Abstract
Background
It has been unclear whether supplemental probiotics therapy improves clinical outcomes in type 2 diabetic patients. This meta-analysis aimed to summarize the effect of probiotics on glucose and lipid metabolism and C-reactive protein (CRP) from 12 randomized controlled trials (RCTs).
Material/Methods
An up-to-date search was performed for all relevant RCTs up to April 2016 from PubMed, Embase, and Cochrane Library. Standardized mean difference (SMD) and weighted mean difference (WMD) were calculated for a fixed-effect and random-effect meta-analysis to assess the impact of supplemental probiotics on fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), fasting insulin, homeostasis model assessment of insulin resistance (HOMA-IR), lipid profile, and CRP level.
Results
A total of 12 studies (684 patients) were entered into the final analysis. The effect of probiotics was significant on reducing HbA1c level (standardized mean difference [SMD], −0.38; confidence interval [CI], −0.62 to −0.14, P=0.002; I2=0%, P=0.72 for heterogeneity), fasting insulin level (SMD, −0.38; CI −0.59 to −0.18, P=0.0003; I2=0%, P=0.81 for heterogeneity), and HOMA-IR (SMD, −0.99; CI −1.52 to −0.47, P=0.0002; I2=86%, P<0.00001 for heterogeneity). Pooled results on effects of probiotics on FPG, CRP, or lipid profile were either non-significant or highly heterogeneous.
Conclusions
This meta-analysis demonstrated that probiotics supplementation was associated with significant improvement in HbA1c and fasting insulin in type 2 diabetes patients. More randomized placebo-controlled trials with large sample sizes are warranted to confirm our conclusions.
MeSH Keywords: Diabetes Mellitus, Type 2; Meta-Analysis; Probiotics
Background
Diabetes mellitus (DM) is becoming a serious international public health problem. As reported by the International Diabetes Federation (2013), the world-wide diabetic population is now 382 million and will reach 592 million by 2035. Diabetes has cost 548 billion USD and led to 5.1 million deaths by the end of 2013 [1]. The pathogenesis of type 2 diabetes (T2D) involves both genetic and environmental factors [2–4], among which gut microorganisms play an important role [5,6]. The human gut hosts trillions of microorganisms, including thousands of bacterial species [7], affecting a large number biological functions and metabolism in humans [8]. Cani et al. first demonstrated the direct role of gut bacteria in insulin resistance in 2007 [9]. They found that a high-fat diet increased certain gut bacterial species that generate higher levels of lipopolysaccharide, triggering the progression of insulin resistance [9]. Later studies also found that the gut microbiota contributes to glucose hemostasis through numbers of different bacterial metabolites [10]. More importantly, administration of probiotics in a mouse model effectively inhibited gluconeogenesis in type 2 diabetes [11], indicating its glucose-lowering effect might contribute to its inhibition of tumorigenesis [12,13]. Randomized controlled trials in humans have also shown potential benefits of probiotics in type 2 diabetes. Previous systematic reviews have evaluated the effect of probiotics on blood glucose, insulin, and C-reactive protein (CRP) in type 2 diabetes; however, they had a small number of cases and lacked convincing evidence [14–16]. Therefore, we aimed to summarize the effect of probiotics on type 2 diabetes by conducting a meta-analysis.
Material and Methods
This study protocol was established based on the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions [17].
Study selection
All randomized controlled trials (RCTs) investigating the effect of probiotics as a dietary supplementation on glucose and lipid metabolism and inflammatory markers in patients with type 2 diabetes mellitus were eligible for enrolment in this meta-analysis. We excluded the studies presented only as abstracts with no subsequent full report of findings, on-going clinical studies, quasi-randomized study design, review papers, non-English literature, studies involving patients with GDM, type 1 diabetes mellitus (T1DM), and any other metabolic diseases such as obesity or hypercholesterolemia.
Search strategy
We searched PubMed, MEDLINE, EMBASE, and Cochrane library databases up to April 2016. Data from newly available studies were also accessed by searching editorials and web-based information. The terms for searching were: (‘probiotics’) AND (‘supplementation’) AND (‘type 2 diabetes mellitus’) AND (‘glycemic-related parameters’) AND (‘inflammatory markers’) AND (randomized OR blind OR placebo OR meta-analysis). We also attempted to contact the investigators if their clinical endpoints were not reported.
