Abstract
Background/Aims
To evaluate the era of direct acting antivirals (DAAs), we must understand the treatment patterns and outcomes of interferon-based therapy for hepatitis C virus (HCV) infection. We aimed to elucidate the treatment rate, factors affecting treatment decisions, and efficacy of interferon-based therapy in a real-world setting.
Methods
This nationwide cohort study included 1,191 newly diagnosed patients with chronic HCV infection at seven tertiary hospitals in South Korea. Subjects were followed retrospectively until March 2015, which was just before the approval of DAA therapy.
Results
In total, 48.2% and 49.3% of the patients had HCV genotypes 1 and 2, respectively. Interferon-based therapy was initiated in 541 patients (45.4%). The major reasons for no treatment included ineligibility (18.9%), concern about adverse events (22.3%), cost (21.5%), and an age >75 years (19.5%). Interferon-based therapy was discontinued (18.5%) mainly due to adverse events (n=66). The intent-to-treat analysis found that the sustained virologic response (SVR) rate was 58.3% in genotype 1 patients and 74.7% in non-genotype 1 patients.
Conclusions
Approximately one-third of newly diagnosed HCV patients in South Korea received interferon-based therapy and showed a suboptimal SVR rate. Diagnosis of patients at younger ages and with a less advanced liver status and reducing the DAA therapy cost may fulfill unmet needs.
Keywords: Hepacivirus, Therapeutics, Peginterferon alfa, Ribavirin, Sustained virologic response
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is the leading cause of cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC). More than 185 million people around the world have been infected with HCV. Of those infected with HCV, 350,000 die each year, making HCV infection a major public health concern worldwide.1,2
The global distribution of HCV prevalence and genotype has shown significant variation across regions.3 Moreover, interferon-based therapy for HCV infection has shown differences in outcomes and treatment efficacy that are associated with epidemiological and racial variations. According to previous studies, the sustained virologic response (SVR) rate of pegylated interferon (pegIFN) plus ribavirin (RBV) therapy is higher in Asian HCV patients compared to that of Caucasian HCV patients.4–6 Nevertheless, actual treatment initiation rates were documented to be as low as less than 10% in many countries.7,8
Recently, potent direct acting antiviral agents (DAAs) have been adopted into clinical practice. However, the high cost of DAA therapy precludes its wide application in resource-restrained regions. To estimate the potential economic effect of DAAs, precise information on the characteristics of untreated chronic hepatitis C patients, reasons for no treatment, and treatment efficacy of interferon-based therapy in a real-world setting are needed. The present study aimed to elucidate the treatment rate, factors affecting treatment decision, and efficacy of pegIFN and RBV therapy (PR therapy) in South Korea.
MATERIALS AND METHODS
1. Patients
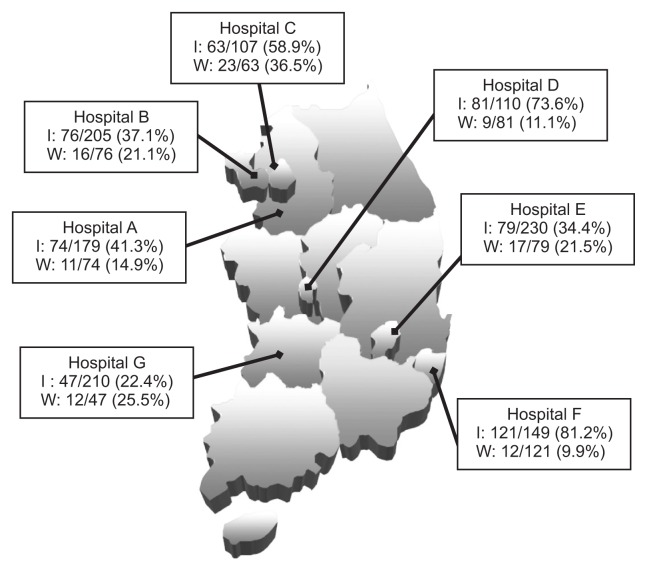
A total of 1,191 newly diagnosed, treatment naive patients with chronic HCV infection aged >18 years and positive for HCV RNA were included in this observational, multicenter cohort study. Subjects were enrolled between January 2008 and December 2011 and were observed until December 2013 at seven tertiary hospitals in South Korea. This nationwide study included institutions located in the capital area (Seoul National University Bundang Hospital, Soonchunhyang University Bucheon Hospital, and Inje University Ilsan Paik Hospital), three different metropolitan cities (Inje University Pusan Paik Hospital in Busan, Chungnam National University Hospital in Daejeon, and Keimyung University Hospital in Daegu), and the Jeolla province (Chonbuk National University Hospital) (Fig. 1). This study was approved by the Institutional Review Board of the seven hospitals, and written informed consent was waived because this study was based on the retrospective review of existing medical record data. All data were de-identified prior to analysis.
