Abstract
Background
Mizoribine (MZR) is an immunosuppressive drug used in Japan for treating patients with lupus nephritis and nephrotic syndrome and has been also reportedly effective in patients with immunoglobulin A (IgA) nephropathy. However, to date, few randomized control studies of MZR are performed in patients with IgA nephropathy. Therefore, this prospective, open-label, randomized, controlled trial aimed to investigate the efficacy and safety of adding MZR to standard treatment in these patients, and was conducted between April 1, 2009, and March 31, 2016, as a multicenter study.
Methods
Patients were randomly assigned (1:1) to receiving standard treatment plus MZR (MZR group) or standard treatment (control group). MZR was administered orally at a dose of 150 mg once daily for 12 months.
Results
Primary outcomes were the percentage reduction in urinary protein excretion from baseline and the rate of patients with hematuria disappearance 36 months after study initiation. Secondary outcomes were the rate of patients with proteinuria disappearance, clinical remission rate, absolute changes in estimated glomerular filtration rate from baseline, and the change in daily dose of prednisolone. Forty-two patients were randomly assigned to MZR (n = 21) and control groups (n = 21). Nine patients in MZR group and 15 patients in the control group completed the study. No significant differences were observed between the two groups with respect to primary and secondary outcomes.
Conclusion
The addition of MZR to standard treatment has no beneficial effect on reducing urinary protein excretion and hematuria when treating patients with IgA nephropathy.
Keywords: Hematuria, Immunoglobulin A nephropathy, Mizoribine
Introduction
Immunoglobulin A (IgA) nephropathy is characterized by microhematuria and proteinuria with a histology consisting of IgA deposition in the glomerulus [1]. This disease is the most common form of chronic glomerulonephritis and progresses to end-stage renal disease in 10% to 20% of patients within 10 years post-diagnosis [2].
The Kidney Disease Improving Global Outcomes guidelines recommend renin–angiotensin system inhibitors in patients with overt proteinuria (> 1 g/day), and furthermore, systemic corticosteroids in those with estimated glomerular filtration rate (eGFR) > 50 mL/min/1.73 m2 despite supportive care [3]. However, the use of renin–angiotensin system inhibitors have been reportedly reduced urinary protein excretion by only 30% to 50% [4,5], and relapse of proteinuria after corticosteroid therapy has been frequently observed [6].
Mizoribine (MZR), which is categorized as an immunosuppressive drug, has been used in Japan in patients with lupus nephritis and steroid-resistant nephrotic syndrome [7]. In addition, MZR was effective in patients with IgA nephropathy in a clinical setting [8–10]. However, randomized control studies of MZR administration in patients with IgA nephropathy are scarce [10,11]. Therefore, we conducted a multicenter, randomized, open-label, parallel-design study to investigate the efficacy and safety of MZR administration in patients with IgA nephropathy in this study.
Methods
Patients
Inclusion criteria in this study were: (1) patients diagnosed with primary IgA nephropathy by renal biopsy; (2) urinary protein excretion > 0.5 g/day; and (3) age > 16 years. Exclusion criteria were: (1) MZR hypersensitivity; (2) leukocyte count < 3,000/mm3; (3) pregnant patients or patients desiring to be pregnant; (4) patients receiving renal replacement therapy; and (5) use of other immunosuppressants.
Study design
This study was performed as a multicenter, randomized, open-label, parallel-design study conducted between April 1, 2009, and March 31, 2016, in Saitama Medical Center, Jichi Medical University, Saiseikai Kawaguchi General Hospital, Saitama Social Insurance Hospital, and Dokkyo University Koshigaya Medicak Center, in Saitama, Japan. Patient enrollment period was performed between April 1, 2009, and March 31, 2013.