Selection criteria
Two independent authors (KY and XZ) identified eligible articles and a third investigator (QH) resolved any disagreements. The process of study selection is shown in Figure 1. The study selection process was based on preferred reporting items for systematic reviews and meta-analyses (PRISMA) [18].
Figure 1.
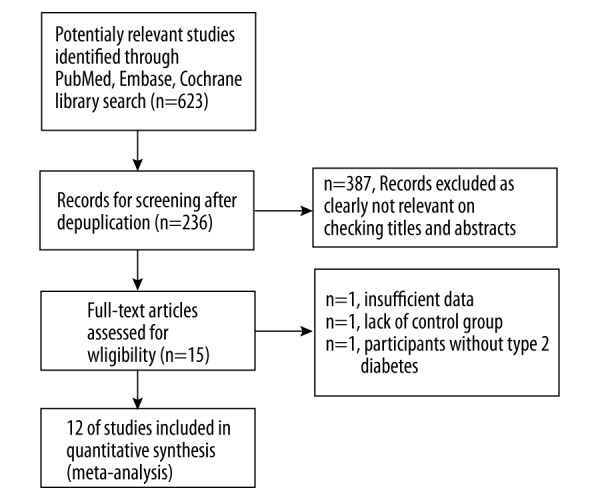
PRISMA 2009 flow diagram.
Study quality assessment
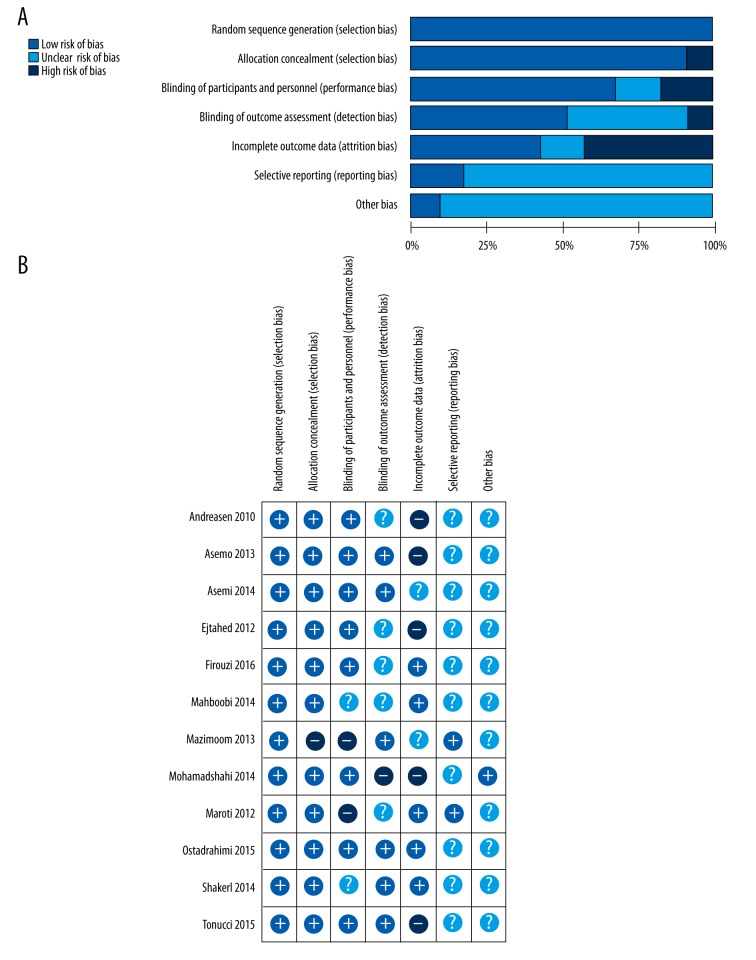
Data were extracted, including baseline information of the study population, types of probiotics administration, and clinical outcomes. The study quality was determined according to the Cochrane Handbook [17] (Figure 2). Randomization was assessed and considered adequate for 2 out of 12 trials.
Figure 2.
Risk of bias graph (A) and risk of bias summary (B) in 12 randomized controlled trials.
Clinical endpoints
The main clinical endpoints in this study were FPG, HbA1c, fasting insulin level, homeostasis model assessment of insulin resistance (HOMA-IR), CRP, and the levels of triglycerides, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
Data synthesis and analysis
RevMan 5.3 (Nordic Cochrane Centre) was used for data synthesis and analysis. A fixed-effects or random-effects meta-analysis was performed for the standardized mean difference (SMD) or weighted mean difference (WMD), with 95% confidence intervals for continuous outcomes. All reported P values were two-sided, with a significance level set at P<0.05. Heterogeneity of studies was calculated by I2 statistics and an I2 of 0–25%, 25–50%, and 50–75% were considered as low, moderate, and high levels of heterogeneity, respectively.
