Abstract
BACKGROUND
Cryolipolysis of the arms has been shown to be an effective but somewhat time-consuming process.
OBJECTIVE
The study evaluated safety and efficacy of a contoured cup cryolipolysis applicator for reduction of arm fat. The prototype was designed to maximize tissue contact with the cooling surface to improve comfort, while reducing treatment time by 25 minutes.
MATERIALS AND METHODS
Both arms were treated using a prototype device that delivered treatment in 35 minutes at −11°C. Photographic and ultrasound documentation was captured at baseline and 12 weeks post-treatment. Efficacy was assessed by photo review and measurement of fat reduction in ultrasound images. Immediately after 1, 4, and 12 weeks post-treatment, clinical assessments were performed to evaluate treatment areas and sensory alterations.
RESULTS
Thirty women were enrolled and completed treatments to both arms. Ultrasound measurements found mean fat layer reduction of 3.2 mm with an SD of 2.7 mm. Blinded independent photo review found 85.2% correct identification of baseline photographs by at least 2/3 of reviewers. There were no unanticipated adverse device effects. Four study subjects experienced numbness in the treatment area beyond the 12-week visit that subsequently resolved without intervention.
CONCLUSION
These data suggest that the CoolCup prototype applicator provides rapid, safe, and effective arm treatment.
Cryolipolysis (CoolSculpting System; ZELTIQ Aesthetics, Pleasanton, CA) is a safe and effective noninvasive body contouring procedure. Most commonly used to treat the abdomen and flanks, cryolipolysis is also highly tolerable for treatment of areas including the back, submental area, inner thighs, and outer thighs.1–12
Cryolipolysis treatment of the arms has been shown to be effective but requires careful applicator placement and patient monitoring during the procedure to ensure safety.13,14 In a small study of 7 subjects who were treated unilaterally in the arms using a flat parallel plate vacuum applicator (CoolFit), it was found that while the procedure was deemed safe and effective, 4 incidents of transient numbness and paresthesia occurred.13 In a different study of 6 subjects for cryolipolysis arm treatments using the flat parallel plate vacuum applicator, it was reported that one study subject was unable to complete the study because of pain and numbness during treatment.14 These unwanted side effects are likely caused by compression of the ulnar nerve by the applicator, particularly near the medial condyle on the humerus.
For most cryolipolysis treatments, tissue is pulled by vacuum suction between 2 parallel cooling plates for a treatment duration of 60 minutes. To reduce treatment time and improve patient comfort, efforts have been made to improve the efficiency of tissue cooling. The cryolipolysis applicator has been redesigned to create a contoured cup surface to maximize tissue contact with the cooling surface. A contoured, cooled cup had been successfully developed for small volume fat reduction, such as in the submental area.10 Subsequently, a prototype contoured cup applicator (−11°C for 35 minutes) was investigated with a standard cryolipolysis applicator (−10°C for 60 minutes) in a flank cryolipolysis study and found to have equivalent safety and efficacy with greater patient preference.15 This study uses a similar contoured cup prototype applicator with a flat body contour suitable for treating the arm. The safety and efficacy of arm treatment were evaluated using a prototype applicator which reduced treatment time by 42%.
Previous arm studies investigated cryolipolysis using a flat parallel plate applicator in small populations.13,14 This is the first study of cryolipolysis for subcutaneous fat reduction in the arms with a large patient population.
Materials and Methods
This was a prospective, multicenter, open label, interventional cohort study. The study was registered at ClinicalTrials.gov (identifier NCT02669329). The protocol was approved by Quorum Review Independent Review Board.
The bilateral treatment study evaluated the prototype cryolipolysis applicator with a flat contour. The prototype device was created by modifying an existing parallel plate cryolipolysis vacuum applicator with a flat body contour (CoolFit). A machined metal insert was installed in the modified applicator to create a cooled, contoured cup surface (Figure 1). The contoured cup allowed the tissue to fully seat against the cooled treatment surfaces. Each subject received one −11°C, 35-minute cooling cycle to each arm delivered using a prototype medium volume contoured cup vacuum applicator (CoolCup).
Figure 1.