Fig. 1.
Treatment initiation (I) and withdrawal (W) rates for chronic hepatitis C infection using an interferon-based regimen at seven participating institutions located nationwide in South Korea.
Hospital A, Seoul National University Bundang Hospital; Hospital B, Soonchunhyang University Bucheon Hospital; Hospital C, Inje University Ilsan Paik Hospital; Hospital D, Chungnam National University College of Medicine; Hospital E, Keimyung University School of Medicine; Hospital F, Inje University Busan Paik Hospital; Hospital G, Chonbuk National University Hospital.
The diagnosis of chronic hepatitis C was based on the persistence of anti-HCV and HCV RNA for more than 6 months regardless of aminotransferase levels. Liver cirrhosis was defined by histological examination or by one or more clinical findings of portal hypertension:9 (1) cirrhotic appearance of the liver with splenomegaly on imaging study (ultrasonography, computed tomography or magnetic resonance image); (2) thrombocytopenia (platelet <120,000/mm3); (3) presence of esophagogastric varices on endoscopy; (4) presence of ascites; and (5) presence of portosystemic encephalopathy. Decompensated liver cirrhosis was defined as the presence of significant jaundice (total bilirubin >2 mg/dL), ascites, variceal bleeding, or portosystemic encephalopathy. HCC was diagnosed based on histological findings or typical imaging characteristics as defined by the Korean Liver Cancer Study Group and the National Cancer Center guidelines,10 which are similar to AASLD guidelines.
Baseline comorbidities (obesity, cancer, thyroid disease, psychiatric disease, cerebrovascular disease, cardiovascular disease, kidney disease, and diabetes) were described, and the comorbidity index was calculated by the Charlson Comorbidity Index,11 which has been validated in many diverse patient cohorts including those with liver cirrhosis.
2. Data collection for treatment pattern and efficacy of antiviral therapy
During the study period, DAA was not available in Korea, so all anti-HCV treatments were interferon-based therapies, which included either conventional/pegIFN or RBV or both after the index date. Clinical factors related to treatment initiation were retrieved from each patient’s medical chart and were reviewed by trained investigators. For those not-treated against HCV, their reasons for no treatment were categorized into “ineligible,” which indicated the attending physician did not recommend antiviral treatment, “unwillingness,” which meant the patient refused antiviral treatment against the physician’s recommendation, and “unknown.” These three categories (ineligible, unwillingness, and unknown) were mutually exclusive, but more than one detailed reason could be listed under each category. Investigators were regularly educated during the study period on the standardized review of medical records.
The definition of treatment efficacy followed the clinical practice guideline for hepatitis C by the Korean Association for the Study of the Liver (KASL).12 Briefly, a rapid virological response (RVR) is defined as undetectable HCV RNA level when measured by a sensitive assay with a lower detection limit of <50 IU/mL at treatment week 4. An early virological response (EVR) is defined as undetectable HCV RNA (complete EVR, cEVR) or a ≥2 log reduction in HCV RNA compared to the baseline level (partial EVR, pEVR). An end-of-treatment response (ETR) is defined as undetectable HCV RNA at the end of treatment (EOT). SVR is defined as undetectable HCV RNA at 24 weeks after completion of pegIFN-based treatment. Viral breakthrough (VB) refers to the reappearance of HCV RNA during treatment after an adequate virological response, and relapse is defined as the reappearance of HCV RNA after EOT. Measurement of adverse events was described based on the medical chart review by the attending physician. Owing to the limitation of retrospective design, the grade of adverse events could not be evaluated.
3. Statistical analysis
Analyses were conducted by descriptive statistics for the treatment patterns and outcomes. Counts and percentages for patients who initiated antiviral treatment, patients who did not initiate antiviral treatment, and reasons for not initiating treatment were reported. To compare characteristics of subjects who initiated treatment and of those who did not receive treatment, Student t-test for normal continuous variables, Mann-Whitney U test for nonnormal continuous variables, and chi-square for dichotomous variable were performed. A p-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using Stata version 14.0 (StataCorp, College Station, TX, USA).
RESULTS
1. Patient characteristics
The baseline characteristics of 1,191 patients newly diagnosed with HCV RNA positivity were summarized in Table 1. The mean age was 57.5 years and 50.5% were females. Of the total of 904 patients (75.9%) who tested for HCV genotype, 436 (48.2%) were genotype 1, 446 (49.3%) genotype 2, eight (0.9%) genotype 3, three (0.3%) genotype 4, and 11 (1.2%) genotype 6. Of the 436 genotype 1 patients, 18 (4.3%), 382 (87.6%), and 14 (3.2%) were subcategorized as type 1a, 1b, and 1c, respectively (Table 1). There were 477 (49.8%) patients with high viral loads above 800,000 IU/mL, and most patients (896, 75.2%) were diagnostically categorized as having chronic hepatitis. Regarding comorbidities, the median comorbidity score was 1.94±1.8, and the most common non-liver related comorbid disease was diabetes mellitus (150, 12.6%) (Table 1).