Results
Study description
Originally, a total of 623 RCT studies were searched and 12 RCTs satisfied the inclusion criteria (Table 1). Table 1 shows the baseline characteristics of the 12 included RCTs [19–30]. Hariri (2015) [31] and Yan (2015) [32] were very small trials and their clinical endpoints were different. Therefore, they were not included in this analysis.
Table 1.
Study characteristics of 12 randomized control trials in this meta-analysis.
| Studies | Participants (P/C) | Country | Design | Age (P/C) | Intervention | Weeks | Measure outcones |
|---|---|---|---|---|---|---|---|
| Andreasen 2010 | Type 2 diabetes mellitus (21/24 adult patients) | Denmark | Randomized, placebo-controlled, double blinded trial | 55±14/ 60±13 | L.acidophilus NCFM | 4 | HOMA-IR, CRP |
| Asemi 2013 | Type 2 diabetes mellitus (27/27 patients) | Iran | Randomized, placebo-controlled, double blinded trial | 50.5±9.8/ 52.6±7.1 | L. acidophilus (2×109 CFU), L. casei (7×109 CFU), L. rhamnosus (1.5×109 CFU), L. bulgaricus (2×108 CFU), B. breve (2×1010 CFU), B. longum (7×109 CFU), S. thermophilus (1.5×109 CFU), and 100 mg fructo-oligosaccharide | 8 | FPG, HbA1c, insulin, total cholesterol, triglycerides, LDL-C, HDL-C |
| Asemi 2014 | Type 2 diabetes mellitus (62/62 patients) | Iran | Randomized, double-blinded, crossover controlled clinical trial | 53.1±8.7/ 52.6±4.1 | Probiotic viable and heat resistant Lactobacillus sporogenes (1×107 CFU), 0.04 g inulin (HPX) as prebiotic with 0.38 g isomalt, 0.36 g sorbitol and 0.05 gstevia as sweetener per 1 g | 6 | FPG, insulin, total cholesterol, triglycerides, LDL-C, HDL-C |
| Ejtahed 2012 | Type 2 diabetes mellitus (30/30 patients) | Iran | Double-blinded, randomized controlled clinical trial | 50.9±7.7/ 51.0±7.3 | 300 g/d of probiotic yogurt containing L. acidophilus La5 and B. lactis Bb 12 | 6 | FPG, insulin, HbA1c |
| Firouzi 2016 | Type 2 diabetes mellitus (48/53 patients) | Malaysia | Randomized, double-blinded, parallel-group controlled clinical trial | 52.9±9.2/ 54.2±8.3 | Provideda 3×1010 dose of six viable microbial cell preparation strains: three strains from the genus Lactobacillus, Firmicutes phyla (Lactobacillus acidophilus, Lactobacilluscasei, Lactobacillus lactis) and three strains from the genus Bifidobacterium and Actinobacteriaphyla (Bifidobacterium bifidum, Bifidobacterium longum and Bifidobacteriuminfantis) | 12 | FPG, insulin, HOMA-IR, HbA1c, total cholesterol, triglycerides, LDL-C, HDL-C |
| Mahaboobi 2014 | Type 2 diabetes mellitus (28/27 paitients) | Iran | Randomized, double-blinded, controlled clinical trial | 51.0±1.4/ 50.4±1.3 | Probiotic capsules contained 7×109 colonyforming unit (CFU) L. casei, 2×109 CFU L. Acidophilus, 1.5×109 CFU L. rhamnosus, 2×108 CFU L. bulgaricus, 2×1010 CFU B. breve, 7×109 CFU B. longum, 1.5×1010 CFU S. thermophilus | 8 | Total cholesterol, triglycerides, LDL-C, HDL-C |
