Abstract
Bacteria exposed to host serum are subject to the antibacterial effects to the complement system. However, pathogenic microorganisms have evolved mechanisms of evading this immune attack. We have previously demonstrated that at least two R. conorii antigens, RC1281/Adr1 and OmpB β-peptide, contribute to the evasion of complement-mediated killing by binding the complement regulatory proteins vitronectin and factor H. RC1282/Adr2, a protein related to Adr1, is predicted to share similar structural features, suggesting that this protein may also contribute to evasion of complement-mediated killing. Interestingly, the R. prowazekii Adr1 and Adr2(RP828) proteins were originally found to interact with host cell surface proteins, suggesting their putative roles as adhesins in this pathogenic rickettsial species. In this study, we expressed both R. conorii and R. prowazekii Adr2 on the surface of a non-adherent, serum-sensitive strain of E. coli to examine the potential role of this protein to mediate evasion of complement-mediated killing and adherence to host cells. We demonstrate that, similar to R. conorii Adr1, R. conorii and R. prowazekii Adr2 are sufficient to mediate serum resistance and to promote interaction with the host complement regulator vitronectin. Furthermore, we demonstrate that expression of Adr2 in a non-adherent strain of E. coli is insufficient to mediate adherence to cultured mammalian endothelial cells. Together, our data demonstrate that the R. conorii and R. prowazekii Adr2 protein does not participate in the interactions with mammalian cells, but rather, participates in the evasion of killing by complement.
Introduction
Rickettsia conorii is a small Gram-negative, obligate-intracellular bacterium, and the etiological agent of endemic Mediterranean spotted fever (MSF). MSF is transmitted by an arthropod vector, and subsequent dissemination of the microbe throughout a human host results in the development of a characteristic maculopapular dermal rash [1]. Although this disease is commonly referred to as a milder version of the spotted fever group rickettsioses, lack of timely medical treatment can prove fatal [2]. Seroprevelance in affected canine populations despite increased use of anti-tick treatments, cyclic re-emergence of disease, and risk of fatal clinical complications in infected persons demonstrate that this pathogen is an active threat to human health [3–5].
Rickettsial pathogens must employ several mechanisms to persist in the presence of opsonophagocytosis or host bactericidal complement-mediated lysis while in blood circulation, during invasion of target cells, and subsequent intracellular proliferation. Spotted fever group (SFG) Rickettsia sp. express a multitude of membrane-bound proteins which have been demonstrated not only to play integral roles in rickettsial pathogenesis through processes of adherence and invasion of host cells, but confer resistance to anti-bacterial host responses [6–14]. Among the characterized outer membrane proteins, Rickettsia conorii RC1281/Adr1 was demonstrated to recruit host regulatory complement protein vitronectin in order to confer resistance to serum-mediated killing [9]. Its paralog, Adr2(RC1282), has been identified in all pathogenic species in the genus Rickettsia, and is implicated in the entry of R. prowazekii into non-phagocytic mammalian cells [12, 15]. However, its putative role in SFG rickettsiae, such as R. conorii, has yet to be elucidated.
We have previously demonstrated that R. conorii remains viable in the presence of serum complement proteins, and that depletion of one of these regulatory proteins, such as factor H and vitronectin, is not sufficient to completely ablate serum sensitivity [10, 11]. These findings indicate that mechanisms of redundancy exist within the rickettsial genome, and additional membrane bound proteins may contribute in the serum-resistance phenotype in a similar fashion.
Results
Adr2 is conserved among pathogenic species of Rickettsia
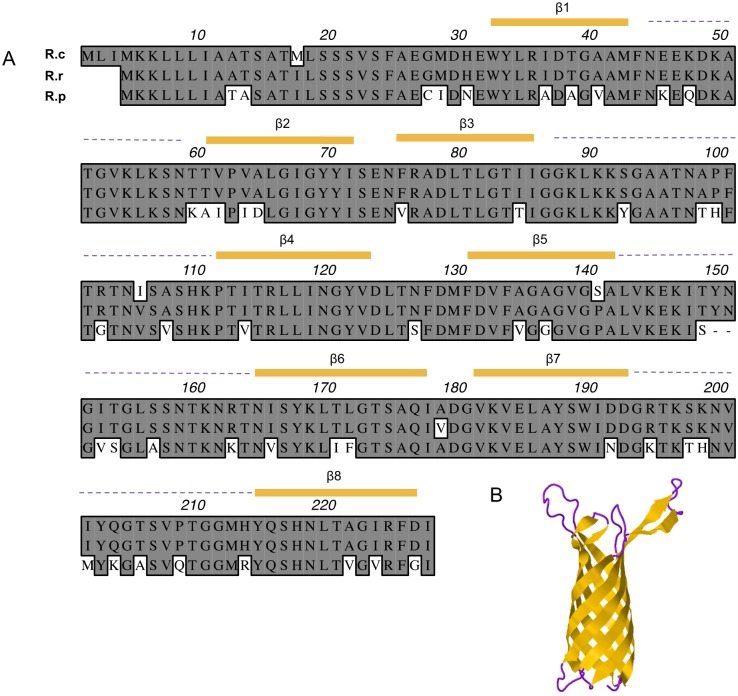
Alignment of amino acid profiles for R. conorii, R. rickettsii, and R. prowazekii Adr2 is shown in Fig 1A. Analysis of R. conorii Adr2 sequence demonstrated sequence similarities to proteins belonging to the superfamily OMPA [16]. These proteins are characterized by having trans-membrane beta-barrels, which are thought to form a pore-like structure at the outer membrane [17]. The Phyre2 protein structure-modeling algorithm predicts that two of the highest confidence structural models are based on threading against the N. gonorrhea NspA protein (PDB: d1p4ta) and the Opa60 protein (PDB: c2nlha). The model for R. conorii Adr2 predicts a protein that contains 8 outer membrane-spanning β-sheets and 4 surface exposed peptides (Fig 1B). Although the similarity between the deduced amino acid sequences of Adr1 and Adr2 in R. conorii is approximately 41%, both predicted structures based on the Phyre2 algorithm are virtually identical, suggesting that both proteins may share functional similarities [15].