Table 1.
Comparison of Baseline Characteristics between Korean Patients with Chronic HCV Infection Treated and Not Treated with Interferon-Based Therapy
| Total (n=1,191) | No antiviral Tx (n=650) | Antiviral Tx (n=541) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr | 57.5 (13.8) | 62.8 (13.4) | 51.2 (11.3) | <0.001 |
| >75 | 118 (9.9) | 113 (17.4) | 5 (0.9) | <0.001 |
| Sex | ||||
| Male | 590 (49.5) | 336 (51.7) | 254 (47.0) | 0.103 |
| Female | 601 (50.5) | 314 (48.3) | 287 (53.1) | |
| BMI, kg/m2 (n=574) | 23.5±3.3 | 23.2±3.2 (n=266) | 23.8±3.3 (n=308) | 0.676 |
| Virologic data | 0.15 | |||
| Genotype | ||||
| 1 | 433 (48.2)* | 182 (50.0)* | 251 (46.9)* | |
| 1, not subcategorized | 19 (4.4) | 8 (4.4) | 11 (4.4) | |
| 1a | 18 (4.2) | 6 (3.3) | 12 (4.8) | |
| 1b | 380 (87.8) | 162 (89.0) | 218 (86.9) | |
| 1b/2a | 2 (0.5) | 1 (0.6) | 1 (0.4) | |
| 1c | 14 (3.2) | 5 (2.8) | 9 (3.6) | |
| 2 | 444 (49.4)* | 171 (47.0)* | 273 (51.0)* | |
| 2, not subcategorized | 88 (19.8) | 31 (18.1) | 57 (20.9) | |
| 2a | 134 (30.2) | 62 (36.3) | 72 (26.4) | |
| 2ac | 207 (46.6) | 77 (45.0) | 130 (47.6) | |
| 2b | 11 (2.5) | 1 (0.6) | 10 (3.7) | |
| 2c | 2 (0.5) | 0 | 2 (0.7) | |
| 2/2ac | 2 (0.5) | 0 | 2 (0.7) | |
| 3 | 8 (0.9)* | 2 (0.5)* | 6 (1.1)* | |
| 4 | 3 (0.3)* | 1 (0.3)* | 2 (0.4)* | |
| 6 | 11 (1.2)* | 8 (2.2)* | 3 (0.6)* | |
| Not tested | 292 (24.4) | 286 (44.0) | 6 (1.1) | |
| HCV RNA viral load, IU/mL (n=957) | 773,000 (98,400–3,579,570) | 655,000 (60,700–2,940,000) | 953,000 (140,000–3,980,000) | 0.002 |
| <400,000 | 384 (40.1) | 217 (43.9) | 167 (36.1) | 0.013 |
| >800,000 | 477 (49.8) | 230 (46.6) | 247 (53.4) | 0.036 |
| Severities of liver disease | ||||
| Liver disease | <0.001 | |||
| Chronic hepatitis | 896 (75.2) | 418 (64.3) | 478 (88.4) | |
| Liver cirrhosis, compensated | 154 (12.9) | 101 (15.5) | 53 (9.8) | |
| Liver cirrhosis, decompensated | 42 (3.5) | 36 (5.5) | 6 (1.1) | |
| Hepatocellular carcinoma | 99 (8.3) | 95 (14.6) | 4 (0.7) | |
| Liver transplantation | 0 | |||
| Child-Pugh class | <0.001 | |||
| A | 1,043 (87.7) | 524 (80.7) | 519 (95.9) | |
| B | 138 (11.6) | 116 (17.9) | 22 (4.1) | |
| C | 9 (0.8) | 9 (1.4) | 0 | |
| MELD score (n=847) | 5.34 (2.82–8.17) | 6.11 (3.02–8.97) | 4.77 (2.36–7.23) | 0.007 |
| Comorbidities | ||||
| Charlson comorbidity score | 1.94±1.77 | 2.42±2.14 | 1.35±0.89 | <0.001 |
| Comorbid diseases | ||||
| Myocardial infarction | 7 (0.6) | 6 (0.9) | 1 (0.2) | 0.098 |
| Heart failure | 8 (0.7) | 6 (0.9) | 2 (0.4) | 0.246 |
| Peripheral vascular disease | 36 (3.0) | 20 (3.1) | 16 (3.0) | 0.091 |
| Dementia | 4 (0.3) | 3 (0.5) | 1 (0.2) | 0.413 |
| Cerebrovascular disease | 18 (1.5) | 13 (2.0) | 5 (0.9) | 0.131 |
| Chronic pulmonary disease | 27 (2.3) | 22 (3.4) | 5 (0.9) | 0.005 |
| Connective tissue disease | 2 (0.2) | 0 | 2 (0.4) | 0.12 |
| Peptic ulcer | 34 (2.8) | 25 (3.8) | 9 (1.7) | 0.025 |
| Mild chronic liver disease | 1,091 (91.9) | 556 (85.5) | 535 (98.9) | <0.001 |
| Diabetes mellitus without complication | 124 (10.4) | 77 (11.8) | 47 (8.7) | 0.078 |
| Hemiplegia | 1 (0.1) | 1 (0.2) | 0 | 0.362 |
| Chronic kidney disease | 47 (3.9) | 43 (6.6) | 4 (0.7) | <0.001 |
| Diabetes mellitus with complication | 26 (2.2) | 19 (2.9) | 7 (1.3) | 0.056 |
| Solid tumor | 137 (11.5) | 120 (18.4) | 17 (3.1) | <0.001 |
| Leukemia | 1 (0.1) | 1 (0.2) | 0 | 0.362 |
| Lymphoma | 1 (0.1) | 1 (0.2) | 0 | 0.362 |
| Severe chronic liver disease | 109 (9.1) | 99 (15.1) | 10 (1.8) | <0.001 |
| Tumor with metastasis | 18 (1.5) | 18 (2.8) | 0 | <0.001 |
Data are presented as mean±SD, number (%), or median (interquartile range).