| Mazloom 2013 | Type 2 diabetes mellitus (16/18 paitients) | Iran | Randomized, single-blinded, controlled clinicaltrial | 55.4±8.0/ 51.8±10.2 | The lactobacillus probiotics contained L. acidophilus, L. bulgaricus, L. bifidum, and L. casei | 6 | FPG, Insulin, HOMA-IR, HbA1c, total cholesterol, triglycerides, LDL-C, HDL-C |
| Mohamadshahi 2014 | Type 2 diabetes mellitus (16/18 paitients) | Iran | Randomized, double-blinded, controlled clinicaltrial | Mean age 51 | 300 g probiotic yogurt containing 3.7×106 cfu/mg of both L. acidophilus La-5 and B. lactis Bb-12 | 8 | FPG, insulin, HOMA-IR, HbA1c, total cholesterol, triglycerides, LDL-C, HDL-C |
| Moroti 2012 | Type 2 diabetes mellitus (10/10 patients) | Brazil | Randomized, placebo-controlled, double blinded trial | 55.5±2.0/ 56.9±1.7 | Symbiotic shake containing 4×108 UFC/100 mL L. acidophillus, 4×108 UFC/100 mL B. bifidum and 1 g/100 mL of fructooligosaccharides | 4 | FPG, total cholesterol, triglycerides, HDL-C |
| Ostadrahimi 2015 | Type 2 diabetes mellitus (30/30 paitients) | Iran | Randomised, placebo-controlled, double blinded trial | Range from 35 to 65 | Probiotic fermented milk (kefir) containing L. casei, L. acidophilus and Bifidobacteria | 8 | FPG, HbA1c, total cholesterol, triglycerides, LDL-C, HDL-C |
| Shakeri 2014 | Type 2 diabetes mellitus (26/26 paitients) | Iran | Randomized, placebo-controlled, double blinded trial | 52.3±8.2/ 53.1±7.5 | Probiotic bread contained L. sporogenes (1×108 CFU) per 1 g | 8 | FPG, total cholesterol, triglycerides, LDL-C, HDL-C |
| Tonucci 2015 | Type 2 diabetes mellitus (23/22 paitients) | Brazil | Randomized, placebo-controlled, double blinded trial | 51.8±6.6/ 51.0±7.2 | Fermented milk containing Lactobacillus acidophilus La-5 and B. animalissubsplactis BB-12 (109 colony-forming units/d, each) | 6 | FPG, insulin, HOMA-IR, HbA1c, total cholesterol, triglycerides, LDL-C, HDL-C |
Age was presented as mean ±SD or as otherwise indicated; P/C – patient/control; HOMA-IR – homeostasis model assessment of insulin resistance; CRP – C-reactive protein; FPG – fasting plasma glucose; HbA1c – glycated hemoglobin; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol.
Probiotics and glucose metabolism
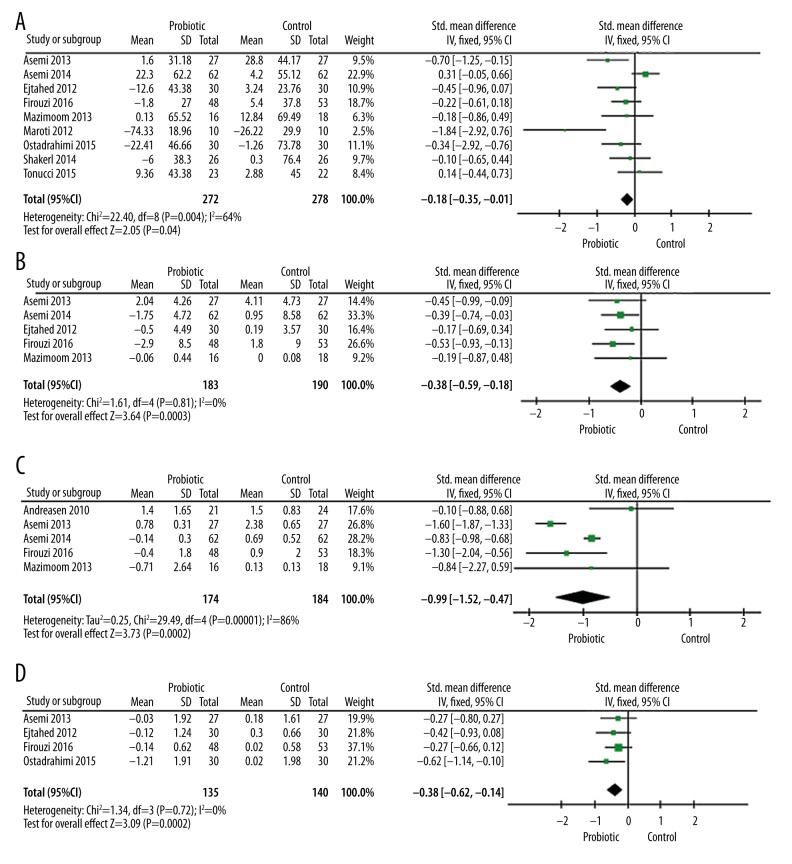
The pooled results of probiotics and glucose metabolism are presented in Figure 3. Nine studies reported the effect of probiotics on fasting plasma glucose levels. As shown in Figure 3A, 7 trails demonstrated significantly decreased glucose levels in the probiotics group, with a pooled standardized mean difference of −0.18 mg/dl (95% CI −0.35, −0.01; P=0.04). However, there was also significant heterogeneity (I2=64%, P=0.004).