Randomization was done at the registration center of Jichi Medical University using a computer-based allocation program. Eligible patients were randomly assigned (1:1) to receive standard treatment plus MZR (MZR group) or standard treatment (control group). The concomitant use of immunosuppressants other than MZR was not permitted during the study period, although other drugs including corticosteroid, antiplatelet agents, and antihypertensive agents were permitted. Patients in the MZR group received 150 mg MZR once daily orally in the morning for 12 months. Patients were followed every three months for 36 months. All participants provided written informed consent to participate in the study. This study was approved by the institutional review board of Saitama Medical Center, Jichi Medical University, Japan (RIN B08-27), and conforms to the provisions of the Declaration of Helsinki (as revised in Tokyo in 2004).
At each three month examination, blood pressure, urinary protein excretion, serum creatinine, and urinary sediment with red blood cells were collected. We used spot urine samples for urinalysis at each medical examination. The eGFR values were calculated using the Japanese guidelines equation [12] described below:
where S-Cr is the serum creatinine concentration (mg/dL).
In patients with this study, hematuria disappearance was defined as the number of red blood cells in urinary sediments < 5 using high power field. Proteinuria disappearance was defined as levels < 0.3 g/day. Furthermore, clinical remission was defined as the disappearance of proteinuria and hematuria [13].
Study outcomes
The primary outcomes of this study were the percentage reduction in urinary protein excretion from baseline and rate of patients with the disappearance of hematuria at 36 months. The secondary outcomes were the rate of patients with the disappearance of proteinuria, clinical remission rate, absolute changes in eGFR from baseline, and the change in daily dose of prednisolone.
Statistics
Statistical analysis was performed using JMP® 11 (SAS Institute Inc., Cary, NC, USA). Data were expressed as means ± standard deviation for continuous variables, and as counts and percentages for categorical variables. Comparisons of component ratios were performed using Fisher’s exact test. Comparisons between the two groups were performed using the Mann-Whitney U-test. Differences of P < 0.05 were considered statistically significant. Based on a previous report [14], we assumed that the difference in mean urinary protein excretion between the two groups would be 160 mg/day and standard deviation, 200 mg/day, at 36 months. Twenty patients in each group were needed to establish the superiority of the test treatment for the primary endpoint with 80% power at a 2.5% one-sided significance level. This study was planned to enroll 30 patients per group to account for dropouts. We set the significance level at 5% (two-sided) and reported two-sided P-values for secondary outcomes, and carried out an intention-to-treat analysis using last observation carried forward method for missing data due to dropouts.
Results
Patient characteristics
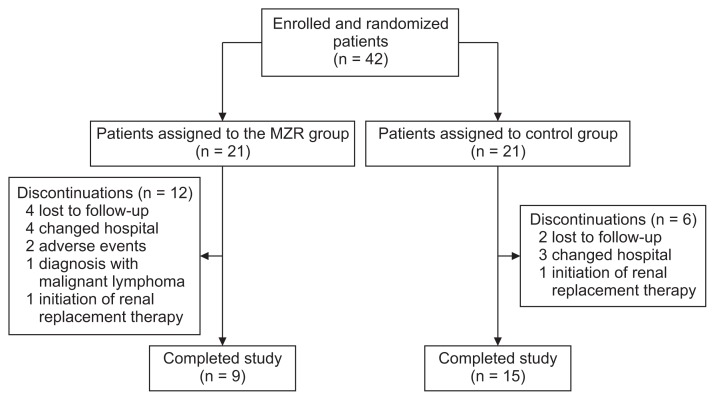
Forty-two patients met the criteria for study inclusion, provided written informed consent, and were randomly assigned to the MZR group (n = 21) and control group (n = 21) (Fig. 1). One patient in the control group received MZR after randomization; however, this patient was analyzed as part of the control group in the intention-to-treat analysis. Patients’ baseline characteristics are summarized in Table 1. There were no significant differences for all clinical parameters between the two groups. In particular, Japanese histological grades assessed by the Special IgA Nephropathy Study Group classification [15] were not significantly different between the two groups, nor was histological grades assessed by Oxford classification [16]. In most patients, prednisolone was administered at an initial dose of 0.5 mg/kg/day on alternate days, and tapered over a minimum of 6 months, as described by Pozzi et al [6]. However, higher dose prednisolone was administered to patients with nephrotic-range proteinuria.
