Abstract
Purpose
The aim of the study was to assess the effectiveness, tolerability, and safety of oral ketamine as an antidepressant treatment in adults with treatment-resistant depression.
Methods
We reviewed retrospective data on 22 patients with treatment-resistant depression, who failed at least 3 adequate antidepressant treatment trials and 1 adequate trial of repetitive transcranial magnetic stimulation; subsequently, they received open-label treatment with oral ketamine, commenced at a dose of 50 mg every 3 days, titrated up by 25 mg every 3 days, according to response and tolerability. The primary outcome measure was the Beck Depression Inventory II, which was used to rate subjective mood improvement at baseline and then at each follow-up visit. Data about adverse effects related to ketamine and a self-harm risk assessment were also obtained.
Findings
Over the course of treatment, 18% of the patients showed greater than 50% reduction in the Beck Depression Inventory II scores, 14% reported partial improvement in mood symptoms, while 45% had no response to ketamine and 23% showed a mild worsening in their depressive symptoms. The most frequent adverse effects were acute dissociation, dizziness, blurred vision, numbness and sedation. Neither serious adverse effects, nor any cases of abuse or dependence were observed.
Conclusions
Although this case series found oral ketamine to be safe and well tolerated, the findings also showed rather modest effectiveness of oral ketamine in treatment-resistant depression, with only approximately 30% reporting some benefit and approximately 70% reporting no change or worsening of mood. However, bearing in mind the limitations of this small, open-label case series, further exploration of the effectiveness of oral ketamine is warranted.
Key Words: depression, ketamine, case series, safety, tolerability
Treatment-resistant depression (TRD) is a serious, disabling illness with a significant impact on social and occupational outcomes.1 Given that at least one third of patients with major depressive disorder (MDD) do not achieve remission with conventional, monoaminergic antidepressant medications,2 new treatment strategies are urgently needed. The dissociative anesthetic, ketamine, has received increasing attention as a novel intervention in TRD, distinguished from other agents in part via its putative mechanism of action as an N-methyl-d-aspartate receptor antagonist and in part by reports of extremely rapid-onset of (<24 h) antidepressant action.3 Regarding pharmacology, ketamine has a relatively short elimination half-life of 2 to 3 hours and may be dosed intravenously, subcutaneously, intranasally, or orally, with oral bioavailability of approximately 20% to 25% and intranasal bioavailability of approximately 45%.4 Further details on the pharmacology, dosing, and adverse effects of ketamine are available in a recent review.5
Rapid antidepressant effects of ketamine in MDD have been recognized for more than 15 years; in 2000, Berman et al6 conducted the first randomized, double-blind crossover study of intravenous ketamine (0.5 mg/kg) versus saline solution, in unipolar or bipolar patients in a major depressive episode. All 8 patients who were treated with ketamine had significant improvement in depressive symptoms, and 1 patient maintained mood improvement for 2-week postinfusion.6
In a subsequent double-blind crossover study in TRD, 12 (71%) of the 17 participants receiving a single ketamine infusion had a 50% or more reduction in 21-item Hamilton Depression Rating Scale scores on the first day, compared with none (0%) of the 14 participants treated with placebo. Six (35%) individuals continued to exhibit improved scores for at least 1 week.7 The next landmark study involving 73 TRD patients across 2 sites demonstrated rapid antidepressant effects of ketamine compared with midazolam as an active control. The treatment response rate at 24 hours was 64% for the ketamine group, compared with 28% in the midazolam group, with a significant difference of 8 points on Montgomery-Åsberg Depression Rating Scale (MADRS) scores at end point between ketamine and midazolam groups. The ketamine group continued to show a significant reduction on MADRS score for a 1-week period postinfusion compared with midazolam, with gradual loss of benefit after day 7.8
A subsequent meta-analysis of 9 randomized controlled trials of intravenous ketamine (192 subjects with MDD and 34 with BD) reported significant improvement on depression scores for ketamine compared with placebo. Two of the 9 trials evaluated the duration of ketamine's effect, which seemed to last 2 to 3 days for a single dose.9 Serafini et al10 also carried out a systematic review of 24 studies involving 416 subjects with TRD and reported that ketamine has rapid antidepressant and antisuicidal effects.
