Abstract
Objectives:
We assessed the association between the timing of pregnancy with the risk of postpartum virologic failure and loss from HIV care in South Africa.
Design:
This is a retrospective cohort study of 6306 HIV-positive women aged 15–49 at antiretroviral therapy (ART) initiation, initiated on ART between January 2004 and December 2013 in Johannesburg, South Africa.
Methods:
The incidence of virologic failure (two consecutive viral load measurements of >1000 copies/ml) and loss to follow-up (>3 months late for a visit) during 24 months postpartum were assessed using Cox proportional hazards modelling.
Results:
The rate of postpartum virologic failure was higher following an incident pregnancy on ART [adjusted hazard ratio 1.8, 95% confidence interval (CI): 1.1–2.7] than among women who initiated ART during pregnancy. This difference was sustained among women with CD4+ cell count less than 350 cells/μl at delivery (adjusted hazard ratio 1.8, 95% CI: 1.1–3.0). Predictors of postpartum virologic failure were being viremic, longer time on ART, being 25 or less years old and low CD4+ cell count and anaemia at delivery, as well as initiating ART on stavudine-containing or abacavir-containing regimen. There was no difference postpartum loss to follow-up rates between the incident pregnancies group (hazard ratio 0.9, 95% CI: 0.7–1.1) and those who initiated ART in pregnancy.
Conclusion:
The risk of virologic failure remains high among postpartum women, particularly those who conceive on ART. The results highlight the need to provide adequate support for HIV-positive women with fertility intention after ART initiation and to strengthen monitoring and retention efforts for postpartum women to sustain the benefits of ART.
Keywords: antiretroviral therapy, antiretroviral therapy retention, HIV treatment, postpartum period, pregnancy, South Africa
Introduction
HIV among women of childbearing age remains an important contributor to maternal and child morbidity and mortality [1]. The risk remains high as the HIV prevalence among women attending antenatal care (ANC) in the South African public health sector has remained steady around 30% in the past 10 years [2–4]. In 2015, the amended South African guidelines for the prevention of mother-to-child transmission (PMTCT) of HIV made provisions to initiate all HIV-positive pregnant and breastfeeding women on lifelong antiretroviral therapy (ART) upon HIV diagnosis [5]. Although this policy further increased the ART coverage in this population, long-term ART success will depend on their ability to attain sustained virologic suppression.
The PMTCT programmes play a critical role in the diagnosis and ART initiation of HIV-positive women. However, the programme's strong focus on protecting the baby frames the ART experience of many HIV-positive women [6–9]. As a result, the motivation of women initiated on ART in the context of PMTCT to adhere to ART and remained in HIV care wanes gradually after delivery [10–12]. Although this is well documented among women who initiate ART during pregnancy (prevalent pregnancies at ART initiation), far less has been written about women who conceive while receiving ART (incident pregnancies). The 2013 South African national ANC survey showed that the majority (73.5%) of primiparous women who tested HIV-positive in ANC had prior knowledge of their HIV status but the proportion with prior ART exposure is unclear [2]. Certainly, as South Africa scales up the ‘treat-all’ strategy, many more HIV-positive women will be receiving ART at conception [13,14].
To date, few studies have quantified the postpartum risk of virologic failure because of viral load data limitations in most resource-limited settings. Furthermore, the question of whether initiating ART before a pregnancy improves postpartum retention and virologic outcomes has not been sufficiently explored. Understanding predictors of postpartum virologic failure and loss from HIV care among treatment experienced women who conceive on ART is necessary to inform interventions to improve postpartum retention in care and ART adherence among women with fertility intentions after ART initiation.
In this article, we aim to assess whether the timing of ART initiation in relation to pregnancy start has some bearing on the risk of virologic failure and the risk of becoming lost to follow-up (LTFU) in the first 2 years postpartum.
Methods
Study population
We conducted a cohort study using prospectively collected, anonymized medical records of HIV-infected adult women (18–49 years old at ART initiation) initiated on a standard first-line ART regimen according to South African guidelines between 1 January 2004 and 31 December 2013, at three nongovernmental organizations (NGO) and seven public ART clinics that receive technical support from right to care, a nonprofit organization in Johannesburg, South Africa. The first South African ART guidelines in 2004 made provisions for one first-line regimen including stavudine (d4T), lamivudine (3TC), efavirenz (EFV) or nevirapine (NVP) in the case of contraindications to EFV [15]. In 2010, the standard first-line regimens were updated to substitute d4T with tenofovir (TDF) as the first option for new patients, include zidovudine (ZDV) as part of first-line ART in which TDF was contraindicated and use emtricitabine (FTC) as an alternative for 3TC [16]. In 2013, abacavir (ABC) was added as an option in cases of contraindications to TDF, ZDV and d4T in first-line ART [17].
Clinical data from the ART clinics were captured on site and stored in an electronic patient-management system, TherapyEdge-HIV (Advanced Biologic Laboratories, SA, Luxembourg). Additional clinical and laboratory data were obtained from electronic records held by the National Health Laboratory Services. Data were fully anonymized for analysis. The final analytic data set included only women who had at least one recorded viral load measurement during the period of observation.
The primary exposure event was having a first (ever) pregnancy lasting at least 7 months and ending before 31 December 2013 that resulted in a live birth. Unexposed women (no recorded pregnancy) were matched to exposed women on age at ART initiation, year of ART initiation and time on ART at delivery. Time on ART for exposed women was measured from ART initiation to the date of delivery. The observation period for unexposed women (pseudo-postpartum period) began at the delivery date of their matched exposed woman.
The data set was closed on 31 December 2015, allowing for 24 months of postpartum (or equivalent period on ART for the unexposed) follow-up. Up to two unexposed women were matched to each exposed woman. Ethics approval for the data review was obtained from the Human Research Ethics Committee of the University of Witwatersrand (M140201) as well as Boston University Institutional Review Board (H-29768).
Analytic variables
The secondary exposure variable was created by further categorizing exposed women by when, in relation to the pregnancy start date, ART was initiated as unexposed women (no recorded pregnancy), women conceived while receiving ART (incident pregnancy) and women who initiated ART during pregnancy (prevalent pregnancy).
The primary outcome was virologic failure (defined as having two consecutive viral load measurements >1000 copies/ml, and the first failing viral load occurring at least 3 months after the date of delivery) in the first 24 months postpartum or pseudo-postpartum for unexposed women (six). The secondary outcome was postpartum LTFU from HIV care, defined as being at least 3 months late for a scheduled visit during the observation period.
Potential confounders included were demographic variables as well as clinical and laboratory variables measured closest to the date of delivery either in the last trimester of the pregnancy or no more than 3 months after delivery or baseline for the unexposed, including BMI, CD4+ cell counts, ART regimen and haemoglobin (Hb). BMI was categorized as underweight (BMI < 18.5), normal (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30) and obese (BMI ≥ 30). Anaemia was defined as an Hb value below 11.5 g/dl. CD4+ (measured in cells/μl) was categorized as less than199, 200–350 and more than 350 cells/μl. Viral suppression was defined as having a viral load measurement less than 50 copies/ml.