HCV, hepatitis C virus; Tx, treatment; BMI, body mass index; MELD, Model for End-Stage Liver Disease.
Percentage was calculated among subjects tested to determine the HCV genotype.
2. Treatment rate, its related factors, and comorbidity
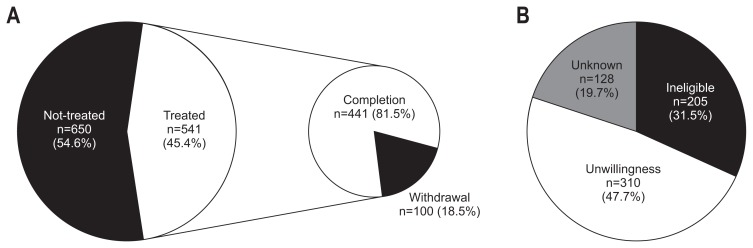
Interferon-based antiviral treatments were initiated for 541 patients (45.4%) with chronic HCV infection (Fig. 2A). Treatment initiation and withdrawal rates were significantly different between hospitals (p<0.001, respectively) (Fig. 1). The comparison of clinical characteristics of the 541 treated and 650 untreated patients were summarized in Table 1. Mean age at diagnosis of treated patients (51.2±11.3 years) was significantly younger than that of untreated patients (62.8±13.4 years) (p<0.001), and only five (0.9%) among the treated patients were more than 75 years old. Gender and genotype did not affect treatment decision. Higher viral load was observed in treated patients than in untreated patients (median, 9.53×105 IU/mL vs 6.55×105 IU/mL, respectively, p=0.002).
Fig. 2.
Treatment patterns of 1,191 Korean patients with chronic hepatitis C infection in the interferon-based therapy era. (A) Overall treatment initiation and withdrawal rates. Detailed information is provided in Table 3. (B) Reasons for no treatment for chronic hepatitis C. Detailed information is provided in Table 2.
Ineligible, contraindicated for antiviral therapy as judged by the attending physician; Unwillingness, patient refused antiviral treatment against the physician’s recommendation.
Liver disease status was a significant factor for treatment decision (p<0.001) (Table 1). Of treated patients, 478 (88.4%) had chronic hepatitis and 53 (9.8%) had compensated cirrhosis. In other words, 53.3% of chronic hepatitis patients received antiviral treatment, but treatment initiation rate fell with disease progression: 34.4% in compensated liver cirrhosis, 14.3% in decompensated liver cirrhosis, and 4% in HCC patients. Similarly, the proportion of Child-Pugh class B or C patients was significantly different between the treated and nontreated groups (p<0.001). A total of six decompensated liver cirrhosis patients received antiviral therapy. They were all of “decompensated” status due to the presence of ascites, which was controlled with diuretics. They had no other evidences of decompensation such as jaundice, variceal bleeding or encephalopathy.
Charlson comorbidity score of the treated group was significantly lower than that of the untreated group (1.35±0.89 vs 2.42±2.14, respectively, p<0.001). Among 150 patients with diabetes mellitus with or without complications, 54 (36%) were treated with PR therapy. The number of subjects with myocardial infarction, heart failure or cerebrovascular diseases, and thus the number of subjects likely to use drugs such as statins or digitalis, which have significant interactions with most DAAs, were seven (0.6%), eight (0.7%), and 18 (1.5%), respectively.