There have been relatively few studies concerning the crucial question of whether repeated doses of ketamine are capable of exerting a sustained antidepressant effect over time. Singh et al11 conducted a multicenter, double-blind study to assess the efficacy of intravenous ketamine administered twice versus thrice weekly in TRD patients. A total of 68 subjects were randomized into 1 of 4 arms in a double-blind trial; they received either intravenous ketamine or placebo either 2 or 3 times weekly for up to 4 weeks. Both dosing regimens of ketamine were comparable in efficacy, which persisted for 15 days.11
There is also a paucity of information about other routes of administration for ketamine: can it reliably exert antidepressant effects when administered by nonintravenous routes, such as oral or intranasal? This issue is of translational importance, given the high prevalence of TRD and the short duration of effect of individual ketamine doses. If the treatment requires patients to attend clinics twice weekly for intravenous sessions for extended periods of time, then cost, resources, and inconvenience may limit the practical utility of ketamine in real-world settings.
Subsequently, some investigators have examined the antidepressant efficacy of ketamine via intranasal or oral routes. In 1 study, intranasal ketamine (50 mg) was compared with intravenous saline in a randomized, double-blind crossover design involving 20 unipolar TRD patients. Eight (44%) of 18 completers in the ketamine group met response criteria after 24 hours, compared with 1 (6%) of 18 in the placebo group; effects persisted for 48-hour posttreatment, in line with the durability of intravenous administration.12 So far, there have been no reports regarding sustained efficacy for repeated doses of intranasal ketamine.
Regarding oral ketamine, 0.5 mg/kg was administered to hospice patients receiving palliative care, who also had co-occurring depressive and anxiety symptoms based on Hospital Anxiety and Depression Scale. Eight of the 14 patients completed the 28-day trial, whereas 6 patients dropped out at different time points, 4 of them withdrew because of no response to ketamine after day 14, and 2 withdrew because of different reasons, which were unrelated to ketamine treatment. All 8 subjects who completed this open-label trial reported significant improvement in mood and anxiety symptoms. In addition, no serious adverse events were observed.13 In another report, oral ketamine was administered as an augmentation strategy in 2 patients with chronic suicidal ideation and at least 2 significant past suicide attempts. Within 24 hours of the first treatment, both patients showed significant reduction on the total MADRS score and on the suicide item. Remission was sustained with repeated treatment every 2 to 4 weeks, with no adverse events reported.14
Still, less evidence is available regarding the real-world incidence of adverse outcomes that could potentially ensue during ketamine treatment outside the research setting. Based on effects seen among recreational ketamine users, these adverse outcomes range from the potential for misuse, diversion, or dependence,15 to the possibility of emergent psychotic symptoms with repeated use,16 or urinary complications such as “ketamine bladder” with sustained use.17 Although reviews on safety in the research setting have been encouraging,18 it is unclear whether such findings translate to the clinical setting or to patients receiving treatment at home. Oral ketamine has been prescribed fairly commonly for home use in patients with chronic pain, following guidelines for dosing,19 safety, and efficacy.20,21 However, such data are not yet available in the setting of MDD.
In summary, despite 15 years or more of research on the antidepressant properties of ketamine, the treatment has not entered widespread use outside research settings, and a number of important translational questions remain unresolved. First, does oral ketamine reliably exert comparable effects to intravenous administration? Second, do the antidepressant properties of ketamine persist with repeated dosing over timescales of weeks to months? Third, do adverse outcomes (eg, misuse, dependence, psychosis, or urinary complications) ensue when ketamine treatment is provided outside the research setting? To explore these questions, data from real-world effectiveness studies may be informative.
CASE SERIES
Here, we report on a case series of 22 patients with TRD who received rescue open-label treatment with a course of oral ketamine at Toronto Western Hospital, University Health Network, Toronto, Canada, between July 2013 and November 2015. Access to retrospective data on these patients was approved by the Research Ethics Board, University Health Network, Toronto, Ontario, Canada. The objectives of this review are (1) to assess acute and sustained effectiveness of oral ketamine and (2) to evaluate safety and tolerability of oral ketamine.
