Abstract
Direct-acting antivirals for hepatitis C virus infection may revolutionize treatment among persons with substance use disorders. Despite persons with substance use disorders having the highest hepatitis C virus prevalence and incidence, the vast majority have not engaged into care for the infection. Previously, interferon-based treatments, with substantial side effects and the propensity to exacerbate mental health conditions, were major disincentives to pursuit of care for the infection. Direct-acting antivirals with viral eradication rates of >90%, significantly improved side effect profiles, and shorter treatment duration are dramatic improvements over prior treatment regimens that should promote widespread hepatitis C virus care among persons with substance use disorders. The major unmet need is strategies to promote persons with substance use disorders engagement into care for hepatitis C virus. Although physical integration of treatment for substance use and co-occurring conditions has been widely advocated, it has been difficult to achieve. Telemedicine offers an opportunity for virtual integration of behavioral and medical treatments that could be supplemented by conventional interventions such as hepatitis C virus education, case management, and peer navigation. Furthermore, harm reduction and strategies to reduce viral transmission are important to cease reinfection among persons with substance use disorders. Widespread prescription of therapy for hepatitis C virus infection to substance users will be required to achieve the ultimate goal of global virus elimination. Combinations of medical and behavioral interventions should be used to promote persons with substance use disorders engagement into and adherence with direct-acting antiviral-based treatment approaches. Ultimately, either physical or virtual colocation of hepatitis C virus and substance use treatment has the potential to improve adherence and consequently treatment efficacy.
Key Words: HCV, persons with substance use disorders, HCV treatment
BACKGROUND ON HEPATITIS C VIRUS INFECTION
Chronic infection with hepatitis C virus is a major cause of liver disease and is the most common chronic blood-borne infection in the United States. Over 180 million people globally are affected by hepatitis C virus, representing a major global health problem.1 The prevalence of the disease varies significantly depending upon geographic region and country, with the higest prevalence in Central and East Asia and in North Africa/Middle East. Epidemiological studies have reported that between 3 and 4 million people in the United States have chronic hepatitis C virus infection.1,2 However, an even larger number, possibly >5 million, may have chronic infection when accounting for high-risk populations including the homeless, incarcerated individuals, and people who inject drugs.3
A large percentage of hepatitis C virus–infected individuals in the United States remain undiagnosed and are at risk of progressive liver disease.4 Consequently, the health care burden related to hepatitis C virus is projected to continue to increase with associated increases in the prevalence of cirrhosis, liver failure, and hepatocellular carcinoma.5,6 Hepatitis C virus is currently the leading indication for liver transplantation in developed countries,7,8 and ∼700,000 people globally die every year from hepatitis C virus–related liver diseases,9 As highly effective antiviral therapy for hepatitis C virus infection is now available, efforts to diagnose hepatitis C virus and to identify treatment-eligible candidates is an increasingly important priority, particularly among persons with substance use disorders.
As hepatitis C virus is a blood-borne pathogen, viral transmission as a consequence of illicit substance use is the most common acquisition risk factor in developed countries.7,10 Hepatitis C virus seroprevalence among persons who inject drugs is extremely high, reaching ≥60% in most countries.11 Likewise, the yearly incidence of hepatitis C virus in these individuals ranges from 5% to as high as 45%,12 with a higher frequency among younger individuals and recent initiates to injection drug use.13,14 As a result, injection drug use accounts for 68% to 80% of all hepatitis C virus infections in the developed world.7 Therefore, targeting persons who inject drugs for hepatitis C virus prevention and treatment is critically important to its global elimination. Several groups have recently highlighted and continue to forcefully advocate for the importance of efforts to reduce the risk of hepatitis C virus transmission among persons who inject drugs.15,16
NATURAL HISTORY OF HEPATITIS C VIRUS INFECTION
Acute hepatitis C virus infection is typically asymptomatic. Although spontaneous recovery occasionally occurs, the majority (up to 85%) progresses to chronic infection. Once chronic infection is established, progressive liver disease can result in cirrhosis in up to 30% of individuals within 20 years. Factors associated with rapid disease progression include chronic alcohol consumption, coexisting nonalcoholic fatty liver disease, and coinfection with hepatitis B or human immunodeficiency virus (HIV), which can result in intermittent elevation of alanine aminotransferase levels.17,18 Chronic hepatitis C virus infection has a major impact on survival, leading to an increase in both liver-related and non–liver-related mortality among those at any stage of hepatitis C virus–associated liver disease.19 Cirrhotic individuals are at increased risk of liver cancer and can progress relatively quickly from asymptomatic, clinically compensated disease to overt decompensation with portal hypertension characterized by the onset of ascites, hepatic encephalopathy, or variceal hemorrhage.20 The development of liver cancer or clinical decompensation is associated with a significant change in prognosis and an acute increase in mortality risk.
EVOLUTION OF HEPATITIS C VIRUS TREATMENT
Recent therapeutic advances have fundamentally changed the approach to treatment of hepatitis C virus. Unlike HIV infection, successful long-term clearance of hepatitis C virus after therapy is defined by achievement of a sustained virologic response, which is a durable endpoint and is equivalent to viral eradication. Viral eradication is associated with improved health-related quality of life, fibrosis regression, reduction in liver-related and all-cause mortality, and decreased risk of events related to cardiovascular disease, kidney disease, stroke, and malignancy.21–23 In the setting of advanced liver disease, successful treatment can significantly reduce the risk of clinical decompensation, liver cancer, need for liver transplantation, and mortality, independent of comorbidities or other risk factors.24,25
Since shortly after the discovery of hepatitis C virus in 1989 and until very recently, interferon α was the cornerstone of treatment for chronic hepatitis C. Interferon-based regimens were associated with significant side effects and led to viral eradication in only ∼50% of treated patients.26,27 In 2011, the first direct-acting antivirals, telaprevir, and boceprevir, received regulatory approval and were utilized in combination with interferon and ribavirin resulting in viral eradication in ∼70% of treatment-naive patients.28,29 In late 2014, the approval of interferon-free, all-oral treatment regimens was granted.30,31
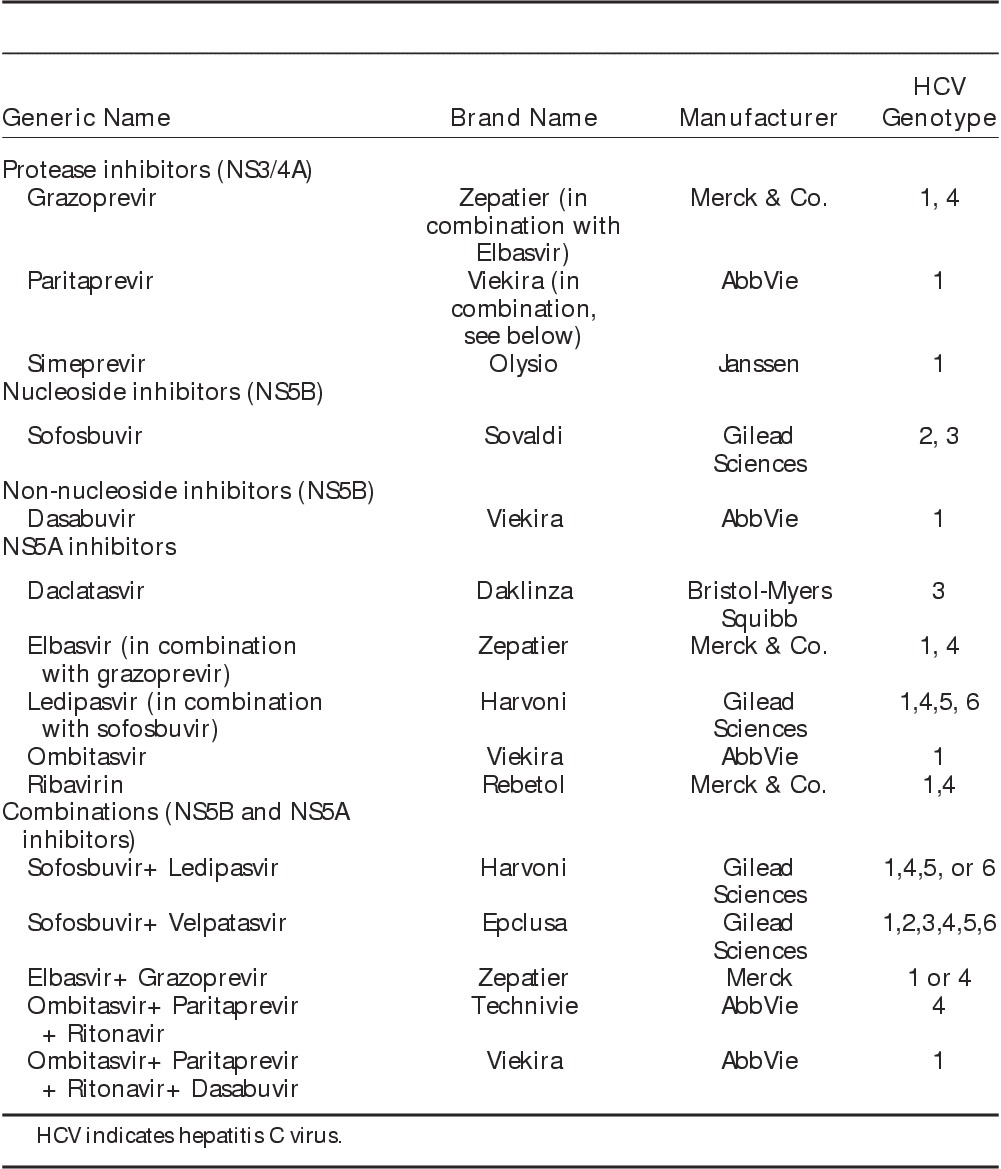
The first, widely prescribed direct-acting antiviral combination approved was composed of the hepatitis C virus NS5A inhibitor, ledipasvir, and the NS5B polymerase inhibitor, sofosbuvir, coformulated in a fixed-dose, single tablet (Harvoni, Gilead Sciences). The combination is typically prescribed for either 8 or 12 weeks (depending upon hepatitis C virus RNA level) for genotype 1, it has superior efficacy with eradication rates of at least 90%, a high barrier to resistance, and minimal side effects.32,33 Other all oral direct-acting antiviral regimens for treatment of hepatitis C virus genotype 1 include the combination of 3 agents, the NS5A inhibitor, ombitasvir, the NS5B polymerase inhibitor, dasabuvir, and the NS3/4A protease inhibitor, paritaprevir, together with the antiretroviral drug, ritonavir (Viekira Pak, AbbVie), which is included in the regimen for its ability to boost paritaprevir concentrations.34 Another frequently prescribed direct-acting antiviral combination is the coformulation of the NS3/4A protease inhibitor, grazoprevir, with the NS5A inhibitor, elbasvir (Zepatier, Merck).35 The combination of sofosbuvir and daclatasvir has been approved for treatment of hepatitis C virus genotypes 2 and 3.36 Most recently, the pan-genotypic combination of sofosbuvir with the NS5A inhibitor, velpatasvir, has been approved (Epclusa, Gilead Sciences).37 The availability of interferon-free, direct-acting antiviral regimens has led to significant improvements in treatment efficacy, in which viral eradication can be achieved in over 90% of patients regardless of hepatitis C virus genotype (Table 1).38
TABLE 1.