3. Reasons for not treating chronic hepatitis C patients with interferon-based antiviral agents
The reasons for not treating patients were summarized in Fig. 2B and Table 2. Among 650 untreated patients, 205 (31.9%) were judged to be ineligible for antiviral treatment using PR regimen by their physician. Of the 205 ineligible patients, 100 (48.8%) had advanced liver disease (61 HCC and 39 decompensated LC), and 13 subjects had contraindications for PR therapy and accounted for only 2% of untreated patients. Of 310 patients (48.2%) who were unwilling to undergo therapy (unwillingness), 140 (43.6%) refused antiviral treatment due to burden of cost (about $300–400 of the patients’ shares per month for pegIFN, RBV, and blood tests for treatment monitoring) and 145 (45.2%) due to fear of adverse events during antiviral treatment. A total of 127 patients (19.5%) were not indicated for antiviral treatment due to old age. As each physician’s decision to treat elderly patients (>75 years old) with chronic HCV infection was inconsistent, advanced age (>75 years old) was included in both categories of reason-for-no-treatment, and it accounted for 78 of ineligible (39.8%) and 49 of unwillingness cases (15.3%).
Table 2.
Factors Associated with Anti-HCV Treatment Initiation in the Interferon-Based Therapy Era in Korean Patients
| Reason for no antiviral treatment | HCV patients treated with interferon-based therapy (n=650) |
|---|---|
| Ineligible (not recommended by physician) | 205 (31.5) |
| Old age (>75 yr) | 78 (38.0) |
| Liver cancer including hepatocellular carcinoma | 61 (29.8) |
| Decompensated liver cirrhosis | 39 (19.0) |
| Hypersensitivity to interferon | 0 |
| Uncontrolled psychiatric illness or depression | 1 (0.5) |
| Untreated thyroid illness | 2 (1.0) |
| Poorly controlled diabetes mellitus | 3 (1.5) |
| Autoimmune disease | 1 (0.5) |
| Severe heart disease | 3 (1.5) |
| Severe lung disease | 3 (1.5) |
| Severe kidney disease | 7 (3.4) |
| Severe bone marrow dysfunction | 0 |
| Pregnancy or unwilling to comply with contraception | 3 (1.5) |
| Unwillingness (recommended, but refused by patient) | 310 (47.7) |
| High cost | 140 (45.2) |
| Fear for adverse events during antiviral treatment | 145 (46.8) |
| Old age (>75 yr) | 49 (15.8) |
| Limited accessibility to hospital | 10 (3.2) |
| Unknown | 128 (19.7) |
Data are presented as number (%).
HCV, hepatitis C virus.
4. Treatment pattern of interferon-based therapy in a real-life setting
Of 541 treated patients, three received conventional interferon combined with RBV therapy in early 2008. However almost all patients 527 (44.2%) underwent pegIFN-based therapy: 11 pegIFN-α-2a (2.0%), seven pegIFN-α-2b (1.3%), 309 pegIFN-α-2a with RBV (57.1%), 193 pegIFN-α-2b with RBV (35.7%), and seven pegIFN-α-2a/b with RBV (1.3%) (Table 3).
Table 3.
Treatment Pattern for Patients with Chronic Hepatitis C Infection in South Korea
| Treatment pattern | No. (%) (n=1,191) |
|---|---|
| Treatment initiation | 541 (45.4) |
| Treatment regimen | |
| Conventional IFN+ribavirin | 3 (0.6) |
| PegIFN-α-2a or 2b | 18 (3.3) |
| PegIFN-α-2a or 2b+ribavirin | 509 (94.1) |
| Treatment schedule | |
| Standard* | 476 (88.0) |
| Shorten | 59 (10.9) |
| Extended | 6 (1.1) |
| Treatment withdrawal | 100 (18.5) |
| Reason for Tx withdrawal | |
| Adverse events | 66 (66.0) |
| No achievement of early virologic response | 15 (15.0) |
| Viral breakthrough | 1 (1.0) |
| F/U loss during Tx | 18 (18.0) |
IFN, interferon; pegIFN, pegylated IFN; Tx, treatment; F/U, follow-up.
Standard treatment was defined as 48 weeks for genotypes 1/4 and 24 weeks for genotypes 2/3/6.
Although response guided therapy is recommended by KASL guideline,12 88% of subjects were treated based on a standard schedule (48 weeks for genotype 1/4 and 24 weeks for genotype 2/3/6). Shortened and extended therapies were performed in 59 (10.9%) and six subjects (1.1%), respectively (Table 3). A total of 100 patients (18.5%) withdrew from antiviral treatment during scheduled therapy, and the major reason for the withdrawal was adverse events (66.0%) during antiviral therapy and no achievement of early virologic response (15.0%) (Table 3).