Patient Characteristics
The patients (13 females, 9 males) had a mean age of 39 years. All participants met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for MDD, currently in a major depressive episode without psychotic features (based on the Mini-International Neuropsychiatric Interview screening tool).22 Fifteen participants (68%) had a comorbid psychiatric diagnosis, most frequently generalized anxiety disorder (27%), and 10 (46%) had a medical comorbidity. Patients were either referred by their outpatient psychiatrist or primary care physician and were offered ketamine under the rationale of having exhausted or declined alternative interventions in TRD; all had failed to respond to at least 3 pharmacological interventions and at least 1 adequate trial of repetitive transcranial magnetic stimulation at our clinic, 16 patients (73%) had refused electroconvulsive therapy, and 6 patients (27%) had previously failed to respond to electroconvulsive therapy. Thirteen (59%) had not benefited from cognitive behavioral therapy or mindfulness-based cognitive therapy. Twelve patients (55%) had previously failed to respond to lithium, 13 patients (59%) had previously failed to respond to an monoamine oxidase inhibitor, and 17 patients (77%) had previously failed to respond to an atypical (dopaminergic antagonist) agent. Clinical ratings were performed weekly at each clinic visit, and dose adjustments were performed on the basis of tolerability and clinical response. Participants were allowed to remain on ongoing medications or psychological treatment, without dose adjustment during the trial. Concomitant psychotropic medication classes included selective serotonin reuptake inhibitor (n = 3), serotonin/norepinephrine reuptake inhibitor (n = 5), tricyclic antidepressant (n = 2), bupropion (n = 2), trazodone (n = 3), and atypical (dopaminergic antagonist) agent (n = 6). Patients were not offered treatment if an axis 1 diagnosis other than MDD was considered primary or if there was a significant axis 2 diagnosis or a history of substance dependence or psychotic illness.
Treatment Approach
Before initiating ketamine, patients discussed with the prescriber the nature of ketamine as an anesthetic agent under investigation for off-label use as an antidepressant, as well as discussing the potential risks, benefits, and alternatives to off-label use of ketamine for depression. Ketamine was prescribed as compounded 25-mg capsules and obtained by the patients from a compounding pharmacy in the Toronto area. The treatment approach was derived from a recommended oral ketamine strategy in chronic pain.19 The initial dose of oral ketamine was taken under prescriber supervision at the clinic, and subsequent doses were taken at home during nighttime, once every 3 days to minimize overall dosage and to avoid tachyphylaxis associated with daily dosing. The dose commenced at 50 mg and was titrated upward by 25 mg at each dose (ie, every 3 days), until reaching 1 of the following 3 end points: (1) improvement in depressed mood on the day after the dose, (2) a lack of tolerability of the dose, without improvement in mood, or (3) no improvement in mood despite reaching a dose of 300 mg. If the latter 2 end points occurred, ketamine treatment was discontinued and the patient considered a nonresponder. In the case of the former end point, the dose was maintained or titrated upward by 1 or 2 increments to maximize the antidepressant effect. Where ketamine was tolerated, the minimum duration of treatment was 4 weeks. During follow-up visits, information about subjective improvement, subjective mood ratings on the Beck Depression Inventory II (BDI-II), adverse effects related to ketamine and a self-harm risk assessment were collected.
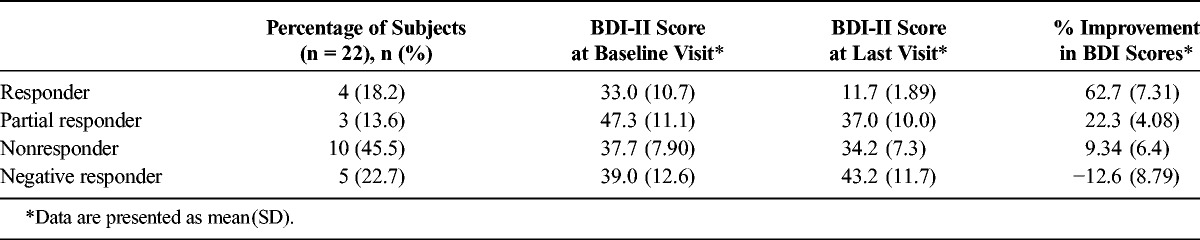
Mood Outcomes
Patients reached a mean (SD) dose of 222(72) mg, with a distribution as follows: 100 mg (2 patients), 150 mg (5 patients), 175 mg (1 patient), 225 mg (4 patients), 250 mg (4 patients), and 300 mg (6 patients). Over the course of dose titration as described previously, 4 of the 22 patients (18%) achieved more than 50% reduction in mood symptoms and 3 patients (14%) showed an improvement of 20% to 50% in mood symptoms. Ten (45%) of the 22 patients showed less than 20% improvement in mood, with a mean BDI-II score of 37.7 at baseline and 34.2 at the time of treatment discontinuation due to lack of effect. Five (23%) of the 22 patients showed a slight worsening in mood over the course of the titration. The mean BDI-II scores at baseline and at last visit for each group are summarized in Table 1. There was no significant correlation between baseline BDI-II score and percent improvement after treatment. We did not identify any relationship between ketamine response and concomitant psychotropic medication regimen. Among responders/partial responders, documentation of continued efficacy ranged from 15 weeks to 2 years from the onset of treatment.