Summary of Direct-acting Antiviral Regimens
Changes to the Therapeutic Regimen Enhance Uptake by Persons With Substance Use Disorders
Historically, hepatitis C virus–infected individuals who actively inject drugs as well as those who have transitioned to opioid replacement therapy were rarely treated, despite professional society guidelines and recommendations that advocated the opposite.39–41 Recent advances in hepatitis C virus therapeutics; however, have fundamentally changed the approach to treatment of persons with substance use disorders. Principal among these was elimination of interferon from the therapeutic regimen. Because of a similarity in symptomatology between the adverse effects of interferon and that of methadone withdrawal, many persons with substance use disorders reported that interferon was the major deterrent to pursuit of hepatitis C virus treatment. In addition, concerns regarding persons with substance use disorders likely adherence to the therapeutic regimen and the potential to develop resistant-associated variants to the first generation direct-acting antivirals, dampened provider’s enthusiasm to treat hepatitis C virus among persons with substance use disorders.28,29,42
Interferon elimination also removed its potential exacerbation of mental health conditions that are frequent co-occurrences among persons with substance use disorders, which could reduce treatment adherence and might be reasons for treatment failure.43,44 Shorter treatment duration, 2 to 3 months as opposed to 6 or 11 months, is also a potential benefit of direct-acting antivirals that can increase treatment adherence among persons with substance use disorders. Although some drug-drug interactions exist, particularly with specific antiretroviral agents, concomitant use of direct-acting antiviral therapy with either methadone or buprenorphine is safe and effective. Although these interventions should increase direct-acting antiviral treatment among persons with substance use disorders, new restrictions on hepatitis C virus treatment provision particularly among persons with substance use disorders have arisen, such as restrictions established by third-party payers including the presence of active substance use and restricting treatment to those with late-stage liver disease, that is, advanced fibrosis and cirrhosis.45,46
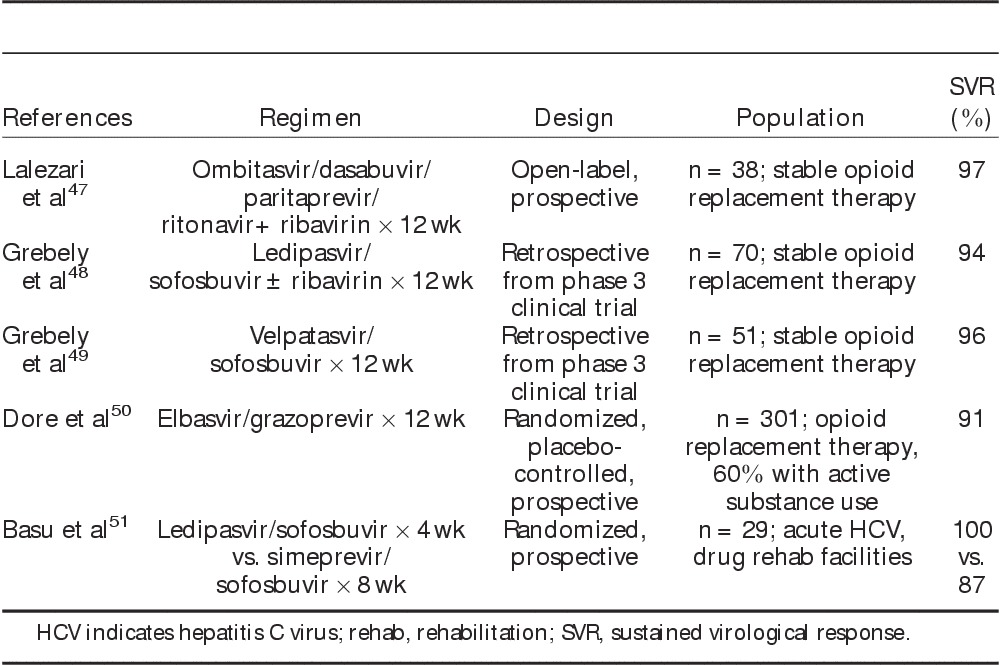
Several studies have now demonstrated high rates of viral eradication associated with direct-acting antiviral therapy in persons with substance use disorders, both in the setting of stable opioid replacement therapy and in those with recent hepatitis C virus exposure due to active drug use (Table 2). An open-label, prospective study of the 3 drug direct-acting antiviral combination regimen ombitasvir, dasabuvir, paritaprevir, and ritonavir plus ribavirin in patients with genotype 1 hepatitis C virus on a stable regimen of opioid replacement therapy reported no differences in antiviral efficacy and pharmacokinetics between those receiving methadone versus buprenorphine, with an overall sustained viral response of 97%.47 In addition, retrospective data derived from large-scale phase 3 clinical trials of sofosbuvir-based therapy involving ledipasvir/sofosbuvir and velpatasvir/sofosbuvir revealed no differences in efficacy, adherence, or adverse events in those receiving opioid replacement therapy versus others, with viral eradication rates of 94% to 96%.48,49 A randomized, double-blind, placebo-controlled phase III study of 12 weeks of elbasvir/grazoprevir in persons with substance use disorders on opiate agonist therapy was notable for an overall viral eradication rate of 91% in genotypes 1, 4, and 6 despite active drug use in greater than one-half of patients.50 An additional prospective study identified persons with substance use disorders presenting with recent hepatitis C virus exposure associated with active injection drug use, in which a shortened duration of direct-acting antiviral therapy of 4 weeks of ledipasvir/sofosbuvir or 8 weeks of simeprevir/sofosbuvir led to viral eradication in 93% by intention-to-treat and 100% by per-protocol analysis.51 The high efficacy associated with a short duration of direct-acting antiviral therapy in early hepatitis C virus infection suggests a cost benefit in identifying and treating recent hepatitis C virus exposures in persons with substance use disorders.
TABLE 2.
Studies Involving Direct-acting Antiviral Therapy for Genotype 1 Hepatitis C in People Who Inject Drugs47–51
New Barriers to Hepatitis C Virus Treatment Uptake by Persons With Substance Use Disorders
The high costs of new hepatitis C virus treatments have resulted in the implementation of onerous requirements to obtain approval for direct-acting antivirals in the United States. In some cases, 6 months of documented abstinence from illicit substance use or excessive alcohol intake, in addition to enrollment in a formal substance use treatment program, may be mandated. Additional requirements may include written informed consent stipulating that patients will be adherent to the therapeutic regimen. The importance of maximizing adherence and the risk of reinfection continue to be concerns for medical providers who are largely unfamiliar with the issues of treatment of persons with substance use disorders. In actuality, though, high adherence and low rates of reinfection have been observed among persons with substance use disorders irrespective of injection drug use before or during treatment with interferon-based regimens.52,53 More recent data involving direct-acting antiviral regimens have also noted high adherence rates, with no difference in efficacy, adherence, and adverse events in patients receiving opioid replacement therapy versus others.47–50 Similarly, as described below, reinfection after successful viral eradication remains exceedingly low.50,54,55 As many persons with substance use disorders are uninsured, underfunded, and without social support, the onerous cost of hepatitis C virus treatment and payers’ requirements for direct-acting antiviral approval can become major obstacles to their treatment.
STATUS OF US FEDERAL GOVERNMENT STRATEGIES TO END THE HEPATITIS C VIRUS EPIDEMIC
Surveillance programs have identified significant health disparities attributed to viral hepatitis, in which more than one-half of individuals with chronic hepatitis C virus have incomes lower than twice the poverty level and less than a high school education. Racial disparities also exist, in which American Indians and Alaska Natives have the highest rate of new hepatitis C virus infections, whereas African Americans account for 25% of people with chronic hepatitis C virus but comprise only 11% of the US population.56 In addition to persons with substance use disorders, other vulnerable populations with a high prevalence of hepatitis C virus infection include individuals with HIV infection (∼20%), HIV-infected drug users (60% to 90%), incarcerated individuals (33%), and the homeless (up to 70%).3,57 After declining (2000 to 2005) or remaining stable (2006 to 2010), the number of cases of acute hepatitis C virus doubled between 2011 and 2014.58 In the period between 2010 and 2013, the greatest increase occurred among those aged 20 to 29 years mostly among young, nonminority, persons who inject drugs and many of whom also use oral prescription opioid drugs.58,59 The largest increases in acute hepatitis C virus occurred among the states in central Appalachia (Kentucky, Tennessee, Virginia, and West Virginia) among individuals aged 18 to 29 years old, predominantly white, equally female and male, and who resided in nonurban or suburban areas.60 Some areas within the United States seem to be particularly vulnerable to outbreaks of HIV and hepatitis C virus among persons with substance use disorders. As reported by the Centers for Disease Control, at least 220 counties are at risk for hepatitis C virus, with associated increases in unemployment rates, prescription opioid sales, and overdose deaths being factors responsible for the increase.61
To address emerging data indicating a significant increase in hepatitis C virus exposure risk among persons with substance use disorders and the large proportion of new hepatitis C virus infections occurring in this population, new guidance on the use of syringe services programs has been developed. In 2015, the US Congress passed legislation to allow federal funds to be used to support some components of syringe services program. On the basis of this change, funds from several federal agencies can be used after “determination of need” is established. Key initiatives associated with this change in policy are integration of HIV and hepatitis C virus testing into syringe services programs, promoting linkage to care, and facilitating referrals to substance abuse treatment.62 Additional educational programs have been implemented with a focus on hepatitis C virus awareness and screening. Hopefully, these funds will result in several improvements related to hepatitis C virus since in 2013, less than one-quarter of all reporting substance use treatment facilities offered hepatitis C virus screening, representing missed opportunities for screening and linkage to care, and highlighting major gaps in health care provider education.63
In response to the rising incidence of acute hepatitis C virus in the United States and in recognition of the long-term impact of viral hepatitis on the overall health care burden in the United States, a national Viral Hepatitis Action Plan has been established with the intent of providing a pathway to achieve viral hepatitis elimination in this country.64 Specific goals outlined in the Viral Hepatitis Action Plan include the prevention of new hepatitis B and hepatitis C infections, reductions in mortality and improvements in the health of individuals living with viral hepatitis, reductions in viral hepatitis health disparities, and to coordinate, monitor, and report on the implementation of viral hepatitis actions. Although the Viral Hepatitis Action Plan provides a framework for setting goals, priorities, and measurable targets, it is recognized that these goals cannot be achieved through federal action alone and that active involvement and innovation from a broad mix of nonfederal stakeholders from various sectors, both public and private, is essential.