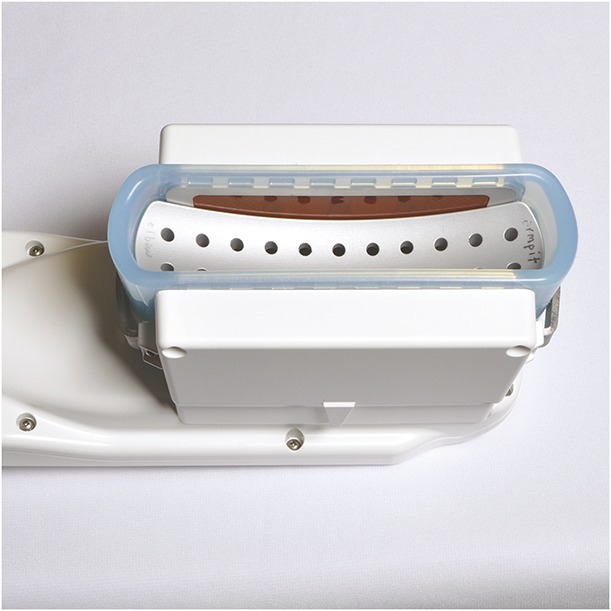
Prototype CoolCup was created by inserting a machined metal cup into a modified parallel plate cryolipolysis applicator.
Eligible subjects were men or women, between 22 and 65 years of age, and with clearly visible subcutaneous arm fat. Subject selection guidelines included a distinct bulge of fat in the arm at least 14 cm from the elbow, with soft, pliable tissue of sufficient volume for treatment, and elbow to axilla length of at least 20 cm to minimize risk of ulnar nerve involvement during treatment. Exclusion criteria included patients that had a previous fat reduction procedure or implants in or near the treatment area, previous surgery in the arms, known history of cryoglobulinemia, cold urticaria, cold agglutinin disease, or paroxysmal cold hemoglobinuria, Raynaud disease, bleeding disorder, or current medications which may increase the risk of bruising, history of carpal tunnel syndrome, compartment syndrome, or deep vein thrombosis in the upper extremities, and having an active implanted device such as a pacemaker, defibrillator, or drug delivery system. For the duration of the study, subjects were instructed to avoid implementing major diet or exercise routine changes to maintain their weight within ±5% of baseline measurement.
The efficacy end point was defined as 75% or greater correct identification of the pretreatment images by at least 2/3 of the blinded, independent physician reviewers with expertise in the areas of dermatology and/or plastic surgery.
The primary safety end point was defined by monitoring the incidence of device- and/or procedure-related unanticipated adverse device effects. Immediately after treatment and at the 4- and 12-week follow-up visits, clinical assessments were performed to evaluate the treatment areas. Clinical assessments involved visual assessment of the treatment site and querying the study subjects about any changes in sensation that they might be experiencing. One week post-treatment, subjects were also contacted by telephone or email to assess the condition of the treatment areas. Anticipated treatment side effects, such as bruising, erythema/purpura, edema/swelling, numbness, and tingling at the treatment site, were evaluated. Subjects were assessed throughout the study for adverse events.
The study was statistically powered for a minimum of 24 subjects to meet a 55% correct photograph identification criterion. With 48 photograph pairs, the study results have 1 − β = 80% statistical power to declare 75% statistically larger than 55% (H0: π1 < π0 vs H1: π1 > π0 where π1 = 0.75 and π0 = 0.55) when using a 1-sided exact binomial test with α = 0.025 statistical significance. To account for attrition, an additional 6 subjects were recruited in the study, for a total of 30 study subjects.
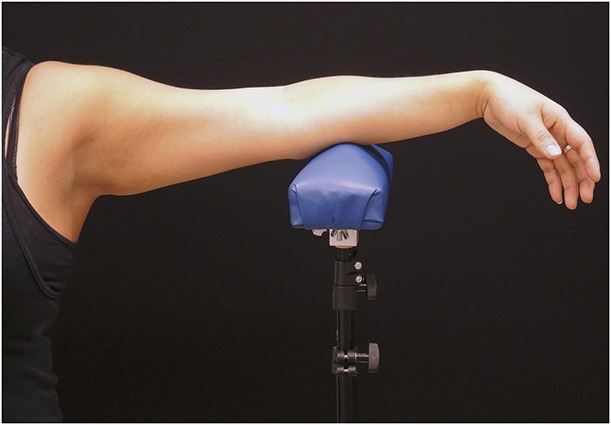
Photographs were taken at pretreatment and 12-week post-treatment visits by the same photographer to ensure consistent photographic methods. At the baseline and follow-up visits, photographs were acquired using a standardized photography set-up (Nikon D810, Nikon 60 mm lens, 2 DynaLite strobes set to 125 W/s, black backdrop) to ensure consistency, Figure 2.
Figure 2.

