activity of metalloproteinase and MMP-13 specifically caused the enzymatic digestion of type 2 collagen, therefore ECM degradation was studied by quantifying the COL2A1 levels which are released into culture medium and the remaining content present in cartilage matrix using Sircol collagen Assay Kit (Biocolor Ltd.).
Cartilage explants of equal sizes were cut from femoral condyle of OA cartilage, weighed and cultured in 24-well plate in basal medium for 24 h. At the end of 24h, cartilage explants were serum starved overnight and then treated with Wogonin (10–50 μM) for 2 hours in fresh medium without serum, and then stimulated with IL-1β (25 ng/ml) for 72 h and supernatant were collected. The remaining COL2A1 levels in cartilage explants was also measured that require additional extraction step using acid-pepsin treatment (4°C, 16 h) followed by collagen concentration step as per manufacturer protocol. Briefly, 100 μl of extracted samples were mixed with Sirius red dye containing sulfonic acid, which reacts specifically with the basic side chain groups of COL2A1, for 30 minutes at room temperature. After centrifuging for 10 minutes at 12,000 rpm, the unbound dye was removed, and the dye bound to COL2A1 was redissolved in 0.5N NaOH. Absorbance was measured at 540 nm using Synergy H1 multi-mode plate reader. Concentrations were calculated using a standard curve generated with standards provided by the manufacturer.
Sulfated-glycosaminoglycan (s-GAG) release assay
Aggrecan degradation due to enzymatic activity of ADAMTS results into release of s-GAG which are quantified in culture medium of cartilage explants treated with Wogonin (10–50 μM) and then stimulated with IL-1β (25 ng/ml, 72 h) using the DMMB assay as described earlier [27]. Briefly, 200 μl of DMMB dye was added to 50 μl of diluted cultured medium and absorbance of the solution was measured at 540 nm using the Synergy H1 multi-mode plate reader. Chondroitin sulfate C from shark cartilage was used to generate standard curve.
Histopathological examination
Cartilage matrix depletion due to IL-1β-induced activation of matrix degrading proteases was determined by Safranin-O staining of cartilage explants. The cartilage explants were treated with Wogonin (10–50 μM) and then stimulated with IL-1β (25 ng/ml, 72 h). At the end of the experiment, the explant were fixed in 10% neutral buffer formalin for 24h, decalcified in 10% EDTA (pH 7.4) for 72 h, dehydrated with series of alcohols and then embedded in paraffin wax. The sections (6 μm) were prepared and stained with safranin-O dye as described previously [28]. The cartilage sections were imaged by a Nikon eclipse Ti inverted microscope.
Cellular uptake of Wogonin
Cellular uptake of Wogonin in human OA chondrocytes was performed by LC-MS/MS analysis using a UHPLC system connected to a triple quadrupole mass spectrometer (LC-MS 8040; Shimadzu, Kyoto, Japan) equipped with an electrospray ionization source. The uptake was quantitated using the ESI ion source in multiple reaction monitoring and negative ion mode (MRM-) by monitoring transition pairs m/z 283.00 (precursor ion)/162.9 (product ion). The following instrument settings were used for MRM analysis: heat block temperature, 400 °C; DL temperature, 250 °C; nebulizing gas (N2), 3L/min; drying gas (N2), 15L/min; collision energy, 35.0; dwell time, 100 msec. A calibration curve was prepared using pure Wogonin dissolved in methanol at the concentrations of 0.001, 0.005, 0.01, 0.1 and 1mg/ml.
The cell lysate for mass spectrometric analysis for Wogonin was prepared as described earlier [29]. Briefly, after the treatment with Wogonin (50–100 μM) for 4 or 24h, OA chondrocytes were washed three times with phosphate-buffered saline, lysed with lysis buffer (10 mM Tris-HCl (pH 9.6), 1% Triton X-100,150 mM NaCl, 5 mM EDTA) and deproteinized with an equivalent amount of acetonitrile. After centrifugation for 5 minutes at 10,000xg, the supernatant was used for MRM- analysis using LC-MS/MS system.
Statistical Analyses
The values are presented as the Mean±SD and the statistically significant difference between the experimental groups and controls were analyzed using one-way ANOVA followed by post hoc analyses using the Tukey test. Unless otherwise noted, each experiment was repeated three times using three independent samples. P<0.05 was considered to be statistically significant.
Results
Wogonin did not affect the viability of human OA chondrocytes
To confirm the chondrogenic phenotype of monolayer culture of human OA chondrocytes, we measured the expression of chondrocyte marker genes, and data showed that COL2A1 and ACAN were highly expressed while the expression of COL10A1 was low indicating that the chondrocyte’s phenotype was maintained at the time of analyses (Fig. 1 in [30]). We first examined the effect of Wogonin on the viability of human OA chondrocytes. Compared with untreated control, treatment of OA chondrocytes with Wogonin (10–50 μM, 24 h) had no effect on chondrocytes viability (Fig. 2A in [30]). Further, absence of cytotoxic effect of Wogonin was confirmed by PI staining and flowcytometric histograms of PI stained cells revealed that OA chondrocytes incubated with Wogonin (50 μM) for 24 h did not show any increase in apoptosis (pre-G1 peak) compared to untreated control chondrocytes (Fig. 2B in [30]).
Wogonin inhibited the expression and production of IL1-β-induced inflammatory mediators in human OA chondrocytes
Inflammation is recognized as an important component of OA pathogenesis [3]. We determined whether Wogonin had any effect on IL-1 β induced expression of IL-6, iNOS and COX-2 in OA chondrocytes. OA Chondrocytes were pretreated with Wogonin (10–50 μM) for 2 h and then stimulated with IL-1 β for 24 h and gene and protein expression of selected inflammatory mediators was determined by TaqMan assay and immunoblotting assay respectively. As reported previously [18, 24], gene expression data showed that treatment of OA chondrocytes with IL-1β significantly increased the mRNAs expression of IL-6, iNOS and COX-2 as compared to untreated control OA chondrocytes (Fig. 1A). Interestingly, pretreatment of OA chondrocytes with Wogonin significantly inhibited the expression of these inflammatory mediators in a dose dependent manner (Fig. 1A). Western blot analysis demonstrated that protein levels of IL-6, iNOS and COX-2 were also significantly suppressed by pre-treatment of human OA chondrocytes with Wogonin in agreement with the mRNA expression data (Fig. 1B). Additionally, we also measured the secreted levels of IL-6 in the culture supernatant of treated OA chondrocytes using ELISA and found that treatment with Wogonin completely blocked the secretion of IL-6 in response to IL-1β by OA chondrocytes (Fig. 1C). COX-2 mediates its inflammatory effect through production of prostaglandin E2 (PGE2), which is known to play a significant role in the inhibition of proteoglycan synthesis and stimulation of matrix degradation in OA chondrocytes [31]. Therefore, we examined the effect of Wogonin on IL-1β-induced PGE2 production in the supernatants of control and experimental cultures and the result showed that IL-1β-mediated production of PGE2 was also significantly blocked by Wogonin in OA chondrocytes (Fig. 1D). Further, we also measured the level of NO, the effector molecule of iNOS for its catabolic and inflammatory effects [32]. Our result showed that treatment of OA chondrocytes with Wogonin significantly blocked the IL-1β induced production of NO in a dose dependent manner (Fig. 1E). Taken together, these results suggest that Wogonin exhibits potent anti-inflammatory effects through the suppression of expression and production of inflammatory mediators in OA chondrocytes under pathological conditions.
Figure 1. Wogonin (WG) inhibited the IL1-β induced inflammatory mediators in human OA chondrocytes.