GOALS OF INTERVENTIONS TO END THE HEPATITIS C VIRUS EPIDEMIC
Screening
Hepatitis C virus is primarily transmitted through parenteral exposure, and injection drug use is currently the principal risk factor for transmission of hepatitis C virus infection.10 Other risk factors include transmission via blood transfusion, noninjection drug use, and unprofessional tattoos.65 Consequently, hepatitis C virus screening recommendations have been designed to identify populations with the greatest risk of viral acquisition. In 1998, the Centers for Disease Control and Prevention recommended that all persons who inject drugs be screened for hepatitis C virus.66 In 2012, the hepatitis C virus screening recommendations were revised to shift focus from risk factor based to age-based screening based upon the observation that up to 40% of hepatitis C virus–infected individuals were not aware of their diagnosis.67 Consequently, hepatitis C virus screening recommendations were refocused to emphasize screening among individuals born between 1945 and 1965, a population with extremely high rates of undiagnosed hepatitis C virus infection.68 Although the birth cohort comprises up to 75% of prevalent hepatitis C virus infections in the United States, injection drug use accounts for the vast majority of incident hepatitis C virus infections.10,69 The prevalence of chronic hepatitis C virus within cohorts of persons who use injection drugs can reach as high as 30% within the first year and increases to >50% within 5 years of habitual injection drug use, with further increases in prevalence resulting from continued substance use.70
Despite these recommendations, hepatitis C virus screening rates and linkage to care remain low in the United States, especially among persons with substance use disorders. Overall, only 1 in 3 patients with chronic hepatitis C virus are referred to care and as few as 1 in 10 patients have undergone antiviral therapy with interferon-based regimens.71 Adherence to hepatitis C virus evaluation and treatment recommendations among persons with substance use disorders is even lower. To ultimately reduce the transmission of hepatitis C virus, efforts to screen persons who inject drugs will be critical in identifying infected individuals and to implementation of educational programs, to initiate transition to opioid replacement therapy, and to identify potential candidates for antiviral therapy.
Prevention of Transmission and Reinfection
As no hepatitis C virus vaccine currently exists, the only truly effective means of hepatitis C virus prevention is limiting the potential for viral exposure. In addition to needle sharing, other transmission risks exist in persons who inject drugs, including contact with hepatitis C virus-contaminated blood through preparation equipment, highlighting the need for education on safe injection practices and other transmission routes. Drug administration via mucosal routes, such as intranasal cocaine use, is also considered a risk factor for viral acquisition, although significantly less efficient than via blood contamination.72 Hepatitis C virus RNA levels are typically at least 10-fold higher than HIV RNA, leading to increased virulence and increased opportunities for viral acquisition per exposure.73 Interventions to control HIV and consequent reductions in HIV transmission have not translated into equivalent reductions in hepatitis C virus transmission.74 Thus, hepatitis C virus prevalence is higher than HIV prevalence and chronic hepatitis C virus is more virulent than HIV. Mortality due to hepatitis C virus, particularly among those aged 55 to 64 years, has been increasing, surpassing that due to HIV and all 60 other reportable infectious diseases combined.75
Needle exchange programs have led to a decrease in the use of contaminated needles; however, access to these programs varies widely. Ultimately, a combined reduction in the number of contacts and a reduction in the probability of transmission per contact through safe injection practices and equipment, regular testing within networks of persons who inject drugs, and successful viral eradication through antiviral therapy could have major impacts on decreasing the overall hepatitis C virus prevalence and the risk of exposure within this population.
One of the major reasons for low hepatitis C virus treatment uptake among active substance users is providers’ misconceptions about poor treatment adherence as well as the false impression that there is a high rate of reinfection in this patient population. Published data, however, counter each of these assertions. A recent meta-analysis and systemic review that evaluated studies of active injectors treated with pegylated-interferon and ribavirin reported a rigorous adherence to therapy in 82% of patients and pooled rates of reinfection were 2.1% and 2.4% per year.21,55 In addition, as mentioned above, high adherence and low reinfection rates have been identified in studies of direct-acting antiviral therapy among persons with substance use disorders.
Hepatitis C Virus Transmission Among Men Who Have Sex With Men
Among certain populations of persons with substance use disorders, namely men who have sex with men, rates of hepatitis C virus infection have reached epidemic proportions in North America,76–79 Europe,80–84 Australia,85 and Asia.86,87 This new epidemic of hepatitis C virus among HIV-infected men who have sex with men has been due to sexual transmission, with only rare injection drug use, although noninjection drug use, especially with methamphetamine, seems to have fueled the epidemic in many urban centers.88,89 In the decade as the first recognition of this epidemic, hepatitis C virus incidence increased among HIV-infected men who have sex with men globally, with incidence of primary hepatitis C virus as high as 4%,90 and annual reinfection rates as high as 15%,91,92 in some cohorts. We therefore need a better understanding of routes and mechanisms of transmission among these men to develop prevention strategies to decrease the infection rate.
Seven case-controlled studies have been performed to identify risk factors for hepatitis C virus acquisition among HIV-infected men who have sex with men in the United States and Europe.90,93–98 Overall, the most frequently identified risk factors for hepatitis C virus acquisition were unprotected anal intercourse (5 of 7 studies), fisting (4 of 5 studies), group sex (3 of 5 studies), and drug use (3 of 5 studies). One of the early studies, in New York City found that sex while high on methamphetamine was associated with an adjusted odds ratio of 29. Although an early study from London did not find hepatitis C virus acquisition associated with drug use, subsequent studies from England evaluating drug use among men who have sex with men have shown a high proportion of men who have sex with men using drugs to have sex, which they have called “chemsex,” as well as an association with hepatitis C virus infection.99 The actual source of hepatitis C virus that is transmitted has been controversial as the hepatitis C virus field has been focused on blood as the infecting agent through injection drug use and investigators seem loath to accept the likelihood that other infection sources could be responsible for virus transmission during sex, where neither fists nor penises bleed during insertion into the rectum. Hepatitis C virus has long been known to present in semen, and has again recently been shown to be present in one-third to one-half of HIV-infected men who have sex with men, further evidence that the hepatitis C virus-containing fluid that infects anal receptive partners is most plausibly semen.100,101 Hepatitis C virus in semen could be absorbed through the rectal mucosa that was mildly abraded by the action of the inserted penis in the absence of more serious rectal trauma, possibly enhanced by changes induced by rectal douching, and possibly further enhanced by the mucosa compromised by the CD4+ T-cell depletion caused by primary HIV infection. The epidemiological findings of fisting and group sex as risk factors further suggest fomite/fomite-like transmission through, or direct infection of unprotected penises or fists. Rectal shedding of hepatitis C virus has just recently been demonstrated at sufficient concentration to explain transmissions in these settings without positing visible rectal bleeding, which rarely occurs in any case.102 With this emerging understanding of sex among HIV-infected men who have sex with men being fueled by mostly noninjection drug use with hepatitis C virus being transmitted by semen and rectal fluid, we can now begin a campaign of education about these elements to the men at risk, as well as the physicians who care for them, as most are currently unaware of these mechanisms of hepatitis C virus transmission among this new risk group.
Treatment of Addiction
Of the estimated 23.5 million Americans who are currently addicted to alcohol and/or other drugs, only 11% receives treatment for addiction.103 Addressing the underlying addictive disorder and implementing a treatment plan are critical to reducing exposure risk and to obtaining further decreases in transmission of hepatitis C virus in persons with substance use disorders. Changes in the epidemiology of injection drug use can influence patterns of acute hepatitis C virus infection. Over the last decade, the numbers of prescription opioid sales, opioid-related deaths, and opioid treatment admissions have all increased significantly, although data from 2010 to 2013 suggest that the trend has waned.104 Simultaneously, however, heroin use has increased sharply, including not only a rise in the number of heroin users but also in the number of deaths attributed to heroin overdose.105 Consequently, the demographics of hepatitis C virus has changed as a result of changes in the characteristics of people using heroin, which has increased in whites, women, and those living in suburban or rural areas.60,106
Effect of Substance Use Treatment on Pursuit of Hepatitis C Virus Treatment
Engagement in treatment of addiction and integration with primary medical care has been shown to be cost-effective, to improve medical outcomes, and to result in decreased numbers of hospitalizations.107,108 Treatment of addiction may have other health benefits from a psychosocial perspective. Indeed, the combination of opioid replacement therapy and needle exchange has been shown to decrease hepatitis C virus transmission compared with a single intervention.109 Specifically with regard to hepatitis C virus, engagement of active persons with substance use disorders into hepatitis C virus care could serve as a gateway into the health care system. Among patients on opioid replacement therapy, those who self-report hepatitis C virus seropositivity and those with higher levels of knowledge about the virus and its management have demonstrated increased willingness to undergo hepatitis C virus treatment.110 In addition, hepatitis C virus education of persons with substance use disorders results in increases in hepatitis C virus–related knowledge.111 Lack of information about hepatitis C virus has been associated with unwillingness to pursue treatment.112 Thus, hepatitis C virus–related education and ultimately, therapy, could further result in engagement in substance use therapy and the assessment of other comorbidities. Informing persons who inject drugs about hepatitis C virus seropositivity, providing education regarding risk factors for viral transmission, and establishing a linkage to hepatitis C virus care can lead to reductions in both drug use behavior and injection frequency.112–114 Therefore, a diagnosis of hepatitis C virus may offer an opportunity for a “teachable moment,” the concept of utilizing health events to motivate individuals to spontaneously adopt risk-reduction health behaviors.115
Active drug use in the months preceding the initiation of antiviral therapy is not associated with a decline in efficacy116–118; however, the amount or frequency of drug use may influence the degree of adherence to antiviral therapy and therefore efficacy.118–120 In active drug users, interferon-based hepatitis C virus treatment has been shown to result in slightly lower rates of viral eradication.55 In these individuals, treatment of addiction during receipt of interferon-based therapies for hepatitis C virus has been shown to result in higher treatment completion and viral eradication rates, similar to those obtained in registration trials and in the general population.121,122 The extent to which ongoing drug use affects treatment efficacy in patients treated with direct-acting antivirals remains to be determined, although in studies performed to date, no effect has been observed. In HIV infection, however, a meta-analysis has shown that better treatment outcomes are obtained among former drug users, those on opioid substitution therapy and/or psychosocial support.123 In addition, active drug users demonstrated significantly worse medication adherence than did nonusers (79% vs. 63%).124 To eliminate hepatitis C virus, we will need to treat populations such as active injectors. Although the optimal treatment strategy remains to be determined in persons with substance use disorders, interventions will be needed to support them during hepatitis C virus therapy to optimize treatment efficacy. An additional consideration might be the use of directly observed therapy or modified directly observed therapy, an effective strategy demonstrated in interferon-based regimens,119,125 which may also be applied to direct-acting antiviral therapy. In light of challenges associated with treatment of active drug users, systematic approaches will be required to include education, treatment of addiction, transition to opioid replacement therapy if possible, and considerations of directly observed therapy to optimize adherence and treatment efficacy.