Figure 3.
Forest plots for the effect of probiotics on fasting plasma glucose (A), glycated hemoglobin (B), fasting insulin levels (C), and insulin resistance (D) compared to controls in pooled analysis.
Glycated hemoglobin reflects the average blood glucose level in the past 3 months. Four studies compared the change of HbA1c between the probiotics and control groups (Figure 3B). There was a statistically significant reduction in HbA1c in the probiotics group, with a pooled standardized mean difference of −0.38% (95% CI −0.62, −0.14; P=0.002) and a non-significant heterogeneity (I2=0%, P=0.72) compared to control.
Regarding the change in fasting insulin level, 5 studies reported the effect of probiotics on fasting insulin compared to control (Figure 3C). Probiotics significantly reduced fasting insulin levels, with a pooled standardized mean difference of −0.38 (95% CI −0.59, −0.18; P=0.003) and a non-significant heterogeneity (I2=0%, P=0.81). Similarly, 5 studies reported HOMA-IR results (Figure 3D). As a result, probiotics statistically reduced HOMA-IR level (pooled effect of −0.99, 95% CI −1.52, −0.4; P=0.0002). However, there was also significant heterogeneity (I2=86%, P<0.00001).
Probiotics and lipid metabolism
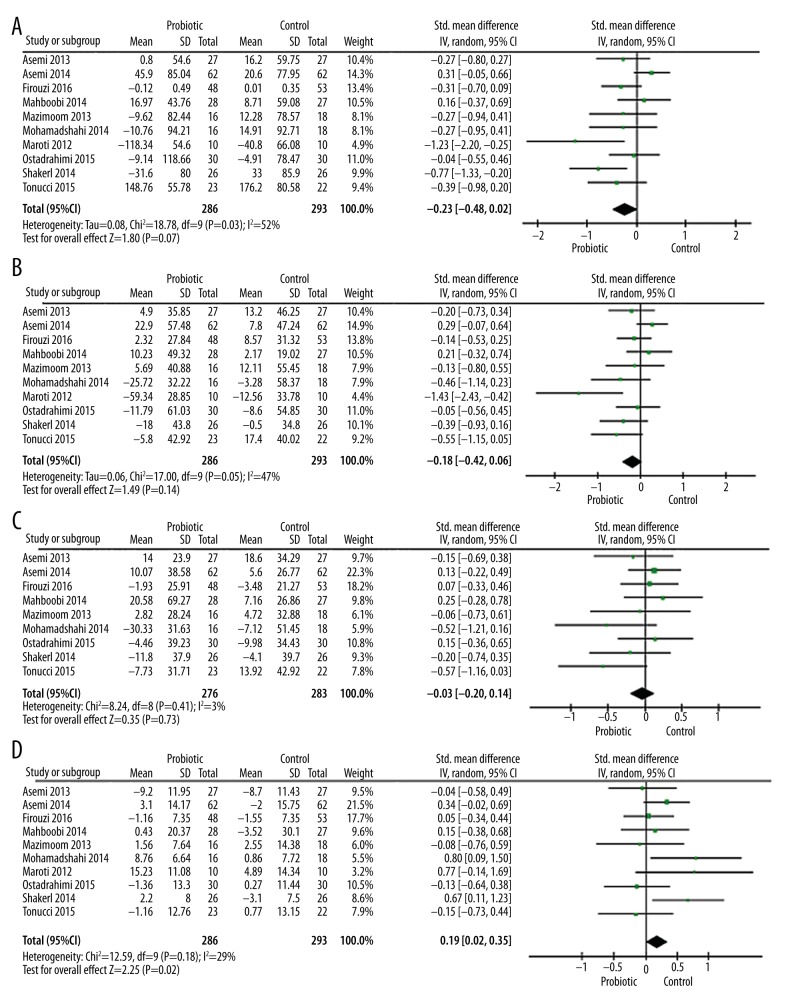
The effect of probiotics on lipid profile is presented in Figure 4. Ten trails were included in this analysis for evaluating the effects of probiotics on triglyceride levels (Figure 4A). Of these, 8 RCTs showed a significant decrease in triglyceride levels in the probiotics group compared to the control group. However, the total effect was found to be non-significant and there was significant heterogeneity (SMD, −0.23; 95% CI −0.48, 0.02; P=0.07; I2=52%, P=0.03 for heterogeneity).