Fig 1. Adr2 is highly conserved among pathogenic rickettsial species.
(A) ClustalW alignment of Adr2 homologs from rickettsial species in both Spotted Fever Group (R. conorii, R. rickettsii) and Typhus Group (R. prowazekii). Predicted β-sheets and surface exposed loops are indicated in yellow and purple dashed lines, respectively. (B) Phyre2 predicted structure of R. conorii Adr2 demonstrates of 8 transmembrane β-sheets configured in a “barrel”-like structure with possible surface exposed loops.
Adr2 is present at the R. conorii outer membrane
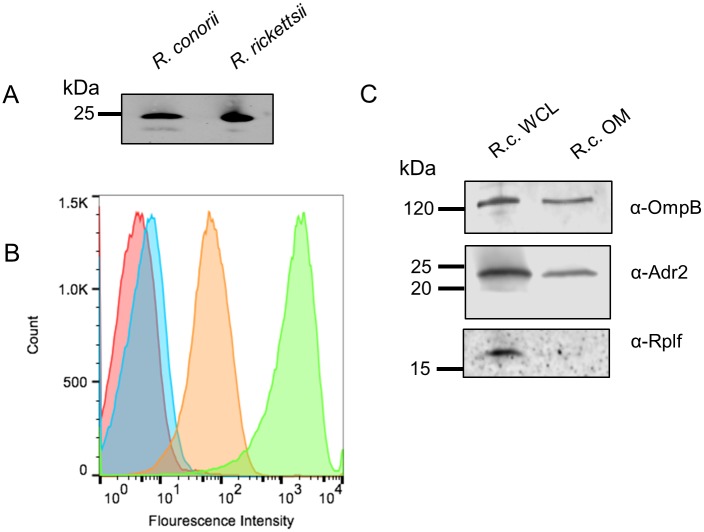
To demonstrate that Adr2 is surface exposed in R. conorii, we produced polyclonal antiserum raised against the predicted surface exposed domains termed loops 1 and 2. Western immunoblot analysis of both R. conorii Malish 7 and R. rickettsii Sheila Smith whole cell protein lysates (WCL) yielded reactive bands at approximately 25 kDa for both species (Fig 2A). To verify surface expression of Adr2, paraformaldehyde-fixed R. conorii cells were stained with anti-Adr2 antibody and a fluorophore-tagged secondary antibody. As shown in Fig 2B, flow cytometric analysis of stained bacteria demonstrated a significant shift in fluorescence intensity (orange trace) compared to untreated bacteria (red trace) or R. conorii labeled with secondary antibody only (blue trace). Additionally, we verified expression of Adr2 in the rickettsial outer membrane by western-blot analysis of isolated R. conorii outer membrane fractionations (Fig 2C). Taken together, these results indicate that Adr2 is present at the surface of R. conorii and that segments of this protein are exposed to the extracellular environment.
Fig 2. Outer-membrane expression of Adr2 in R. conorii.
(A) Western blot analysis using rabbit-anti-Adr2 antiserum confirms the expression in R. conorii Malish 7 and R. rickettsii Sheila Smith whole cell lysate (WCL). (B) Flow cytometry confirmed the expression of Adr2 at the surface of R. conorii. A shift in fluorescence is observed when fixed R. conorii was incubated with primary and secondary antibodies (orange) compared to samples prepared with only primary or secondary (red and blue, respectively). A sample is incubated with Anti-R. conorii serum (green) as a positive control of the flow cytometer. (C) Western blot analysis of R. conorii whole cell lysates (WCL) and outer-membrane(OM) preparations using antibodies against OmpB (mAb 6B6.6) or Adr2 demonstrates the presence of reactive species in both WCL and OM preparations. Anti-RplF (50s ribosomal protein L6) is used a control for cytoplasmic contents.