Treatment as Prevention
The advances in the effectiveness of hepatitis C virus treatment suggest the possibility of global viral eradication. To achieve this goal, however, treatment must be extended to marginalized and disenfranchised populations, such as persons with substance use disorders. As a consequence of the highest hepatitis C virus incidence and prevalence, persons with substance use disorders, especially those who engage in unsafe injection practices, continue to serve as reservoirs for hepatitis C virus transmission.
Recently, the concept of the use of antiviral therapy as a method to prevent hepatitis C virus transmission among persons who inject drugs has been promoted.126–128 Even a small increase in the number of treated persons who inject drugs can result in substantial reductions in hepatitis C virus transmission. It has been estimated that a 12-week interferon-free, oral direct-acting antiviral regimen could reduce hepatitis C virus prevalence by 26% to 75% within 15 years depending upon the number of persons who inject drugs treated annually.129 The same principle of treatment as prevention in the epidemic among HIV-infected men who have sex with men has been considered in similar mathematical modeling in England, suggesting this would be an important intervention.130 On its face, treatment as prevention as the sole intervention to eliminate hepatitis C virus would be unprecedented in the history of infectious diseases, where prevention (in the form of vaccine) has been the only method of eradication to date. In line with this historical observation, a different model of the epidemic in Switzerland showed that significant behavioral changes must be effected before even a decrease in incidence, let alone prevalence, can be accomplished.131 These same factors are likely to be important in considering hepatitis C virus prevention or even elimination among persons who inject drugs. The observation that hepatitis C virus phylogeny is associated with injection networks and that injecting networks substantially impact transmission suggest that targeting treatment to the heaviest injectors and their immediate contacts may be the most cost-effective strategy to reduce viral transmission.132–134 However, challenges remain in the provision of hepatitis C virus treatment to persons who inject drugs (as below) and in minimizing the potential for reinfection among those who continue to inject.129
MODELS OF HEPATITIS C VIRUS TREATMENT DELIVERY TO MARGINALIZED AND DISENFRANCHISED PERSONS WITH SUBSTANCE USE DISORDERS
Substance users are a heterogenous population with different patterns of drug use, frequency, and engagement in the health care system. These variables are important determinants of this population’s engagement in hepatitis C virus management. The substance use paradigm includes users who continue to actively inject, those in early recovery, and those who have a remote history of substance use and who may or may not currently receive treatment for addiction. The frequency of drug use is another consideration, and it can range from active daily use to periods of abstinence with persistent risk of relapse. For substance users in recovery, the type and level of support services provided among substance use treatment programs is another important determinant of engagement in hepatitis C virus management. Multidisciplinary programs offering primary care, behavioral medicine, and social services are more likely to be equipped for hepatitis C virus screening and management.135 Therefore, obstacles to engagement into hepatitis C virus care and treatment as well as approaches to overcome these obstacles will vary for different subpopulations of persons with substance use disorders.
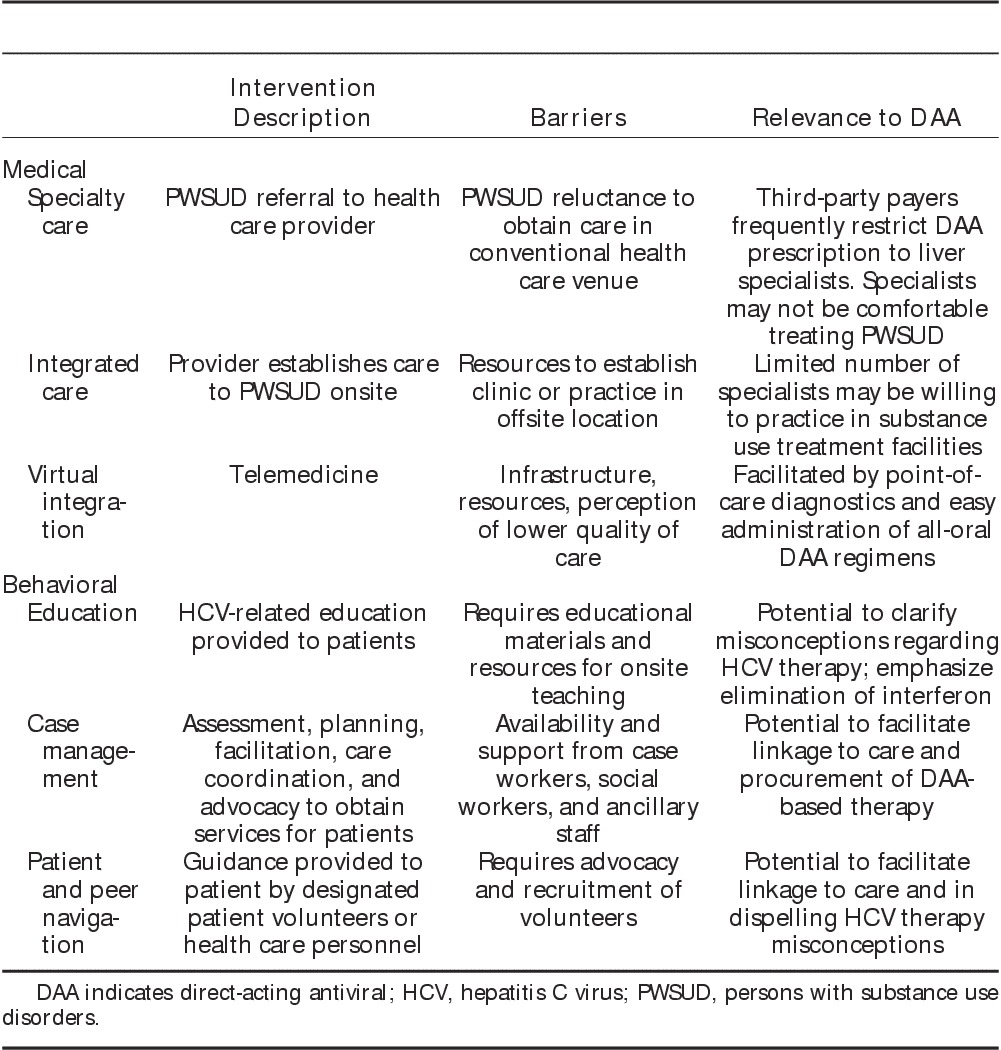
Opportunities to interact with different populations of persons with substance use disorders may vary based on local programs and resources, which may include opioid replacement therapy clinics, needle exchange programs, safe injection facilities, detoxification centers, correctional facilities, or federally qualified health centers (Fig. 1). Efforts to provide outreach to and to deliver integrated care focusing on hepatitis C virus prevention, screening, and treatment directly within these locations may be critical in establishing access to larger numbers of persons with substance use disorders and to achieve a greater impact in reducing the prevalence of hepatitis C virus infection. Medical and behavioral approaches to engage persons with substance use disorders into hepatitis C virus care are summarized below (Table 3).
FIGURE 1.
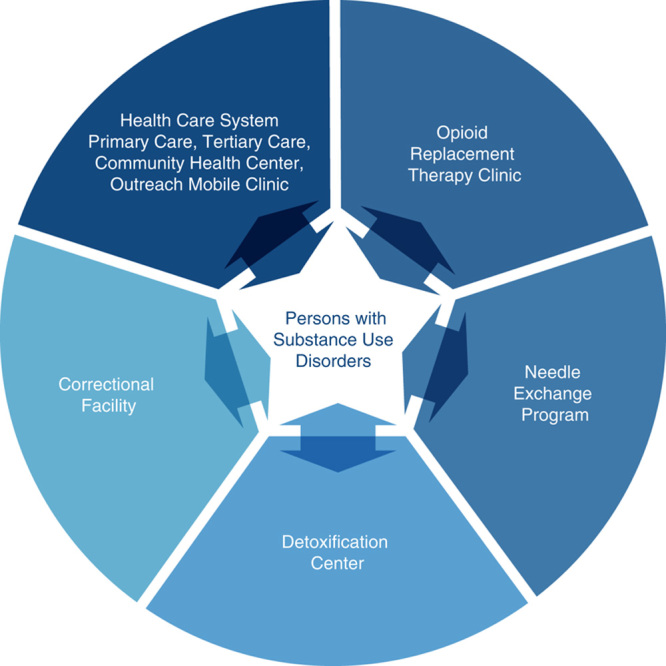
Venues providing access to different populations of persons with substance use disorders. Efforts to provide care to populations of persons with substance use disorders may frequently require expansion beyond the conventional health care setting. The ability to access persons with substance use disorders directly at venues where they habitually congregate or receive treatment could be a means to overcome the stigmatization associated with hepatitis C virus infection and the reluctance to seek care within a conventional health care setting. These alternative sites vary greatly in terms of accessibility and degree of health care services administered and in many cases may require outreach or integration of the hepatitis C virus provider into the particular setting.
TABLE 3.