Follow-up time
Person-time accrued from the date of delivery (considered as baseline time point) for postpartum women or their matched baseline date for unexposed women. Women were followed until the outcome of interest, the last date seen at the clinic during the first 2 years after the baseline date (for those who died, were LTFU or transferred out) or 31 December 2015, whichever came first.
Statistical analysis
Baseline characteristics were described using medians and interquartile ranges (IQRs) for continuous variables and percentages for categorical variables stratified by pregnancy status. Computed incidence of virologic failure or LTFU was expressed as rates per 100 person-years. Cox proportional hazard models were used to explore predictors of postpartum virologic failure and LTFU separately. We then conducted a Cox regression stratified by timing of the first pregnancy to examine whether predictors of postpartum virologic failure or LTFU among women who conceive on ART differ from those of women who initiate ART during pregnancy. Variables associated with virologic failure or LTFU in crude Cox proportional hazards models (P < 0.10) in the postpartum period were included in multivariate models. Also included were variables that changed the hazard ratio for the exposure of interest by more than10% and are known not to be on the causal pathway or colliders. Schoenfeld residuals were used to test for adherence to the proportional hazard assumption. Kaplan–Meier curves were used to compare progression to first virologic failure or LTFU event in the postpartum period among women with high baseline CD4+ (>350 cells/μl) to women with low baseline CD4+ (≤350 cells/μl), stratified by timing of pregnancy. Data analysis was conducted using STATA version 14 (StataCorp, College Station, Texas, USA).
Results
Baseline demographic characteristics of study sample
The data set included 7953 women. From these, 1128 (14.2%) with missing scheduled visits after delivery and 519 (6.5%) with missing postpartum viral load measurements were excluded from the final analytic data set. Among the excluded women, 278 of 1647 (16.9%) were transferred within the first 3 months after delivery, and 44 of 1647 (2.7%) women died within 90 days of delivery.
Table 1 presents the demographic and clinical characteristics of the study sample consisting of 6306 women, 2403 (38.1%) were unexposed, 1953 (31.0%) had an incident pregnancy on ART and 1950 (30.9%) had a prevalent pregnancy at ART initiation. The overall median age at the start of observation was 31.2 years (IQR: 27.7–35.0). The prevalent pregnancy group was slightly younger at delivery (median 29.7 years, IQR: 26.4–33.6) compared with the incident pregnancy group (median 32.3, IQR: 28.9–35.6). Furthermore, the proportion of school attenders with at least a grade 12 level of education was markedly higher among women with an incident pregnancy (67.9%) than women with a prevalent pregnancy (50.3%). Unemployment rates were similar across exposure categories with an average rate of 61.9%.
Table 1.
Demographic and baseline clinical characteristics of the study sample.
| Women with no recorded pregnancy | Women with incident pregnancy | Women with prevalent pregnancy | Total | |
| n (col %) | n (col %) | n (col %) | n (col %) | |
| N = 2403 | N = 1953 | N = 1950 | N = 6306 | |
| Average follow-up time, mean years (SD) | 1.6 (0.6) | 1.7 (0.5) | 1.5 (0.6) | 1.6 (0.6) |
| Race | ||||
| Black | 2334 (98.3) | 1917 (98.6) | 1896 (98.6) | 6147 (98.5) |
| Other | 41 (1.7) | 28 (1.4) | 27 (1.4) | 96 (1.5) |
| Age at delivery/baseline | ||||
| Under 25 | 289 (12.0) | 120 (6.1) | 323 (16.6) | 732 (11.6) |
| 25–29.9 | 669 (27.8) | 518 (26.5) | 689 (35.3) | 1876 (29.7) |
| 30–39.9 | 1327 (55.2) | 1223 (62.6) | 867 (44.5) | 3417 (54.2) |
| 40–49.9 | 118 (4.9) | 91 (4.7) | 71 (3.6) | 280 (4.4) |
| Highest level of education at ART initiation | ||||
| Primary school | 213 (9.2) | 143 (7.6) | 121 (6.4) | 477 (7.9) |
| Some secondary school | 528 (22.8) | 260 (13.8) | 525 (27.9) | 1313 (21.6) |
| Grade 12 completed | 971 (42.0) | 801 (42.7) | 627 (33.4) | 2399 (39.5) |
| Postmatric | 48 (2.1) | 53 (2.8) | 27 (1.4) | 128 (2.1) |
| No schooling | 552 (23.9) | 621 (33.1) | 580 (30.9) | 1753 (28.9) |
| Employment status at ART initiation | ||||
| Employed | 838 (36.8) | 680 (36.1) | 770 (41.9) | 2288 (38.1) |
| Unemployed | 1441 (63.2) | 1203 (63.9) | 1069 (58.1) | 3713 (61.9) |
| ART site | ||||
| Clinic in hospital complex | 1055 (43.9) | 1037 (53.1) | 510 (26.2) | 2602 (41.3) |
| Local primary care clinic | 949 (39.5) | 438 (22.4) | 593 (30.4) | 1980 (31.4) |
| NGO-run clinic | 398 (16.6) | 478 (24.5) | 847 (43.4) | 1723 (27.3) |
| Initial first-line ART regimen | ||||
| 3TC/FTC + TDF + EFV/NVP | 753 (31.3) | 309 (15.8) | 843 (43.2) | 1905 (30.2) |
| ZDV + 3TC + EFV/NVP | 146 (6.1) | 112 (5.7) | 355 (18.2) | 613 (9.7) |
| d4T + 3TC + EFV/NVP | 1491 (62.0) | 1530 (78.3) | 751 (38.5) | 3772 (59.8) |
| 3TC + ABC + EFV/NVP | 13 (0.5) | 2 (0.1) | 1 (0.1) | 16 (0.3) |
| ART regimen changes by the time of delivery (or equivalent matched time) | ||||
| First-line regimen preserved | 1927 (80.2) | 1049 (53.7) | 1826 (93.6) | 4802 (76.2) |