Adr2 mediates resistance to serum killing in complement
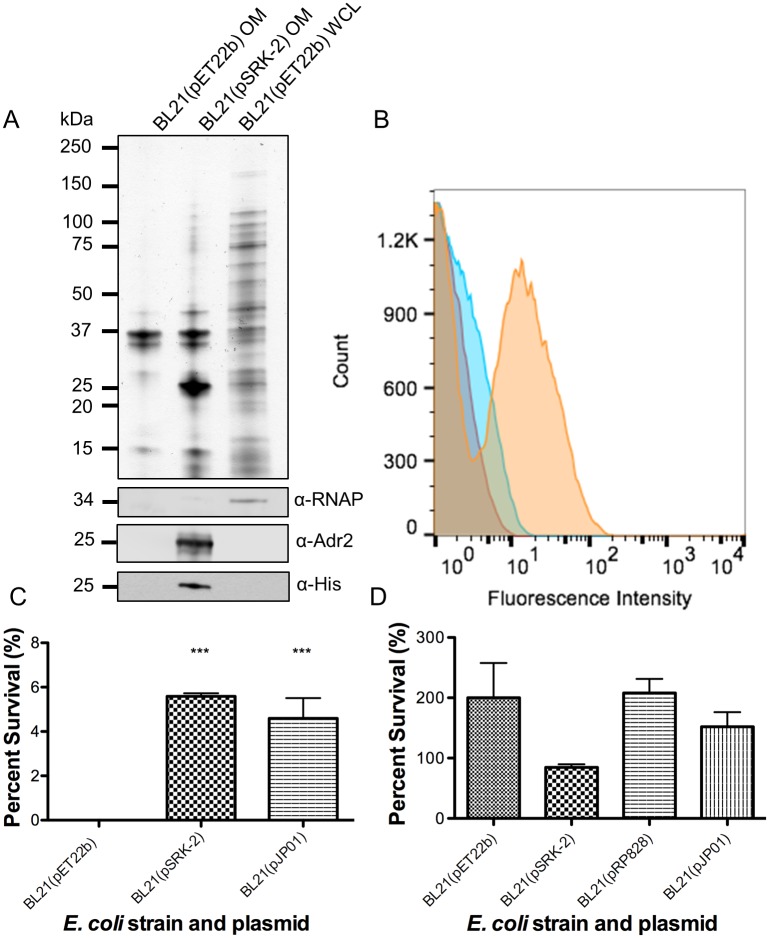
To determine if R. conorii Adr2 is expressed at the outer-membrane of E. coli, we transformed a non-adherent, serum-sensitive strain of E. coli (BL21 (DE3)) with pSRK-2, a pET22b derivative containing an IPTG-inducible promoter driving expression of R. conorii adr2 with a C-terminal His6 tag. Expression of Adr2 in transformed E. coli was verified by the presence of an immune reactive species at the predicted molecular weight within isolated outer membrane (OM) protein fractions (Fig 3A). In addition, surface expression of Adr2 in E. coli was verified by flow cytometry using anti-Adr2 antibodies directed against predicted extracellular domains (Fig 3B). These results demonstrate that expression of Adr2 in E. coli results in proper localization of this protein to the outer-membrane.
Fig 3. Expression of Adr2 at the E. coli outer-membrane is sufficient to mediate resistance to serum-killing.
(A) OM preparations or WCL from E. coli transformed with the empty vector (pET22b) or the plasmid encoding for R. conorii His6-tagged Adr2 are separated by SDS-PAGE. Subsequent silver staining demonstrates similar protein loading and migration. Anti-E. coli RNA Polymerase alpha (RNAP) is used to demonstrate the presence of cytoplasmic contents. (B) Flow cytometry analysis reveals the expression of Adr2 at the surface of E. coli. A shift in fluorescence was observed in BL21(pSRK-2 [R. conorii Adr2]) in cultures incubated with both primary and secondary antibodies (orange) compared to primary(red) or secondary(blue) alone. (C) Expression of R. conorii Adr2(pSRK-2) in E. coli (BL21) is sufficient to mediate serum-killing to normal human serum. R. conorii Adr1(pJP01) is used as a positive control. Results are shown as the mean ± SD (P values: *** = 0.0007). (D) Serum-sensitive E. coli (pET22b) and cultures expressing Adr2 or Adr1 survive when incubated with heat-inactivated normal human serum.
To demonstrate whether the expression of Adr2 could mediate serum resistance, we incubated cultures of Adr2-expressing E. coli with either PBS as a control or normal human serum (NHS) in PBS. Resistance to serum killing was measured by a colony forming unit (CFU)-based assay and is represented as the percentage of bacteria that survive serum challenge versus the same inoculum incubated in PBS [10]. As seen in Fig 3C, bacteria expressing Adr2 were significantly resistant to serum killing compared to bacteria harboring the empty vector. The observed survival was comparable to those previously observed for R. conorii Adr1-expressing E. coli (Fig 3C) and Riley et al., 2014. In contrast, bacteria harboring the empty vector as well as those expressing Adr2 and Adr1 survived when incubated with heat-inactivated NHS (Fig 3D). These results demonstrate that similar to Adr1, Adr2 is sufficient to confer resistance to serum killing when expressed at the outer membrane of E. coli.
Adr2-expressing E. coli binds host complement regulatory protein vitronectin
We next sought to determine how Adr2 contributes to the observed serum-resistance phenotype. We hypothesized that based on structural and functional similarities to Adr1, Adr2 would similarly bind a soluble complement regulatory protein such as vitronectin. To test this hypothesis, we incubated Adr2-expressing E. coli with NHS, co-sedimented bacteria and any interacting proteins, washed away any unbound proteins with PBS, and then prepared whole cell protein lysates. Bacterial proteins and any interacting serum proteins were separated by SDS-PAGE. Western immunoblot analysis revealed a reactive species at approximately 75 kDa in lysates of Adr2-expressing E. coli, but not in control E. coli lysates harboring the empty vector (Fig 4) when probed with an anti-vitronectin antibody. These results demonstrate that Adr2 interacts with vitronectin and this acquisition correlates to the observed serum-resistance phenotype.
Fig 4. R. conorii Adr2 binds complement regulatory protein vitronectin.
(A) E. coli BL21 harboring the empty vector pET22b, the plasmid encoding R. conorii His6-tagged Adr2 (pSRK-2) or R. conorii His-tagged Adr1 (pJPO1) were incubated with normal human serum. Western immunoblot analysis revealed a reactive band at approximately 75 kDa in all lanes except for the empty vector when probed with antibody raised against vitronectin. Expression of both Adr2 and Adr1 was confirmed by anti-His6, and equal loading was demonstrated with anti-E. coli RNA Polymerase alpha (RNAP). (B) Western immunoblot analysis of multimeric vitronectin binding to E. coli expressing Adr1 derivative mutant (pAF17) or R. conorii Adr2. Expression of both the Adr1 derivative and Adr2 was confirmed by anti-His6, and equal loading was verified using anti-E. coli RNA polymerase alpha (RNAP).