TABLE 1.
Mood Outcomes for Oral Ketamine
Adverse Effects
The most frequently reported adverse event was acute dissociation (a subjective report of feeling disconnected from one's thoughts or emotions, or a subjective sense of time passing more slowly or quickly than normal, or out-of-body experiences) in the period from 30 to 120 minutes after taking the ketamine dose (41%). Although 1 subject reported acute visual hallucinations during the dissociative period, these were not accompanied by delusions or thought disorder and did not persist beyond the period of the acute dose. Other commonly reported adverse events were dizziness (23%), blurred vision (18%), numbness (14%), sedation (4%), nausea (4%), and insomnia (4%). Two subjects reported transient suicidal ideation that was not associated with self-harm or suicidal attempt. One participant reported lower urinary tract symptoms (mainly polyuria and dysuria), which occurred after 6 months of ketamine treatment; this was investigated via urological consultation and determined to be unrelated to the ketamine. This patient subsequently reinitiated ketamine without a recurrence of urological symptoms. Other adverse effects previously reported with ketamine (corneal edema, hepatic injury, changes in blood pressure, or memory impairment) were not observed or reported. Regarding propensity for ketamine abuse or dependence, no patient in this series reported developing ketamine cravings or urges to use ketamine beyond the prescribed amount or more frequently than prescribed. There were no pharmacy reports of requests for early prescription refills, repeated reports of “lost” supply, or other objective suggestions of overuse for any of the 22 patients.
DISCUSSION
Although ketamine has shown promising antidepressant effects in the research literature for more than 15 years, so far there has been much less literature on its potential to be used in real-world practice. Off-label regimens of oral ketamine (liquid or compounded capsules) for home use are fairly common in the management of chronic pain, raising the question of whether similar regimens might be applicable in the management of TRD. Although definitive answers to this question must await the rigor of a formal randomized controlled trial, in the interim, case reports and case series may be of interest.
In the present case series, approximately 30% reported some clinical benefit, whereas approximately 70% had no benefit or felt worse. On the other hand, adverse effects were less severe than those reported in the research literature. In addition, none of the patients in this series showed evidence of overuse, abuse, or dependence, nor were there any cases of urinary, hepatic, or ophthalmic adverse effects or evidence of psychosis or other serious psychiatric sequelae.
There are a number of important limitations to the present report that should inform future work. First, all of patients in this series had highly resistant forms of depression, with failure of multiple treatment modalities (multiple antidepressant medications, neuromodulation, and in most cases cognitive psychotherapy). Second, adherence to the prescribed regimen could not be confirmed for at-home dosing regimens. Third, because this report concerns a series of clinical cases rather than a randomized controlled trial, standardized clinician ratings of mood symptom were not consistently obtained. Fourth, a series of 22 patients is insufficiently large to assess the incidence of rare but serious adverse effects (medical or psychiatric) ensuing from the treatment.
Notwithstanding these limitations, it would be premature to rule out the potential clinical usefulness of “chronic pain–like” at-home regimens of oral ketamine in the setting of depression. However, a reasonable conclusion from the present work is that while such regimens may not engender harmful effects in most TRD patients, they may not achieve marked improvement in most TRD patients either.