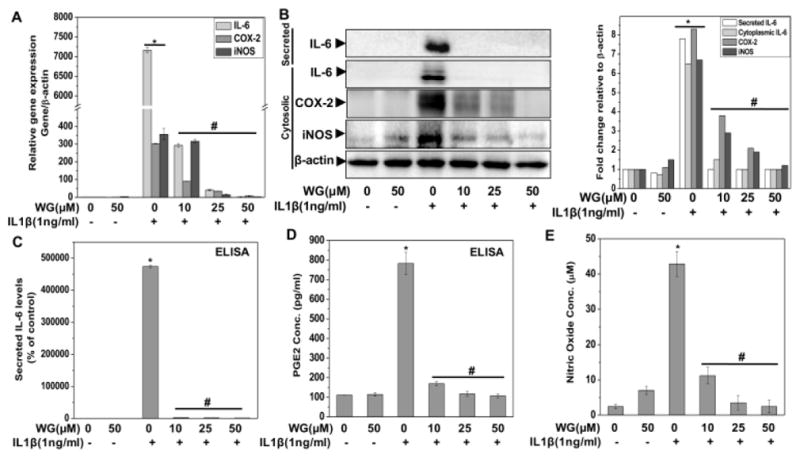
Primary human OA chondrocytes were pre-treated with Wogonin (10–50 μM) for 2 h followed by treatment with IL-1β (1 ng/ml) for 16 h. At the end of treatment culture supernatant were collected and chondrocytes were harvested. Cell lysate were prepared using RIPA buffer for immunoblot analysis or RNA were isolated for real time PCR analysis. (A) Expression of IL-6, COX-2 and iNOS was measured by quantitative PCR using the TaqMan assay system (Life Technologies). β-actin was used as endogenous expression control. (B) Protein expression of secreted IL-6, cytosolic IL-6, COX-2 and iNOS was investigated by immunoblotting using antibodies against indicated protein. β-actin was used as a control for equal loading. Specific signal intensities were quantified by ImageJ software. (C–D) Secreted levels of IL-6 (C) and PGE2 production (D) were measured in the culture supernatant by ELISA (E) Production of NO was estimated in supernatant using Griess assay as described in methods. Bar graph represents mean±SD from two subjects. *p≤0.01, as compared to control, #≤0.01, as compared to IL-1β.
Wogonin inhibited the IL-1β induced oxidative stress in human OA chondrocytes
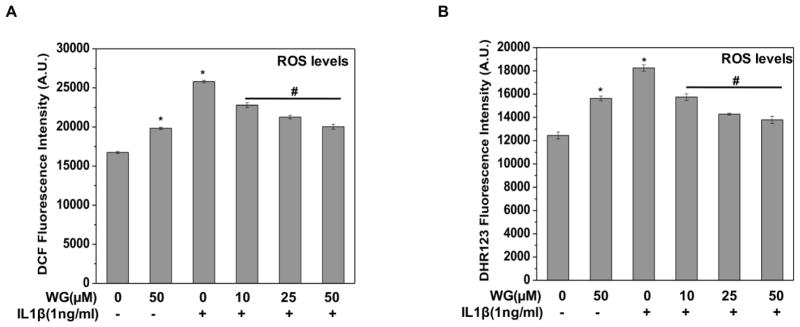
Oxidative stress has recently been implicated in OA pathogenesis because of its role in chondrocytes apoptosis and ECM degradation [33, 34]. Therefore, we determined whether Wogonin has suppressive effect on IL-1β-induced oxidative stress in OA chondrocytes. The level of oxidative stress was determined by measuring the ROS levels using oxidation-sensitive dye H2DCF-DA and DHR-123 and result showed that IL-1β treatment significantly increased the ROS production in OA chondrocytes whereas, pretreatment of OA chondrocytes with Wogonin significantly suppressed the IL-1β-mediated generation of ROS in a dose dependent manner (Fig. 2A, B). These results indicated that Wogonin could effectively repress IL-1β-induced oxidative stress by suppressing the production of ROS in OA chondrocytes.
Figure 2. Wogonin (WG) inhibited the IL-1β induced oxidative stress in human OA chondrocytes.
Human OA chondrocytes were treated with Wogonin (10–50 μM) for 2 h, then stained with (A) H2DCF-DA (20 μM) or (B) DHR123 (5 μM) for 0.5h at 37°C, and stimulated with IL-1β (1ng/ml) for 5 minutes at 37°C. Fluorescence emission of DCF was measured at 525 nm after excitation at 485 nm and emission of DHR was measured at 536 nm after excitation at 500 nm. Bar graph shows arbitrary fluorescence units indicating ROS levels. Data points represent mean±SD from four replicates. *p≤0.01, as compared to control, #≤0.01, as compared to IL-1β.
Wogonin inhibited the IL-1β induced expression and activity of matrix degrading proteases in human OA chondrocytes
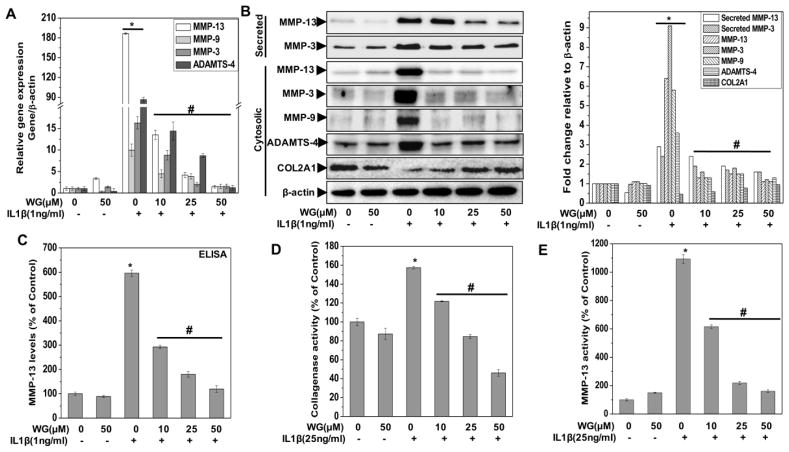
Activation of matrix degrading proteases such as aggrecanases and metalloproteinases (MMPs) is a hallmark of OA pathogenesis [35]. MMP-13, MMP-3, MMP-9 and ADAMTS-4 are produced by chondrocytes in response to IL-1β that contribute to OA pathogenesis. Therefore, we investigated the effect of Wogonin on the IL-1β-induced mRNA and protein expression of these proteases in human OA chondrocytes. Our results showed that IL-1β-stimulation of human OA chondrocytes resulted in a significant up-regulation of mRNA expression of MMP-3, MMP-9, MMP-13 and ADAMTS-4 whereas treatment with the Wogonin resulted in significant suppression of expression of all of these enzymes in a dose dependent manner (Fig 3A). Additionally, Western blot analysis further demonstrated that protein levels of both secreted and cytoplasmic MMP-3, MMP-13, and MMP-9 and ADAMTS-4 were also significantly suppressed by pre-treatment of human OA chondrocytes with Wogonin in a dose dependent manner (Fig. 3B). We also measured the secreted levels of MMP-13 in the culture supernatant of treated chondrocytes using ELISA and result showed that Wogonin treatment of OA chondrocytes significantly blocked the IL-1β mediated secretion of MMP-13 in a dose dependent manner (Fig. 3C).
Figure 3. Wogonin (WG) inhibited the IL-1β induced matrix degradation in human OA chondrocytes and cartilage explants.