Figure 1. Study flow diagram.
MZR, mizoribine.
Table 1.
Baseline characteristics in the MZR and control groups
| Characteristic | MZR group (n = 21) | Control group (n = 21) | P |
|---|---|---|---|
| Age (yr) | 41.6 ± 14.7 | 43.2 ± 19.4 | 0.90 |
| Gender, men/women | 13/8 | 13/8 | 1.00 |
| Systolic blood pressure (mmHg) | 133.8 ± 15.8 | 127.5 ± 26.7 | 0.08 |
| Diastolic blood pressure (mmHg) | 81.7 ± 13.9 | 76.6 ± 15.4 | 0.30 |
| Serum creatinine (mg/dL) | 1.2 ± 1.0 | 1.2 ± 1.1 | 1.00 |
| eGFR (mL/min/1.73 m2) | 67.5 ± 30.4 | 67.4 ± 33.6 | 0.90 |
| Urinary protein excretion (g/gCr) | 0.90 ± 0.82 | 1.11 ± 1.36 | 0.83 |
| Serum albumin (g/dL) | 4.1 ± 0.6 | 4.2 ± 0.3 | 1.00 |
| Uric acid (mg/dL) | 5.7 ± 1.8 | 6.5 ± 1.7 | 0.29 |
| Serum IgA (mg/dL) | 306.7 ± 116.2 | 387.5 ± 190.1 | 0.15 |
| Duration of illness (y) | 4.6 ± 4.2 | 4.7 ± 5.0 | 0.74 |
| Japanese histological grade | |||
| Grade I | 15 (71.4) | 14 (66.7) | 0.79 |
| Grade II | 4 (19.0) | 4 (19.1) | |
| Grade III | 2 (9.5) | 2 (9.5) | |
| Grade IV | 0 (0.0) | 1 (4.8) | |
| Oxford classification | |||
| M0 | 17 (81.0) | 14 (66.7) | 0.48 |
| M1 | 4 (19.0) | 7 (33.3) | |
| E0 | 20 (95.2) | 18 (85.7) | 0.61 |
| E1 | 1 (4.8) | 3 (14.3) | |
| S0 | 9 (42.9) | 12 (57.1) | 0.54 |
| S1 | 12 (57.1) | 9 (42.9) | |
| T0 | 14 (66.7) | 16 (76.2) | 0.73 |
| T1 | 5 (23.8) | 3 (14.3) | |
| T2 | 2 (9.5) | 2 (9.5) | |
| Antiplatelet agents | 15 (71.4) | 17 (80.1) | 0.47 |
| RASI | 16 (76.2) | 17 (80.1) | 0.71 |
| Tonsillectomy | 16 (76.2) | 15 (71.4) | 0.73 |
| Steroid pulse therapy | 16 (76.2) | 16 (76.2) | 1.00 |
Values are presented as mean ± standard deviation, number only, or number (%).
Japanese histological grades are classified based on the percentage of the glomeruli exhibiting cellular or fibrocellular crescents, global sclerosis, segmental sclerosis or fibrous crescents (grade 1: < 25%, grade 2: 25 49%, grade 3: 50 74%, grade 4: ≥ 75%).
eGFR, estimated glomerular ltration rate; IgA, immunoglobulin A; MZR, mizoribine; RASI, renin angiotensin system inhibitors.
MZR was administered 144 ± 22 mg/day for a duration of 397 ± 197 days. In 12 patients, serum concentration 2 hours after MZR administration was 1.80 ± 0.87 μg/mL.