In light of the high prevalence and disability associated with TRD and the impracticality of providing in-hospital intravenous ketamine to most patients who might benefit, more formal studies of nonintravenous ketamine or comparable regimens should be considered a priority. This would include emerging agents that target the N-methyl-d-aspartate receptor with antidepressant effect but with fewer propensities for dissociation or dependence.23 The practical importance of devising safe and effective at-home treatment regimens bears further emphasis in the field. Such work will be essential to determine whether the promising research literature on glutamatergic antidepressants can be translated into meaningful advances in the clinical setting.
ACKNOWLEDGMENT
The authors acknowledge continuing research support from the Ontario Brain Institute to the Canadian Biomarker Integration Network in Depression.
AUTHOR DISCLOSURE INFORMATION
Dr Al Shirawi receives fellowship funding from the Ministry of Health, Sultanate of Oman. Dr Kennedy has received research funding or honoraria from the following sources: Allergan, AstraZeneca, BMS, Brain Cells Inc, Brain Canada, Clera, CIHR, Eli Lilly, Janssen, Lundbeck, Lundbeck Institute, OMHF, Ontario Brain Institute, Pfizer, Servier, St. Jude Medical, Sunovion, and Xian-Janssen. Dr Downar has received research support from CIHR, Brain Canada, the Canadian Biomarker Integration Network in Depression, the Ontario Brain Institute, the Klarman Family Foundation, the Edgstone Foundation, a travel stipend from Lundbeck and from ANT Neuro, and in-kind equipment support for an investigator-initiated trial from MagVenture. No pharmaceutical or industry support was received for any part of this work. The opinions, results and conclusions are those of the authors, and no endorsement by the Ontario Brain Institute is intended or should be inferred. The authors declare no conflicts of interest.
REFERENCES
- 1.Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26:2475–2484. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. [DOI] [PubMed] [Google Scholar]
- 3.Lee EE, Della Selva MP, Liu A, et al. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry. 2015;37:178–184. [DOI] [PubMed] [Google Scholar]
- 4.Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013;19:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanagihara Y, Ohtani M, Kariya S, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos. 2003;24:37–43. [DOI] [PubMed] [Google Scholar]
- 6.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. [DOI] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. [DOI] [PubMed] [Google Scholar]
- 8.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl). 2014;231:3663–3676. [DOI] [PubMed] [Google Scholar]
- 10.Serafini G, Howland RH, Rovedi F, et al. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol. 2014;12:444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816–826. [DOI] [PubMed] [Google Scholar]
- 12.Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Gioannis A, De Leo D. Oral ketamine augmentation for chronic suicidality in treatment-resistant depression. Aust N Z J Psychiatry. 2014;48:686. [DOI] [PubMed] [Google Scholar]
- 15.Morgan CJ, Curran HV, Independent Scientific Committee on Drugs. Ketamine use: a review. Addiction. 2012;107:27–38. [DOI] [PubMed] [Google Scholar]
- 16.Zuccoli ML, Muscella A, Fucile C, et al. Paliperidone for the treatment of ketamine-induced psychosis: a case report. Int J Psychiatry Med. 2014;48:103–108. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Wang Q, Huang Z, et al. Clinical staging of ketamine-associated urinary dysfunction: a strategy for assessment and treatment. World J Urol. 2016;34:1329–1336. [DOI] [PubMed] [Google Scholar]
- 18.Perry EB, Jr, Cramer JA, Cho HS, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl). 2007;192:253–260. [DOI] [PubMed] [Google Scholar]
- 19.Blonk MI, Koder BG, van den Bemt PM, et al. Use of oral ketamine in chronic pain management: a review. Eur J Pain. 2010;14:466–472. [DOI] [PubMed] [Google Scholar]
- 20.Marchetti F, Coutaux A, Bellanger A, et al. Efficacy and safety of oral ketamine for the relief of intractable chronic pain: a retrospective 5-year study of 51 patients. Eur J Pain. 2015;19:984–993. [DOI] [PubMed] [Google Scholar]
- 21.de Godoy MC, Dalmolin GD, Rigo FK, et al. Management of chronic neuropathic pain of different causes with the combination of oral methadone along with ketamine: a report of 18 cases. Eur J Anaesthesiol. 2013;30:638–640. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 23.Burgdorf J, Zhang XL, Nicholson KL, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]