Standardized photography set-up and arm support fixture.
Study subjects were positioned standing with their arms resting on an adjustable height fixture, Figure 3. At the post-treatment visit, the photographer adjusted the fixture to replicate the baseline fixture height for each subject. A live view image was superimposed over each subject's baseline image to facilitate exact arm positioning. Subsequently, photographs taken at the 12-week post-treatment visit were compared with those taken at baseline by a blinded independent panel of 3 physicians. Independent photograph review data were generated by randomizing pretreatment and post-treatment photograph pairs of each subject, then asking the reviewers to determine which image was the pretreatment image.
Figure 3.
Study subjects had photographs and ultrasound images captured with arms resting on a support fixture.
Ultrasound images were acquired at baseline and 12-week post-treatment visits by the same ultrasonographer to ensure consistent imaging methods. The ultrasounds were also obtained with subjects standing with their arms resting on an adjustable height fixture, Figure 3. Ultrasound areas were marked parallel to the length of the arm starting at the area of largest bulge with one additional site marked immediately adjacent either to the left or right of the first ultrasound. A transparent film was applied to each arm to mark the measurement areas and any landmarks (e.g., moles and scars) to facilitate locating the same ultrasound sites in the follow-up visit. Measurements were conducted by placing the transducer (model L38, bandwidth 10–5 MHz; SonoSite Inc., Bothell, WA) over the arm measurement site and capturing the image on an ultrasound device (SonoSite Titan) to a depth of 4.6 cm. Care was taken to avoid adding pressure or negative pressure during measurement. During the follow-up visit, subjects were positioned in the same manner as at baseline. The transparent film was applied and matched to the landmarks on the skin; ultrasound sites were marked on the skin, and posttreatment images were captured. Ultrasound images were postprocessed to measure anatomical features in the pretreatment and post-treatment images, and the fat layer reduction in the treatment area was calculated. A paired t-test was performed to determine statistical significance of the fat layer reduction.
The cryolipolysis arm treatment used a custom-made fixture to ensure standard positioning and patient comfort (Figure 4). The arm support fixture consisted of a stand, applicator cradle, and forearm support. The adjustable stand positioned the applicator at an appropriate height for each study subject. The arm cradle provided stable positioning of the applicator for the duration of treatment. The forearm support provided a comfortable platform for the forearm and reduced pressure on the ulnar nerve. A squeeze ball and wrist rest were supplied for comfort and to promote circulation to the extremity while in a prolonged elevated position. The prototype CoolCup applicator was positioned within the fixture; a protective gel pad (CoolGel) was applied to the skin; the arm was positioned over the vacuum applicator with the edge of the treatment cup as close to the axilla as possible to prevent compression of the ulnar nerve; and vacuum suction was initiated. The forearm support angle was adjusted to comfortably support the forearm and wrist. The vacuum adhered the applicator to the treatment area and the subject was seated throughout the cryolipolysis procedure.
Figure 4.
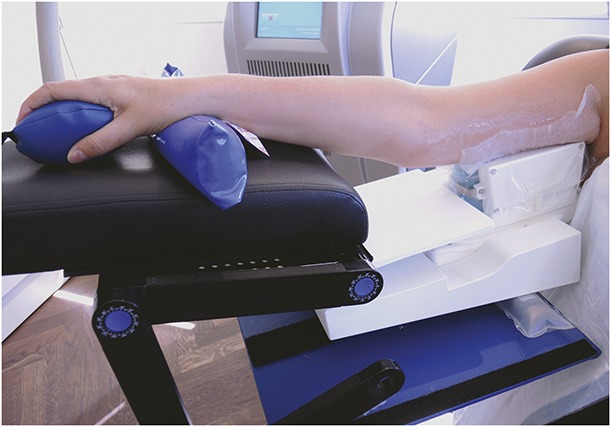
Applicator fixture facilitated standardized positioning and comfort throughout treatment with adjustable arm, forearm, and wrist support.
At the conclusion of the treatment cycle, the vacuum was discontinued and the subject's arm was removed from the applicator, revealing firm, frozen tissue. Immediately after removal of the applicator, infrared (IR) thermography images (FLIR Systems, Wilsonville, OR) were obtained to evaluate the thermal profile across the treatment areas. Next, a 2-minute manual massage of the treatment area was performed. The contralateral arm was then treated using the same applicator and treatment parameters.