Medical and Behavioral Models of Care
Referral to Specialty Care
Although numerous interventions have been used to engage substance users into treatment, most of them fall into 2 distinct categories: referral to a liver or infectious diseases specialist or integration of hepatitis C virus care with substance use treatment. Referral, the most frequently used strategy for hepatitis C virus treatment of persons with substance use disorders, is inefficient and somewhat ineffective due to a variety of reasons including persons with substance use disorders mistrust of the health care system and stigmatization encountered in conventional health care settings. As a result, less than one third of persons with substance use disorders referred to specialty clinics appear for appointments, and <20% of those evaluated for treatment eligibility actually initiates antiviral therapy.112,136–138
Integrated Care
When treatment for substance use disorders and hepatitis C virus care are colocalized, studies have shown that persons with substance use disorders are more likely to pursue an hepatitis C virus evaluation.139–142 To foster adaptation of colocated care, a variety of integrated medical care models have been developed.143 An integrated, multidisciplinary care model, which typically emanates from substance use treatment facilities, has been shown to be extremely effective.144 Similar models have also been developed in prison-based settings, community health centers, and general medical practices. These models utilize members from a variety of health care specialties who are able to address the diverse medical issues affecting persons with substance use disorders. In addition, the majority of opioid replacement therapy programs have psychosocial support with counselors, psychologists, psychiatrists, patient navigators, and peer support programs that may enhance medication adherence, thereby increasing the likelihood of achieving viral eradication. Furthermore, the daily administration of methadone offers an opportunity for modified directly observed hepatitis C virus therapy, which has been shown to be effective in improving therapeutic outcomes.145 Other colocalized care models include an internist-addiction medicine specialist from an opioid replacement therapy program embedded in the viral hepatitis clinic or hepatitis C virus treatment initiation by an addiction medicine specialist onsite in the opioid replacement therapy program.146,147
The development of direct-acting antiviral-based therapy and the provision of noninvasive alternatives to liver biopsy have suggested that treatment might be delivered onsite in the opioid replacement therapy program. In the United States, current requirements for obtaining third-party payer approval for direct-acting antivirals require quantitative hepatitis C virus RNA, hepatitis C virus genotype, and an assessment of hepatic fibrosis. Although the liver biopsy has been considered the gold standard for the assessment of hepatic fibrosis and steatosis, several noninvasive indices have been developed over the past decade. Fibrosure (Laboratory Corporation of America) is one of the most widely utilized indices, especially in the clinical care of patients. Fibrosure is performed on peripheral blood, can be drawn at the point-of-care, and can be used as a replacement for the biopsy. Another noninvasive liver fibrosis technique is vibration-controlled transient elastography, a modified ultrasound-based technique that assesses liver stiffness. Vibration-controlled transient elastography can reliably identify cirrhosis with an area under the receiver operator characteristic curve of 90 or higher, and it provides an estimate of liver stiffness ranging from 1.5 to 75 kPa. Results <5 kPa are considered normal and values >12.7 kPa indicate cirrhosis.148 Vibration-controlled transient elastography has also recently been proposed as an alternative methodology to assess hepatic steatosis,149 especially after the development of the controlled attenuation parameter technology.150,151 A recent meta-analysis of 11 studies comprising 13 cohorts reported that controlled attenuation parameter has reasonable sensitivity and specificity for the detection of steatosis grades 1 to 3.152 A portable vibration-controlled transient elastography machine has recently been approved in the United States that could easily permit onsite assessment of hepatic fibrosis within the opioid replacement therapy program. Thus, liver biopsy is no longer required; liver histology assessments using either blood or vibration-controlled transient elastography can both be performed at the point-of-care.
Virtual Integration
Although colocalized care models have been shown to be effective, few opioid replacement therapy or other types of substance use treatment facilities have the capability to offer hepatitis C virus management onsite.153,154 A survey of 269 US substance use treatment facilities found that only ∼50% had hepatitis C virus–related medical history and physical examination services, whereas only 30% offered hepatitis C virus treatment.155 Similar results have been obtained in a nationwide survey of programs offering hepatitis C virus screening.63 A telehealth approach may be an alternative modality to deliver hepatitis C virus treatment services to opioid replacement therapy programs.156
Telehealth and Telemedicine
Telehealth and telemedicine are a growing field of patient care that involves the use of telecommunication devices to treat patients across space and/or time (Fig. 2A). Telehealth is a more general term than telemedicine, which is specifically defined as synchronous interaction between a patient and physician via a telecommunications device across distance. In contrast, telehealth is a more broadly defined general concept and includes other forms of telecommunication device enabled, patient-physician interactions such as store and forward systems (ie, transmission of videos and digital images such as x-rays and photos through a secure electronic communications environment), remote patient monitoring (ie, personal health and medical data collection from an individual in one location and transferred to a provider in a different location), and mobile health (ie, certain smartphone apps, Fitbit, etc.).157
FIGURE 2.
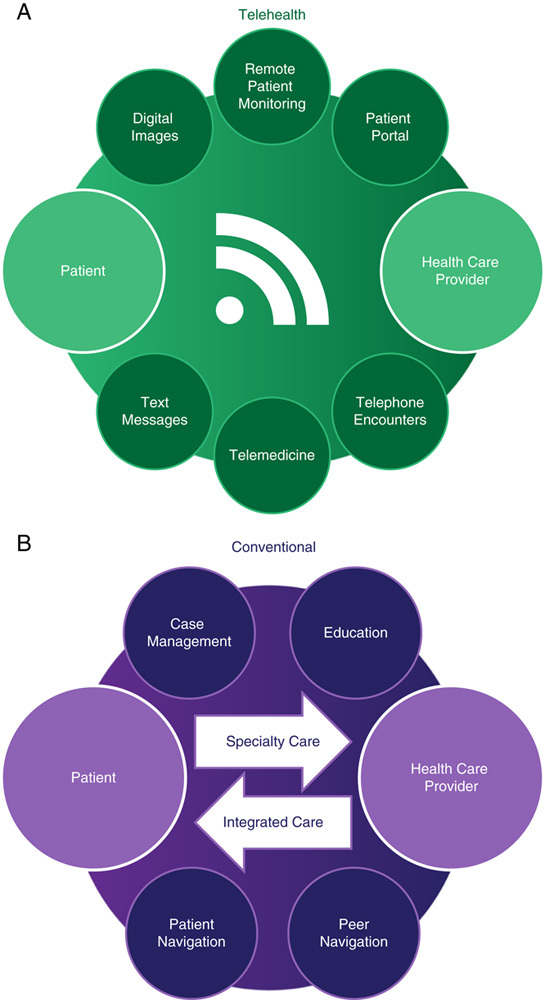
Models of care in telehealth (A) and conventional settings (B). A, In a telehealth model, the patient and the health care provider are each geographically separated and connected using technology, such as through the use of text messages, telephones, or telemedicine (ie, 2-way videoconferencing between a patient and a physician each located remotely). Technology can also be used to transmit data from a physician to a patient, vice versa, or between physicians. For example, a patient portal enables patients to view their test results, remote patient monitoring permits results (ie, glucose or blood oxygenation) to be transmitted from a patient to a physician, and digital images can be transmitted for remote review by a radiologist. B, In conventional health care settings, persons with substance use disorders are typically referred to a health care provider to obtain treatment for hepatitis C virus infection. Alternatively, a health care provider could engage directly with persons with substance use disorders through an integrated care model in which hepatitis C virus care is delivered directly in the venue that offers treatment for substance use. Case management, patient education, peer navigation, and patient navigation are important interventions that can support persons with substance use disorders access to care and adherence.
Telemedicine seems to be a promising alternative for health care delivery that is positioned to grow considerably in the next decade.158 Potential benefits from increased use of telemedicine can result from cost savings, increased patient satisfaction, and increased access to care for difficult-to-engage patient populations. In addition, increased ease of and expanded access for providers who care for these populations is an additional benefit that can accrue. Primary patient populations that could benefit from telemedicine are those who are medically disenfranchised, that is, those who reside in medically isolated locales, rural communities, and those whose treatment and care regimens adapt well to the telemedicine model such as treatment and management of long-term, chronic conditions in which specialty knowledge can be helpful, such as chronic hepatitis C virus.
We propose that persons with substance use disorders and persons who inject drugs with chronic hepatitis C virus infection might also benefit from telemedicine-based treatment of the infection. Colocating hepatitis C virus telemedicine-based treatment in opioid replacement therapy programs has been shown in a pilot study to result in high levels of patient satisfaction and adherence to hepatitis C virus treatment.159,160 In contrast, potential limitations of the telemedicine-based treatment model are infrastructural limitations in the opioid replacement therapy program and lack of broadband connectivity. However, as the technology improves and broadband coverage increases, such as through innovative programs calling for entire New York State coverage by broadband by 2018,161 telemedicine coverage will likely become a more established means of health care delivery.
Additional largely regulatory concerns are: (1) differences in third-party reimbursement for telemedicine services depending upon geography and payer, (2) variations in state-mandated medical license requirements and potential additional certifications required for out-of-state physicians consulting on patients across state boundaries, and (3) concerns about the quality and security of the telemedicine-based care.162
SUPPORT SERVICES TO PROMOTE PERSONS WITH SUBSTANCE USE DISORDERS ENGAGEMENT INTO CARE FOR HEPATITIS C VIRUS INFECTION
As persons with substance use disorders frequently lack knowledge about hepatitis C virus and have coexisting psychosocial and economic difficulties, educational programs and medical interventions with behavioral components may be required to address issues specific to persons with substance use disorders engagement into hepatitis C virus care. Behavioral interventions, such as educational programs, case management, and peer and patient navigators and could also motivate hepatitis C virus screening, enrollment, and retention in hepatitis C virus care among substance users (Fig. 2B).
Educational Interventions
The inclusion of support systems for hepatitis C virus treatment is critical to optimizing efforts to achieve greater awareness, education, and active participation by persons with substance use disorders. Hepatitis C virus–related education has been shown to promote patient engagement into hepatitis C virus care through increased screening, vaccination, and treatment adherence.163 Regular educational interactions delivered by physicians, physician-extenders, counselors, or other health care personnel during hepatitis C virus treatment have played an important role in maximizing treatment adherence. Topics that might be important for persons with substance use disorders are the importance of hepatitis C virus screening, the significance of hepatitis C virus RNA positivity, the long-term complications of chronic infection, and the availability of curative treatment regimens. Educational programs might also specifically target active injectors, promote harm reduction, and facilitate persons with substance use disorders entry into multidisciplinary programs for treatment of both substance use and hepatitis C virus.
