Abstract
Purpose
We evaluate feasibility and repeatability of measures for lipid peroxidation and DNA oxidation in human tears, as well as relationships between outcome variables, and compared our findings to previously reported methods of evaluation for ocular sun exposure.
Methods
A total of 50 volunteers were seen for 2 visits 14 ± 2 days apart. Tear samples were collected from the inferior tear meniscus using a glass microcapillary tube. Oxidative stress biomarkers were quantified using enzyme-linked immunosorbent assay (ELISA): lipid peroxidation by measurement of hexanoyl-lysine (HEL) expression; DNA oxidation by measurement of 8-oxo-2′-deoxyguinosone (8OHdG) expression. Descriptive statistics were generated. Repeatability estimates were made using Bland-Altman plots with mean differences and 95% limits of agreement were calculated. Linear regression was conducted to evaluate relationships between measures.
Results
Mean (±SD) values for tear HEL and 8OHdG expression were 17368.02 (±9878.42) nmol/L and 66.13 (±19.99) ng/mL, respectively. Repeatability was found to be acceptable for both HEL and 8OHdG expression. Univariate linear regression supported tear 8OHdG expression and spring season of collection to be predictors of higher tear HEL expression; tear HEL expression was confirmed as a predictor of higher tear 8OHdG expression.
Conclusions
We demonstrate feasibility and repeatability of estimating previously unreported tear 8OHdG expression. Seasonal temperature variation and other factors may influence tear lipid peroxidation. Support is demonstrated to suggest lipid damage and DNA damage occur concurrently on the human ocular surface.
Keywords: tears, lipid peroxidation, DNA oxidation, oxidative stress, biomarkers
Human tissue damage associated with ultraviolet (UV) radiation exposure is responsible for significant loss of quality of life and approximately 60,000 premature deaths annually.1 While premature deaths are primarily secondary to cutaneous melanoma, the World Health Organization identified three ocular diseases with enough support in the literature to be associated with UV exposure: cortical cataract, pterygium, and squamous cell carcinoma of the cornea and conjunctiva.1 It is thought that many UV exposure–associated diseases involve oxidative stress.2–4
Oxidative stress is known to be an important component of UV-induced damage. In general, oxidative stress occurs when reactive oxygen species (ROS) levels exceed the antioxidant capacity of a biological system. While ROS are a normal part of healthy biological processes, extended levels of elevated oxidative stress leads to tissue injury and molecular damage.5 Levels of oxidative stress can be influenced by UV irradiation, toxic chemicals, and genetic responses.3
UV-induced oxidative stress has been demonstrated to cause protein, lipid, and DNA damage in animal models6,7 and cell culture (Andley U. IOVS 2009;50:ARVO E-Abstract 2537).8 However, to our knowledge human studies showing UV-induced ocular surface oxidative stress are not found in the literature. This void in our knowledge base and the scientific literature is a barrier to progress in the field for development of viable effective methods to reduce or eliminate ocular disease induced by UV exposure.
Although to our knowledge no human studies have demonstrated UV-induced ocular surface oxidative stress, based on animal models it was anticipated that human tears would show increased oxidative stress markers with higher amounts of UV exposure. We assessed oxidative stress by examining levels of damaged lipid and DNA products. Specifically, the purpose of this study was to evaluate feasibility and repeatability measurements of hexanoyl-lysine (HEL) levels and 8-hydroxy-2′-deoxyguanosine (8OHdG) levels for in vivo human tear samples, and to evaluate relationships for these measures with previously reported methods of evaluation for ocular sun exposure and conjunctival ultraviolet autofluorescence (UVAF).9
Methods
Subjects
A total of 50 volunteers were seen for 2 visits 14 ± 2 days apart. Designed as a feasibility study, inclusion and exclusion criteria were liberal. All adults 18 years and older were included. Only those who self-reported current pregnancy or lactation, or sporadic use of topical ocular medications were excluded from the study. Before initiation of the study, this research was approved by The Ohio State University Institutional Review Board. Participants signed an informed consent document following the tenets of the Declaration of Helsinki. After in-person screening for ocular pathology by slit-lamp examination, a brief medical history questionnaire, and the Ocular Surface Disease Index (OSDI, Allergan, Parsippany-Troy Hills, NJ, USA)10 were completed, in addition to sun exposure questionnaires.11,12 Tear samples then were collected from each eye.