| First-line drug substitution | 346 (14.4) | 619 (31.7) | 50 (2.6) | 1015 (16.1) |
| Switched to second-line ART | 56 (2.3) | 219 (11.2) | 15 (0.8) | 290 (4.6) |
| Treatment interrupted | 74 (3.1) | 66 (3.4) | 59 (3.0) | 199 (3.2) |
| Among those who interrupted ART | ||||
| Overall length of treatment interruption, median months (IQR) | 12.7 (6.6–21.2) | 5.8 (3.9–11.4) | 5.7 (2.7–14.1) | 8.0 (4.1–16.2) |
| Period of ART interruption in pregnancy, median months (IQR) | 4.6 (1.4–9.0) | 0.6 (0–4.5) | 0 (0–0.5) | 1.0 (0–5.0) |
| Period of interruption after delivery, median months (IQR) | 6.3 (2.4–13.6) | 4.7 (3.0–7.3) | 6.3 (2.0–14.8) | 5.4 (2.4–12.8) |
| New regimen after treatment interruption | ||||
| 3TC/FTC + TDF + EFV/NVP | 32 (43.2) | 18 (27.7) | 25 (42.4) | 75 (37.9) |
| ZDV + 3TC + EFV/NVP | 3 (4.1) | 9 (13.9) | 6 (10.2) | 18 (9.1) |
| d4T + 3TC + EFV/NVP | 25 (33.8) | 19 (29.2) | 22 (37.3) | 66 (33.3) |
| Second-line (three ARVs with LPVr/ATVr) | 6 (8.1) | 16 (24.6) | 5 (8.5) | 27 (13.6) |
| Other | 8 (10.8) | 3 (4.6) | 1 (1.7) | 12 (6.1) |
| Time on ART before delivery (or equivalent matched time) | ||||
| 3 months or less | 563 (23.4) | 0 | 1390 (71.3) | 1953 (31.0) |
| 4–6 months | 432 (18.0) | 0 | 440 (22.6) | 872 (13.8) |
| 7–12 months | 428 (17.8) | 163 (8.3) | 120 (6.2) | 711 (11.3) |
| 13–24 months | 527 (21.9) | 862 (44.1) | 0 | 1389 (22.0) |
| 25 months or longer | 453 (18.9) | 928 (47.5) | 0 | 1381 (21.9) |
| BMI up to 3 months before or after delivery (or equivalent matched time) | ||||
| Underweight | 135 (7.5) | 21 (1.2) | 19 (1.1) | 175 (3.3) |
| Normal | 876 (48.7) | 570 (31.7) | 553 (31.6) | 1999 (37.4) |
| Overweight | 499 (27.8) | 719 (39.9) | 697 (39.8) | 1915 (35.8) |
| Obese | 288 (16.0) | 490 (27.2) | 483 (27.6) | 1261 (23.6) |
| Anaemic up to 3 months before or after delivery (or equivalent matched time) | ||||
| No | 1103 (70.4) | 1075 (73.7) | 777 (49.5) | 2955 (64.3) |
| Yes | 463 (29.6) | 383 (26.3) | 794 (50.5) | 1640 (35.7) |
| CD4+ cell count up to 3 months before or after delivery (or equivalent matched time) | ||||
| Under 200 | 464 (25.3) | 148 (9.9) | 462 (28.2) | 1074 (21.6) |
| 200–349 | 579 (31.6) | 420 (28.2) | 555 (33.9) | 1554 (31.3) |
| 350 or higher | 789 (43.1) | 923 (61.9) | 622 (38.0) | 2334 (47.0) |
| Unsuppressed viral load (≥50 copies/ml) up to 3 months before or after delivery (or equivalent matched time) | ||||
| No | 1149 (63.1) | 1054 (64.6) | 913 (55.7) | 3116 (61.2) |
| Yes | 672 (36.9) | 577 (35.4) | 726 (44.3) | 1975 (38.8) |
| Viral load ≥400 copies/ml up to 3 months before or after delivery (or equivalent matched time) | ||||
| No | 1464 (80.4) | 1359 (83.3) | 1226 (74.8) | 4049 (79.5) |
| Yes | 357 (19.6) | 272 (16.7) | 413 (25.2) | 1042 (20.5) |
Baseline/equivalent matched time: end of first pregnancy for exposed and equivalent time on ART for matched nonexposed women. 3TC, lamivudine; ABC, abacavir; ARV, antiretroviral; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; LPVr, lopinavir; NGO, nongovernmental organizations; NVP, nevirapine; IQR, interquartile range; TDF, tenofovir; ZDV, zidovudine.
Baseline clinical characteristics of study sample
Overall, 30.2% of women initiated ART on a combination of 3TC/FTC, TDF and EFV or NVP, only 9.7% were initiated on a regimen of ZDV, 3TC and EFV/NVP, and 59.8% were initiated on d4T, 3TC and EFV/NVP. Although the majority (93.6%) of women with prevalent pregnancy and 80.2% of unexposed women remained on their first regimen throughout the pregnancy, only 53.7% of women with incident pregnancies were still on their first regimen with 11.2% permanently switched to second-line ART at delivery. Overall, 39.8% of postpartum (exposed) women had detectable viral RNA (>50 copies/ml) at delivery. Anaemia at delivery was most common in the prevalent pregnancy group (29.6, 26.3 and 50.5% among unexposed women, incident pregnancy and prevalent pregnancy, respectively). Overall, 47.0% had baseline CD4+ at least 350 cells/μl, 43.1% among unexposed women, 61.9% among the incident pregnancy group and 38.0% among prevalent pregnancy group.
Predictors of virologic failure 24 months postpartum
Table 2 and Fig. 1 show rates and predictors of virologic failure in the study sample. Study patients were followed up for an average of 1.6 years (SD 0.6). Overall, 510 (8.1%) women experienced virologic failure in the 10 091.5 person-years of observation [incidence rate 5.1/100 person-years, 95% confidence interval (CI): 4.6–5.5]. Among exposed (postpartum) women, 9.4% experienced a virologic failure compared with 8.2% in the unexposed group. The crude incidence rate of failure was highest among the incident pregnancy group (5.7/100 person-years, 95% CI: 5.0–6.6) and lower among the prevalent pregnancy group (4.2/100 person-years, 95% CI: 3.5–5.0). Although only 16.9% of women with unsuppressed baseline viral load went on to fail in the postpartum period, they were 42.4 times more likely to fail (95% CI: 20.2–89.0) compared with those who were suppressed at delivery.
Table 2.
Predictors of virologic failure 24 months postpartum among HIV-positive women receiving antiretroviral therapy in Johannesburg, South Africa.