Primary and secondary outcomes
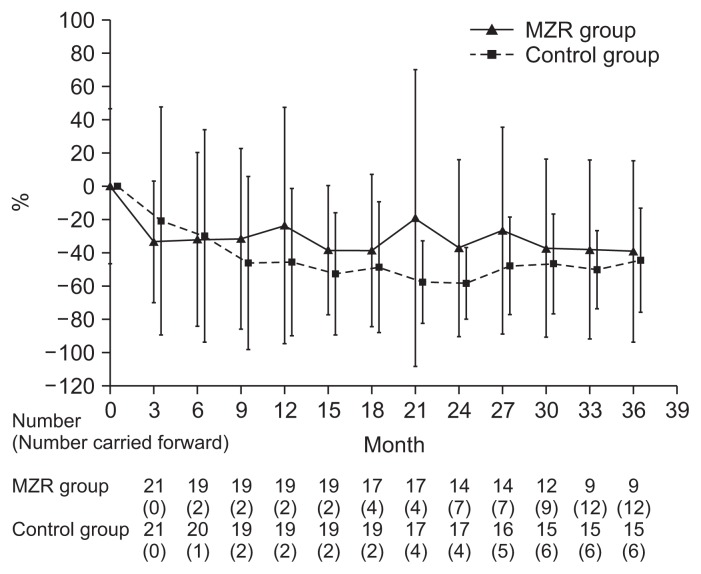
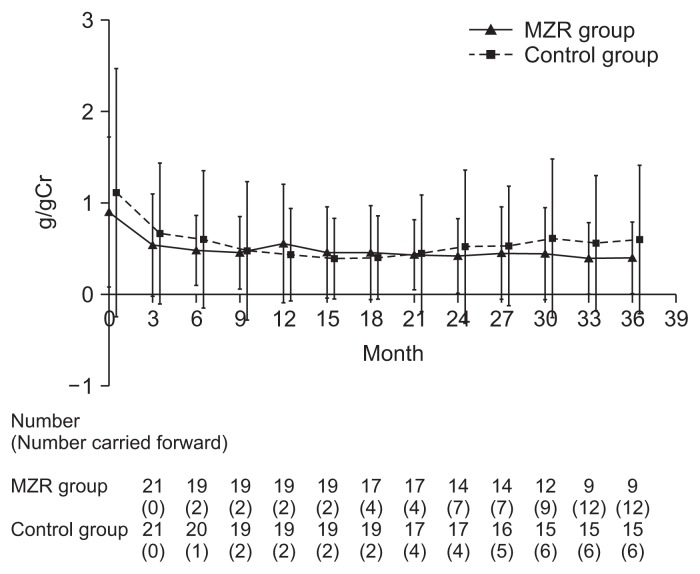
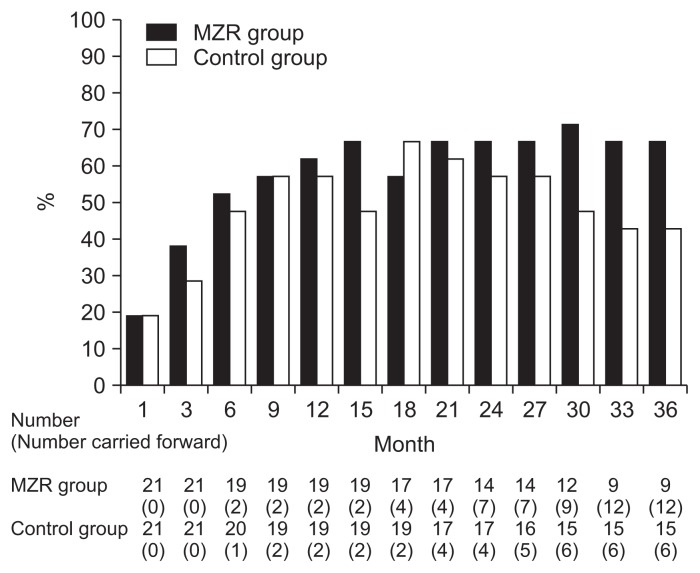
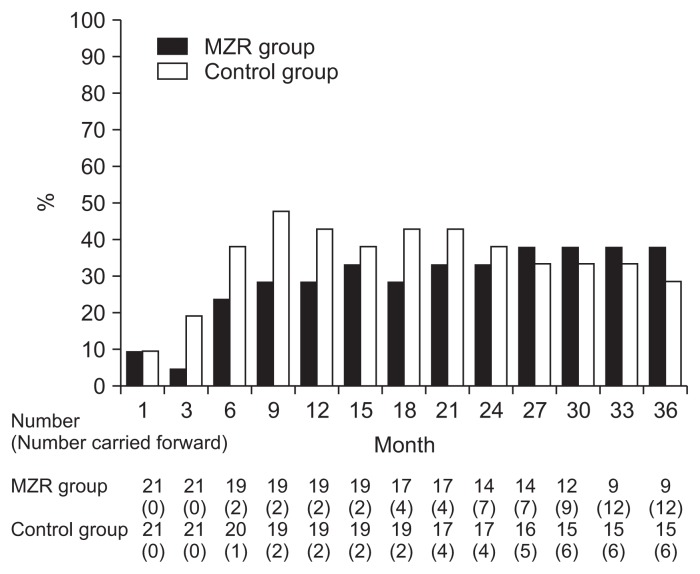
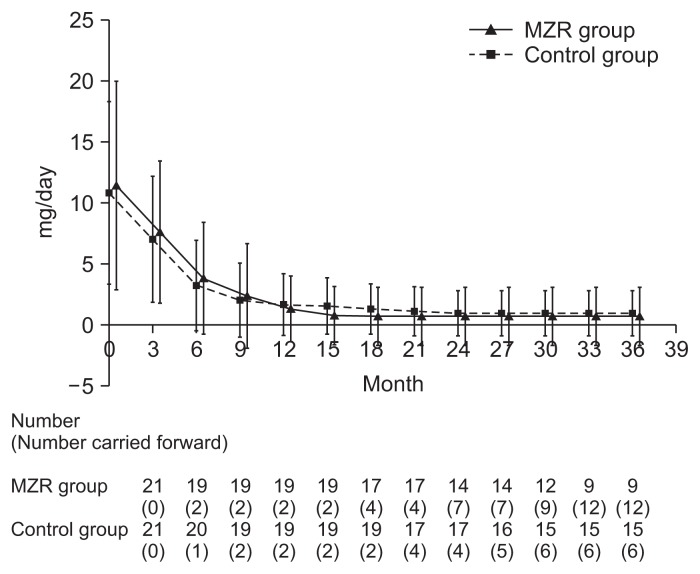
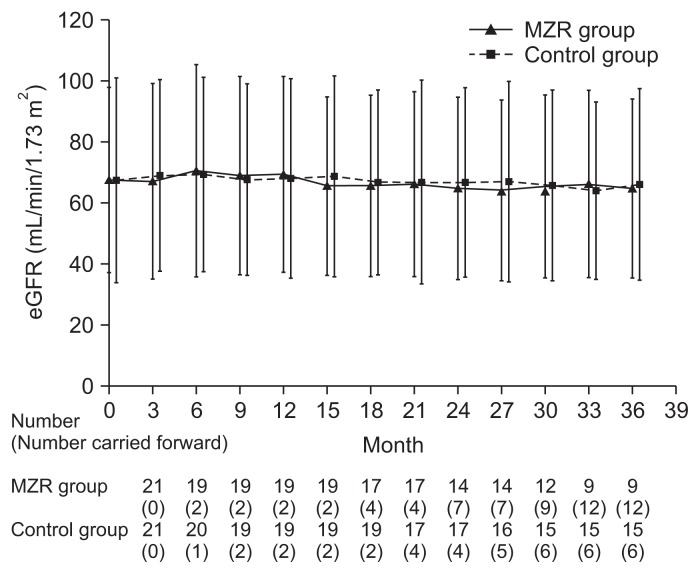
Percentage reductions in urinary protein excretion from study initiation to 36 months are shown in Fig. 2. There were no significant differences in urine protein excretion between the two groups during the study (Fig. 3). Among patients who completed the study, the rate of hematuria disappearance was significantly higher in the MZR group than the control group at 30 months (100% vs. 53.3%, P < 0.05) and 36 months (88.9% vs. 46.7%, P < 0.05). However, the intention-to-treat analysis demonstrated no significant difference in the hematuria disappearance rate between groups (Fig. 4). Furthermore, no significant differences were observed in the rate of patients with proteinuria disappearance, clinical remission (Fig. 5), the change in daily dose of prednisolone (Fig. 6), and in particular, absolute change in eGFR (Fig. 7) between the groups.