Procedural pain was assessed for each arm during and immediately post-treatment, before discharge and at the 1-week, 4-week, and 12-week follow-up visits using a scale of 0 (no pain) to 10 (worst possible pain). Clinical assessment of the treatment sites was performed immediately post-treatment and at the follow-up visits. Bruising, erythema/purpura, edema/swelling, numbness, and tingling at the treatment site were evaluated and any other reported side effects were also assessed and recorded.
Results
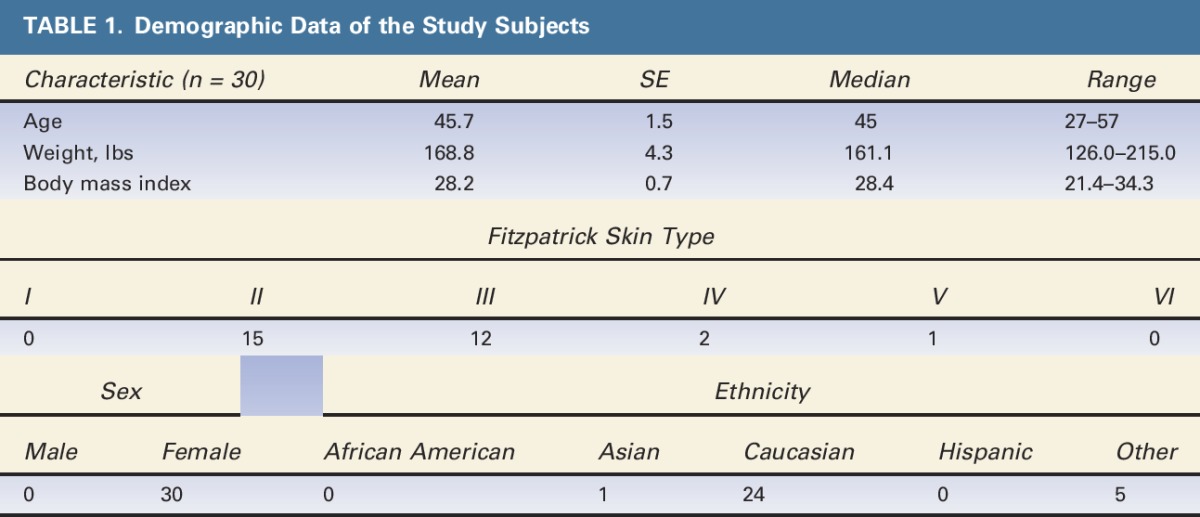
Thirty patients were enrolled and completed treatment. Demographic data are shown in Table 1. All subjects completed the 12-week follow-up; however, one subject (RIV-006) did not have 12-week ultrasounds and standardized photographs taken. Twenty-seven subjects remained within the allowed ±5% weight change limit; 3 subjects (CAR-016, RIV-006, and RIV-013) were excluded from treatment efficacy analysis because of weight change beyond ±5% from baseline.
TABLE 1.
Demographic Data of the Study Subjects
Immediate post-treatment photographs are shown in Figures 5 and 6 after removal of the arm from the applicator. The post-treatment images in Figure 5 demonstrate the firm, solidified “butter stick” tissue before post-treatment massage. As shown by the IR images obtained immediately after applicator removal (Figure 6), the CoolCup applicator produced a uniform cooling profile because the entire surface area of the treated arm was in contact with the contoured cooling cup. Treatments with traditional cryolipolysis applicators typically produce localized cooling at the parallel plates.15 The prototype insert used in this study transformed the parallel plate applicator into a cooled cup and produced a uniform cooling profile across the entire treatment area on the arm.
Figure 5.
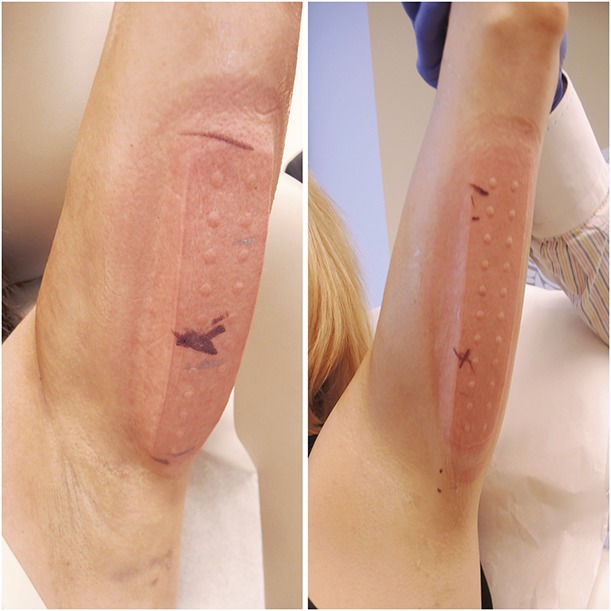
Immediate post-treatment photographs showing firm “butter stick” treatment areas from the CoolCup applicator. (Left) Subject RIV-007. (Right) Subject RIV-012.