Figure 4.
Forest plots for the effect of probiotics on triglycerides (A), total cholesterol (B), low-density lipoprotein cholesterol (C), and high-density lipoprotein cholesterol (D) compared to controls in pooled analysis.
Ten studies evaluated the effects of probiotics on total cholesterol levels (Figure 4B). Eight of these were included in this meta-analysis, and there was a significant decrease in total cholesterol level in the probiotics group compared to the control group. However, the overall effect was non-significant and the heterogeneity was marginally significant (SMD, −0.18; 95% CI −0.42, 0.06; P=0.14; I2=47%, P=0.05 for heterogeneity). The effect of probiotics on low- and high-density lipoprotein cholesterol are presented in Figures 4C and 4D. Among 9 RCTs included (Figure 4C), 5 studies showed a significant decrease in LDL-C levels in the probiotics group. However, no significance in the overall effect was found between the probiotics group and the control group (SMD, −0.03; 95% CI −0.20, 0.14; P=0.73, I2=3%, P=0.41 for heterogeneity). Regarding the effect of probiotics on HDL-C levels (Figure 4D), the total effect was marginally significant (SMD, 0.19; 95% CI 0.02, 0.35; P=0.02) and a moderate level of heterogeneity was also found (I2=29%, P=0.18).
Probiotics and C-reactive protein level
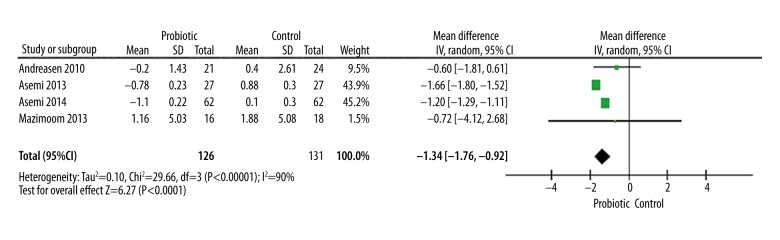
In this meta-analysis, we also investigated the effect of probiotics on CRP level and found 4 studies reported these effects (Figure 5). Probiotics significantly reduced CRP level, with a pooled mean difference of −1.34 mg/l (95% CI −1.76, −0.92; P<0.00001) and significant heterogeneity (I2=90%, P<0.00001).
Figure 5.
Forest plot for the effect of probiotics on C-reactive protein compared to controls.
Discussion
T2D is a metabolic disease characterized by hyperglycemia and insulin resistance, and associated with metabolic disturbance of blood lipids [1–3]. It causes severe pain to patients and imposes a heavy burden on families and society. In recent years, many studies have reported that probiotics have variety of effects on metabolic disturbance in T2D [10,11]. Therefore, we systematically analyzed these studies and evaluated the effect of probiotics on glucose and lipid metabolic profiles in T2D.
In this meta-analysis evaluating 12 randomized control trials with a total population of 684 diabetes patients, we demonstrated that probiotics supplementation significantly reduced glucose level and alleviated insulin resistance. However, the effects of probiotics on lipid metabolism and CRP level were not convincing.
Previous meta-analyses with smaller numbers of studies have concluded that probiotics improve insulin resistance and reduce the level of glycated hemoglobin [14–16]. A most recent meta-analysis, with 11 RCTs and 614 subjects, also demonstrated similar results [14]. They found that probiotics supplementation significantly reduced FPG, HbA1c, insulin, and HOMA-IR in diabetic patients. Our study further confirmed these findings; however, our pooled results on fasting glucose and HOMA-IR demonstrated a high level of heterogeneity, indicating that further RCTs with larger populations are needed for confirmation of these results.