Figure 2.
The changes of percentage reduction in urinary protein excretion in the mizoribine (MZR) and control group.
Figure 3.
The changes of urinary protein excretion in the mizoribine (MZR) and control group.
Figure 4.
The changes in the rate of patients with hematuria disappearance in the mizoribine (MZR) and control group.
Figure 5.
The changes in the rate of patients with clinical remission in the mizoribine (MZR) and control group.
Figure 6.
The changes in daily dose of prednisolon in the mizoribine (MZR) and control group.
Figure 7.
The absolute changes in estimated glomerular filtration rate (eGFR) in the mizoribine (MZR) and control group.
Adverse events
Two patients were withdrawn from the MZR group due to adverse events (hepatotoxicity and low-grade fever). No patient died during this study. Twelve patients in the MZR group and 6 patients in the control group dropped out before the end of this study. The reasons for dropouts were: lost to follow-up (4 patients in MZR group and 2 patients in control group), changed hospital (4 patients in MZR group and 3 patients in control group), diagnosed with malignant lymphoma (1 patient in MZR group), adverse events (2 patients in MZR group), and initiation of renal replacement therapy (1 patient in MZR group and 1 patient in control group).
Discussion
In this study, we investigated the efficacy and safety of MZR administration compared with standard treatment in patients with IgA nephropathy, and found that the hematuria disappearance rate during the late phase of the study was significantly higher in patients in the MZR group who completed the study than in patients in the control group. However, this significant difference in hematuria disappearance rate between the two groups was disappeared by intention-to-treat analysis in this study.
MZR is an immunosuppressive agent that inhibits purine synthesis by inhibiting inosine monophosphate dehydrogenase expression and suppressing the proliferation of lymphocytes [7]. This agent was previously reported to represent renoprotective effects, including inhibition of mesangial proliferation and improvement of urinary protein excretion [17]. Furthermore, a multicenter randomized control study demonstrated that treatment with angiotensin II receptor blockers (ARB) combined with MZR significantly reduced urinary protein excretion, compared with ARB treatment alone in adult patients with IgA nephropathy [10]. Unfortunately, our study could not show a significant change in the reduction of proteinuria between the MZR and control group. Several reasons would be considered to explain the clinical differences between our results and those from previous reports. First, approximately 80% of all patients in our study underwent aggressive, standard treatment of IgA nephropathy using tonsillectomy and steroid pulse therapy, which have been shown to be as effective as multidrug therapy in pediatric patients with diffuse IgA nephropathy [18]. In addition, a recent small-scale randomized control trial indicated that the addition of MZR to steroid-pulse therapy did not have an additional effect on urinary protein excretion and renal function in patients with progressive IgA nephropathy [11]. Furthermore, most patients in our study showed mild histological damage; therefore, a large-scale trial in patients with severe pathological findings may be needed to detect a significant difference in efficacy of MZR administration. Second, approximately 40% of patients enrolled dropped out from this study; therefore, the statistical power of our analysis was possible to be reduced to confirm clinical differences between groups. Third, the clinical efficacy of MZR administration is associated with peak serum concentrations [19]. Specifically, a peak MZR level of 3 to 5 μg/mL obtained 2 to 3 hours after oral administration is needed to obtain a good response [20,21]. In our study, serum MZR concentration 2 hours after administration was 1.80 ± 0.87 μg/mL in 12 patients; therefore, MZR dosage may have been insufficient in this study.