| Virologic failure | Person-years | Rate per100 PY (95% CI) | Total sample | Women with incident pregnancy | Women with prevalent pregnancy | ||
| Crude HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | ||||
| Total sample | 510 (8.1) | 10 091.5 | 5.1 (4.6–5.5) | ||||
| Timing of pregnancy | |||||||
| No recorded pregnancy | 198 (8.2) | 3870.1 | 5.1 (4.5–5.9) | 1 | 1 | ||
| Incident pregnancy | 188 (9.6) | 3284.9 | 5.7 (5.0–6.6) | 1.1 (0.9–1.4) | 1.3 (1.0–1.8) | ||
| Prevalent pregnancy | 124 (6.4) | 2936.4 | 4.2 (3.5–5.0) | 0.8 (0.7–1.0) | 0.7 (0.5–1.0) | ||
| Age at baseline | |||||||
| Under 25 | 73 (10.0) | 1085.6 | 6.7 (5.3–8.5) | 1 | 1 | 1 | 1 |
| 25–29.9 | 166 (8.8) | 2947.8 | 5.6 (4.8–6.6) | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.9 (0.5–1.7) | 0.6 (0.3–1.3) |
| 30–39.9 | 257 (7.5) | 5583.2 | 4.6 (4.1–5.2) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.6 (0.4–1.1) | 0.9 (0.5–1.7) |
| 40–49.9 | 14 (5.0) | 473.2 | 3.0 (1.8–5.0) | 0.4 (0.2–0.8) | 0.3 (0.1–0.7) | 0.2 (0.1–0.8) | 0.4 (0.1–1.7) |
| Highest level of education at ART initiation | |||||||
| Primary school | 43 (9.0) | 744.2 | 5.8 (4.3–7.8) | 1 | |||
| Some secondary school | 96 (7.3) | 1974.3 | 4.9 (4.0–5.9) | 0.8 (0.6–1.2) | |||
| Grade 12 completed | 203 (8.5) | 3875.3 | 5.2 (4.6–6.0) | 0.9 (0.7–1.3) | |||
| Postmatric | 11 (8.6) | 210.5 | 5.2 (2.9–9.4) | 0.9 (0.5–1.8) | |||
| No schooling or unknown | 141 (8.0) | 2908.2 | 4.8 (4.1–5.7) | 0.8 (0.6–1.2) | |||
| Employment status at ART initiation | |||||||
| Employed | 160 (7.0) | 3777.8 | 4.2 (3.6–4.9) | 1 | 1 | 1 | 1 |
| Unemployed | 333 (9.0) | 5847.9 | 5.7 (5.1–6.3) | 1.3 (1.1–1.6) | 1.2 (0.9–1.5) | 1.0 (0.7–1.4) | 1.2 (0.8–1.9) |
| ART site | |||||||
| Clinic in hospital complex | 219 (8.4) | 4258.7 | 5.1 (4.5–5.9) | 1 | |||
| Local primary care clinic | 167 (8.4) | 3012.6 | 5.5 (4.8–6.5) | 1.1 (0.9–1.3) | |||
| NGO-run clinic | 124 (7.2) | 2819.2 | 4.4 (3.7–5.2) | 0.9 (0.7–1.1) | |||
| First ever ART regimen | |||||||
| TDF + 3TC/FTC + EFV/NVP | 130 (6.8) | 2845.0 | 4.6 (3.8–5.4) | 1 | 1 | 1 | 1 |
| ZDV + 3TC + EFV/NVP | 44 (7.2) | 1001.8 | 4.4 (3.3–5.9) | 1.0 (0.7–1.3) | 1.0 (0.7–1.6) | 0.9 (0.3–3.0) | 1.2 (0.6–2.3) |
| d4T + 3TC + EFV/NVP | 332 (8.8) | 6221.6 | 5.3 (4.8–5.9) | 1.2 (0.9–1.4) | 0.7 (0.4–1.2) | 2.3 (1.2–4.4) | 0.2 (0.1–0.8) |
| ABC + 3TC + EFV/NVP | 4 (25.0) | 23.1 | 17.3 (6.5–46.1) | 3.8 (1.4–10.3) | 0.9 (0.2–3.5) | 5.5 (1.1–27.0) | – |
| ART regimen changes by delivery/baseline | |||||||
| First-line regimen preserved | 377 (8.0) | 7600.6 | 5.0 (5.9–5.5) | 1 | 1 | 1 | 1 |
| First-line drug substitution | 74 (7.3) | 1730.9 | 4.3 (3.4–5.4) | 0.9 (0.7–1.1) | 0.8 (0.6–1.2) | 1.0 (0.7–1.5) | 0.6 (0.1–2.5) |
| Switched to second-line ART | 27 (9.3) | 446.4 | 6.0 (4.1–8.8) | 1.2 (0.8–1.8) | 0.8 (0.5–1.3) | 1.0 (0.6–1.7) | |
| Treatment interrupted | 32 (16.1) | 313.6 | 10.2 (7.2–14.4) | 2.1 (1.4–2.9) | 1.1 (0.7–1.9) | 1.2 (0.5–2.6) | 0.7 (0.2–2.9) |
| Time on ART before delivery/baseline | |||||||
| 3 months or less | 136 (7.0) | 2973.5 | 4.6 (3.9–5.4) | 1 | 1 | 1 | |
| 4–6 months | 61 (7.0) | 1333.2 | 4.6 (3.6–5.9) | 1.0 (0.8–1.3) | 1.6 (1.1–2.3) | 1.8 (1.0–3.1) | |
| 7–12 months | 59 (8.3) | 1160.5 | 5.1 (3.9–6.6) | 1.0 (0.7–1.4) | 1.4 (0.9–2.2) | 1 | 3.0 (1.4–6.2) |
| 13–24 months | 147 (10.6) | 2300.8 | 6.4 (5.4–7.5) | 1.1 (0.8–1.5) | 2.0 (1.3–3.1) | 1.8 (1.0–3.5) | |
| 25 months or longer | 107 (7.7) | 2323.5 | 4.6 (3.8–5.6) | 1.4 (1.1–1.8) | 1.7 (1.1–2.9) | 1.5 (0.7–2.9) | |
| BMI (up to 3 months before or after baseline) | |||||||
| Underweight | 15 (8.6) | 265.9 | 5.6 (3.4–9.4) | 1.1 (0.6–1.8) | |||
| Normal | 171 (8.6) | 3190.3 | 5.4 (4.6–6.2) | 1 | |||
| Overweight | 138 (7.2) | 3076.3 | 4.5 (3.8–5.3) | 0.8 (0.7–1.1) | |||
| Obese | 104 (8.2) | 2017.9 | 5.2 (4.3–6.2) | 1.0 (0.8–1.2) | |||
| Anaemic (up to 3 months before or after delivery/baseline) | |||||||
| No | 227 (7.7) | 4867.3 | 4.7 (4.1–5.3) | 1 | 1 | 1 | 1 |
| Yes | 160 (9.8) | 2537.3 | 6.3 (5.4–7.4) | 1.4 (1.1–1.7) | 1.2 (0.9–1.5) | 1.6 (1.1–2.3) | 1.0 (0.7–1.6) |
| CD4+ cell count (up to 3 months before or after delivery/baseline) | |||||||
| Under 200 | 149 (13.9) | 1637.7 | 9.1 (7.7–10.7) | 1 | 1 | 1 | 1 |
| 200–349 | 153 (9.8) | 2447.0 | 6.3 (5.3–7.3) | 0.7 (0.5–0.9) | 0.6 (0.4–0.9) | 1.1 (0.7–1.7) | 0.8 (0.5–1.4) |
| 350 or higher | 129 (5.5) | 3740.7 | 3.4 (2.9–4.1) | 0.4 (0.3–0.5) | 0.2 (0.1–0.4) | 0.4 (0.3–0.7) | 0.5 (0.3–0.9) |
| CD4+ recovery from date of ART initiation to date of delivery | |||||||
| Decline/no change | 105 (9.5) | 1684.0 | 6.2 (5.1–7.5) | 1 | 1 | 1 | 1 |
| <99 cell/μl increase | 85 (12.0) | 1098.3 | 7.7 (6.3–9.6) | 1.2 (0.9–1.7) | 1.5 (1.1–2.1) | 1.1 (0.5–2.1) | 1.6 (0.8–3.0) |
| 100–200 cell/ μl increase | 95 (8.8) | 1698.3 | 5.6 (4.6–6.8) | 0.9 (0.7–1.2) | 1.2 (0.8–1.7) | 0.7 (0.4–1.4) | 1.3 (0.6–2.6) |
| >200 cell/μl increase | 146 (7.0) | 3344.6 | 4.4 (3.7–5.1) | 0.7 (0.5–0.9) | 1.3 (0.9–2.0) | 1.0 (05–2.0) | 1.4 (0.6–3.4) |
| Unsuppressed viral load (≥50 copies/ml) up to 3 months before or after delivery (or equivalent matched time) | |||||||
| No | 97 (3.1) | 5080.1 | 1.9 (1.6–2.3) | 1 | 1 | 1 | 1 |
| Yes | 333 (16.9) | 2908.6 | 11.4 (10.2–12.7) | 6.0 (4.8–7.5) | 42.4 (20.2–89.0) | 6.1 (4.2–8.9) | 74.4 (11.6–477.5) |
3TC, lamivudine; ABC, abacavir; aHR, adjusted hazard ratio; ART, antiretroviral therapy; CI, confidence interval; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; HR, hazard ratio; NGO, nongovernmental organizations; NVP, nevirapine; PY, person-years; TDF, tenofovir; ZDV, zidovudine.