As seen in the amino acid alignment of R. conorii Adr2 and its homologs, sequence homology is greater amongst pathogenic SFG rickettsial species, but is still significant amongst members of the TG rickettsiae, including R. prowazekii (97.8% to 88.1% respectively). We hypothesized that because of this putative protein structural conservation, the R. prowazekii Adr2 protein would be sufficient to interact with Vn and provide resistance to complement-mediated killing. As predicted, Vn acquisition and serum resistance was observed at levels comparable to R. conorii Adr2 (Fig 5A and 5C). We further assessed the acquisition of Vn by determining whether Adr2 could directly bind Vn in absence of serum proteins. Cultures expressing Adr2, and E. coli harboring pAF17, a plasmid encoding for a non-Vn-binding Adr1 derivative, were incubated with multimeric Vn and whole cell lysates were subsequently prepared, separated by SDS-PAGE, and analyzed by western blot [18]. Probing with an anti-vitronectin antibody revealed a reactive band at approximately 75 kDa in lysates of Adr2 expressing E. coli, but not in the lanes containing pAF17 lysate (Figs 4B and 5B). These results demonstrate that direct Vn binding by Adr2 is a phenotype conserved across both SFG and TG rickettsial species.
Fig 5. R. prowazekii Madrid E Adr2 confers acquisition of vitronectin and resistance in serum.
(A) E. coli BL21(DE3) harboring the empty vector pET22b or the plasmid encoding R. prowazekii Madrid E His6-tagged Adr2 (pRP828) were incubated with normal human serum. Western immunoblot analysis revealed a reactive band at approximately 75 kDa in the lane with R. prowazekii Adr2 only when probed with antibody raised against vitronectin. Expression of Adr2 was confirmed by anti-His6, and equal loading was demonstrated with anti-E. coli RNA Polymerase alpha (RNAP). (B) Western immunoblot analysis of multimeric vitronectin binding to E. coli expressing Adr1 derivative mutant (pAF17) or R. conorii Adr2. Expression of both the Adr1 derivative and Adr2 was confirmed by anti-His6, and equal loading was verified using anti-E. coli RNA polymerase alpha (RNAP). (C) Expression of R. prowazekii Adr2 in E. coli BL21(DE3) is sufficient to mediate resistance in the presence of serum. Results are shown as the mean ± SD (P value: ** = 0.0024) and E. coli harboring R. conorii Adr1 (pJP01) was used as a positive control. (D) OM preparations or WCL from E. coli transformed with the empty vector (pET22b) or the plasmid encoding for R. prowazekii His6-tagged Adr2 are separated by SDS-PAGE. Subsequent silver staining demonstrates similar protein loading and migration. Anti-His6 was used to demonstrate expression of R. prowazekii Adr2. A reactive band at approximately 25 kDa was observed when probed with anti-R. conorii Adr2.
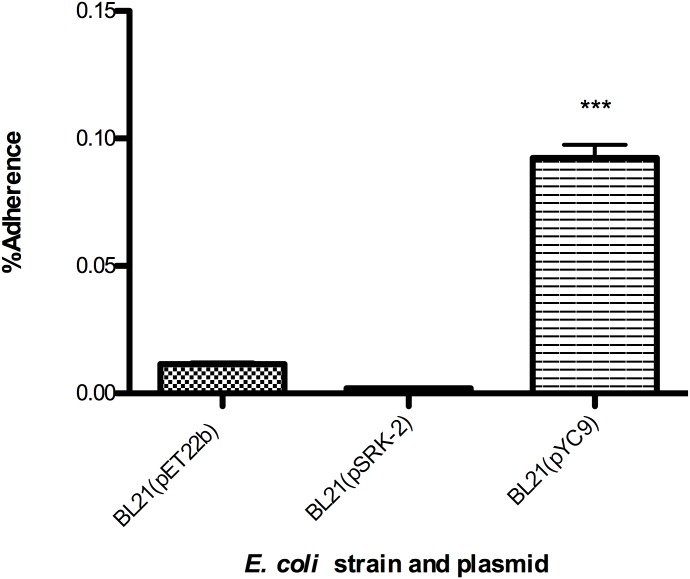
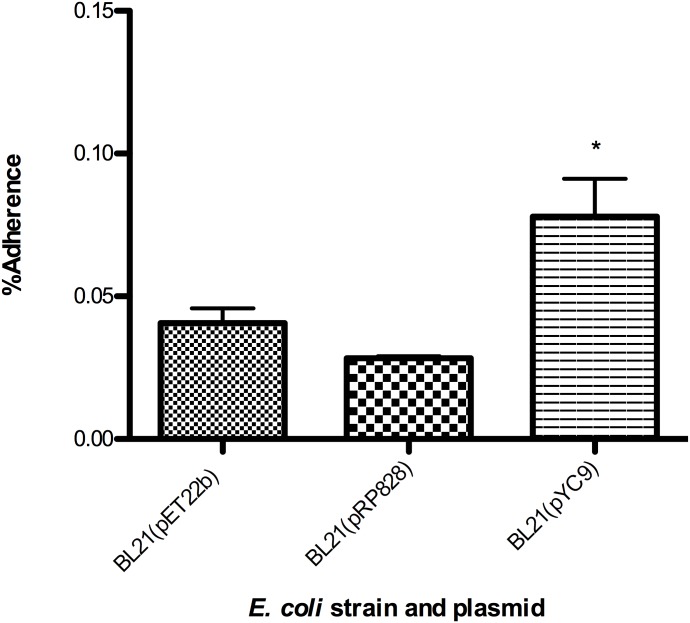
Recombinant E. coli expressing R. conorii Adr2 are not adherent to mammalian cells
Previous studies have demonstrated that incubation of pathogenic Typhus Group (TG) rickettsial species with antiserum produced against Adr2 results in an overall reduction in infection of endothelial host cells [12, 15]. These findings suggested that R. prowazekii Adr2 may function as a potential adhesin and interacts with still unidentified human cell surface receptors. To test whether the R. conorii Adr2 homologue could similarly function as an adhesin, we employed a well-established heterologous expression system to examine the ability of Adr2-expressing E. coli to adhere to mammalian cells in the absence of serum proteins [6–11, 19, 20]. E. coli cultures expressing Adr2 were applied to confluent monolayers of human endothelial-like cell culture model (EA.hy926) cells and centrifuged to induce contact. Bacteria expressing the R. conorii OmpB protein (pYC9) or containing an empty vector (pET22b) were used as positive and negative controls, respectively. Following a brief incubation period, the cells were thoroughly washed free of non-adherent bacteria, and potential Adr2-mediated adherence determined by enumeration using a CFU-based quantification