No significant relationships between probiotics intake and improved lipid metabolism were found in our study, despite the fact that the present meta-analysis in general participants suggested probiotics intake significantly reduced total cholesterol and LDL-C levels [33,34]. The underlying reason might be the difference in participant characteristics between the present study and other studies. Hence, our statistical power for detecting an effect of probiotics on lipid metabolism in type 2 diabetic patients may be lower. Interestingly, neither of these 2 studies found any relationships between probiotics and levels of triglycerides or HDL-C. Future clinical trials and animal studies are warranted to elucidate the effect of probiotics on lipid metabolism.
Our results also demonstrated insufficient evidence on probiotics reducing CRP levels, with a high level of heterogeneity. CRP is an important inflammatory marker for diabetes progression and complications [35,36]. A previous meta-analysis also presented non-significant effects of probiotics on CRP levels [15]. These results suggest that although probiotics have an important role in intestinal immunological modulation [37], the evidence for an effect on CRP level is scarce. More inflammatory markers screening may help expand our understanding of the regulation of probiotics on immunological modulation.
The mechanism of these effects of probiotics remains unclear. One possibility was pointed out by Le Chatelier et al., who suggested the role of gut bacterial species richness in body weight and fat content in humans, which may further lead to other adiposity-related metabolic disorders [38]. Therefore, increasing the diversity of gut bacterial species by taking probiotics may have the reverse effect in alleviating metabolic disorders. On the other hand, animal studies have also provided insights [39–41]. Naito et al. showed that obese mice fed Lactobacillus casei strain Shirota had better insulin resistance through decreasing plasma levels of lipopolysaccharide-binding protein, a marker of endotoxemia [39], rather than reducing abdominal fat. Chen et al. demonstrated that in rats fed a high-fat diet, supplementation with Bifidobacterium longum led to reduced intestinal inflammatory activity index [40], which may also be the underlying mechanism by which probiotics affect glucose and lipid metabolism.
Limitations
Our study has several limitations. First, the doses of probiotics as dietary supplementation in the 12 included RCTs were not identical; therefore, it was not possible to determine the optimal dose for diabetic patients. Secondly, the researchers in these studies expected patients to have higher compliance, which contributes to positive results, producing a potentially higher selection bias. Finally, due to the excessive attention of researchers, the strength of the results is overestimated and the reliability of the results is reduced.
Conclusions
The present meta-analysis demonstrated that probiotics supplementation significantly reduced glucose level and alleviated insulin resistance, thereby potentially improving the clinical prognosis of type 2 diabetes. The evidence that probiotics improve lipid profiles in type 2 diabetic patients was not convincing. These results may provide evidence for encouraging use of probiotics in patients with type 2 diabetes mellitus. However, more randomized placebo-controlled trials with larger sample sizes are warranted to confirm these conclusions.
Footnotes
Disclosures
The authors declare they have no conflicts of interest regarding this study.
Source of support: This work was supported by the Graduate Student Research Innovation Fund of Three Gorges University (SDYC2016090) and Science and Technology Research and Development Project of Yichang City (A11301-15)
References
- 1.Hirst M. Diabetes in 2013. The new figures. Diabetes Res Clin Pract. 2013;102(3):265. doi: 10.1016/j.diabres.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes CJ. Type 2 diabetes – a matter of beta-cell life and death? Science. 2005;307(5708):380–84. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofe CR, Feng L, Zephyr D, et al. Fruit and vegetable intake, as reflected by serum carotenoid concentrations, predicts reduced probability of polychlorinated biphenyl-associated risk for type 2 diabetes: National Health and Nutrition Examination Survey 2003–2004. Nutr Res. 2014;34(4):285–93. doi: 10.1016/j.nutres.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burcelin R, Serino M, Chabo C, et al. Gut microbiota and diabetes: From pathogenesis to therapeutic perspective. Acta Diabetol. 2011;48(4):257–73. doi: 10.1007/s00592-011-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–67. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 7.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care. 2010;33(10):2277–84. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 10.Delzenne NM, Cani PD, Everard A, et al. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia. 2015;58(10):2206–17. doi: 10.1007/s00125-015-3712-7. [DOI] [PubMed] [Google Scholar]