During antiviral therapy, RVR was measured in 70.4% of genotype 1 and 64.1% of genotype 2 patients. EVR measurement was performed in 78.6% of genotype 1 and 46.5% of genotype 2 patients. Even ETR and SVR were not measured in 17.4% and 22.9% of genotype 1 and 12.8% and 17.2% of genotype 2, respectively (data not shown). Therefore, response monitoring was insufficient in the real-life setting.
5. Treatment efficacy of interferon-based therapy in a real-life setting
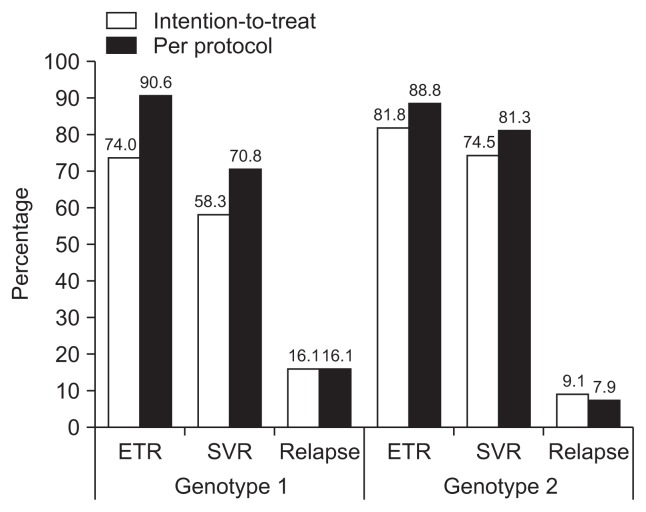
Among 254 genotype 1 patients who received interferon at least once (intention-to-treat group), 192 (75.6%) completed their scheduled therapy (per-protocol group). Response rates in intention-to-treat genotype 1 patients were 37.4% (RVR), 76.4% (EVR; 61.4% cEVR, 15.0% pEVR), 74.0% (ETR), and 58.3% (SVR). VB during therapy was detected in 3.5%, and relapse was observed in 16.1% after PR therapy (Fig. 3, Supplementary Table 1). Null response to antiviral treatment was observed in 11.8% of genotype 1 patients. As per-protocol, RVR rate was 41.1%, EVR 84.4%, ETR 90.6%, SVR 70.8%, and relapse rate was 16.1% (Fig. 3, Supplementary Table 1).
Fig. 3.
Virologic response rates of interferon-based therapy for Korean patients with chronic hepatitis C infection. Detailed response rates are described in Supplementary Table 1.
ETR, end of treatment response rate; SVR, sustained virologic response rate.
In 275 genotype 2 patients, intention-to-treat response rates were 52.7% (RVR), 46.2% (EVR; 41.5% cEVR, 4.7% pEVR), 81.8% (ETR), and 74.5% (SVR). VB was detected in 1.5%, and relapse rate was 9.1% (Fig. 3, Supplementary Table 1). Of genotype 2 patients, 35 (12.7%) withdrew from antiviral treatment before scheduled duration. As per-protocol, RVR rate was 56.3%, EVR 47.5%, ETR 88.8%, SVR 81.3%, and relapse rate was 7.9%. Null response to antiviral treatment was observed in 5.4% of genotype 2 patients (Fig. 3, Supplementary Table 1).
Adverse events of any grade during interferon-based therapy were frequently observed in 455 patients (84.1%). Frequencies of adverse events during antiviral treatment in this study population were listed in Table 4.
Table 4.
Adverse Events during Interferon-Based Treatment for Patients with Chronic Hepatitis C Infection in South Korea
| Adverse event | No. (%) (n=455) |
|---|---|
| Dyspepsia, nausea | 135 (29.7) |
| Flu like symptoms | 111 (24.4) |
| Itching | 102 (22.4) |
| Alopecia | 95 (20.9) |
| Rash | 82 (18.0) |
| Anemia | 77 (16.9) |
| Depression | 56 (12.3) |
| G/W, fatigue | 53 (11.6) |
| Neutropenia | 52 (11.4) |
| Insomnia | 51 (11.2) |
| Dizziness | 45 (9.9) |
| Anorexia | 40 (8.8) |
| Headache | 40 (8.8) |
| Thrombocytopenia | 30 (6.6) |
| Dyspnea | 18 (4.0) |
| Eye discomfort | 13 (2.9) |
| Oral mucositis | 9 (2.0) |
| Thyroid dysfunction | 8 (1.8) |
| Menstrual change | 6 (1.3) |
| Diarrhea | 5 (1.1) |
| Hearing disturbance | 2 (0.4) |
Data are presented as number (%).
G/W, general weakness.