Case Management
Case management involves a collaborative process of assessment, planning, facilitation, care coordination, and advocacy in an effort to obtain services to meet an individual’s health needs. As substance users may face limited economic and social support, case management may play a central role in the coordination of care within a complex health care system and could facilitate hepatitis C virus screening as well as linkage to care for substance users. Case management could also be instrumental in the acquisition of hepatitis C virus medications for substance users through providing assistance with obtaining health insurance and enrollment in medication reimbursement programs. Once antiviral therapy is initiated, case managers can also provide guidance with maintaining adherence to the treatment regimen and consistent follow-up with provider appointments.
The impact of care coordination can be substantial in establishing linkage to care and optimizing the potential for delivery of care for hepatitis C virus infection care to persons with substance use disorders. A prospective randomized-controlled trial of patients attending opioid replacement therapy clinics found that individuals randomized to an intervention arm in which they received onsite screening, education, counseling, and case management had a significantly greater likelihood of follow-up for an evaluation for hepatitis C virus infection, which occurred in 65% in contrast with only 37% of controls.164 Efforts to target populations at risk within community health centers and needle exchange programs involving onsite rapid hepatitis C virus screening tests with immediate blood draws for hepatitis C virus RNA and linkage to care with patient navigators, have reported follow-up for an evaluation and care in the majority of hepatitis C virus-positive individuals screened.165
Patient and Peer Navigation
Patient navigation promotes initiation, linkage, and retention in medical care through the guidance and support of health care personnel. Peer navigation involves patients, many of whom may have been treated for hepatitis C virus infection, providing guidance regarding hepatitis C virus management to their peers. Both patient and peer navigation aim to facilitate patient access to and transition into the health care system so that they receive standard of care treatment in a timely and efficient manner.166 Navigators are similar to case managers, except that the navigator’s responsibilities are more narrowly defined as a result of focusing exclusively on health care processes.167
The benefits of navigation include improvements in access to care, increased adherence to care programs, increased independence, reduced social isolation, improved quality of care, and increased patient satisfaction.168,169 In addition, navigation can lead to a reduction in overall health care costs and fewer hospitalizations. As navigators are frequently laypeople rather than licensed medical professionals, the cost is usually modest in comparison with other interventions.170 Health navigation is most effective when targeted to patient groups, such as substance users, who are not highly engaged in medical care due to perceived or actual barriers.167,170,171
CONCLUSIONS
Recent advances in hepatitis C virus therapy have revolutionized treatment approaches to marginalized and disenfranchised populations such as persons with substance use disorders. Many previously existent barriers to engagement into hepatitis C virus care among this population were attributed to interferon; interferon elimination from the therapeutic regimen has been enthusiastically celebrated among this population. Simultaneously, however, new obstacles to widespread provision of direct-acting antivirals have emerged, principally related to the cost of medications.
To achieve the goal of universal elimination of hepatitis C virus infection or, at a minimum, its elimination from developed countries, new initiatives are required to engage substance users into therapy for hepatitis C virus infection. The combination of medical interventions, such as colocalized treatment for hepatitis C virus and substance use, with behavioral interventions, such as case management, peer navigation, and education, will likely have the greatest effect at engaging the largest number of persons with substance use disorders into therapy for hepatitis C virus infection. To halt virus transmission, harm reduction techniques, such as needle exchange programs, and opioid replacement therapy, must also be included. Expansion of treatment availability to venues involved in provision of medical care to persons with substance use disorders, such as substances use treatment facilities, correctional facilities, and psychiatric institutions, could substantially increase the number of treated individuals. Broad collaboration between specialists of diverse fields is required to increase persons with substance use disorders access to health care and to develop novel care models to increase direct-acting antiviral-based treatment uptake and adherence. By utilizing multicomponent interventions, substance users can likely be successfully and fully engaged into care for hepatitis C virus infection.
ACKNOWLEDGMENTS
The authors acknowledge the writing assistance of Anthony Martinez, MD, Manoj Kumar, MD, and Marija Zeremski, PhD on an earlier version of this article. The authors also acknowledge Corinna Dan, RN and Lynn Taylor, MD, for insightful discussions and the writing and editorial assistance of C. Elliot Frank.
CME ACTIVITY
To claim 1.0 AMA PRA Category 1 CreditTM for this activity, please visit:
http://www.chronicliverdisease.org/TripleE
Target Audience
This educational program is targeted at physicians and other healthcare providers who specialize in the management of patients with addictive disorders.
Learning Objectives
Upon completion of this activity, the participants should be better able to:
Review the importance of Hepatitis C screening, diagnosis, and linkage to care
Describe new developments in HCV that have eliminated many of the previous barriers to pursuit of HCV therapy in individuals with substance use disorders
Develop strategies for the engagement of patients with substance use disorders into HCV care.
Accreditation Statement
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education through the joint providership of the Annenberg Center for Health Sciences at Eisenhower and the Chronic Liver Disease Foundation (CLDF). The Annenberg Center for Health Sciences at Eisenhower is accredited by the ACCME to provide continuing medical education for physicians.
The Annenberg Center for Health Sciences at Eisenhower designates this activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure of Conflicts of Interest
All faculty and staff involved in the planning or presentation of continuing education activities provided by Annenberg Center for Health Sciences at Eisenhower (ACHS) are required to disclose to the audience any real or apparent commercial financial affiliations related to the content of the presentation or enduring material. Full disclosure of all commercial relationships must be made in writing to the audience prior to the activity. John Bayliss, VP, Business Development, Annenberg Center, spouse is an employee of Amgen, Inc; all other staff at the Annenberg Center for Health Sciences at Eisenhower and Chronic Liver Disease Foundation (CLDF) have no relationships to disclose.
Learner Assurance Statement
The Annenberg Center for Health Sciences at Eisenhower is committed to resolving all conflicts of interest issues that could arise as a result of prospective faculty members’ significant relationships with drug or device manufacturer(s). The Annenberg Center for Health Sciences at Eisenhower is committed to retaining only those speakers with financial interests that can be reconciled with the goals and educational integrity of the CME activity.
Footnotes
Research reported in this article was partially funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (IHS-1507-31640), the Troup Fund of the Kaleida Health Foundation, the Chronic Liver Disease Foundation, and through unrestricted educational grants from Merck, AbbVie, and Gilead Sciences. The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
S.A.G.: Speaker’s Bureau—AbbVie, Gilead, Merck; Advisory board—AbbVie. D.S.F.: reports owning stock in Gilead Sciences. A.H.T.: Grant/Research Support—Merck, Gilead, Abbott, AbbVie, Intercept, Tobira, Conatus; Committee/Advisor—Merck, Abbott Diagnostics, AbbVie, Chronic Liver Disease Foundation; Speaker’s Bureau—Chronic Liver Disease Foundation.
REFERENCES
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. [DOI] [PubMed] [Google Scholar]
- 2.Edlin BR, Eckhardt BJ, Shu MA, et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. [DOI] [PubMed] [Google Scholar]
- 4.Colvin HM, Mitchell AE. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. 2010. Available at: www.cdc.gov/hepatitis/pdfs/iom-hepatitisandlivercancerreport.pdf. Accessed January 22, 2017.
- 5.Denniston MM, Klevens RM, McQuillan GM, et al. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. [DOI] [PubMed] [Google Scholar]
- 7.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. [DOI] [PubMed] [Google Scholar]
- 8.Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973–978. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Hepatitis C fact sheet no. 164. 2016. Available at: www.who.int/mediacentre/factsheets/fs164. Accessed January 21, 2017.
- 10.Williams IT, Bell BP, Kuhnert W, et al. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Arch Intern Med. 2011;171:242–248. [DOI] [PubMed] [Google Scholar]
- 11.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grebely J, Matthews GV, Lloyd AR, et al. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: feasibility and future requirements. Clin Infect Dis. 2013;57:1014–1020. [DOI] [PubMed] [Google Scholar]
- 13.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15:543–549. [DOI] [PubMed] [Google Scholar]
- 14.Roy E, Boudreau JF, Boivin JF. Hepatitis C virus incidence among young street-involved IDUs in relation to injection experience. Drug Alcohol Depend. 2009;102:158–161. [DOI] [PubMed] [Google Scholar]
- 15.Brahm J, Castera L, Hou J, et al. Joint society statement for the elimination of viral hepatitis. Hepatology. 2016;64:1031–1032. [DOI] [PubMed] [Google Scholar]
- 16.Murthy VH. Surgeon General’s letter on opiod use. Letter to US Physicians. 2016. Available at: www.turnthetiderx.org. Accessed September 23, 2016.
- 17.Massard J, Ratziu V, Thabut D, et al. Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol. 2006;44:S19–S24. [DOI] [PubMed] [Google Scholar]
- 18.Zeremski M, Dimova RB, Pillardy J, et al. Fibrosis Progression in patients with chronic hepatitis C virus infection. J Infect Dis. 2016;214:1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. [DOI] [PubMed] [Google Scholar]
- 20.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 21.Simmons B, Saleem J, Heath K, et al. Long-term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis. 2015;61:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu YC, Ho HJ, Huang YT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. [DOI] [PubMed] [Google Scholar]
- 23.Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis. 2015;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516. [DOI] [PubMed] [Google Scholar]
- 25.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. [DOI] [PubMed] [Google Scholar]
- 26.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. [DOI] [PubMed] [Google Scholar]
- 27.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. [DOI] [PubMed] [Google Scholar]
- 29.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya A, Perry CM. Simeprevir: first global approval. Drugs. 2013;73:2093–2106. [DOI] [PubMed] [Google Scholar]
- 31.Peter J, Nelson DR. Optimal interferon-free therapy in treatment-experienced chronic hepatitis C patients. Liver Int. 2015;35(suppl 1):65–70. [DOI] [PubMed] [Google Scholar]
- 32.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. [DOI] [PubMed] [Google Scholar]
- 33.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493. [DOI] [PubMed] [Google Scholar]
- 34.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. [DOI] [PubMed] [Google Scholar]
- 35.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163:1–13. [DOI] [PubMed] [Google Scholar]
- 36.Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. [DOI] [PubMed] [Google Scholar]
- 38.Feld JJ, Foster GR. Second generation direct-acting antivirals—do we expect major improvements? J Hepatol. 2016;65:S130–S142. [DOI] [PubMed] [Google Scholar]
- 39.American Association for the Study of Liver Diseases. (AASLD)-Infectious Diseases Society of America (IDSA). Recommendations for testing, managing, and treating hepatitis C. 2016. Available at: www.hcvguidelines.org. Accessed October 12, 2016.