assay. As shown in Fig 6, there is no significant difference in the ability Adr2-expressing bacteria to adhere to endothelial cells when compared to E. coli harboring the empty vector, pET22b. In contrast, E. coli expressing the R. conorii protein, Sca5/OmpB [6] are adherent to these cells. In addition, we investigated whether the R. prowazekii Adr2 protein when expressed in a heterologous E. coli host was sufficient to mediate adherence to mammalian endothelial cells. As shown in Fig 7, similar to what was observed for the R. conorii homologue, the R. prowazekii Adr2 is not sufficient to confer adherence to mammalian cells when expressed at the outer-membrane of E. coli. Together, these data demonstrate that while independent expression of the R. conorii Adr2 and the R. prowazekii Adr2 proteins at the surface of E. coli is sufficient to contribute to evasion of complement-mediated killing, neither Adr2 protein is sufficient to mediate adherence to mammalian cells.
Fig 6. Adherence potential of R. conorii Adr2 expressing E. coli.
Expression of R. conorii Adr2 in E. coli (BL21) is not sufficient to mediate cell adherence to EA.hy926 cells. The results are expressed as percentages of recovered adherent bacteria based on the total bacterial input. Results are shown as the mean ± SD (P values: *** <0.0001).
Fig 7. Adherence potential of R. prowazekii Adr2 expressing E. coli.
Expression of R. prowazekii Adr2 in E. coli (BL21) is not sufficient to mediate cell adherence to EA.hy926 cells. The results are expressed as percentages of recovered adherent bacteria based on the total bacterial input. Results are shown as the mean ± SD (P values: * = 0.0123).
Materials and methods
Amino acid alignment and protein modelling
Amino acid sequences for Adr2 from R. conorii Malish 7 (gene identification number, gi: 499284784), R. rickettsii Sheila Smith (gi:157801598), R. prowazekii Madrid E (gi: 3861354) were aligned using ClustalW (Mac Vector 14.1). The R. conorii Adr2 sequence was analyzed by the Phyre2 protein structural prediction software. Secondary structures were predicted using Phyre2 modeling software.
Antibodies
Anti-Adr2 polyclonal antiserum was generated from immunization of rabbits with peptides CNEEKDKATGVKLKSNTT, CKKSGAATNAPFTRTNISASHK, CTGLSSNTKNRTNISY, and CELAYSWIDDGRTKSKN corresponding to Adr2 amino acids 44–60, 90–110, 153–167, and 184–199 (loops 1, 2, 3, and 4 respectively) (Yenzym Antibodies). Total antibodies were recovered from antiserum utilizing HiTrapProteinA columns (GE Healthcare). Concentrations of the resulting antibodies were determined using Bradford assay. Polyclonal antibodies against R. conorii RplF have been described elsewhere [9].
Plasmid assembly
Amplification of adr2 from R. conorii Malish 7 genomic DNA flanked by a BamHI restriction site was accomplished using the primers 5’-GGATCCTGCAACAATGTTGTCTTCTA-3’ and 5’-GGATCCATATATCAAATCTTATACCG-3’. The resulting adr2 DNA fragment was cloned into pCR2.1, digested with BamHI (New England Biolabs, Ipswich, MA), and ligated into the vector pET22b resulting in the construction of pSRK-2. pRP828 was constructed similarly using the primers 5’-GGATCCAATTTTATCTTCTAGTGTATCG-3’ and 5’-GGATCCGATATACCAAATCTTACACCTACTGT-3’. Construction of pJPO1, pAF17, pYC9 is described elsewhere [6, 9, 18]. A list of plasmids used in this study can be found in S1 Table.
Culture of bacterial and mammalian cells
EA.hy926 and Vero (ATCC) cells were cultured in Dulbecco’s Modified Eagle Medium (Life Technologies) supplemented with 10% heat-inactivated FBS, 1% sodium pyruvate (Mediatech), and 1% MEM Non-Essential Amino Acids (Mediatech) at 37°C in 5% CO2 until used for experimentation.
R. conorii Malish 7 was propagated in Vero cells, needle-lysed and sucrose-gradient purified as previously described [21]. The resulting titers were enumerated as previously described [7]. E. coli BL21(DE3)(pET22b, pJP01, pYC9, pSRK-2) were grown overnight at 37°C in LB supplemented with carbenicillin. The bacteria were diluted 1:10 in fresh media, grown to OD600 ~0.5, and induced with 0.5mM IPTG for 4 h at 37°C. BL21(DE3)(pJP01) received 0.6mM IPTG.