Figure 6.
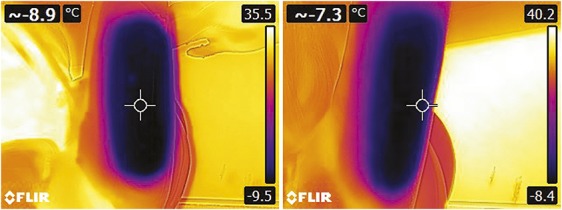
Immediate post-treatment IR thermography images show uniform cooling from the CoolCup applicator. (Left) Subject CAR-015. (Right) Subject CAR-012. IR, infrared.
Figures 7–10 show representative subjects at baseline and at 12-week post-treatment. Visible reduction in arm volume is demonstrated from the pre- and posttreatment photographs. From the independent photograph review, 3 blinded, independent physicians reviewed the 27 subjects' photographs in randomized pairs to assess the photographs for visible reduction of fat in the treatment area. Two reviewers were board-certified plastic surgeons and 1 reviewer was a board-certified dermatologist.
Figure 7.
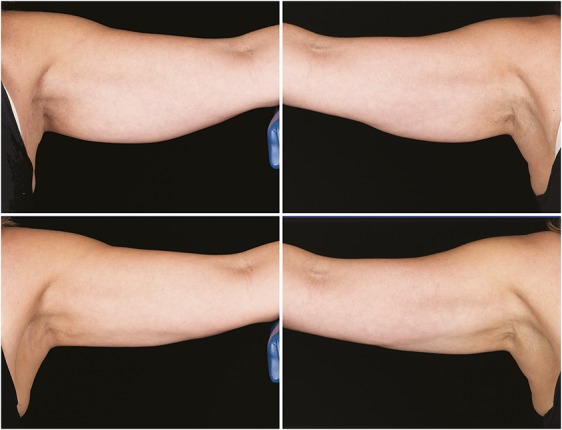
Baseline top row, left and right side, 12-week post-treatment bottom row, left and right side, photographs of a 57-year-old woman. Weight change +0.2 lbs. (+0.1%) from baseline. Subject RIV-007. Procedure by Dr. Rivers.
Figure 10.
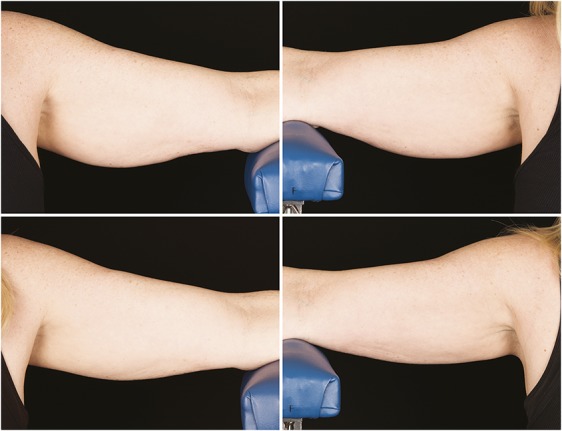
Baseline top row, left and right side, 12-week post-treatment bottom row, left and right side, photographs of a 52-year-old woman. Weight change +0.2 lbs. (+0.1%) from baseline. Subject CAR-011. Procedure by Drs. Carruthers and Humphrey.
Figure 8.
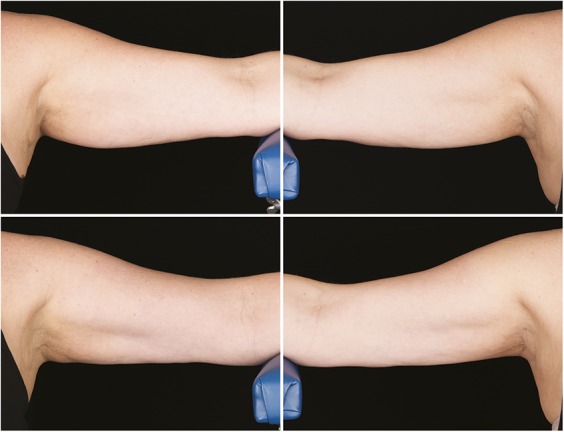
Baseline top row, left and right side, 12-week post-treatment bottom row, left and right side, photographs of a 52-year-old woman. Weight change +1.0 lbs. (+0.6%) from baseline. Subject CAR-007. Procedure by Drs. Carruthers and Humphrey.