- 40.European Association for Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199–236. [DOI] [PubMed] [Google Scholar]
- 41.Grebely J, Robaeys G, Bruggmann P, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26:1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vermehren J, Sarrazin C. The role of resistance in HCV treatment. Best Pract Res Clin Gastroenterol. 2012;26:487–503. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Subero M, Diez-Quevedo C. Mental disorders in HIV/HCV coinfected patients under antiviral treatment for hepatitis C. Psychiatry Res. 2016;246:173–181. [DOI] [PubMed] [Google Scholar]
- 44.Schaefer M, Sarkar R, Diez-Quevedo C. Management of mental health problems prior to and during treatment of hepatitis C virus infection in patients with drug addiction. Clin Infect Dis. 2013;57(suppl 2):S111–S117. [DOI] [PubMed] [Google Scholar]
- 45.Barua S, Greenwald R, Grebely J, et al. Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163:215–223. [DOI] [PubMed] [Google Scholar]
- 46.Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state medicaid programs. Ann Intern Med. 2015;163:226–228. [DOI] [PubMed] [Google Scholar]
- 47.Lalezari J, Sullivan JG, Varunok P, et al. Ombitasvir/paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J Hepatol. 2015;63:364–369. [DOI] [PubMed] [Google Scholar]
- 48.Grebely J, Mauss S, Brown A, et al. Efficacy and safety of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic HCV genotype 1 infection receiving opioid substitution therapy: analysis of phase 3 ION trials. Clin Infect Dis. 2016;63:1405–1411. [DOI] [PubMed] [Google Scholar]
- 49.Grebely J, Dore GJ, Zeuzem S, et al. Efficacy and safety of sofosbuvir/velpatasvir in patients with chronic hepatitis C virus infection receiving opioid substitution therapy: analysis of phase 3 Astral trials. Clin Infect Dis. 2016;63:1479–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dore GJ, Altice F, Litwin AH, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165:625–634. [DOI] [PubMed] [Google Scholar]
- 51.Basu PP, Shah NJ, Farhat S, et al. SLAM C study sofosbuvir and ledipasvir versus sofosbuvir and simeprevir combination therapy in the management of acute hepatitis C: a randomized open label prospective clinical pilot study (Abstract). Presented at the 25th Annual Conference of the Asian Pacific Association for the Study of the Liver, Tokyo, Japan. 2016.
- 52.Grady BP, Schinkel J, Thomas XV, et al. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis. 2013;57(suppl 2):S105–S110. [DOI] [PubMed] [Google Scholar]
- 53.Grebely J, Matthews GV, Hellard M, et al. Adherence to treatment for recently acquired hepatitis C virus (HCV) infection among injecting drug users. J Hepatol. 2011;55:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons B, Saleem J, Hill A, et al. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57(suppl 2):S80–S89. [DOI] [PubMed] [Google Scholar]
- 56.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altice FL, Kamarulzaman A, Soriano VV, et al. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams DA, Thomas KR, Jajosky RA, et al. Summary of notifiable infectious diseases and conditions—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;63:1–152. [DOI] [PubMed] [Google Scholar]
- 59.Adams DA, Gallagher KM, Jajosky RA, et al. Summary of notifiable diseases United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;59:1–111. [PubMed] [Google Scholar]
- 60.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64:453–458. [PMC free article] [PubMed] [Google Scholar]
- 61.Van Handel MM, Rose CE, Hallisey EJ, et al. County-level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr. 2016;73:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Department of Health and Human Services. Implementation guidance to support certain components of syringe services programs. 2016. Available at: www.aids.gov/pdf/hhs-ssp-guidance.pdf. Accessed January 21, 2017.
- 63.United States Department of Health and Human Services—Substance Abuse and Mental Health Services Administration. National Survey of Substance Abuse Treatment Services (N-SSATS): 2013. 2014. Available at: www.samhsa.gov/data/sites/default/files/2013_N-SSATS_National_Survey_of_Substance_Abuse_Treatment_Services/2013_N-SSATS_National_Survey_of_Substance_Abuse_Treatment_Services.html. Accessed October 23, 2016.
- 64.United States Department of Health and Human Services—Office of HIV/AIDS and Infectious Disease Policy. Action plan for the prevention, care, and treatment of viral hepatitis, 2014-2016. 2015. Available at: www.aids.gov/pdf/viral-hepatitis-action-plan.pdf. Accessed January 21, 2017.
- 65.Moyer VA. US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. [DOI] [PubMed] [Google Scholar]
- 66.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 67.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 68.Smith BD, Morgan RL, Beckett GA, et al. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward JW. The epidemiology of chronic hepatitis C and one-time hepatitis C virus testing of persons born during 1945 to 1965 in the United States. Clin Liver Dis. 2013;17:1–11. [DOI] [PubMed] [Google Scholar]
- 70.Hagan H, Pouget ER, Des Jarlais DC, et al. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168:1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holmberg SD, Spradling PR, Moorman AC, et al. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez A, Talal AH. Noninjection drug use: an under-appreciated risk factor for hepatitis C virus transmission. Liver Int. 2008;28:757–760. [DOI] [PubMed] [Google Scholar]
- 73.Paintsil E, He H, Peters C, et al. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis. 2010;202:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Des Jarlais DC, Perlis T, Arasteh K, et al. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990-2001. AIDS. 2005;19(suppl 3):S20–S25. [DOI] [PubMed] [Google Scholar]
- 75.Ly KN, Hughes EM, Jiles RB, et al. Rising mortality associated with hepatitis C virus in the United States, 2003-2013. Clin Infect Dis. 2016;62:1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ristig MB, Tebas P, Gandy I, et al. Cure of acute hepatitis C in HIV co-infection? J Clin Gastroenterol. 2004;38:303. [DOI] [PubMed] [Google Scholar]
- 77.Luetkemeyer A, Hare CB, Stansell J, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor LE, Holubar M, Wu K, et al. Incident hepatitis C virus infection among US HIV-infected men enrolled in clinical trials. Clin Infect Dis. 2011;52:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghosn J, Pierre-Francois S, Thibault V, et al. Acute hepatitis C in HIV-infected men who have sex with men. HIV Med. 2004;5:303–306. [DOI] [PubMed] [Google Scholar]
- 81.Gotz HM, van Doornum G, Niesters HG, et al. A cluster of acute hepatitis C virus infection among men who have sex with men—results from contact tracing and public health implications. AIDS. 2005;19:969–974. [DOI] [PubMed] [Google Scholar]
- 82.Gilleece YC, Browne RE, Asboe D, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. J Acquir Immune Defic Syndr. 2005;40:41–46. [DOI] [PubMed] [Google Scholar]
- 83.Vogel M, Bieniek B, Jessen H, et al. Treatment of acute hepatitis C infection in HIV-infected patients: a retrospective analysis of eleven cases. J Viral Hepat. 2005;12:207–211. [DOI] [PubMed] [Google Scholar]
- 84.Bottieau E, Apers L, Van Esbroeck M, et al. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001-2009. Euro Surveill. 2010;15:19673. [PubMed] [Google Scholar]
- 85.Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2009;48:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun HY, Chang SY, Yang ZY, et al. Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors. J Clin Microbiol. 2012;50:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishikane M, Watanabe K, Tsukada K, et al. Acute hepatitis C in HIV-1 infected Japanese cohort: single center retrospective cohort study. PLoS One. 2014;9:e100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fierer DS. Epidemic of sexually transmitted hepatitis C virus infection among HIV-infected men. Curr Infect Dis Rep. 2010;12:118–125. [DOI] [PubMed] [Google Scholar]
- 89.Kaplan-Lewis E, Fierer DS. Acute HCV in HIV-infected MSM: modes of acquisition, liver fibrosis, and treatment. Curr HIV/AIDS Rep. 2015;12:317–325. [DOI] [PubMed] [Google Scholar]
- 90.Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55:1408–1416. [DOI] [PubMed] [Google Scholar]
- 91.Lambers FA, Prins M, Thomas X, et al. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS. 2011;25:F21–F27. [DOI] [PubMed] [Google Scholar]
- 92.Ingiliz P, Martin TC, Rodger A, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol. 2016;66:282–287. [DOI] [PubMed] [Google Scholar]
- 93.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–991. [DOI] [PubMed] [Google Scholar]
- 94.Centers for Disease Control and Prevention (CDC). Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men—New York City, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60:945–950. [PubMed] [Google Scholar]
- 95.Schmidt AJ, Rockstroh JK, Vogel M, et al. Trouble with bleeding: risk factors for acute hepatitis C among HIV-positive gay men from Germany—a case-control study. PLoS One. 2011;6:e17781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Witt MD, Seaberg EC, Darilay A, et al. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984-2011. Clin Infect Dis. 2013;57:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Apers L, Vanden Berghe W, De Wit S, et al. Risk factors for HCV acquisition among HIV-positive MSM in Belgium. J Acquir Immune Defic Syndr. 2015;68:585–593. [DOI] [PubMed] [Google Scholar]
- 98.Vanhommerig JW, Lambers FA, Schinkel J, et al. Risk factors for sexual transmission of hepatitis C virus among human immunodeficiency virus-infected men who have sex with men: a case-control study. Open Forum Infect Dis. 2015;2:ofv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hegazi A, Lee MJ, Whittaker W, et al. Chemsex and the city: sexualised substance use in gay bisexual and other men who have sex with men attending sexual health clinics. Int J STD AIDS. 2017;28:362–366. [DOI] [PubMed] [Google Scholar]
- 100.Bradshaw D, Lamoury F, Catlett B, et al. A comparison of seminal hepatitis C virus (HCV) RNA levels during recent and chronic HCV infection in HIV-infected and HIV-uninfected individuals. J Infect Dis. 2015;211:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turner SS, Gianella S, Yip MJ, et al. Shedding of hepatitis C virus in semen of human immunodeficiency virus-infected men. Open Forum Infect Dis. 2016;3:ofw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foster AL, Gaisa MM, Hijdra RM, et al. Shedding of hepatitis C virus into the rectum of HIV-infected men who have sex with men. Clin Infect Dis. 2017;64:284–288. [DOI] [PubMed] [Google Scholar]
- 103.Partnership™ for Drug-free Kids. The partnership at DrugFree.org. New data show millions of Americans with alcohol and drug addiction could benefit from health care reform. 2010. Available at: www.drugfree.org/untaxed/new-data-show-millions-of. Accessed January 1, 2014.