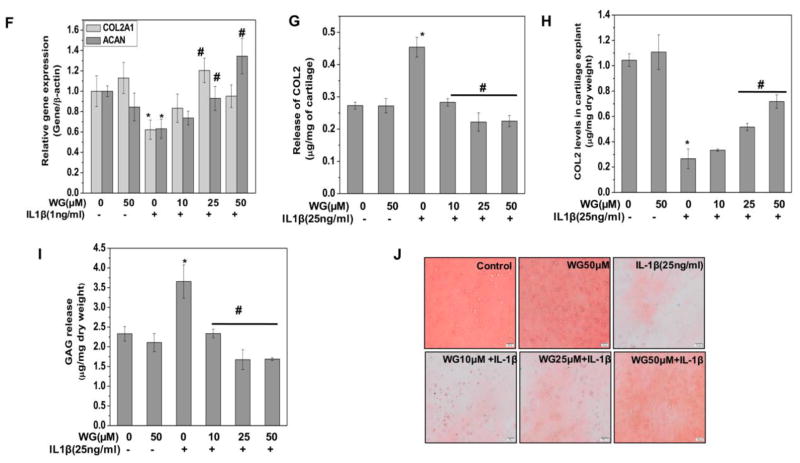
Human OA chondrocytes were pre-treated with Wogonin (10–50 μM) for 2 h followed by treatment with IL-1β (1 ng/ml) for 16 h. At the end of treatment culture supernatant were collected and chondrocytes were harvested. Cell lysate were prepared using RIPA buffer for immunoblot analysis or RNA were isolated for real time PCR analysis. (A) Expression of MMP-3, MMP-9, MMP-13 and ADAMTS-4 was measured by quantitative PCR using the TaqMan assay system (Life Technologies). β-actin was used as endogenous expression control. (B) Protein expression of secreted MMP-13, MMP-3, cytosolic MMP-13, MMP-9, MMP-3, ADAMTS-4 and COL2A1 was investigated by immunoblotting using antibodies against indicated protein. β-actin was used as a control for equal loading. Specific signal intensities were quantified by ImageJ software. (C) Secreted level of MMP-13 production was measured in the culture supernatant using ELISA. The data was expressed relative to untreated controls. (D) Collagenase/gelatinase activity in the culture supernatant was measured using method described in method section. Supernatant were collected from OA chondrocytes (1×106/well of 12-well plate in 0.5 ml) treated with Wogonin (10–50 μM) for 2 h and then stimulated with IL-1β (25 ng/ml) for 16 h. Activity was expressed relative to untreated controls. (E) MMP-13 activity in the culture media activated using APMA (1 mM, 40 minutes) was determined by fluorogenic peptide assay using commercially available kit (AnaSpec). Supernatant were collected from OA chondrocytes (1×106/well of 6-well plate) treated with Wogonin (10–50 μM) for 2 h and then stimulated with IL-1β (25 ng/ml) for 16 h. Activity was expressed relative to untreated controls. (F) Expression of COL2A1 and ACAN was measured by quantitative PCR using the TaqMan assay system (Life Technologies). β-actin was used as endogenous expression control. (G) The release of COL2A1 levels, (H) COL2A1 content in cartilage explants and (I) release of s-GAG levels was estimated in supernatants isolated from culture of OA cartilage explants treated with Wogonin (10–50 μM) for 2 h followed by treatment with IL-1β (25 ng/ml) for 72 h. (J) Histopathological section of cartilage explants treated with Wogonin (10–50 μM) for 2 h followed by treatment with IL-1β (25 ng/ml) for 72 h, fixed in 4% PFA and embedded in paraffin. Explant sections (6 μm) were stained with Safranin O and fast green and imaged using Nikon eclipse Ti inverted microscope. The scale bar is shown as 100 μm. Bar graph represents mean±SD from three subjects. Immunoblot results are representatives of two blots performed on samples obtained from two individuals *p≤0.01, as compared to control, #≤0.01, as compared to IL-1β.
To determine whether this inhibition of collagenase expression also influenced the collagenase activity, culture supernatants of treated chondrocytes were assayed using fluorescein-conjugate DQ-gelatin as substrate and result showed that IL-1β treatment significantly increased the collagenase activity as compared to untreated control. Interestingly, treatment of OA chondrocytes with Wogonin significantly inhibited the IL-1β induced collagenase activity in a dose dependent manner (Fig. 3D). Since this assay evaluate the activity of mixture of collagenases and lack the specificity for specific MMPs, we particularly determined the activity of MMP-13, a major collagenase involved in cartilage degradation, using fluorescence-based assay. Results showed that Wogonin treatment of OA chondrocytes significantly inhibited the IL-1β induced increased MMP-13 activity in a dose dependent manner (Fig. 3E). Taken together these results suggest that Wogonin could be an effective inhibitory agent for the suppression of matrix degrading proteases in OA chondrocytes.
Wogonin rescued the IL-1β induced suppression of COL2A1 and ACAN expression in human OA chondrocytes
We further explored whether Wogonin can rescue the expression of COL2A1 and ACAN in OA chondrocytes stimulated with IL-1β. As shown previously [36], IL-1β stimulation significantly down regulated the mRNA and protein expression of ACAN and COL2A1 in OA chondrocytes (Fig. 3B, 3F). However, pretreatment with Wogonin significantly enhanced the mRNA and protein expression of COL2A1 and ACAN in IL-1β-stimulated OA chondrocytes (Fig. 3B, 3F). Altogether, these data indicate that Wogonin may exerts cartilage protective effects through up-regulating the expression of cartilage anabolic factors COL2A1 and ACAN in human OA chondrocytes under pathological conditions.
Wogonin inhibited the IL-1β induced matrix degradation in human OA cartilage explants
Since cartilage degradation in OA occurred by collagenase mediated depletion of major components of ECM including type 2 collagen and aggrecan, we explored whether Wogonin had any effect on the levels of COL2A1 and ACAN in an in vitro model of human cartilage degradation. We measured the release of COL2A1 in the supernatants of human cartilage explants treated with and without Wogonin and stimulated with IL-1β. Our result showed that IL-1β treatment resulted in significant release of COL2A1 in the culture medium indicating the matrix degradation. However, Wogonin treatment significantly blocked the IL-1β mediated release of COL2A1 in the culture medium a dose dependent manner indicating that Wogonin possessed cartilage matrix protective ability (Fig. 3G). We further measured the COL2A1 content in the cartilage explants and result showed that Wogonin treatment significantly inhibited the IL-1β mediated depletion of COL2A1 content in the explants in a dose dependent manner (Fig. 3H).
We also measured the aggrecan degradation using similar in vitro explant model of cartilage degradation. Our result showed that treatment of cartilage explants with IL-1β resulted in significantly increased s-GAG release in the culture medium compared to un-stimulated controls (Fig. 3I). However, the IL-1β-induced release of s-GAG in the culture medium was significantly inhibited by the Wogonin treatment in a dose dependent manner (Fig. 3I).
We also measured the loss of GAG in the cartilage explants by evaluating the proteoglycan content of cartilage matrix using Safranin-O/Fast-Green staining of cartilage explants treated with Wogonin and stimulated with IL-1β. The histological results depicted in Fig. 3J, showed the depletion of proteoglycan content in IL-1β treated explants tissue as indicated by diminished staining of proteoglycans. Interestingly, pretreatment of cartilage explants with Wogonin inhibited the IL-1β mediated loss of proteoglycans from the matrix as indicated by the intense staining similar to the controls (Fig. 3J). Taken together these results provide support that Wogonin could be a potent cartilage protective agent and could be used for effective suppression of IL-1β mediated degradation of OA cartilage matrix.