Bacterial fractionation
Outer membrane (OM) fractionations of R. conorii Malish 7 or E. coli BL21 (DE3) harboring either pET22b or pSRK-2 were prepared using sarkosyl solubilization as previously described [9, 22]. The isolated OM fractions were separated on SDS-PAGE and immunoblotted with the polyclonal rabbit anti-Adr2 described above and secondary antibody IRDye donkey anti-rabbit 800CW. In order to verify the efficiency of the fractionation, the lysates were probed for E. coli cytoplasmic contents using antibodies against E. coli RNA polymerase α-subunit. (4RA2, Neoclone). Additionally, R. conorii fractionations were probed with RplF antibody to verify the presence of cytosolic elements as previously described [9].
Binding of host complement regulatory proteins
Induced E. coli BL21(DE3) cultures harboring pET22B, pSRK-2, or pJP01 were prepared as described above, and serum binding assays were performed as previously described [9]. Briefly, the bacterial cultures were incubated in either PBS or 50% NHS/PBS over ice in order to prevent complement-mediated killing. The samples were washed thoroughly with ice-cold PBS, lysed in 2X SDS-PAGE loading buffer, and separated by SDS-PAGE and then transferred to nitrocellulose membranes. Separated proteins on the nitrocellulose membranes were probed for vitronectin binding by western immunoblot analysis using rabbit anti-vitronectin (Complement Tech), and secondary antibody goat anti-rabbit-HRP(Sigma). Expression R. conorii Adr2 was confirmed using anti-His antibody (Genescript). Binding of multimeric vitronectin was assessed as previously described [18]. Cultures expressing Adr2 or Adr1 derivative protein were incubated with 25 ng/mL of multimeric vitronectin (Innovative Research) at room temperature for 1 hr. The samples were washed with PBS, separated by SDS-PAGE and analyzed by western immunoblot using the aforementioned anti-vitronectin (Complement Tech) and secondary goat anti-rabbit-HRP antibodies(Sigma). Expression of proteins was verified using mouse anti-His (Genescript).
Cell adherence assay
Cell adherence assays were performed as previously described [6, 7]. Briefly, induced cultures of E. coli BL21 (DE3) harboring pET22B, pSRK-2, pRP828, or pYC9 were added to a confluent monolayer of EA.hy926 cells in serum-free media. To initiate adherence, a low speed centrifugation of 200xg for 5 min was performed and followed with a 20 min incubation at 37°C in the presence of 5% CO2. Cells were washed three times in PBS to remove non-adherent bacteria, and subsequently lysed with 0.1% Triton X-100 in sterile H2O. The resulting lysates were serially diluted in PBS and plated on LB agar to enumerate adhered bacteria. Results were expressed as a percentage of recovered bacteria based on the input inoculum.
Serum resistance
E. coli BL21(DE3) cultures harboring pET22B, pSRK-2, or pJP01 were grown as described above. Cultures were washed with PBS and approximately 106 colony forming units (cfu) were resuspended in either 200μl of PBS or 50% NHS/PBS and incubated for 1 h at 37°C. Following the incubation, the cultures were serially diluted in PBS and plated on LB-agar plates. The results were expressed as bacteria recovered (cfu) in PBS and 50%NHS/PBS following the incubation period and plotted on a logarithmic scale. These data represent independent triplicate samples, and the experiment was performed a minimum of three times.
Flow cytometry analysis of Adr2
R. conorii or E. coli (BL21(DE3)) harboring pSRK-2 fixed with 4% PFA were used to probe for the presence of Adr2. Surface expression was detected using anti-Adr2 L1/L2 and secondary goat anti-rabbit-AlexaFluor488 (Molecular Probes). Bacteria were analyzed with a BD cytometer using FITC (488 nm excitation, 530/30 nm emission) and FloJo software.
Statistical analysis of results
Data obtained for determination of serum resistance and cell adherence were analyzed by one-way ANOVA where significance was determined for p < 0.05.
Discussion
In the present study, we describe an additional factor, Adr2, in two pathogenic rickettsial species that is sufficient to mediate resistance to serum-mediated killing [9, 10, 23]. We have demonstrated that recombinant Adr2 proteins from both R. conorii (RC1282) and R. prowazekii (RP828) are localized to the outer membrane when expressed in E. coli and are sufficient to confer resistance to innate deleterious effects of serum. We have additionally demonstrated the acquisition of fluid-phase host complement-regulatory vitronectin (Vn) by these expressed outer-membrane proteins.
Initial proteomic-based analysis of R. prowazekii(RP828) and R. conorii Adr2(RC1282) described a protein modeled after the Neisseria meningitidis NspA [24]. This initial analysis depicted a protein containing 10 trans-membrane β-strands and five extracellular domains. In contrast, our analysis using the Phyre2 online protein structural prediction server revealed a putative structure containing 8 trans-membrane regions and four extracellular domains (loops). However, it should noted that both of the predicted models reported were dependent on different algorithmic modeling software and are not based upon derived crystal structures [25]. Despite advances in crystallography studies to date, there are only a few solved crystal structures of proteins from rickettsial species [26–28]. As such, neither of these predicted models can be discarded until crystal structures of Adr2 proteins are solved.
Interestingly, while NspA was originally speculated to function similarly to the related opacity proteins (Opas) as an adhesin, recent data has revealed that NspA also functions as a serum-resistance factor by binding complement regulatory protein, Factor H (fH) [29]. We have also demonstrated the deposition of another regulatory protein, vitronectin (Vn), to surface-expressed R. conorii Adr1 and that this interaction is crucial to survival of this pathogen in the presence of normal human serum. Vitronectin is a multi-faceted regulatory protein that exists in human extracellular matrix and serum. Within the serum, Vn plays a role in cell adhesion, hemostasis, and regulating the formation of the terminal complement complex which results in the lysis of target cells [30]. We have demonstrated that both SFG and TG rickettsial species likely possess redundancy in the mechanisms by which this class of pathogens subverts complement-mediated killing [7]. Elucidating the various strategies evolved by pathogenic bacteria in order to evade this innate immune clearance from the blood circulation may reveal conserved proteins that could function as candidates for therapeutic intervention, which has recently been described [13, 14].