- 11.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110(22):9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isik A, Karavas E, Peker K, et al. Male Mondor’s disease is a rare entity. Breast J. 2016;22(6):700–1. doi: 10.1111/tbj.12657. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Buys NJ. Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: A meta-analysis of randomized placebo-controlled trials. Br J Nutr. 2016;115(7):1167–77. doi: 10.1017/S0007114516000076. [DOI] [PubMed] [Google Scholar]
- 15.Kasinska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: A meta-analysis. Pol Arc Med Wewn. 2015;125(11):803–13. doi: 10.20452/pamw.3156. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina. 2016;52(1):28–34. doi: 10.1016/j.medici.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104(12):1831–38. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]
- 20.Asemi Z, Zare Z, Shakeri H, et al. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 21.Asemi Z, Khorrami-Rad A, Alizadeh SA, et al. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2014;33(2):198–203. doi: 10.1016/j.clnu.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, et al. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin Nutr. 2015 doi: 10.1016/j.clnu.2015.11.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Firouzi S, Majid HA, Ismail A, et al. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur J Nutr. 2016 doi: 10.1007/s00394-016-1199-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–43. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Shakeri H, Hadaegh H, Abedi F, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701. doi: 10.1007/s11745-014-3901-z. [DOI] [PubMed] [Google Scholar]
- 26.Ostadrahimi A, Taghizadeh A, Mobasseri M, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran J Public Health. 2015;44(2):228–37. [PMC free article] [PubMed] [Google Scholar]
- 27.Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran J Med Sci. 2013;38(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 28.Moroti C, Souza Magri LF, de Rezende Costa M, et al. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29. doi: 10.1186/1476-511X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahboobi S, Iraj B, Maghsoudi Z, et al. The effects of probiotic supplementation on markers of blood lipids, and blood pressure in patients with prediabetes: A randomized clinical trial. Int J Prev Med. 2014;5(10):1239–46. [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamadshahi M, Veissi M, Haidari F, et al. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: A randomized controlled clinical trial. J Res Med Sci. 2014;19(6):531–36. [PMC free article] [PubMed] [Google Scholar]
- 31.Hariri M, Salehi R, Feizi A, et al. The effect of probiotic soy milk and soy milk on anthropometric measures and blood pressure in patients with type II diabetes mellitus: A randomized double-blind clinical trial. ARYA Atheroscler. 2015;11(Suppl 1):74–80. [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Q, Li X, Feng B. The efficacy and safety of probiotics intervention in preventing conversion of impaired glucose tolerance to diabetes: study protocol for a randomized, double-blinded, placebo controlled trial of the Probiotics Prevention Diabetes Programme (PPDP) BMC Endocr Disord. 2015;15:74. doi: 10.1186/s12902-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho YA, Kim J. Effect of probiotics on blood lipid concentrations: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94(43):e1714. doi: 10.1097/MD.0000000000001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann Med. 2015;47(6):430–40. doi: 10.3109/07853890.2015.1071872. [DOI] [PubMed] [Google Scholar]
- 35.Del Cañizo Gómez FJ, Fernández Pérez C, Moreno Ruiz I, et al. Microvascular complications and risk factors in patients with type 2 diabetes. Endocrinol Nutr. 2011;58(4):163–68. doi: 10.1016/j.endonu.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Sinha SK, Shaheen M, Rajavashisth TB, et al. Association of race/ethnicity, inflammation, and albuminuria in patients with diabetes and early chronic kidney disease. Diabetes Care. 2014;37(4):1060–68. doi: 10.2337/dc13-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rupa P, Mine Y. Recent advances in the role of probiotics in human inflammation and gut health. J Agric Food Chem. 2012;60(34):8249–56. doi: 10.1021/jf301903t. [DOI] [PubMed] [Google Scholar]
- 38.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–46. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 39.Naito E, Yoshida Y, Makino K, et al. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110(3):650–57. doi: 10.1111/j.1365-2672.2010.04922.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen JJ, Wang R, Li XF, Wang RL. Bifidobacterium longum supplementation improved high-fat-fed-induced metabolic syndrome and promoted intestinal Reg I gene expression. Exp Biol Med (Maywood) 2011;236(7):823–31. doi: 10.1258/ebm.2011.010399. [DOI] [PubMed] [Google Scholar]
- 41.Tang F, Chan E, Lu M, et al. Calpain-1 mediated disorder of pyrophosphate metabolism contributes to vascular calcification induced by oxLDL. PLoS One. 2015;10(6):e0129128. doi: 10.1371/journal.pone.0129128. [DOI] [PMC free article] [PubMed] [Google Scholar]