Wogonin did not inhibit the IL-1 β induced activation of MAPKs and NF-κB in human OA chondrocytes
We next determined the molecular mechanism responsible for the observed chondroprotective effects of Wogonin. Recent literatures suggest that IL-1β mediated expression of MMPs has been regulated through the activation of MAPKs including ERK, p38 kinase, and JNK signal transduction molecules [37]. Therefore, we determined whether Wogonin mediate its chondroprotective effects through the suppression of MAPKs by measuring the phosphorylation levels of ERK, JNK and p38 in IL-1β stimulated OA chondrocytes. Results illustrated that stimulation of OA chondrocytes with IL-1β activates the phosphorylation of p38, JNK and ERK1/2 within 15 minutes of treatment (Fig. 3A in [30]). Surprisingly, pretreatment of OA chondrocytes with Wogonin for 2 h did not inhibit the phosphorylation of p38, JNK and ERK1/2 upon stimulation with IL-1β (Fig. 3A in [30]). These results indicated that Wogonin exerts chondroprotective effect without modulating the activation of MAPK signaling pathways in OA chondrocytes under pathological conditions.
We next examined the involvement of NF-κB transcription factor, as IL-1β induced expression of catabolic mediators such IL-6, COX-2 and iNOS are transcriptionally regulated by NF-κB [18, 24]. Stimulation of OA chondrocytes with IL-1β induce proteasomal degradation of IκBα, the inhibitory protein of NF-κB, resulting in the release and translocation of NF-κB protein to the nucleus [24]. Therefore, we evaluated the effect of Wogonin on the degradation of IκBα and found that stimulation of OA chondrocytes with IL-1β alone caused degradation of IκBα within 15 minutes of treatment, and was not blocked by treatment with Wogonin indicating that Wogonin-mediated suppression of IL-6, COX-2, iNOS and MMPs was not due to the inhibition of the activation of NF-κB in IL-1β-stimulated OA chondrocytes (Fig. 3B in [30]). Altogether these results suggest that Wogonin exerts anti-inflammatory and chondroprotective effects independent of MAPKs and NF-κB signaling pathways in OA chondrocytes under pathological conditions.
Wogonin modulated the cellular redox status of human OA chondrocytes
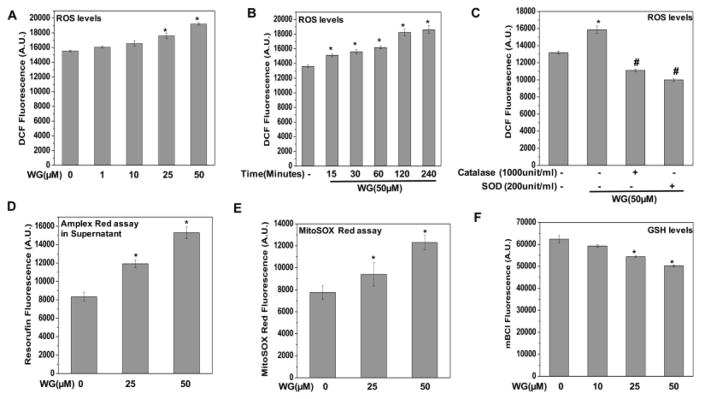
Although Wogonin inhibited the IL-1β induced ROS generation, results as shown in Fig. 2A, B indicated that Wogonin treatment alone elevated the basal ROS levels in OA chondrocytes. To explore these findings in details, we performed dose and time kinetics studies, and the result showed that treatment of OA chondrocytes with Wogonin elevated the basal ROS levels in a concentration-dependent and time dependent manner as measured by DCFH2-DA staining (Fig. 4A, B). To identify the nature of the ROS induced by Wogonin in OA chondrocytes, catalase or SOD were used prior to addition of Wogonin and it was observed that Wogonin mediated increase in DCFH2-DA fluorescence was significantly suppressed in the presence of both catalase and SOD suggesting the involvement of H2O2 and O2− (Fig. 4C). Since H2O2 freely crosses cell membranes, probably through the aquaporins [23], therefore externally added catalase can exert both intracellular and extracellular effects on H2O2 level by neutralizing the H2O2 crossing out the cell membrane. Further, we specifically measured H2O2 levels in the supernatant of Wogonin treated chondrocytes using Amplex red assay and result showed that treatment with Wogonin induced the generation of H2O2 in OA chondrocytes (Fig. 4D). We also measured the mitochondrial O2− generation using MitoSOX red assay and Wogonin treatment for 0.5h increased the levels of O2− in a dose dependent manner in human OA chondrocytes (Fig. 4E). These results suggest that Wogonin treatment increased the basal ROS levels by inducing the generation of H2O2 and O2− in human OA chondrocytes.
Figure 4. Wogonin (WG) modulated the cellular redox status of human OA chondrocytes.
(A, B) Wogonin elevated basal ROS levels in OA chondrocytes. Human OA chondrocytes were stained with H2DCF-DA (20 μM) for 0.5 h at 37°C, and then (A) treated with Wogonin (10–50 μM) for 1 h or (B) treated with Wogonin (10–50 μM) for indicated time points at 37°C. ROS generation was estimated by measuring fluorescence emission at 535 nm. Bar graph shows arbitrary fluorescence units indicating ROS levels. (C–E) Wogonin induced production of H2O2 and O2− in OA chondrocytes. (C) OA chondrocytes were incubated with catalase (1000 unit/ml) or SOD (200 unit/ml) for 1 h, stained with H2DCF-DA (20 μM) for 0.5 h at 37°C and then treated with Wogonin (50 μM) for 1 h at 37°C. Fluorescence emission was measured at 535 nm. Bar graph shows arbitrary fluorescence units indicating ROS levels. (D) The generation of H2O2 in the supernatant of OA chondrocytes treated with Wogonin (25, 50 μM, 1 h) was estimated using Amplex red assay as described in method section. (E) The production of O2− in OA chondrocytes stained with MistoSOX red (5 μM) and then treated with Wogonin (25, 50 μM, 1 h) was estimated by measuring the fluorescence emission at 580 nm following the excitation at 510 nm. (F) Wogonin depleted cellular GSH levels in OA chondrocytes. OA chondrocytes were treated with Wogonin (10–50μM, 1h) and then stained with mBCl (40μM, 0.5 h) and GSH levels were estimated by measuring fluorescence emission at 490 nm, following the excitation at 394 nm. Bar graph represents mean±SD from two subjects. *p≤0.01, as compared to control, #≤0.01, as compared to Wogonin treated cells.
Cellular redox status is maintained by equilibrium between levels of cellular ROS and GSH, the most abundant thiol antioxidant responsible for detoxifying the ROS [22]. This process also consumes GSH and therefore, in neutralizing the ROS generation induced by Wogonin in chondrocytes, there would be a concomitant decrease in the GSH levels in Wogonin treated chondrocytes. To test this hypothesis, we measured the chondrocyte GSH levels using fluorescence based assay and the results showed that treatment of OA chondrocytes with Wogonin for 1h decreased the cellular GSH levels in a dose dependent manner (Fig. 4F). Taken together these results (Figure-4) suggest that Wogonin modulated the cellular redox status in OA chondrocytes by generating ROS which depleted the cellular GSH levels.