Figure 9.
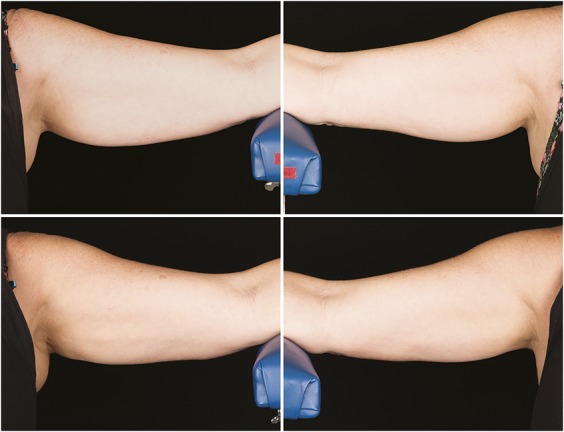
Baseline top row, left and right side, 12-week post-treatment bottom row, left and right side, photographs of a 57-year-old woman. Weight change −1.4 lbs. (−0.9%) from baseline. Subject RIV-015. Procedure by Dr. Rivers.
Removing the subjects who had weight change beyond ±5% from the treatment visit, there were 54 arms (27 subjects, 2 photograph pairs per arm) available for efficacy analysis. The overall correct identification rate was 85.2% (p < .0001) by at least 2/3 of reviewers. The primary efficacy end point of at least 75% correct identification of the pretreatment images by at least 2/3 of reviewers was met.
Ultrasound images were analyzed to calculate fat layer reduction. After excluding 3 subjects who were outside the ±5% weight change limit, results are presented for the 54 arms included in the efficacy analysis. The ultrasound measurement of the CoolCup treatment areas showed a mean fat layer reduction of 3.2 mm, with an SD of 2.7 mm. The ultrasound measurements of fat layer reduction ranged from an increase of 2.5 mm to a reduction of 8.5 cm. Reduction in fat layer was statistically significant (p < .0001).
Pain was assessed on a scale from 0 to 10 during and after treatment, and pain score data are summarized in Table 2. The intratreatment pain scores for all the 60 arms treated were averaged and the overall average pain score was 1.0, with an SD of 1.2. By the 12-week post-treatment visit, all subjects reported a pain score of 0.
TABLE 2.
Summarized Pain Scores Show Average Pain 1.0 During Treatment With Full Resolution at the 12-Week Visit
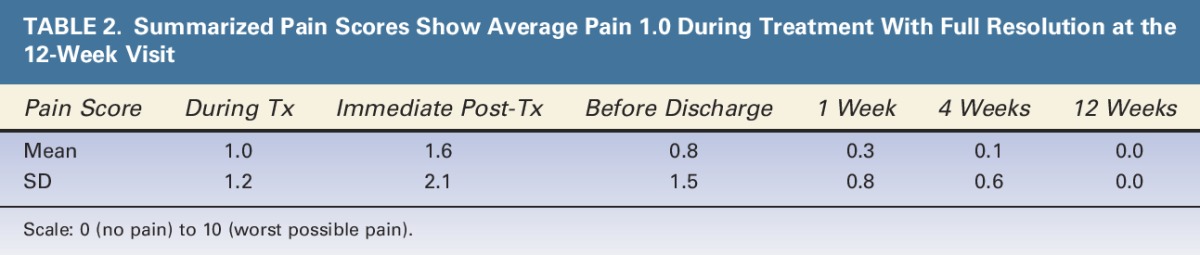
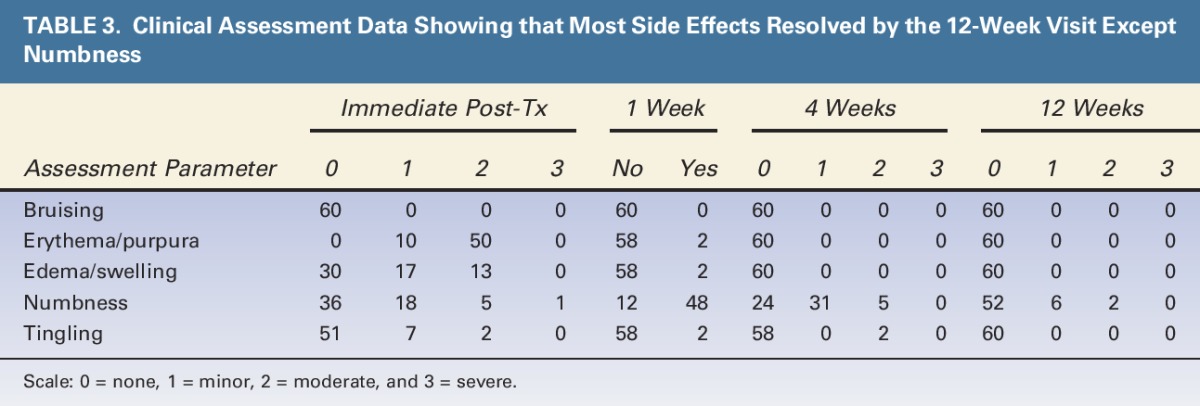
Table 3 summarizes clinical assessments immediately after treatment, and at 1, 4, and 12 weeks post-treatment using the scale 0 = none, 1 = minor, 2 = moderate, and 3 = severe. Immediately after treatment, the most common effects within the treatment area were erythema, edema, numbness, and tingling. By the 12-week post-treatment visit, all side effects had resolved without intervention except for some cases of numbness in the treatment area. The treatment area numbness for these subjects was not clinically significant, did not disrupt normal activities, and spontaneously resolved without intervention. Including the aforementioned prolonged numbness, there were a total of 10 device- and/or procedure-related adverse events. Four subjects reported prolonged numbness (bilateral numbness in 3 subjects, unilateral in 1 subject) with a duration longer than 12 weeks. One subject reported bilateral mild erythema that resolved 15 days posttreatment. One subject reported minor tingling in the fourth and fifth fingers of her left hand immediately after device removal, which resolved within approximately 20 minutes post-treatment.
TABLE 3.
Clinical Assessment Data Showing that Most Side Effects Resolved by the 12-Week Visit Except Numbness
The primary safety end point for the study was satisfied; there were no device- or procedure-related serious adverse events, and no unanticipated adverse device effects occurred during the study.
Discussion