DISCUSSION
This study demonstrated that less than half of Korean patients with newly diagnosed chronic HCV infection who visited a tertiary hospital received anti-HCV therapy in the interferon-based era. The major reasons for no treatment were ineligibility due to medical condition (18.9%), concern for adverse events (22.3%), cost issue (21.5%), and old age (>75 years) (19.5%). About one-third of HCV patients completed interferon-based antiviral therapy, and the real-life SVR rate was suboptimal. These unmet needs found in the interferon-based treatment era point towards the already-coming DAA therapy era.
Previous studies regarding treatment initiation for chronic hepatitis C are few, especially in Asian regions, where HCV is overshadowed by the more prevalent hepatitis B virus infection. A recent U.S. study including 101,444 Veterans showed that treatment initiation rate was very low (7.4%), and those with genotype 2/3, non-white race, and Medicare or other insurances were correlated with high treatment initiation rates.13 In a recent Taiwan study, the treatment rate was 13.7% in the estimated HCV-viremic population of 554,361.8 The treatment initiation rate reported in this study was much higher than in previous reports. This may be because of the study’s enrollment of patients from hepatology clinics in tertiary hospitals, which may be settings more conducive to treatment initiation. Nonetheless, less than two-thirds of patients with chronic HCV infection visited tertiary hospitals in Korea.14 Thus, the actual treatment initiation rate in the whole Korean chronic hepatitis C population would be much lower than our report.
The most important reasons for no treatment (ineligibility) were old age and advanced liver disease such as decompensated liver cirrhosis or liver cancer. Despite the concern of limited indication of interferon-based therapy, the proportion of patients who could not be treated owing to contraindication of interferon or RBV was only 11.6%. Similarly, a previous U.S. population study reported that 15.2% of HCV-infected patients had at least one contraindication to PR therapy.15 According to the study, hepatic decompensation (1.2%) was the third most common contraindication following bipolar disorder (6.5%) and anemia (5.9%), which were defined by ICD-9 diagnosis codes using the electric medical record. Therefore, increasing efforts to discover asymptomatic, young chronic hepatitis C patients before hepatic disease progression are expected to improve treatment initiation rates and subsequent successful treatment.
Fear of adverse events was a major reason behind patient refusal to be treated with interferon-based treatment (46.8%). Actually, the majority of the patients (84%) experienced various adverse events during pegIFN/IFN plus RBV treatment in the present study, and it was higher than that reported in clinical trials as expected.16,17 However, most of the adverse events were manageable with supportive care; thus 82% of patients with adverse events were able to continue antiviral treatment. The treatment discontinuation rate in the present study was similar to other real-world withdrawal rates18 and randomized controlled trials.16,17 Medical cost was also a major obstacle to antiviral treatment initiation. Even though the cost of PR is much cheaper than DAA cost, it may be unaffordable to 21.5% of South Korean patients. The current monthly cost for PR in South Korea is about $500, and patients usually pay $150–200 per month after government insurance coverage.19 Thus, reasonable cost for DAA therapy is of utmost importance for DAA accessibility in Korea as well as in other regions.
Interestingly, genotype did not significantly affect treatment decision in the Korean population (58.3% in GT 1 vs 61.7% in GT 2, p=0.302, data now shown), which was not consistent with previous studies.13,20 In this multicenter study, the decision to initiate treatment was based on each physician’s preference, which may have put different emphasis on genotype when deciding to initiate antiviral treatment. This may explain the wide range of treatment initiation rates seen between hospitals (34.4% to 93.9% in genotype 1, 33.3% to 92.3% in genotype 2), leading to no significant difference in treatment initiation rates between genotypes.
Response rates of PR therapy had been reported in many previous studies.14,18 Considering the favorable IL-28B genotype profiles found in about 90% of Koreans,21 overall response rate for genotype 1 patients in the present study was better than those of Western countries. However, SVR rates (58.3% in genotype 1, 74.5% in genotype 2) in the present study were not as improved compared to previous reports before 2008 (~70% in genotype 1, 80% to 90% in genotype non-1).22
This study was the first and the largest nationwide study to estimate the treatment initiation rate and real-life efficacy of interferon-based therapy in South Korea. Moreover, real-world practice patterns and follow-up loss rates with interferon-based anti-HCV therapy were described for a period immediately before the era of DAA. However, the present study had several limitations. First, all data were collected in tertiary hospitals. Thus the treatment initiation rate may have been overestimated. Secondly, reasons for patient refusal of antiviral treatment could not be described in 36.1% of not-treated patients due to limited information. Additionally, a patient’s given reason might not correctly reflect the true background reason for refusing antiviral treatment including financial difficulties. Third, subjective adverse events may have been lost to record due to the retrospective design of the present study. Lastly, RVR and EVR were not measured in 30.3% and 21.9% of genotype 1 subjects, so these on-treatment response rates were not representative of actual rates, but they reflected real-world practice patterns of Korean physicians.