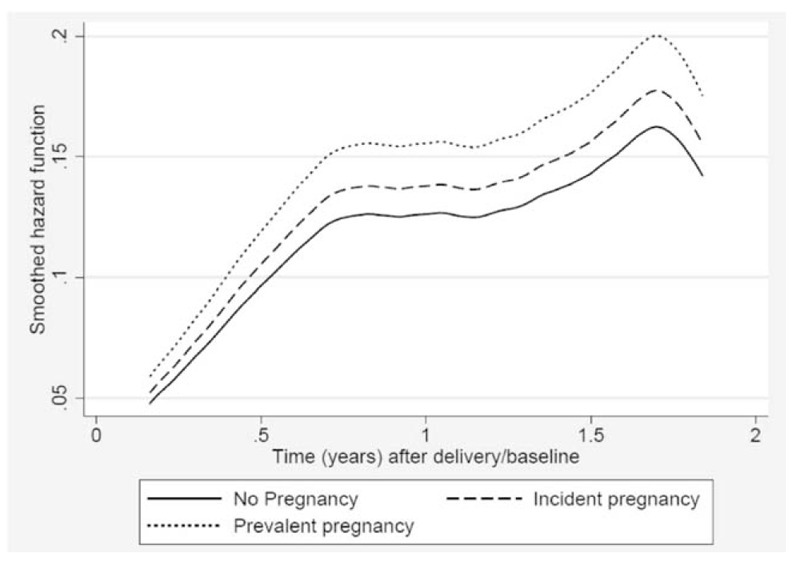
Fig. 1.

Instantaneous probability of virologic failure during the first 24 months postpartum among HIV-positive women on antiretroviral therapy in Johannesburg, South Africa.
After adjusting for baseline demographics and baseline viral suppression, women in the incident pregnancy group were at higher risk of virologic failure compared with unexposed women [adjusted hazard ratio (aHR) 1.3, 95% CI: 1.0–1.8] and to those with a prevalent pregnancy at ART initiation (aHR 1.8, 95% CI: 1.1–2.7), particularly among those with baseline CD4+ cell count less than 350 cells/μl. The hazard for virologic failure was highest in the first 12 months postpartum and decreased with time (aHR 1.8, 95% CI: 1.1–3.0) (Fig. 1). Overall, the risk of failure decreased with older age but increased with longer duration on ART before delivery/baseline.
When the model was stratified by pregnancy group (incident vs. prevalent pregnancy), we found that, although baseline CD4+ cell count and time on ART remained important predictors across pregnancy groups, age was only influential among women in the incident pregnancy group. Although not statistically significant, women who conceived on ART and delivered after receiving ART for longer than 12 months (13–24 months, aHR 1.8, 95% CI: 1.0–3.5) were more likely to experience a failure compared with those who initiated ART less than 12 months before delivery. Furthermore, women who initiated ART on regimens that included d4T (aHR 2.3, 95% CI: 1.2–4.4) or ABC (aHR 5.5, 95% CI: 1.1–27.0) were more likely to fail compared with patients who were receiving TDF-based regimens. Increased risk of postpartum virologic failure was also associated with being anaemic at baseline (aHR 1.6, 95% CI: 1.1–2.3).
In the prevalent pregnancy group, longer antenatal ART duration also increased the risk of postpartum virologic failure (aHR 3.0, 95% CI: 1.4–6.2 for those with ≥7 months of antenatal ART compared with ≤3 months). Similarly, to the incident pregnancy group, among the prevalent pregnancy group, low CD4+ cell count at delivery predicted postpartum virologic failure (aHR 0.5, 95% CI: 0.3–0.9 for those with baseline CD4+ ≥ 350 cells/μl compared with CD4+ < 200 cell/μl).
Predictors of being loss to follow-up by 24 months postpartum
Table 3 presents rates and predictors of becoming LTFU by 24 months postpartum. When the LTFU analysis was restricted to women with at least one viral load measure on record, 1482 (23.5%) had become LTFU at a rate of 14.7/100 person-years (95% CI: 14.0–15.5) compared with 29.3% at a rate of 19.4/100 person-years (95% CI: 18.6–20.3) when all 6825 women with at least one scheduled visit after delivery were included. To ensure comparability between the sample in the virologic failure model, all further LTFU regression analyses were conducted on the restricted sample of 6306 representing 10 077.4 person-years (mean 1.5 years, SD 0.7) of observation time. The proportion of LTFU was lower among women with incident pregnancies (19.5% compared with 27.7% in the prevalent pregnancy group). Women in the prevalent pregnancy group had a slightly higher risk of becoming LTFU compared with unexposed women (aHR 1.2, 95% CI: 1.0–1.5) and a similar risk as women who conceived on ART (aHR 0.9, 95% CI: 0.7–1.1). The hazard of becoming LTFU increased sharply in the first 12 postpartum months and remained relatively stable until about 18 months postpartum (Fig. 2).
Table 3.
Predictors of being lost to follow-up at 24 months postpartum among HIV-positive women receiving antiretroviral therapy in Johannesburg, South Africa.