As seen in the Adr2 alignment in Fig 1A, the predicted extracellular motifs are well conserved among SFG and TG rickettsial species. Interestingly, the predicted Adr2 extracellular moieties 3 and 4 contain an overabundance of positively charged residues (23% and 18% respectively), as has been previously reported for the predicted extracellular loops 3 and 4 of Adr1 [7]. The analogous regions in R. conorii Adr1 were sufficient to not only confer serum resistance, but also demonstrated the ability to sequester Vn when expressed in E. coli. Indeed, a recent study examining Vn binding by various human pathogenic bacteria revealed a potential role of electrostatic interactions in this crucial protein-protein interaction [31]. Whether the lysine and other positively charged residues within extracellular Adr2 domains also play a role in Adr2-Vn binding and subsequent serum resistance is an area of current investigation.
Serum analysis of patients with active R. conorii infections reveals an increase in markers of complement activation [32]. We posit that, even in the face of an activated complement cascade, Rickettsia species are resistant to complement-mediated killing [9–11, 18]. Accordingly, the studies described herein contribute to our understanding of rickettsial resistance to complement mediated killing. Future analysis will be needed to elucidate the relationship between complement activation and rickettsial pathogenesis.
It has previously been reported that both R. prowazekii Adr2 and R. conorii Adr2 interact with unidentified surface-exposed mammalian cell proteins, suggesting that Adr2 may function as an adhesion [25]. In this work, we examined the potential of both Adr2 proteins to mediate adhesion to host cells using our gain-of-function E. coli heterologous expression system. When either Adr2 from R. conorii or R. prowazekii is expressed at the surface of E. coli, this protein does not promote adherence to host cells as compared to E. coli harboring an empty vector. It is possible that in E. coli, recombinant Adr2 proteins do not fold into the same structure at the outer-membrane as on the surface of native rickettsial species. However, we would argue that while possible, this scenario is not likely as recombinant Adr2 proteins assemble at the outer-membrane and retain the ability to mediate interactions with Vn and contribute to serum resistance. Recombinant Adr2 does not mediate adherence using this experimental system; however, this phenotype does not preclude additional functions of Adr2 at the surface of both intact R. conorii and R. prowazekii cells. Whether the putative adhesin function or the Vn-binding function of Adr2 proteins contribute to pathogenesis remains to be elucidated.
Supporting information
(TIF)
Acknowledgments
The authors would like to thank Abbie Fish, Dr. Isaura Simoes, and Pedro Curto for their helpful comments and technical expertise. We would also like to acknowledge members in and support from The Center for Experimental Infectious Disease Research COBRE.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded in part by awards from The National Institues of Health, National Institute of Allergy and Infectious Diseases (AI072606 and AI10392) to JJM. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Walker DH, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Micro. 2008;6(5):375–86. [DOI] [PubMed] [Google Scholar]
- 2.Rovery C, Brouqui P, Raoult D. Questions on Mediterranean spotted fever a century after its discovery. Emerg Infect Dis. 2008;14(9):1360–7. doi: 10.3201/eid1409.071133 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espejo E, Andrés M, Pérez J, PRAT J, Guerrero C, Muñoz MT, et al. Prevalence of antibodies to Rickettsia conorii in human beings and dogs from Catalonia: a 20-year perspective. Epidemiology & Infection. 2016; FirstView:1–6. doi: 10.1017/S0950268816000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espejo-Guerrero A, V-G F., Torres-Cuenca M.; Garcia-Magallón B. [Mediterranean Spotted Fever: epidemiological Assessment in Spain during the Period 2009–2012]. Revista española de salud pública. 2015;89(3):321–8. doi: 10.4321/S1135-57272015000300009 [DOI] [PubMed] [Google Scholar]
- 5.Crespo P, Seixas D, Marques N, Oliveira J, da Cunha S, Melico-Silvestre A. Mediterranean spotted fever: case series of 24 years (1989–2012). Springerplus. 2015;4:272 doi: 10.1186/s40064-015-1042-3 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan YG, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol. 2009;11(4):629–44. doi: 10.1111/j.1462-5822.2008.01279.x ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley SP, Goh KC, Hermanas TM, Cardwell MM, Chan YG, Martinez JJ. The Rickettsia conorii autotransporter protein Sca1 promotes adherence to nonphagocytic mammalian cells. Infect Immun. 2010;78(5):1895–904. doi: 10.1128/IAI.01165-09 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillman RD, Baktash YM, Martinez JJ. OmpA-mediated rickettsial adherence to and invasion of human endothelial cells is dependent upon interaction with alpha2beta1 integrin. Cell Microbiol. 2013;15(5):727–41. doi: 10.1111/cmi.12068 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley SP, Patterson JL, Nava S, Martinez JJ. Pathogenic Rickettsia species acquire vitronectin from human serum to promote resistance to complement-mediated killing. Cell Microbiol. 2014;16(6):849–61. doi: 10.1111/cmi.12243 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley SP, Patterson JL, Martinez JJ. The rickettsial OmpB beta-peptide of Rickettsia conorii is sufficient to facilitate factor H-mediated serum resistance. Infect Immun. 