In conclusion, less than half of newly diagnosed HCV patients in South Korea initiated interferon-based antiviral therapy, one third of the patients completed the therapy, and the real-life SVR rate was suboptimal. Early diagnosis of HCV infection in young age or in nonadvanced stages of liver disease as well as DAA therapy available at a reasonable cost may fulfill these un-met needs.
Supplementary Data
ACKNOWLEDGEMENTS
This study was funded by Bristol-Myers-Squibb, and a grant for the Chronic Infectious Disease Cohort Study (Korea HCV Cohort Study, 4800-4859-304) from the Korea Centers for Disease Control and Prevention. The authors are grateful to the devoted research coordinators (Da-Seul Lee, Dawoon Jeong, Jiyoung Paik, Ye Young Lee, Ae Ran An, Hyoyoung Kang, Hyeyoung Gye, and Sujin Lee).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21(Suppl 1):60–89. doi: 10.1111/jvh.12249. [DOI] [PubMed] [Google Scholar]
- 4.Lyoo K, Song MJ, Hur W, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peginterferon plus ribavirin. J Clin Virol. 2011;52:363–366. doi: 10.1016/j.jcv.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Jeong SH, Jung YK, Yang JW, et al. Efficacy of peginterferon and ribavirin is associated with the IL28B gene in Korean patients with chronic hepatitis C. Clin Mol Hepatol. 2012;18:360–367. doi: 10.3350/cmh.2012.18.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung YK, Kim JH, Ahn SM, et al. Role of interleukin 28B-related gene polymorphisms in chronic hepatitis C and the response to antiviral therapy in Koreans. J Clin Gastroenterol. 2013;47:644–650. doi: 10.1097/MCG.0b013e3182896abf. [DOI] [PubMed] [Google Scholar]
- 7.Hawks L, Norton BL, Cunningham CO, Fox AD. The hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23:473–478. doi: 10.1111/jvh.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu ML, Yeh ML, Tsai PC, et al. Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: a nationwide survey in Taiwan. Medicine (Baltimore) 2015;94:e690. doi: 10.1097/MD.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suk KT, Baik SK, Yoon JH, et al. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012;18:1–21. doi: 10.3350/kjhep.2012.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, Park JW, Choi BI. 2014 KLCSG-NCC Korea Practice Guidelines for the management of hepatocellular carcinoma: HCC diagnostic algorithm. Dig Dis. 2014;32:764–777. doi: 10.1159/000368020. [DOI] [PubMed] [Google Scholar]
- 11.Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48:214–220. doi: 10.1002/hep.22341. [DOI] [PubMed] [Google Scholar]
- 12.Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol. 2016;22:76–139. doi: 10.3350/cmh.2016.22.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundlapalli AV, Nelson RE, Haroldsen C, Carter ME, LaFleur J. Correlates of initiation of treatment for chronic hepatitis C infection in United States veterans, 2004–2009. PLoS One. 2015;10:e0132056. doi: 10.1371/journal.pone.0132056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung YK, Kim JH. Is peginterferon and ribavirin therapy effective in Korean patients with chronic hepatitis C? Clin Mol Hepatol. 2013;19:26–28. doi: 10.3350/cmh.2013.19.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talal AH, LaFleur J, Hoop R, et al. Absolute and relative contra-indications to pegylated-interferon or ribavirin in the US general patient population with chronic hepatitis C: results from a US database of over 45000 HCV-infected, evaluated patients. Aliment Pharmacol Ther. 2013;37:473–481. doi: 10.1111/apt.12200. [DOI] [PubMed] [Google Scholar]
- 16.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 17.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 18.Borroni G, Andreoletti M, Casiraghi MA, et al. Effectiveness of pegylated interferon/ribavirin combination in ‘real world’ patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2008;27:790–797. doi: 10.1111/j.1365-2036.2008.03657.x. [DOI] [PubMed] [Google Scholar]
- 19.Health Insurance Review & Assessment Service. Reimbersement guide for current available drugs [Internet] Wonju: Health Insurance Review & Assessment Service; c2016. [cited 2017 Mar 15]. Available from: http://www.hira.or.kr. [Google Scholar]
- 20.Lee SS, Jeong SH, Jang ES, et al. Treatment rate and factors related to interferon-based treatment initiation for chronic hepatitis C in South Korea. J Med Virol. 2016;88:275–281. doi: 10.1002/jmv.24335. [DOI] [PubMed] [Google Scholar]
- 21.Rangnekar AS, Fontana RJ. IL-28B polymorphisms and the response to antiviral therapy in HCV genotype 2 and 3 varies by ethnicity: a meta-analysis. J Viral Hepat. 2013;20:377–384. doi: 10.1111/jvh.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SY, Rim MY, Yo IK, et al. Efficacy of peginterferon and riba-virin combination therapy of chronic hepatitis C: a pooled analysis. Korean J Gastroenterol. 2012;60:306–314. doi: 10.4166/kjg.2012.60.5.306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.