Microscopic hematuria is a potent risk factor for progression to end-stage renal failure in addition to proteinuria [22]. Disappearance of hematuria was reportedly a powerful predictor of renal prognosis in patients with progressive IgA nephropathy after administration of corticosteroids and immunosuppressant agents [23]. However, a recent randomized control trial showed that the addition of immunosuppressants to renin–angiotensin system inhibitors improved the disappearance rate of hematuria, but did not attenuate the rate of decline in renal function [24]. Thus, the relationship between renal prognosis and hematuria disappearance in patients with IgA nephropathy remains controversial. In our study, the hematuria disappearance rate in the MZR group reached > 60% in the late phase of the study, whereas those in the control group were around 40–50%. Although the increase in the hematuria disappearance rate with MZR administration may lead to the preservation of renal function in patients with IgA nephropathy, the length of our study was too short to support that conclusion. Therefore, long-term studies are needed to evaluate long-term renal function, including eGFR, in patients.
Previous report indicated that MZR administration had fewer severe adverse events than other immunosuppressants [25]. Indeed, in this study, there were no serious adverse events among patients in the MZR group. However, mild liver dysfunction developed in one patient, and a low-grade fever in another patient, both of which promptly disappeared after MZR discontinuation.
This study had several limitations. First, we used an open-label design, which may have introduced interpretive bias. Second, the sample size was small and there were relatively large number of dropouts during the study that could have reduced the statistical power to detect differences in study outcomes. Therefore, long-term studies will be needed to assess the efficacy of MZR for preserving renal function and improving renal prognosis in patients with IgA nephropathy.
In conclusion, the addition of MZR to standard treatment has no beneficial effect on reducing urinary protein excretion and hematuria when treating patients with IgA nephropathy.
Acknowledgments
The authors thank all patients and clinicians who participated in the study. They also thank Asahi Kasei Pharma Corporation for the measurements of the blood levels of MZR.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24:179–196. doi: 10.1016/j.semnephrol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 4.Li PK, Leung CB, Chow KM, Cheng YL, Fung SK, Mak SK, Tang AW, Wong TY, Yung CY, Yung JC, Yu AW, Szeto CC HKVIN Study Group. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47:751–760. doi: 10.1053/j.ajkd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Sekizuka K, Ebihara I, Koide H. Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgA nephropathy. Am J Nephrol. 2000;20:373–379. doi: 10.1159/000013619. [DOI] [PubMed] [Google Scholar]
- 6.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163. doi: 10.1097/01.ASN.0000103869.08096.4F. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki Y. Mizoribine: a new approach in the treatment of renal disease. Clin Dev Immunol. 20092009:681482. doi: 10.1155/2009/681482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko T, Hirama A, Ueda K, Fujino T, Utsumi K, Iino Y, Katayama Y. Methylprednisolone pulse therapy combined with mizoribine following tonsillectomy for immunoglobulin A nephropathy: clinical remission rate, steroid sparing effect, and maintenance of renal function. Clin Exp Nephrol. 2011;15:73–78. doi: 10.1007/s10157-010-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokunaga M, Tamura M, Kabashima N, Serino R, Shibata T, Matsumoto M, Miyamoto T, Miyazaki M, Furuno Y, Nakamata J, Takeuchi M, Abe H, Okazaki M, Otsuji Y. Mizoribine for relapsed proteinuria in an adult IgA nephropathy patient. Clin Nephrol. 2008;70:445–446. doi: 10.5414/CNP70445. [DOI] [PubMed] [Google Scholar]