This study investigated the safety and efficacy of the CoolCup prototype contoured cup cryolipolysis applicator for noninvasive fat reduction in the arm. The prototype applicator in this study was created by modifying a CoolFit applicator with a contoured metal insert that increased direct tissue contact with the cooling surface, thus allowing the reduction of the treatment duration from 60 to 35 minutes to achieve the same treatment efficacy. As shown in a previous flank treatment study using a similar contoured cup modification to a parallel plate applicator with a curved body contour (CoolCore), the prototype applicator produced equivalent safety and efficacy to the traditional applicator with higher patient preference because of its shorter treatment time and reduced skin tension and bruising.15
Previously published arm cryolipolysis studies have been CoolFit applicator studies with small patient populations.13,14 These pilot studies investigated the flat body contour applicator in a rarely reported upon treatment site, but the sample sizes were small. This study of n = 30 subjects treated bilaterally is statically powered to demonstrate treatment efficacy. Independent photograph review found 85.2% correct identification of baseline photographs by at least 2/3 of reviewers, and ultrasound measurements determined that the mean fat layer reduction was 3.2 mm. Treatment results may further improve beyond the 12-week follow-up in this study. As is often seen in clinical practice, optimal cryolipolysis treatment efficacy may be achieved more than 3 months post-treatment.
This study investigated the safety of arm cryolipolysis using a prototype applicator with lower vacuum skin tension and shorter treatment duration. The aforementioned 2 arm cryolipolysis publications had relatively small patient populations and observed several incidents of numbness or pain during or after treatment. In the unilateral arm study of n = 7 subjects with the CoolFit applicator, there were 4 incidents of transient numbness and paresthesia and 2 incidents of mild postprocedural pain, but all resolved without intervention within 2 weeks.13 In the study of n = 5 subjects treated bilaterally in the arms, one subject was unable to tolerate the pain during treatment on her right upper arm.14 This study with the prototype applicator found similar side effects. None of the patients were unable to complete their treatment cycles because of pain; the mean procedural pain score of 1.0 on a scale from 0 to 10 suggests that the treatment is highly tolerable. Treatment side effects, such as erythema, edema, and tingling, were typically mild and self-resolving by the final 12-week follow-up; some cases of prolonged treatment area numbness were reported beyond the 12-week follow-up, but this has been seen in previous studies such as the inner thigh cryolipolysis study which had one case of mild numbness that resolved at 132 days post-treatment.7 For arm treatment, prolonged paresthesia may be minimized by ensuring the ulnar nerve is not compressed by the applicator during treatment. In this study, potential study subjects were screened to ensure elbow to axilla length of at least 20 cm and the applicator was positioned as close to the axilla as possible to minimize the risk of ulnar nerve compression. This study demonstrated that arms could be safely treated by cryolipolysis using a prototype contoured cup applicator with 42% reduction in treatment time.