- 104.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. [DOI] [PubMed] [Google Scholar]
- 105.Cicero TJ, Ellis MS, Harney J. Shifting patterns of prescription opioid and heroin abuse in the United States. N Engl J Med. 2015;373:1789–1790. [DOI] [PubMed] [Google Scholar]
- 106.Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–826. [DOI] [PubMed] [Google Scholar]
- 107.Laine C, Hauck WW, Gourevitch MN, et al. Regular outpatient medical and drug abuse care and subsequent hospitalization of persons who use illicit drugs. JAMA. 2001;285:2355–2362. [DOI] [PubMed] [Google Scholar]
- 108.Weisner C, Mertens J, Parthasarathy S, et al. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–1988. [DOI] [PubMed] [Google Scholar]
- 110.Zeremski M, Dimova RB, Zavala R, et al. Hepatitis C virus-related knowledge and willingness to receive treatment among patients on methadone maintenance. J Addict Med. 2014;8:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeremski M, Zavala R, Dimova RB, et al. Improvements in HCV-related knowledge among substance users on opioid agonist therapy after an educational intervention. J Addict Med. 2016;10:104–109. [DOI] [PubMed] [Google Scholar]
- 112.Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93:141–147. [DOI] [PubMed] [Google Scholar]
- 113.Bruneau J, Zang G, Abrahamowicz M, et al. Sustained drug use changes after hepatitis C screening and counseling among recently infected persons who inject drugs: a longitudinal study. Clin Infect Dis. 2014;58:755–761. [DOI] [PubMed] [Google Scholar]
- 114.Aspinall EJ, Weir A, Sacks-Davis R, et al. Does informing people who inject drugs of their hepatitis C status influence their injecting behaviour? Analysis of the Networks II study. Int J Drug Policy. 2014;25:179–182. [DOI] [PubMed] [Google Scholar]
- 115.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18:156–170. [DOI] [PubMed] [Google Scholar]
- 116.Sylvestre DL, Litwin AH, Clements BJ, et al. The impact of barriers to hepatitis C virus treatment in recovering heroin users maintained on methadone. J Subst Abuse Treat. 2005;29:159–165. [DOI] [PubMed] [Google Scholar]
- 117.Dore GJ, Hellard M, Matthews GV, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138:123–135. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robaeys G, Grebely J, Mauss S, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis. 2013;57(suppl 2):S129–S137. [DOI] [PubMed] [Google Scholar]
- 119.Grebely J, Raffa JD, Meagher C, et al. Directly observed therapy for the treatment of hepatitis C virus infection in current and former injection drug users. J Gastroenterol Hepatol. 2007;22:1519–1525. [DOI] [PubMed] [Google Scholar]
- 120.Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–747. [DOI] [PubMed] [Google Scholar]
- 121.Dimova RB, Zeremski M, Jacobson IM, et al. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49:561–573. [DOI] [PubMed] [Google Scholar]
- 123.Malta M, Magnanini MM, Strathdee SA, et al. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14:731–747. [DOI] [PubMed] [Google Scholar]
- 124.Hinkin CH, Barclay TR, Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Litwin AH, Soloway IJ, Cockerham-Colas L, et al. Successful treatment of chronic hepatitis C with triple therapy in an opioid agonist treatment program. Int J Drug Policy. 2015;26:1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin NK, Vickerman P, Foster GR, et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol. 2011;54:1137–1144. [DOI] [PubMed] [Google Scholar]
- 127.Martin NK, Vickerman P, Hickman M. Mathematical modelling of hepatitis C treatment for injecting drug users. J Theor Biol. 2011;274:58–66. [DOI] [PubMed] [Google Scholar]
- 128.Martin NK, Hickman M, Hutchinson SJ, et al. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(suppl 2):S39–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin NK, Thornton A, Hickman M, et al. Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis. 2016;62:1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salazar-Vizcaya L, Kouyos RD, Zahnd C, et al. Hepatitis C virus transmission among human immunodeficiency virus-infected men who have sex with men: modeling the effect of behavioral and treatment interventions. Hepatology. 2016;64:1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rolls DA, Daraganova G, Sacks-Davis R, et al. Modelling hepatitis C transmission over a social network of injecting drug users. J Theor Biol. 2012;297:73–87. [DOI] [PubMed] [Google Scholar]
- 133.Sacks-Davis R, Daraganova G, Aitken C, et al. Hepatitis C virus phylogenetic clustering is associated with the social-injecting network in a cohort of people who inject drugs. PLoS One. 2012;7:e47335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hellard M, Rolls DA, Sacks-Davis R, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60:1861–1870. [DOI] [PubMed] [Google Scholar]
- 135.Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J. 2013;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schackman BR, Teixeira PA, Beeder AB. Offers of hepatitis C care do not lead to treatment. J Urban Health. 2007;84:455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeremski M, Zibbell JE, Martinez AD, et al. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol. 2013;19:7846–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Friedmann PD, D’Aunno TA, Jin L, et al. Medical and psychosocial services in drug abuse treatment: do stronger linkages promote client utilization? Health Serv Res. 2000;35:443–465. [PMC free article] [PubMed] [Google Scholar]
- 140.Umbricht-Schneiter A, Ginn DH, Pabst KM, et al. Providing medical care to methadone clinic patients: referral vs on-site care. Am J Public Health. 1994;84:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moussalli J, Delaquaize H, Boubilley D, et al. Factors to improve the management of hepatitis C in drug users: an observational study in an addiction centre. Gastroenterol Res Pract. 2010;2010:261472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evon DM, Simpson K, Kixmiller S, et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am J Gastroenterol. 2011;106:1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(suppl 2):S56–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bonkovsky HL, Tice AD, Yapp RG, et al. Efficacy and safety of peginterferon alfa-2a/ribavirin in methadone maintenance patients: randomized comparison of direct observed therapy and self-administration. Am J Gastroenterol. 2008;103:2757–2765. [DOI] [PubMed] [Google Scholar]
- 146.Martinez AD, Dimova R, Marks KM, et al. Integrated internist—addiction medicine—hepatology model for hepatitis C management for individuals on methadone maintenance. J Viral Hepat. 2012;19:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.European Association for Study of the Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–264. [DOI] [PubMed] [Google Scholar]
- 149.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. [DOI] [PubMed] [Google Scholar]
- 150.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. [DOI] [PubMed] [Google Scholar]
- 151.Shi KQ, Tang JZ, Zhu XL, et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol. 2014;29:1149–1158. [DOI] [PubMed] [Google Scholar]
- 152.Wang Y, Fan Q, Wang T, et al. Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med. 2015;8:17654–17663. [PMC free article] [PubMed] [Google Scholar]
- 153.Bini EJ, Kritz S, Brown LS, Jr, et al. Hepatitis B virus and hepatitis C virus services offered by substance abuse treatment programs in the United States. J Subst Abuse Treat. 2012;42:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bini EJ, Kritz S, Brown LS, Jr, et al. Barriers to providing health services for HIV/AIDS, hepatitis C virus infection and sexually transmitted infections in substance abuse treatment programs in the United States. J Addict Dis. 2011;30:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brown LS, Jr, Kritz S, Goldsmith RJ, et al. Health services for HIV/AIDS, HCV, and sexually transmitted infections in substance abuse treatment programs. Public Health Rep. 2007;122:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.US Department of Health and Human Services. Report to Congress: e-health and telemedicine. 2016. Available at: https://aspe.hhs.gov/sites/default/files/pdf/206751/TelemedicineE-HealthReport.pdf. Accessed January 22, 2017. [DOI] [PubMed]
- 158.Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154–161. [DOI] [PubMed] [Google Scholar]
- 159.Talal A, Andrews P, McLeod A, et al. Integrated, co-located, telemedicine-based treatment approaches for hepatitis C virus (HCV) management for individuals on opioid agonist treatment (abstract #SAT-115). J Hepatol. 2016;64:S747. [Google Scholar]
- 160.Talal AH, Andrews P, McLeod A, et al. Telemedicine-based hepatitis C virus (HCV) management for individuals on opioid agonist treatment (OAT). Hepatology. 2016;63:475a-a. [Google Scholar]
- 161.New York State Broadband Program Office. Broadband for all. 2016. Available at: www.ny.gov/programs/broadband-all. Accessed September 29, 2016.
- 162.Daniel H, Sulmasy LS, Health and Public Policy Committee of the American College of Physicians Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. 2015;163:787–789. [DOI] [PubMed] [Google Scholar]
- 163.Shah HA, Abu-Amara M. Education provides significant benefits to patients with hepatitis B virus or hepatitis C virus infection: a systematic review. Clin Gastroenterol Hepatol. 2013;11:922–933. [DOI] [PubMed] [Google Scholar]
- 164.Masson CL, Delucchi KL, McKnight C, et al. A randomized trial of a hepatitis care coordination model in methadone maintenance treatment. Am J Public Health. 2013;103:e81–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ford MM, Jordan A, Johnson N, et al. Check Hep C: a community-based approach to hepatitis C diagnosis in high risk populations (abstract). Presented at the 66th Annual Meeting of the American Association for the Study of Liver Diseases, San Francisco, CA. 2015.
- 166.Farrisi D, Dietz N. Patient navigation is a client-centered approach that helps to engage people in HIV care. HIV Clin. 2013;25:1–3. [PubMed] [Google Scholar]
- 167.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(suppl 1):S49–S58. [DOI] [PubMed] [Google Scholar]
- 168.Health Canada. Investigation and assessment of the Navigator role in meeting the information, decisional and educational needs of women with breast cancer in Canada. 2002. Available at: http://publications.gc.ca/collections/Collection/H39-663-2002E.pdf. Accessed July 9, 2014.
- 169.AIDS Coalition of Nova Scotia. A case for patient navigation for people with HIV/AIDS. 2006. Available at: http://novascotia.ca/aids/documents/Case_Patient_Navagation.pdf. Accessed July 2, 2014.
- 170.Byers T. Assessing the value of patient navigation for completing cancer screening. Cancer Epidemiol Biomarkers Prev. 2012;21:1618–1619. [DOI] [PubMed] [Google Scholar]
- 171.Tobias CR, Cunningham W, Cabral HD, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDS. 2007;21:426–434. [DOI] [PubMed] [Google Scholar]