- 10.Xie Y, Huang S, Wang L, Miao L, Zhang A, Li Y, Wu X, Wang L, Liu S, Lie C, Chen P, Chen X. Efficacy and safety of mizoribine combined with losartan in the treatment of IgA nephropathy: a multicenter, randomized, controlled study. Am J Med Sci. 2011;341:367–372. doi: 10.1097/MAJ.0b013e318207e02d. [DOI] [PubMed] [Google Scholar]
- 11.Masutani K, Tsuchimoto A, Yamada T, Hirakawa M, Mitsuiki K, Katafuchi R, Hirakata H, Kitazono T, Tsuruya K West Japan Study Group for Therapy of IgA Nephropathy Investigators. Comparison of steroid-pulse therapy and combined with mizoribine in IgA nephropathy: a randomized controlled trial. Clin Exp Nephrol. 2016;20:896–903. doi: 10.1007/s10157-016-1226-3. [DOI] [PubMed] [Google Scholar]
- 12.Japan Nephrology Society. Special issue: Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi. 2012;54:1034–1191. [PubMed] [Google Scholar]
- 13.Suzuki Y, Matsuzaki K, Suzuki H, Sakamoto N, Joh K, Kawamura T, Tomino Y, Matsuo S. Proposal of remission criteria for IgA nephropathy. Clin Exp Nephrol. 2014;18:481–486. doi: 10.1007/s10157-013-0849-x. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki Y, Hosoya M, Suzuki J, Onishi N, Takahashi A, Isome M, Nozawa R, Suzuki H. Efficacy of multidrug therapy combined with mizoribine in children with diffuse IgA nephropathy in comparison with multidrug therapy without mizoribine and with methylprednisolone pulse therapy. Am J Nephrol. 2004;24:576–581. doi: 10.1159/000082202. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura T, Joh K, Okonogi H, Koike K, Utsunomiya Y, Miyazaki Y, Matsushima M, Yoshimura M, Horikoshi S, Suzuki Y, Furusu A, Yasuda T, Shirai S, Shibata T, Endoh M, Hattori M, Katafuchi R, Hashiguchi A, Kimura K, Matsuo S, Tomino Y. Study Group Special IgA Nephropathy: A histologic classification of IgA nephropathy for predicting long-term prognosis: emphasis on end-stage renal disease. J Nephrol. 2013;26:350–357. doi: 10.5301/jn.5000151. [DOI] [PubMed] [Google Scholar]
- 16.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 17.Nagaoka R, Kaneko K, Ohtomo Y, Yamashiro Y. Mizoribine treatment for childhood IgA nephropathy. Pediatr Int. 2002;44:217–223. doi: 10.1046/j.1328-8067.2001.01532.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki Y, Takano K, Suyama K, Isome M, Suzuki H, Sakuma H, Fujiki T, Suzuki H, Hosoya M. Efficacy of tonsillectomy pulse therapy versus multiple-drug therapy for IgA nephropathy. Pediatr Nephrol. 2006;21:1701–1706. doi: 10.1007/s00467-006-0272-6. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda T, Hirose S, Tanabe N, Sato H, Nakatsue T, Ajiro J, Wada Y, Murakami S, Hasegawa H, Ito S, Sakatsume M, Nakano M, Gejyo F. Mizoribine therapy for patients with lupus nephritis: the association between peak mizoribine concentration and clinical efficacy. Mod Rheumatol. 2007;17:206–212. doi: 10.3109/s10165-007-0567-x. [DOI] [PubMed] [Google Scholar]
- 20.Nishi E, Kameda H, Ogawa H, Nagasawa H, Takei H, Okuyama A, Kurasawa T, Kondo T, Nishimura K, Shirai Y, Sakai R, Ito T, Takeuchi T, Amano K. Efficacy of weekly mizoribine pulse therapy in refractory lupus nephritis. Mod Rheumatol. 2013;23:97–103. doi: 10.3109/s10165-012-0645-6. [DOI] [PubMed] [Google Scholar]
- 21.Fujieda M, Ishihara M, Morita T, Hayashi A, Okada S, Ohta T, Sakano T, Wakiguchi H. Effect of single-dose oral mizoribine pulse therapy twice per week for frequently relapsing steroid-dependent nephrotic syndrome. Clin Nephrol. 2012;78:40–46. [PubMed] [Google Scholar]
- 22.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, Liu Z. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27:1479–1485. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 23.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 24.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J STOP-IgAN Investigators. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–2236. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 25.Tanabe K, Tokumoto T, Ishikawa N, Kanematsu A, Oshima T, Harano M, Inui M, Yagisawa T, Nakajima I, Fuchinoue S, Takahashi K, Toma H. Long-term results in mizoribine-treated renal transplant recipients: a prospective, randomized trial of mizoribine and azathioprine under cyclosporine-based immunosuppression. Transplant Proc. 1999;31:2877–2879. doi: 10.1016/S0041-1345(99)00599-0. [DOI] [PubMed] [Google Scholar]