| LTFU | Person-years | Rate per 100 PY (95% CI) | Total sample | Women with incident pregnancy | Women with prevalent pregnancy | ||
| Crude HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | ||||
| Total sample | 1482 (23.5) | 10077.4 | 14.7 (14.0–15.5) | ||||
| Timing of pregnancy | |||||||
| No recorded pregnancy | 561 (23.3) | 3873.6 | 14.5 (13.3–15.7) | 1 | 1 | ||
| Incident pregnancy | 380 (19.5) | 3281.5 | 11.6 (10.5–12.8) | 0.8 (0.7–0.9) | 1.0 (0.8–1.3) | ||
| Prevalent pregnancy | 541 (27.7) | 2922.3 | 18.5 (17.0–20.1) | 1.3 (1.2–1.5) | 1.2 (1.0–1.5) | ||
| Age at baseline | |||||||
| Under 25 | 216 (29.5) | 1073.7 | 20.1 (17.6–23.0) | 1 | 1 | 1 | 1 |
| 25–29.9 | 462 (24.6) | 2945.1 | 15.7 (14.3–17.2) | 0.8 (0.7–0.9) | 0.8 (0.7–1.0) | 1.3 (0.7–2.3) | 0.8 (0.6–1.2) |
| 30–39.9 | 741 (21.7) | 5591.9 | 13.3 (12.3–14.2) | 0.6 (0.6–0.7) | 0.7 (0.6–0.9) | 1.1 (0.6–1.8) | 0.7 (0.5–1.0) |
| 40–49.9 | 63 (22.5) | 464.9 | 13.6 (10.6–17.3) | 0.7 (0.5–0.9) | 0.9 (0.6–1.3) | 1.0 (0.4–2.2) | 0.9 (0.5–1.6) |
| Highest level of education at ART initiation | |||||||
| Primary school | 115 (24.1) | 750.7 | 15.3 (12.8–18.4) | 1 | 1 | 1 | 1 |
| Some secondary school | 414 (31.5) | 2006.4 | 20.6 (18.7–22.7) | 1.4 (1.1–1.7) | 1.5 (1.1–2.1) | 1.5 (0.8–2.9) | 1.6 (1.0–2.7) |
| Grade 12 completed | 497 (20.7) | 3884.2 | 12.8 (11.7–14.0) | 0.8 (0.7–1.0) | 0.9 (0.7–1.2) | 0.8 (0.5–1.4) | 0.8 (0.5–1.4) |
| Postmatric | 25 (19.5) | 208.7 | 12.0 (8.1–17.7) | 0.8 (0.5–1.2) | 1.1 (0.6–2.0) | 1.0 (0.4–2.5) | 1.2 (0.4–3.2) |
| No schooling or unknown | 372 (21.2) | 2855.9 | 13.0 (11.8–14.4) | 0.8 (0.7–1.0) | 1.5 (1.1–2.0) | 1.4 (0.8–2.4) | 1.5 (0.9–2.6) |
| Employment status at ART initiation | |||||||
| Employed | 491 (21.5) | 3784.9 | 13.0 (11.9–14.2) | 1 | 1 | 1 | 1 |
| Unemployed | 894 (24.1) | 5829.3 | 15.3 (14.4–16.4) | 1.2 (1.1–1.3) | 1.2 (1.0–1.4) | 1.1 (0.8–1.5) | 1.2 (1.0–1.6) |
| ART site | |||||||
| Clinic in hospital complex | 546 (21) | 4232.1 | 12.9 (11.9–14.0) | 1 | 1 | 1 | 1 |
| Local primary care clinic | 595 (30.1) | 3030.6 | 19.6 (18.1–21.3) | 1.6 (1.4–1.7) | 1.3 (1.1–1.5) | 1.5 (1.1–2.1) | 1.1 (0.7–1.7) |
| NGO-run clinic | 341 (19.8) | 2813.7 | 12.1 (10.9–13.5) | 0.9 (0.8–1.1) | 0.6 (0.5–0.7) | 0.6 (0.4–1.0) | 0.8 (0.4–1.5) |
| First ever ART regimen | |||||||
| 3TC/FTC + TDF + EFV/NVP | 634 (33.3) | 2882.8 | 22.0 (20.3–23.8) | 1 | 1 | 1 | 1 |
| ZDV + 3TC + EFV/NVP | 173 (28.2) | 977.8 | 17.7 (15.2–20.5) | 0.8 (0.7–0.9) | 0.9 (0.7–1.1) | 1.2 (0.6–2.4) | 1.3 (0.9–1.9) |
| d4T + 3TC + EFV/NVP | 672 (17.8) | 6192.0 | 10.9 (10.1–11.7) | 0.5 (0.4–0.5) | 1.1 (0.8–1.6) | 1.4 (0.6–3.1) | 1.7 (0.9–3.1) |
| 3TC + ABC + EFV/NVP | 3 (18.8) | 24.8 | 12.1 (3.9–37.5) | 0.5 (0.2–1.7) | 1.1 (0.7–1.7) | 68.3 (6.9–673.2) | |
| ART regimen changes by delivery/baseline | |||||||
| First-line regimen preserved | 1126 (25.3) | 6973.8 | 16.1 (15.2–17.1) | 1 | 1 | 1 | 1 |
| First-line drug substitution | 132 (17.6) | 1263.8 | 10.4 (8.8–12.4) | 0.6 (0.5–0.8) | 0.9 (0.7–1.1) | 0.8 (0.6–1.1) | 0.7 (0.3–1.5) |
| Switched to second-line ART | 180 (19.8) | 1514.6 | 11.9 (10.2–13.8) | 0.7 (0.6–0.8) | 1.1 (0.8–1.6) | 1.1 (0.7–1.7) | 0.3 (0.04–2.2) |
| Treatment Interrupted | 44 (22.1) | 325.2 | 13.5 (10.1–18.2) | 0.8 (0.6–1.1) | 1.1 (0.7–1.7) | 1.2 (0.6–2.4) | 2.0 (0.9–4.3) |
| Time on ART before delivery/baseline | |||||||
| 3 months or less | 553 (28.3) | 2943.8 | 18.8 (17.3–20.4) | 1 | 1 | 1 | |
| 4–6 months | 228 (26.1) | 1341.4 | 17.0 (14.9–19.4) | 0.9 (0.8–1.1) | 1.0 (0.8–1.2) | 0.8 (0.6–1.1) | |
| 7–12 months | 155 (21.8) | 1150.6 | 13.5 (11.5–15.8) | 0.7 (0.6–0.8) | 0.8 (0.6–1.1) | 1 | 0.4 (0.2–0.7) |
| 13–24 months | 269 (19.4) | 2317.1 | 11.6 (10.3–13.1) | 0.6 (0.5–0.7) | 0.8 (0.5–1.2) | 0.9 (0.6–1.4) | |
| 25 months or longer | 277 (20.1) | 2324.5 | 11.9 (10.6–13.4) | 0.6 (0.5–0.7) | 0.7 (0.4–1.2) | 0.9 (0.6–1.5) | |
| BMI (up to 3 months before or after baseline) | |||||||
| Underweight | 39 (22.3) | 264.9 | 14.7 (10.8–20.2) | 1.0 (0.7–1.4) | |||
| Normal | 464 (23.2) | 3175.4 | 14.6 (13.3–16.0) | 1 | |||
| Overweight | 457 (23.9) | 3063.6 | 14.9 (13.6–16.3) | 1.0 (0.9–1.2) | |||
| Obese | 324 (25.7) | 2031.5 | 15.9 (14.3–17.8) | 1.1 (0.9–1.3) | |||
| Anaemic (up to 3 months before or after delivery/baseline) | |||||||
| No | 611 (20.7) | 4843.7 | 12.6 (11.7–13.7) | 1 | 1 | 1 | 1 |
| Yes | 420 (25.6) | 2554.1 | 16.4 (14.9–18.1) | 1.3 (1.2–1.5) | 1.1 (1.0–1.3) | 1.1 (0.8–1.5) | 1.1 (0.9–1.4) |
| CD4+ cell count (up to 3 months before or after delivery/baseline) | |||||||
| Under 200 | 278 (25.9) | 1665.0 | 16.7 (14.8–18.8) | 1 | 1 | 1 | 1 |
| 200–349 | 372 (23.9) | 2455.5 | 15.1 (13.7–16.8) | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | 0.9 (0.6–1.4) | 0.8 (0.6–1.1) |
| 350 or higher | 514 (22.0) | 3703.5 | 13.9 (12.7–15.1) | 0.8 (0.7–1.0) | 0.9 (0.7–1.0) | 0.7 (0.5–1.1) | 1.1 (0.8–1.5) |
| Unsuppressed viral load (≥50 copies/ml) up to 3 months before or after delivery (or equivalent matched time) | |||||||
| No | 685 (22.0) | 4972.1 | 13.8 (12.8–14.8) | 1 | 1 | 1 | 1 |
| Yes | 505 (25.6) | 3059.5 | 16.5 (15.2–18.0) | 1.2 (1.1–1.4) | 1.5 (1.1–2.1) | 1.6 (1.2–2.1) | 0.9 (0.7–1.2) |
3TC, lamivudine; ABC, abacavir; aHR, adjusted hazard ratio; ART, antiretroviral therapy; CI, confidence interval; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; HR, hazard ratio; LTFU, loss to follow-up; NGO, nongovernmental organizations; NVP, nevirapine; PY, person-years; TDF, tenofovir; ZDV, zidovudine.