Wogonin activated the redox sensitive transcription factor Nrf2 in human OA chondrocytes and binds to Kelch domain of Keap-1
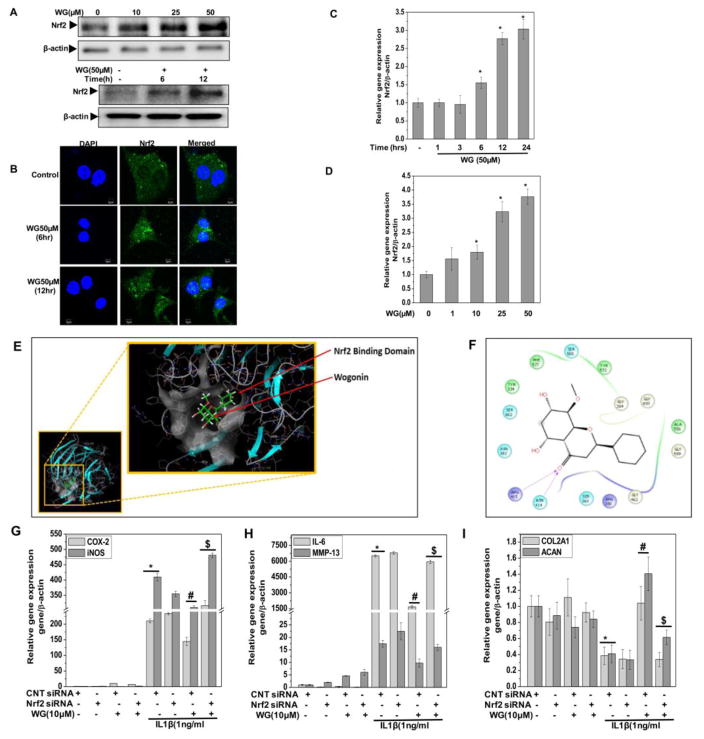
Modulation of cellular redox status is known to affect the redox sensitive transcription factors and other stress related regulatory proteins including Nrf2. Since Wogonin perturbed the redox balance of chondrocytes with low levels of ROS generation, we hypothesized that treatment with Wogonin might be activating the pro-survival pathway by activating the transcription factor Nrf2. Therefore, we measured the activation of Nrf2 in Wogonin treated OA chondrocytes by measuring protein expression using immunoblotting and nuclear translocation of Nrf2 using confocal microscopy. A significant accumulation of Nrf2 in the nucleus was seen after 6h of treatment of chondrocytes with Wogonin (Fig. 5A, B). Wogonin treatment also led to a significant increase in mRNA copy number of Nrf-2 in a dose and time dependent manner (Fig. 5C, D).
Figure 5. Wogonin (WG) activated Nrf2 in human OA chondrocytes.
OA chondrocytes were treated with Wogonin (50 μM) for 6, and 12 h and lysate were prepared from harvested chondrocytes using RIPA lysis buffer. (A) The expression of Nrf2 was investigated by immunoblotting using anti-Nrf2-antibody. β-actin was used as a control for equal loading. (B) Nuclear translocation of Nrf2 in Wogonin treated (50 μM, 6, and 12 h) OA chondrocytes was performed by immunofluorescence using specific antibody on Olympus FV1000 confocal microscope. (C–D) mRNA expression of Nrf2 in OA chondrocytes treated with (C) Wogonin (50 μM) for indicated time points or (D) Wogonin (10–50 μM) for 24h was measured by quantitative PCR using the SYBR® green assay system (Life Technologies). (E) In silico molecular docking of Wogonin with Keap1. Wogonin binds to the Keap1 protein in their Kelch domain, the Nrf2 binding domain. (F) Interactive visualisation of Wogonin binding to Arg415 and Asn414 in Kelch domain of Keap1 protein through hydrogen bonding. (G–I) Genetic ablation of Nrf2 using specific siRNA abrogated the anti-inflammatory and chondroprotective effect of Wogonin in human OA chondrocytes. OA chondrocytes were transfected with siRNA specific for Nrf2 (100 nM) using nucleofection. Forty-eight hours after the transfections, OA chondrocytes were serum starved, treated with Wogonin (10 μM) for 2 h and then stimulated with or without IL-1β (1 ng/ml) for 16 h. (G–I) Gene expression of COX-2, iNOS, IL-6, MMP-13, ADMATS-4, COL2A1 and ACAN was measured by quantitative PCR using the TaqMan assay (Life Technologies). Bar graph represents mean±SD from three subjects. *p≤0.05, as compared to control. For transfection experiments, *p≤0.05, as compared to control siRNA #≤0.05, as compared to control siRNA+IL-1β, $≤0.05, as compared to control siRNA+IL-1β+Wogonin.
To better understand the molecular mechanisms of Wogonin mediated Nrf2 activation, an in silico molecular docking study was performed with Wogonin on the crystal structure of Keap1 Kelch domain to determine if Wogonin can directly inhibit by interfering with the interactions between Keap1 and Nrf2. The molecular docking of Wogonin with Keap1 protein revealed that Wogonin binds to Kelch domain of Keap1 protein efficiently with a glide gscore of −6.240 (Fig. 5E). Wogonin binds to the Kelch domain of Keap1 protein specifically at Arg415 and Asn414 and thus may directly compete and restrict the binding of Nrf2 to Keap1 and thereby prevent its ubiquitination and subsequent degradation leading to activation of Nrf2 (Fig. 5F). Taken together these results suggest that Wogonin treatment of OA chondrocytes lead to the activation of redox sensitive transcription factor Nrf2, at least in part by interfering with its interaction with Keap1.
Genetic ablation of Nrf2 abrogate the anti-inflammatory and chondroprotective effect of Wogonin in human OA chondrocytes
To further substantiate the role of Nrf2 in Wogonin mediated chondroprotection, we specifically depleted Nrf2 in OA chondrocytes which were then treated with Wogonin and stimulated with IL-1β for 16 h. siRNA-mediated depletion of Nrf2 (Fig. 4 in [30]) significantly abrogated the Wogonin-induced suppression of IL-6, COX-2, iNOS, MMP-13 and ADAMTS4 mRNA expression and upregulation of COL2A1 and ACAN expression in OA chondrocytes pre-treated with Wogonin and then stimulated with IL-1β (Fig. 5G–I). These are novel findings and establish a direct role of Nrf2 in Wogonin-mediated suppression of inflammatory and catabolic molecules expression and upregulation of the anabolic molecules in OA chondrocytes under pathological conditions. Taken together these results highlight the potential of Wogonin in inhibiting the joint degradation and disease progression through the activation Nrf2 in human OA chondrocytes. However, this needs to be studied further using in vivo models of the disease.
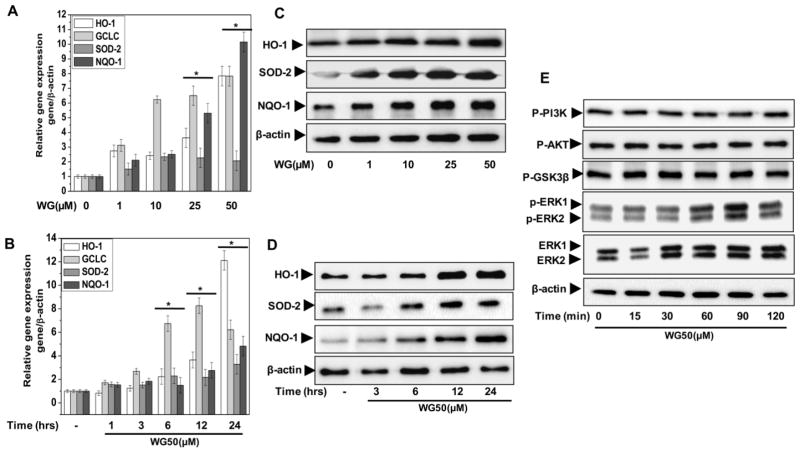
Wogonin upregulated the expression of Nrf2-dependent antioxidant and cytoprotective enzymes in human OA chondrocytes
To determine how Wogonin-mediated Nrf2 activation offers chondroprotection, we examined the effect of Wogonin on the downstream expression of Nrf2 effectors in human OA chondrocytes. These results showed that treatment with Wogonin induced the mRNA expression of SOD-2 and NQO-1 and their downstream target genes GCLC and HO-1 in a dose and time dependent manner in OA chondrocytes (Fig. 6A, B). Corroborating the RNA expression data, there was a time and dose dependent increase was also observed in the protein levels of these molecules in Wogonin treated OA chondrocytes (Fig. 6C, D).