2012;80(8):2735–43. doi: 10.1128/IAI.00349-12 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan YG, Riley SP, Chen E, Martinez JJ. Molecular basis of immunity to rickettsial infection conferred through outer membrane protein B. Infect Immun. 2011;79(6):2303–13. doi: 10.1128/IAI.01324-10 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong W, Xiong X, Qi Y, Jiao J, Duan C, Wen B. Identification of novel surface-exposed proteins of Rickettsia rickettsii by affinity purification and proteomics. PLoS One. 2014;9(6):e100253 doi: 10.1371/journal.pone.0100253 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong W, Xiong X, Qi Y, Jiao J, Duan C, Wen B. Surface protein Adr2 of Rickettsia rickettsii induced protective immunity against Rocky Mountain spotted fever in C3H/HeN mice. Vaccine. 2014;32(18):2027–33. doi: 10.1016/j.vaccine.2014.02.057 . [DOI] [PubMed] [Google Scholar]
- 14.Gong W, Wang P, Xiong X, Jiao J, Yang X, Wen B. Enhanced protection against Rickettsia rickettsii infection in C3H/HeN mice by immunization with a combination of a recombinant adhesin rAdr2 and a protein fragment rOmpB-4 derived from outer membrane protein B. Vaccine. 2015;33(8):985–92. doi: 10.1016/j.vaccine.2015.01.017 . [DOI] [PubMed] [Google Scholar]
- 15.Vellaiswamy M, Kowalczewska M, Merhej V, Nappez C, Vincentelli R, Renesto P, et al. Characterization of rickettsial adhesin Adr2 belonging to a new group of adhesins in alpha-proteobacteria. Microb Pathog. 2011;50(5):233–42. doi: 10.1016/j.micpath.2011.01.009 . [DOI] [PubMed] [Google Scholar]
- 16.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protocols. 2015;10(6):845–58. doi: 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandeputte-Rutten L, Bos MP, Tommassen J, Gros P. Crystal structure of Neisserial surface protein A (NspA), a conserved outer membrane protein with vaccine potential. J Biol Chem. 2003;278(27):24825–30. doi: 10.1074/jbc.M302803200 . [DOI] [PubMed] [Google Scholar]
- 18.Fish AI, Riley SP, Singh B, Riesbeck K, Martinez JJ. The Rickettsia conorii Adr1 Interacts with the C-Terminus of Human Vitronectin in a Salt-Sensitive Manner. Front Cell Infect Microbiol. 2017;7:61 doi: 10.3389/fcimb.2017.00061 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardwell MM, Martinez JJ. The Sca2 autotransporter protein from Rickettsia conorii is sufficient to mediate adherence to and invasion of cultured mammalian cells. Infect Immun. 2009;77(12):5272–80. doi: 10.1128/IAI.00201-09 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchiyama T, Kawano H, Kusuhara Y. The major outer membrane protein rOmpB of spotted fever group rickettsiae functions in the rickettsial adherence to and invasion of Vero cells. Microbes Infect. 2006;8(3):801–9. doi: 10.1016/j.micinf.2005.10.003 . [DOI] [PubMed] [Google Scholar]
- 21.Martinez JJ, Cossart P. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J Cell Sci. 2004;117(Pt 21):5097–106. doi: 10.1242/jcs.01382 . [DOI] [PubMed] [Google Scholar]
- 22.Thanassi DG, Saulino ET, Lombardo M, Roth R, Heuser J, Hultgren S. The PapC usher forms an oligomeric channel: Implications for pilus biogenesis across the outer membrane. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):3146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan YG, Riley SP, Martinez JJ. Adherence to and invasion of host cells by spotted Fever group rickettsia species. Front Microbiol. 2010;1:139 doi: 10.3389/fmicb.2010.00139 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balraj P, Renesto P, Raoult D. Advances in rickettsia pathogenicity. Ann N Y Acad Sci. 2009;1166:94–105. doi: 10.1111/j.1749-6632.2009.04517.x . [DOI] [PubMed] [Google Scholar]
- 25.Renesto P, Samson L, Ogata H, Azza S, Fourquet P, Gorvel JP, et al. Identification of two putative rickettsial adhesins by proteomic analysis. Res Microbiol. 2006;157(7):605–12. doi: 10.1016/j.resmic.2006.02.002 . [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Vonrhein C, Bricogne G, Izard T. Crystal structure of the N-terminal domains of the surface cell antigen 4 of Rickettsia. Protein Sci. 2013;22(10):1425–31. doi: 10.1002/pro.2322 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Gustchina A, Cruz R, Simoes M, Curto P, Martinez J, et al. Structure of RC1339/APRc from Rickettsia conorii, a retropepsin-like aspartic protease. Acta Crystallogr D Biol Crystallogr. 2015;71(Pt 10):2109–18. doi: 10.1107/S1399004715013905 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madasu Y, Suarez C, Kast DJ, Kovar DR, Dominguez R. Rickettsia Sca2 has evolved formin-like activity through a different molecular mechanism. Proc Natl Acad Sci U S A. 2013;110(29):E2677–86. doi: 10.1073/pnas.1307235110 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 2010;6(7):e1001027 doi: 10.1371/journal.ppat.1001027 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schvartz I S D.; Shaltiel S. Vitronectin. The International Journal of Biochemistry & Cell Biology. 1999;31:539–44. [DOI] [PubMed] [Google Scholar]
- 31.Singh B, Su YC, Riesbeck K. Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion. Mol Microbiol. 2010;78(3):545–60. doi: 10.1111/j.1365-2958.2010.07373.x . [DOI] [PubMed] [Google Scholar]
- 32.Otterdal K, Portillo A, Astrup E, Ludviksen JK, Schjalm C, Raoult D, et al. Rickettsia conorii is a potent complement activator in vivo and combined inhibition of complement and CD14 is required for attenuation of the cytokine response ex vivo. Clinical Microbiology and Infection. 2016;22(8):734.e1-.e6. https://doi.org/10.1016/j.cmi.2016.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.