Fig. 2.
Instantaneous probability of becoming loss to follow-up during the first 24 months postpartum among HIV-positive women on antiretroviral therapy in Johannesburg, South Africa.
Women in the incident pregnancy group who received HIV care from an NGO clinic had a lower risk of becoming LTFU (aHR 0.6, 95% CI: 0.4–1.0) compared with those from hospital-based clinics, whereas those who were treated at a local primary care clinic were more likely to become LTFU (aHR 1.5, 95% CI: 1.1–2.1). However, having a baseline CD4+ cell count at least 350 cells/μl was associated with a slightly lower risk of becoming LTFU (hazard ratio 0.7, 95% CI: 0.5–1.1) compared with those with CD4+ less than 200 cells/μl at delivery. In addition, women who were unsuppressed at delivery were 60% more likely to become LTFU (95% CI: 1.2–2.1). In this group, the risk of postpartum LTFU was not associated with age, education, employment status or length of time on ART.
Among women in the prevalent pregnancy group, those who attended some secondary school were more likely to be lost compared with those who only attended primary school (aHR 1.6, 95 CI: 1.0–2.7), and being unemployed marginally increased the risk of becoming LTFU (aHR 1.2, 95% CI: 1.0–1.6). Notably, having received at least 7 months of antenatal ART compared with 3 or less months was associated with a lower risk of becoming LTFU (aHR 0.4, 95% CI: 0.2–0.7). CD4+ cell count and viral suppression at delivery did not predict LTFU in this group.
Discussion
In this article, we show that South African women who conceive while receiving ART are more likely to experience a virologic failure postpartum compared with nonpregnant women and those who initiate ART in pregnancy. Women who initiate ART in pregnancy have a slightly higher risk of becoming lost from care after delivery compared with nonpregnant women. However, there was no difference in the risk of becoming LTFU by 24 months when compared with the incident pregnancy group.
Similar to findings from other sub-Saharan African settings, we found that 39.8% of postpartum women had detectable viral RNA at delivery, and 9.4% experienced a virologic failure in the postpartum period [18–20]. Viral suppression rates decrease gradually from nonpregnant state to postpartum period [18]. Although the PMTCT programme coverage in South Africa has been successful in nearly eliminating MTCT, postpartum virologic suppression is the key for further reductions of postpartum transmission rates [20].
Predictors of postpartum virologic failure among women who conceived on ART included having a CD4+ less than 350 cells/μl at delivery, longer periods on ART before delivery and possible prior experience with adverse drug reaction (initiating ART on regimens including ABC or d4T). Low CD4+ cell count at delivery points to declining health during pregnancy and perhaps even at conception [21–23]. Westreich et al. showed an increased risk of virologic failure in pregnancy among women who conceived on ART, suggesting persistently poorer viral control during pregnancy as well as postpartum [24,25]. The incident pregnancy group was more likely to have had prior ART adherence challenges as demonstrated by the higher proportion switched to second-line ART by the time of delivery compared with the prevalent pregnancy group. This higher risk of failure may also be because of experiences of drug toxicities and treatment fatigue as a consequence of being on ART for longer. Longer period of continuous viral suppression have been associated with lower risk of virologic failure [26,27]. Treatment-experienced women with a longer period of good adherence may be better prepared to handle adherence challenges postpartum. However, women who are already prone to nonadherence may struggle even more postpartum.
In contrast, a similar analysis among HIV-positive women in the United Kingdom found that women who initiated ART in pregnancy were more likely to experience viral rebound at 3 months postpartum compared with those who conceived on ART [27]. The difference may be in improved monitoring of patients on ART before and during pregnancy. Our findings highlight the need to strengthen adherence counselling and viral load monitoring during pregnancy to help identify women in need of additional adherence support postpartum.
The overall proportion of women who became LTFU by 24 months postpartum is considerably lower than previously reported figures in South African settings [28,29]. Over 20% of the original sample was excluded because of missing postpartum viral load data. This may have biased the analysis towards women who are more inclined to enter and remain in postpartum care at the selected clinics. Our results also potentially underestimate rates of programmatic losses from care and virologic failure, which does not bode well for the postpartum risk of MTCT [30]. This is problematic as the data set only covered the pre-Option B+ and ‘treat-all’ policy era (pre-2015), when only women with low CD4+ cell count were initiated on lifelong ART for their own health. Healthier women initiated on lifelong ART under the Option B+ programme and the ‘treat-all’ policy may be even less inclined to adhere and remain in care in the postpartum period [31–34].
Although there was no difference in the risk of becoming LTFU between the incident and prevalent pregnancy groups, the difference in predictors of LTFU could be attributed to the phase of ART experience. The incident pregnancy group most likely consisting of ‘survivors’ of the initial wave of losses after ART initiation and the prevalent pregnancy group are in the early stages of their ART experience. Losses among treatment-experienced women could be related to health system factors such as patient management approaches or monitoring data quality. It is possible that women with low CD4+ cell count and those who were unsuppressed at delivery were sicker, and the referrals to higher level healthcare facilities or even deaths were poorly recorded. In addition, our analysis is limited by the fact that the data consisted of routinely collected medical records with unevenly distributed proportions of missing information. Therefore, further research is needed to understand the differences in LTFU across types of sites to further elaborate on possible retention/data system strengthening interventions for the PMTCT programme.
Losses among the prevalent pregnancy group are associated with personal factors (education, unemployment) that impact on engagement with HIV care both during and after pregnancy. However, women who initiated antenatal ART in the first trimester of pregnancy were more likely to be retained compared with women who initiated in the last trimester or at delivery. This highlights an additional benefit of early ART initiation during pregnancy. Longer ART exposure also ensures that women receive repeat counselling and acquire a better understanding of the risks associated with disengaging from care.
In conclusion, the results highlight the need to strengthen adherence-monitoring efforts during pregnancy to identify women, particularly treatment-experienced women, who will require further support in the postpartum phase of the PMTCT programme. Monitoring and evaluation data must account for transfers across health facilities to better inform retention and follow-up strategies, and for accurate determinations of LTFU rates among postpartum women. Finally, increasing the demand for early ANC and ART initiation among treatment-naive HIV-positive women will also have a positive impact on postpartum LTFU which will, in turn, contribute to the goals of reducing transmission risks and promoting the health of new mothers.
Acknowledgements
The authors gratefully acknowledge the directors and staff of the study sites as well as Right to Care, the Non-Governmental Organization supporting the study sites through a partnership of the South African National and Gauteng provincial Department of Health with the United States Agency for International Development (USAID). Most of all we thank the patients attending the clinics for their continued trust in the treatment provided at the clinic.