Figure 6. Wogonin (WG) upregulated the expression of Nrf2 dependent genes and activated upstream kinase ERK1/2 in human OA chondrocytes.
Human OA chondrocytes were treated with (A, C) Wogonin (10–50 μM) for 24 h or (B, D) Wogonin (50 μM) for indicated time points. At the end of treatment chondrocytes were harvested and cell lysate were prepared using RIPA buffer for immunoblot analysis or RNA were isolated for real time PCR analysis. (A–B) Expression of HO-1, SOD-2, NQO-1 and GCLC was measured by quantitative PCR using the SYBR® green assay system (Life Technologies). β-actin was used as endogenous expression control. (C–D) Protein expression of HO-1, SOD-2, and NQO-1 was investigated by immunoblotting using antibodies against indicated protein. β-actin was used as a control for equal loading. (E) Wogonin activated upstream kinase ERK1/2 in human OA chondrocytes. Human OA chondrocytes were treated with Wogonin (50 μM) for indicated time points and lysate were prepared using RIPA lysis buffer. Activation of MAPKs was investigated by immunoblotting using primary antibodies specific for phospho-ERK1/2, phospho-PI3K, phospho-AKT and phsopho-GSK3β. Expression of total ERK1/2 β-actin was used as control. Immunoblot are representatives of two blots performed on samples obtained from two individuals. Bar graph represents mean±SD from two subjects. *p≤0.05, as compared to control.
Wogonin activated the upstream kinase ERK1/2 in human OA chondrocytes
We further investigated the underlying molecular mechanism of Wogonin-mediated Nrf2 activation by probing the PI3K/AKT/GSK3β signaling axis, as this is an important layer of Nrf2 regulation mediated through cellular redox modulation [38, 39]. However, treatment of OA chondrocytes with Wogonin (0–120 minutes) did not induce the phosphorylation of PI3K, AKT or GSK3β proteins indicating that Wogonin activate Nrf2 independent of PI3K/AKT/GSK3β signaling axis in OA chondrocytes (Fig. 6E). Recent studies have shown that flavonoid-mediated Nrf2 activation involves the phosphorylation of the upstream kinase ERK1/2 [22, 40]. We therefore, determined whether Wogonin treatment induce phosphorylation of ERK1/2 in OA chondrocytes. Our results showed that Wogonin treatment increased the phosphorylation of ERK1/2 significantly in human OA chondrocytes in a time-dependent manner (Fig. 6E). Taken together with the previous findings [22,40] our results suggest that Wogonin likely activated the Nrf2 through the phosphorylation of ERK1/2 in OA chondrocytes.
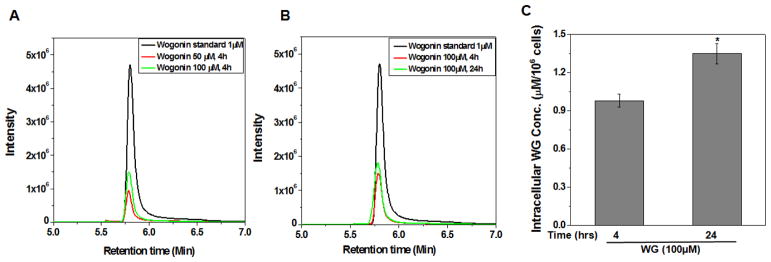
Cellular uptake of Wogonin in human OA chondrocytes
Cellular uptake of Wogonin was examined using multiple reaction monitoring and negative ion mode (MRM-) analysis using LC-MS/MS. Results are shown in Fig. 7A, B and demonstrate that uptake of Wogonin in OA chondrocytes increased in a dose and time dependent manner (Fig. 7A, B). These data were in line with an earlier published report where intracellular uptake of different flavonoids of Scutellaria baicalensis including baicalin and baicalein was studied and only Wogonin was detected inside the RAW 264.7 macrophage cells [29]. The intracellular concentration of Wogonin in treated chondrocytes was quantified using four-point calibration curves prepared with Wogonin standard and MRM- analysis of the extract. Linear calibration curve with good correlation coefficients (R2=0.996) was obtained in the range of 10−1-10−4 mg/ml (Fig. 5 in [30]). Based on our data, the intracellular concentration of Wogonin in OA chondrocytes was determined to be 0.98±0.05 μM/106 chondrocytes in cultures treated with 100 μM of Wogonin for 4 h and 1.35±0.08 μM/106 chondrocytes in cultures treated with 100 μM of Wogonin for 24 h (Fig. 7C). These concentrations are sufficiently high to modulate the cellular response to the inflammatory stimuli. However, a limitation of the present study is that the observed protective effect on chondrocytes may not be attributed to Wogonin only after absorption it is likely to generate several metabolites due to glucuronidation, sulfation, methylation, conjugation and glycosylation of the molecule. Although, we have not considered interactions of possible metabolites of Wogonin with the Nrf2 inhibitor Keap-1in this study, we cannot rule out the possible involvement of these metabolites in playing a role in the observed chondroprotective effects. Therefore, our data suggest that the observed chondroprotective effects could be due to the cumulative effects of Wogonin and its metabolites.
Figure 7. Cellular uptake of Wogonin (WG) to human OA chondrocytes.
OA chondrocytes were treated with WG (50, 100 μM) for 4 or 24 h. Cell lysates were prepared and subjected to LC-MS/MS analysis using the ESI ion source in multiple reaction monitoring and negative ion mode (MRM-) by monitoring transition pairs m/z 283.00 (precursor ion)/162.9 (product ion) as described in method section. MS chromatogram of WG uptake in OA chondrocytes in a (A) dose and (B) time dependent manner. (C) Quantification of Wogonin in the cellular milieu of OA chondrocytes was calculated by four point calibration curves of Wogonin using MRM- analysis. Bar graph represents mean±SD from two subjects. *p≤0.01, as compared to Wogonin (100μM, 4h) treated cells.
Discussion
Cellular response is an outcome of balance between intracellular redox couples such as GSH/GSSG, cysteine/cystine and reduced/oxidized thioredoxin [41]. Modulation of cellular redox status lead to activation of redox sensitive transcription factor Nrf-2, a master regulator of antioxidative stress. These studies were undertaken to test the hypothesis that a natural flavonoid, Wogonin, would protect human OA chondrocytes/cartilage explants against IL-1β-mediated inflammatory and matrix-degrading effects by initiating an adaptive response through the activation of Nrf2/ARE signaling pathway. Our results provide substantial evidence of chondroprotective effects of Nrf2 activation through the suppression of molecular events involved in oxidative stress, inflammation and matrix degradation. In the present study, we also showed for the first time that Wogonin possessed potent chondroprotective and anti-inflammatory effects through the activation of ROS/ERK/Nrf2/HO-1-NQO1-GCLC-SOD2 signaling axis in human OA chondrocytes. Our result indicate that Wogonin, as a natural Nrf2 activator, may be pharmacologically effective against pathogenic events involved in OA progression.