As demonstrated in this bilateral arm study, the evolution of the vacuum cryolipolysis applicator from a parallel plate configuration to a contoured cup resulted in increased tissue contact with the cooling surface, more uniform cooling, shorter treatment duration, and an improved patient experience.
Conclusion
This clinical study of a prototype medium-sized vacuum applicator with a cooled contoured surface indicates that the CoolCup produces safe and effective reduction of subcutaneous fat in the arms. Efficacy was demonstrated by ultrasound measurements showing mean fat layer reduction of 3.2 mm and blinded independent photograph review with 85.2% correct identification of baseline photographs by at least 2/3 of reviewers. Safety was demonstrated by the absence of unanticipated adverse device effects during the study. The prototype applicator reduced treatment time from 60 to 35 minutes and provided safe, effective, and highly tolerable noninvasive fat reduction of the arms.
Footnotes
Supported by ZELTIQ Aesthetics, manufacturer of the CoolSculpting System.
J.D. Carruthers, S. Humphrey, and J.K. Rivers are clinical investigators for ZELTIQ Aesthetics.
References
- 1.Derrick CD, Shridharani SM, Broyles JM. The safety and efficacy of cryolipolysis: a systematic review of available literature. Aesthet Surg J 2015;35:830–6. [DOI] [PubMed] [Google Scholar]
- 2.Ingargiola MJ, Motakef S, Chung MT, Vasconez HC, et al. Cryolipolysis for fat reduction and body contouring: safety and efficacy of current treatment paradigms. Plast Reconstr Surg 2015;135:1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J 2013;33:835–46. [DOI] [PubMed] [Google Scholar]
- 4.Carruthers J, Stevens WG, Carruthers A, Humphrey S. Cryolipolysis and skin tightening. Dermatol Surg 2014;40(Suppl 12):S184–9. [DOI] [PubMed] [Google Scholar]
- 5.Dierickx CC, Mazer JM, Sand M, Koenig S, et al. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg 2013;39:1209–16. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki GH, Abelev N, Tevez-Ortiz A. Noninvasive selective cryolipolysis and reperfusion recovery for localized natural fat reduction and contouring. Aesthet Surg J 2014;34:420–31. [DOI] [PubMed] [Google Scholar]
- 7.Zelickson BD, Burns AJ, Kilmer SL. Cryolipolysis for safe and effective inner thigh fat reduction. Lasers Surg Med 2015;47:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boey GE, Wasilenchuk JL. Fat reduction in the inner thigh using a prototype cryolipolysis applicator. Dermatol Surg 2014;40:1004–9. [DOI] [PubMed] [Google Scholar]
- 9.Stevens WG, Bachelor EP. Cryolipolysis conformable-surface applicator for nonsurgical fat reduction in lateral thighs. Aesthet Surg J 2015;35:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non-invasive reduction of submental fat. Lasers Surg Med 2016;48:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein EF, Bloom JD, Basilavecchio LD, Plugis JM. Non-invasive fat reduction of the flanks using a new cryolipolysis applicator and overlapping, two-cycle treatments. Lasers Surg Med 2014;46:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munavalli GS, Panchaprateep R. Cryolipolysis for targeted fat reduction and improved appearance of the enlarged male breast. Dermatol Surg 2015;41:1043–51. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Jang HW, Kim H, Suh DH, et al. Non-invasive cryolipolysis to reduce subcutaneous fat in the arms. J Cosmet Laser Ther 2016;18:126–9. [DOI] [PubMed] [Google Scholar]
- 14.Wanitphakdeedecha R, Sathaworawong A, Manuskiatti W. The efficacy of cryolipolysis treatment on arms and inner thighs. Lasers Med Sci 2015;30:2165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilmer SL. Prototype CoolCup cryolipolysis applicator with over 40% reduced treatment time demonstrates equivalent safety and efficacy with greater patient preference. Lasers Surg Med 2017;49:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