Funding was provided by USAID under the terms of Cooperative Agreement USAID-674-A-12-00029 to the Health Economics and Epidemiology Research Office and Cooperative Agreement 674-A-00-09-00018-00 to Boston University. The contents of this article are the responsibility of the authors and do not necessarily reflect the views of the funding agencies or participating clinics or patients. The funders had no role in the study design, collection, analysis and interpretation of the data, in manuscript preparation or the decision to publish.
Authors’ contributions: D.O. and T.S. conceptualized the analysis, analysed the data and drafted the article. A.B., L.L. and M.P.F. assisted in the analysis and contributed to the interpretation of the results as well as the article preparations.
Conflicts of interest
There are no conflicts of interest.
References
- 1.UN Joint Programme on HIV/AIDS (UNAIDS). The Gap report. 2013; Geneva: UNAIDS, Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. [Accessed 20 April 2017]. [Google Scholar]
- 2.South African National Department of Health. The 2012 national antenatal sentinel HIV & herpes simplex type-2 prevalence survey in South Africa. Pretoria: National Department of Health; 2012. [Google Scholar]
- 3.South African National Department of Health. National HIV and syphilis antenatal sero-prevalence survey in South Africa 2004. Pretoria: National Department of Health; 2004. [Google Scholar]
- 4.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press; 2014. [DOI] [PubMed] [Google Scholar]
- 5.South African National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria: National Department of Health; 2014. [Google Scholar]
- 6.Chi BH, Sinkala M, Stringer EM, Cantrell RA, Mtonga V, Bulterys M, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS 2007; 21:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffie PA, Ekouevi DK, Chaix ML, Tonwe-Gold B, Clarisse AB, Becquet R, et al. Maternal 12-month response to antiretroviral therapy following prevention of mother-to-child transmission of HIV type 1, Ivory Coast, 2003–2006. Clin Infect Dis 2008; 46:611–621. [DOI] [PubMed] [Google Scholar]
- 8.Westreich D, Eron J, Behets F, van der Horst C, Van Rie A. Survival in women exposed to single-dose nevirapine for prevention of mother-to-child transmission of HIV: a stochastic model. J Infect Dis 2007; 195:837–846. [DOI] [PubMed] [Google Scholar]
- 9.Chi B, Stringer JA, Moodley D. Antiretroviral drug regimens to prevent mother-to-child transmission of HIV: a review of scientific, program, and policy advances for sub-Saharan Africa. Curr HIV/AIDS Rep 2013; 10:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachega J, Uthman O, Anderson J, Peltzer K, Wampold S, Cotton M, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS 2012; 26:2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaz MJ, Barros SM, Palacios R, Senise JF, Lunardi L, Amed AM, et al. HIV-infected pregnant women have greater adherence with antiretroviral drugs than nonpregnant women. Int J STD AIDS 2007; 18:28–32. [DOI] [PubMed] [Google Scholar]
- 12.Henegar CE, Westreich DJ, Maskew M, Miller WC, Brookhart MA, Van Rie A. Effect of pregnancy and the postpartum period on adherence to antiretroviral therapy among HIV-infected women established on treatment. J Acquir Immune Defic Syndr 2015; 68:477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.South African National Department of Health. National policy on HIV preexposure prophylaxis (PrEP) and test and treat (T&T). Pretoria: South African National Department of Health; 2016. [Google Scholar]
- 14.WHO. Consolidated guidelines for the use antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed.Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 15.South African National Department of Health. National antiretroviral treatment guidelines. Pretoria: South African National Department of Health; 2004. [Google Scholar]
- 16.South African National Department of Health. South African antiretroviral treatment guidelines 2010. Pretoria: South African National Department of Health; 2010. [Google Scholar]
- 17.South African National Department of Health. South African antiretroviral treatment guidelines 2013. Pretoria: South African National Department of Health; 2013. [Google Scholar]
- 18.Matthews LT, Ribaudo HB, Kaida A, Bennett K, Musinguzi N, Siedner MJ. HIV-infected Ugandan women on antiretroviral therapy maintain HIV-1 RNA suppression across periconception, pregnancy, and postpartum periods. J Acquir Immune Defic Syndr 2016; 71:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill MM, Hoffman HJ, Bobrow EA, Mugwaneza P, Ndatimana D, Ndayisaba GF. Detectable viral load in late pregnancy among women in the Rwanda Option B+ PMTCT Program: enrollment results from the Kabeho Study. PLoS One 2016; 11:e0168671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myer L, Phillips TK, Hsiao N-Y, Zerbe A, Petro G, Bekker L-G, et al. Plasma viraemia in HIV-positive pregnant women entering antenatal care in South Africa. J Int AIDS Soc 2015; 18:20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takuva S, Maskew M, Brennan AT, Long L, Sanne I, Fox MP. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. J Int AIDS Society 2014; 17:18651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndlovu Z, Chirwa T, Takuva S. Incidence and predictors of recovery from anaemia within an HIV-infected South African Cohort, 2004–2010. Pan Afr Med J 2014; 19:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yotebieng M, Maskew M, Van Rie A. CD4 gain percentile curves for monitoring response to antiretroviral therapy in HIV-infected adults. AIDS 2015; 29:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westreich D, Cole SR, Nagar S, Maskew M, van der Horst C, Sanne I. Pregnancy and virologic response to antiretroviral therapy in South Africa. PLoS One 2011; 6:e22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westreich D, Evans D, Firnhaber C, Majuba P, Maskew M. Prevalent pregnancy, biological sex, and virologic response to antiretroviral therapy. J Acquir Immune Defic Syndr 2012; 60:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenblum M, Deeks SG, van der Laan M, Bangsberg DR. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One 2009; 4:e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huntington S, Thorne C, Newell M-L, Anderson J, Taylor GP, Pillay D, et al. The risk of viral rebound in the year after delivery in women remaining on antiretroviral therapy. AIDS 2015; 29:2269–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouse K, Pettifor A, Shearer K, Maskew M, Bassett J, Larson B, et al. Loss to follow-up before and after delivery among women testing HIV-positive during pregnancy in Johannesburg, South Africa. Trop Med Int Health 2013; 18:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips T, Thebus E, Bekker L-G, Mcintyre J, Abrams EJ, Myer L. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. J Int AIDS Soc 2014; 17:19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Psaros C, Remmert JE, Bangsberg DR, Safren SA, Smit JA. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: Falling off the cliff of the treatment cascade. Curr HIV/AIDS Rep 2015; 12:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, et al. Retention in care under universal antiretroviral therapy for HIV infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS 2014; 28:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieffer MP, Mattingly M, Giphart A, van de Ven R, Chouraya C, Walakira M, et al. Lessons learned from early implementation of option B+: the Elizabeth Glaser Pediatric AIDS Foundation experience in 11 African countries. J Acquir Immune Defic Syndr 2014; 67:S188–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puttkammer N, Domerçant JW, Adler M, Yuhas K, Myrtil M, Young P, et al. ART attrition and risk factors among Option B+ patients in Haiti: a retrospective cohort study. PLoS One 2017; 12:e0173123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancinelli S, Galluzzo CM, Andreotti M, Liotta G, Jere H, Sagno J-B, et al. Virological response and drug resistance 1 and 2 years post-partum in HIV-infected women initiated on life-long antiretroviral therapy in Malawi. AIDS Res Hum Retroviruses 2016; 32:737–742. [DOI] [PubMed] [Google Scholar]