OA, one of the most common aging-related joint diseases, is a heterogeneous complex multifactorial disease [1, 2]. Inflammation and oxidative stress are considered as major mediators of OA pathogenesis. Excessive oxidative stress triggers the activation of signaling pathways leading to apoptosis or necrosis of chondrocytes in OA [33]. Inflammatory molecules that induce mitochondrial dysfunction and high levels of ROS, including the inflammatory cytokines IL-1β, TNF-α, and IL-6, are important mediators of OA pathogenesis [3]. Being master regulator of antioxidative stress, Nrf2 signaling regulates the expression of antioxidant responsive genes and phase II detoxifying enzymes such as NQO1, HO-1, glutathione S-transferase, glutathione peroxidase, glutamate–cysteine ligase, and peroxiredoxin I that counteract the oxidative damage in tissue injury by enhancing the removal of cytotoxic ROS [42]. Several studies have shown an indispensable role of Nrf-2 in the regulation of inflammatory responses and its deficiency had been shown to increase susceptibility to inflammatory disorders [43, 44]. Therefore, we hypothesized that Nrf2 activation by a natural flavonoid may be chondroprotective in OA. We employed an in vitro model of cartilage damage using human OA cartilage explants and chondrocytes to demonstrate the protective role of Wogonin-mediated Nrf2 activation and also elucidated the mechanism of Nrf2 activation by Wogonin in OA chondrocytes under pathological conditions. Wogonin-mediated increase in ROS and decrease in GSH levels were associated with increased activation of Nrf2 and an increased transcription of Nrf2-dependent genes including HO-1, GCLC, SOD-2 and NQO-1 in OA chondrocytes stimulated with IL-1β in vitro (Fig. 4, 5A–D, 6A–D).
By investigation of different upstream kinases leading to Nrf2 activation, we obtained data indicating that Wogonin induced Nrf2 activation was not mediated through the activation of PI3K/AKT/GSK3β signaling axis, but through the activation of the MAP kinase ERK1/2 (Fig. 6E). We also found that treatment with Wogonin induce the generation of ROS in OA chondrocytes and this may be related to the observed activation of ERK1/2 as ROS mediated signaling is considered an important mediator of MAP Kinase activation [45]. The modulation of Nrf2 activation via direct inhibition of Keap1-Nrf2 protein-protein interaction has evolved as an effective molecular target for various diseases. In silico molecular docking study, within the limitations discussed above, suggests that Wogonin specifically binds to Arg415 and Asn414 in Kelch domain of Keap1 protein, thereby directly blocking the binding site of Nrf2 in Keap 1 protein and thus may restrict/interfere with the binding of Nrf2 and lead to its activation (Fig. 5E, F).
Activation of Nrf2 has been shown to inhibit the inflammatory pathways in a variety of inflammatory conditions [46, 47]. A recent study using nutraceuticals showed that protandim and 6-gingerol mediated Nrf2 activation offers protection against IL-1β-induced NO and PGE2 production in OA chondrocytes [48]. Wogonin treatment of OA chondrocytes under stimulation with IL-1β completely inhibited the expression and production of varieties of inflammatory mediators including COX-2, PGE2, iNOS, NO, IL-6 and MMP-13 in human OA chondrocytes (Fig. 1A–E, Fig. 3A, B). To test our hypothesis that activation of Nrf2 mediates the anti-inflammatory effect of Wogonin, genetic ablation of Nrf2 was performed using specific siRNA in human OA chondrocytes. Interestingly, siRNA-mediated depletion of Nrf2 significantly abrogated the Wogonin-mediated suppression of IL-1β-induced expression of inflammatory molecules in OA chondrocytes which supports the involvement of Nrf2/ARE pathway in the observed chondroprotective effects of Wogonin (Fig. 5G, H). We further probed the ability of Wogonin to inhibit molecular pathways involved in matrix degradation, the hallmark of OA. At one end of metabolic axis, Wogonin inhibited the expression, production and enzymatic activities of catabolic mediators including MMP-13, MMP-3, MMP-9, ADAMTS-4, and release of s-GAG and COL2A1 in OA chondrocytes and cartilage explants (Fig. 3A–I). On the other hand, Wogonin treatment elevated the expression of cartilage anabolic factors COL2A1 and ACAN and restores the COL2A1 and proteoglycan contents in the cartilage explants under pathological conditions (Fig. 3G–J). Interestingly, siRNA-mediated depletion of Nrf2 inhibited the suppressive effects Wogonin on the expression of MMP-13 in IL-1β-stimulated OA chondrocytes (Fig. 5H) and abolished the up-regulation of anabolic genes in human OA chondrocytes (Fig. 5I). These results are in agreement with a recent report demonstrating that Nrf2 over-expression reduced the production of MMP-13 in IL-1β stimulated OA chondrocytes [48]. Collectively, these results demonstrate that Wogonin is a potent inducer of anabolic genes in OA chondrocytes in vitro and has the potential to suppress matrix degradation in vivo. However, this need to be tested in whole body models of OA before arriving at a decision.
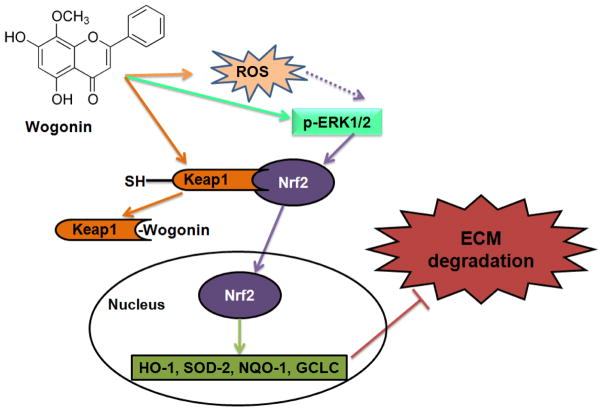
In conclusion, the present study demonstrates that Wogonin exert chondro- and cartilage protection through the suppression of key molecular events involved in oxidative stress, inflammation and matrix degradation in human OA chondrocytes and cartilage explants. Our results also demonstrate that induction of low levels of ROS in OA chondrocytes by Wogonin leads to the modulation of the redox mediated induction Nrf2/ARE pathways via activation of ROS/ERK/Nrf2/HO-1-SOD2-NQO1 signaling axis (Fig. 8). Further, we also highlight the potential of redox modifiers as promising anti-inflammatory and chondroprotective agents. Our study provides novel insights into the development of Nrf2 as a promising candidate for the development of novel therapies for the management of OA.
Figure 8. Schematic representation of proposed mechanism of Wogonin on Nrf2 activation in human OA chondrocytes.
Wogonin treatment caused increased production of ROS and activation of ERK1/2 which leads to activation of redox sensitive transcription factor Nrf2. Wogonin disrupts the Keap1-Nrf2 interaction by directly blocking the binding site of Nrf2 in the Keap-1 protein, thereby competing the Nrf2 binding of Keap1 leading to activation of Nrf2. Activation of Nrf2 lead to the transcription of downstream genes HO-1, NQO-1, SOD-2 and GCLC. Wogonin inhibits matrix degradation through the suppression of MMPs in OA chondrocytes.
Highlights.
Wogonin suppressed the expression and production of inflammatory mediators.
Wogonin suppressed the expression of matrix degrading proteases.
Wogonin enhanced the expression of cartilage anabolic factors COL2A1 and ACAN.
Wogonin modulated the redox homeostasis of OA chondrocytes by disturbing the balance between cellular ROS and GSH levels.
Wogonin activated Nrf2 through induction of ROS/ERK/Nrf2/HO-1-NQO1 signaling axis.
Acknowledgments
This work was supported in part by National Institute of Health grants (RO1-AT-005520; RO1-AT-007373; RO1-AR- 067056; R21-AR- 064890) and funds from the Northeast Ohio Medical University to TMH.
List of abbreviations used
- IL-1β
Interleukin-1 β
- IL-6
Interleukin-6
- MMP
Matrix Metalloproteinase
- ADAMTS
A Disintegrin and Metalloproteinase with Thrombospondin Motifs
- COX-2
Cyclo-oxygenase-2
- iNOS
Inducible Nitric Oxide Synthase
- PGE2
Prostaglandin E2
- COL2A1
Type 2 Collagen
- s-GAG
Sulfated-Glycosaminoglycans
- ACAN
Aggrecan
- GCLC
Glutamate-Cysteine Ligase Catalytic Subunit
- NO
Nitric oxide
- ROS
Reactive Oxygen Species
- ERK
Extracellular signal regulated kinase
- MAPK
Mitogen activated protein kinase
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- NQO1, NAD(P)H
quinone oxidoreductase
- HO-1
Heme Oxygenase-1
- SOD-2
Super Oxide Dismutase-2
- ECM
Extracellular Matrix
- TNF-α
Tumor Necrosis Factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916–26. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146(3):185–96. doi: 10.1016/j.clim.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56(5):1529–36. doi: 10.1002/art.22523. [DOI] [PubMed] [Google Scholar]
- 6.Akhtar N, Haqqi TM. Current nutraceuticals in the management of osteoarthritis: a review. Ther Adv Musculoskelet Dis. 2012;4(3):181–207. doi: 10.1177/1759720X11436238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10(11):549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17(7):363–71. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Cai D, Yin S, Yang J, Jiang Q, Cao W. Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis. Arthritis Res Ther. 2015;17(269) doi: 10.1186/s13075-015-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224(2):171–84. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74(13):1526–39. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 12.Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012;32(4):687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi YS, Lim H, Park H, Kim HP. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression. Biochem Pharmacol. 2003;66(7):1271–8. doi: 10.1016/s0006-2952(03)00463-5. [DOI] [PubMed] [Google Scholar]
- 14.Shen SC, Lee WR, Lin HY, Huang HC, Ko CH, Yang LL, Chen YC. In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E(2) production. Eur J Pharmacol. 2002;446(1–3):187–94. doi: 10.1016/s0014-2999(02)01792-2. [DOI] [PubMed] [Google Scholar]
- 15.Yao J, Zhao L, Zhao Q, Zhao Y, Sun Y, Zhang Y, Miao H, You QD, Hu R, Guo QL. NF-kappaB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014;5:e1283. doi: 10.1038/cddis.2014.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JS, Lee HJ, Lee DY, Jo HS, Jeong JH, Kim DH, Nam DC, Lee CJ, Hwang SC. Chondroprotective Effects of Wogonin in Experimental Models of Osteoarthritis in vitro and in vivo. Biomol Ther (Seoul) 2015;23(5):442–8. doi: 10.4062/biomolther.2015.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan NM, Ansari MY, Haqqi TM. Sucrose, But Not Glucose, Blocks IL1-beta-Induced Inflammatory Response in Human Chondrocytes by Inducing Autophagy via AKT/mTOR Pathway. J Cell Biochem. 2016;26(10):25750. doi: 10.1002/jcb.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haseeb A, Ansari MY, Haqqi TM. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J Orthop Res. 2016;15(10):23262. doi: 10.1002/jor.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mankin HJ. Biochemical and metabolic abnormalities in osteoarthritic human cartilage. Fed Proc. 1973;32(4):1478–80. [PubMed] [Google Scholar]
- 20.Khan NM, Poduval TB. Immunomodulatory and immunotoxic effects of bilirubin: molecular mechanisms. J Leukoc Biol. 2011;90(5):997–1015. doi: 10.1189/jlb.0211070. [DOI] [PubMed] [Google Scholar]
- 21.Khan NM, Poduval TB. Bilirubin augments radiation injury and leads to increased infection and mortality in mice: molecular mechanisms. Free Radic Biol Med. 2012;53(5):1152–69. doi: 10.1016/j.freeradbiomed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Khan NM, Sandur SK, Checker R, Sharma D, Poduval TB, Sainis KB. Pro-oxidants ameliorate radiation-induced apoptosis through activation of the calcium-ERK1/2-Nrf2 pathway. Free Radic Biol Med. 2011;51(1):115–28. doi: 10.1016/j.freeradbiomed.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Henzler T, Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H(2)O(2) across water channels. J Exp Bot. 2000;51(353):2053–66. doi: 10.1093/jexbot/51.353.2053. [DOI] [PubMed] [Google Scholar]
- 24.Haseeb A, Chen D, Haqqi TM. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology (Oxford) 2013;52(6):998–1008. doi: 10.1093/rheumatology/kes363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49(21):6177–96. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa I, Garcia S, Barbier-Chassefiere V, Caruelle JP, Martelly I, Papy-Garcia D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13(9):647–53. doi: 10.1093/glycob/cwg082. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010;18(Suppl 3):S113–6. doi: 10.1016/j.joca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko T, Chiba H, Horie N, Kato T, Kobayashi M, Hashimoto K, Kusama K, Sakagami H. Effect of Scutellariae radix ingredients on prostaglandin E(2) production and COX-2 expression by LPS-activated macrophage. In Vivo. 2009;23(4):577–81. [PubMed] [Google Scholar]
- 30.Khan NM, Haseeb A, Ansari MY, Devarapalli P, Hyanie S, Haqqi TM. Dataset of Wogonin, a natural flavonoid on the viability and activation of NF-κB and MAPKs in IL-1β-stimulated human OA chondrocytes. Data in Brief. 2017 doi: 10.1016/j.dib.2017.03.054. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attur M, Al-Mussawir HE, Patel J, Kitay A, Dave M, Palmer G, Pillinger MH, Abramson SB. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J Immunol. 2008;181(7):5082–8. doi: 10.4049/jimmunol.181.7.5082. [DOI] [PubMed] [Google Scholar]
- 32.Vuolteenaho K, Moilanen T, Knowles RG, Moilanen E. The role of nitric oxide in osteoarthritis. Scand J Rheumatol. 2007;36(4):247–58. doi: 10.1080/03009740701483014. [DOI] [PubMed] [Google Scholar]
- 33.Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–91. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Poulet B, Beier F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res Ther. 2016;18:32. doi: 10.1186/s13075-015-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldring MB. Articular cartilage degradation in osteoarthritis. Hss J. 2012;8(1):7–9. doi: 10.1007/s11420-011-9250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akhtar N, Miller MJ, Haqqi TM. Effect of a Herbal-Leucine mix on the IL-1beta-induced cartilage degradation and inflammatory gene expression in human chondrocytes. BMC Complement Altern Med. 2011;11:66. doi: 10.1186/1472-6882-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura H, Yukitake H, Suzuki H, Tajima Y, Gomaibashi K, Morimoto S, Funabashi Y, Yamada K, Takizawa M. The chondroprotective agent ITZ-1 inhibits interleukin-1beta-induced matrix metalloproteinase-13 production and suppresses nitric oxide-induced chondrocyte death. J Pharmacol Sci. 2009;110(2):201–11. doi: 10.1254/jphs.09076fp. [DOI] [PubMed] [Google Scholar]
- 38.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem. 2007;282(22):16502–10. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 39.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31(6):1121–33. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim EH, Shim B, Kang S, Jeong G, Lee JS, Yu YB, Chun M. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009;126(2):320–31. doi: 10.1016/j.jep.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 41.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45(10):1375–83. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 44.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114(9):1248–59. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta A, Rosenberger SF, Bowden GT. Increased ROS levels contribute to elevated transcription factor and MAP kinase activities in malignantly progressed mouse keratinocyte cell lines. Carcinogenesis. 1999;20(11):2063–73. doi: 10.1093/carcin/20.11.2063. [DOI] [PubMed] [Google Scholar]
- 46.Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181(1):680–9. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abusarah J, Benabdoun HA, Shi Q, Lussier B, Martel-Pelletier J, Malo M, Fernandes JC, Pereira de Souza F, Fahmi H, Benderdour M. Elucidating the Role of Protandim and 6-Gingerol in Protection Against Osteoarthritis. J Cell Biochem. 2016;27(10):25659. doi: 10.1002/jcb.25659. [DOI] [PubMed] [Google Scholar]