Tear Collection
Glass microcapillary tubes (Drummond Microcaps; Sigma-Aldrich Corp., St. Louis, MO, USA) were used to collect tears from the inferior tear meniscus. Tear samples were collected with care to minimize stimulation of tearing and inadvertent collection of cells. After collection, tears were removed from the microcapillary tubes and stored in microcentrifuge tubes at −80°C until analysis.
Ocular Surface Vital Staining
Although the present study was not designed to evaluate a specific ocular disease, such as dry eye, ocular surface vital staining was used to evaluate for possible sample collection–induced ocular surface damage. Following sample collection, 5 μL of nonpreserved fluorescein sodium (Leiter's Compounding Pharmacy, San Jose, CA, USA) was instilled into the inferior cul-de-sac of each eye and the corneas examined for evidence of superficial punctuate keratitis and/or corneal abrasion induced during sample collection. Next, 5 μL of nonpreserved Lissamine green (Leiter's Compounding Pharmacy) was instilled into the inferior cul-de-sac of each eye and the conjunctivas examined for evidence of conjunctival cell loss and/or sample collection induced abrasions.
Ocular Sun Exposure
Ocular sun exposure was estimated over a 2-week time period by questionnaires as described previously.9 To summarize, questionnaires of ocular protection habits and hours spent in outdoor activities over the most recent 2-week time period were administered.11 Each protective measure was assigned a number relative to their ocular exposure–specific effectiveness.13 The outdoor activity questionnaire asked each participant to estimate over the past 2 weeks the number of hours spent doing each of the following activities: driving, gardening, home, walking/light exercise, recreation, water, and leisure.12 The mean total number of hours reported less those spent driving were used in subsequent calculations.
Conjunctival UVAF
Conjunctival UVAF was estimated as reported previously.9 In summary, images were obtained using a camera system designed to acquire visible light emitted from conjunctival tissue following stimulation with UV light. Total UVAF was obtained for each subject by summing nasal and temporal areas of autofluorescence for both eyes.
Quantification of Oxidative Damage by HEL Enzyme-Linked Immunosorbent Assay (ELISA)
Oxidative stress damage was evaluated by measuring lipid peroxidation in tears using a commercially available HEL ELISA14,15 (Northwest Life Science Specialties, LLC, Vancouver, WA, USA). Tear samples from the right and left eyes were pooled by subject for each visit. Following pooling, tear samples were diluted for each subject to 1:20 concentration with 1 × phosphate buffered saline (PBS) before ELISA analysis.
The HEL ELISA protocol was followed according to manufacturer's recommendations. Manufacturer recommended four parameter logistic standard curves were generated using an online software program (Readerfit.com; Hitachi Solutions America, San Bruno, CA, USA) to quantify HEL levels based on the obtained absorbance data.
Quantification of Oxidative Damage by 8OHdG ELISA
DNA damage was measured using a commercially available 8-OHdG ELISA16 (Northwest Life Science Specialties). Tear samples from the right and left eyes were pooled by subject for each visit. Following pooling, tear samples were diluted for each subject to 1:20 concentration with 1 × PBS before ELISA analysis. The protocol for the 8OHdG ELISA was followed according to manufacturer's recommendations. Manufacturer-recommended four parameter logistic standard curves were generated using an online software program (available in the public domain at Readerfit.com) to quantify 8OHdG levels based on the obtained absorbance data.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics 21 (IBM Corporation, Armonk, NY, USA) software. Means, standard deviations, percentages, and frequencies were used to summarize data collected. Thus, normal values are provided to calculate sample size estimates to detect differences between groups for two continuous or dichotomous variables. In addition, repeatability based on test–retest variability for ELISAs was evaluated using Bland-Altman plots. Furthermore, univariate and multivariate linear regression was conducted to evaluate relationships between measures. Statistical significance was accepted at probability level less than 5%.
Results
Subject Characteristics
The study included 50 subjects recruited from The Ohio State University, 58% female, with a mean age of 38 ± 13 (range, 21–64) years. Nine subjects reported outdoor occupations, 41 reported indoor occupations. Four subjects were classified as having mild dry eye at visit one based on their responses to the OSDI. Corrective lenses were worn by 30 subjects: 20 spectacles, 10 contact lenses. Of the 10 subjects who wore contact lenses, only three reported wearing known UV absorbing lenses, three reported wearing known non-UV absorbing lenses, and the remaining four were not aware of the specific brand of contact lenses worn. All subjects completed the study and there were no adverse events.
Descriptive Statistics
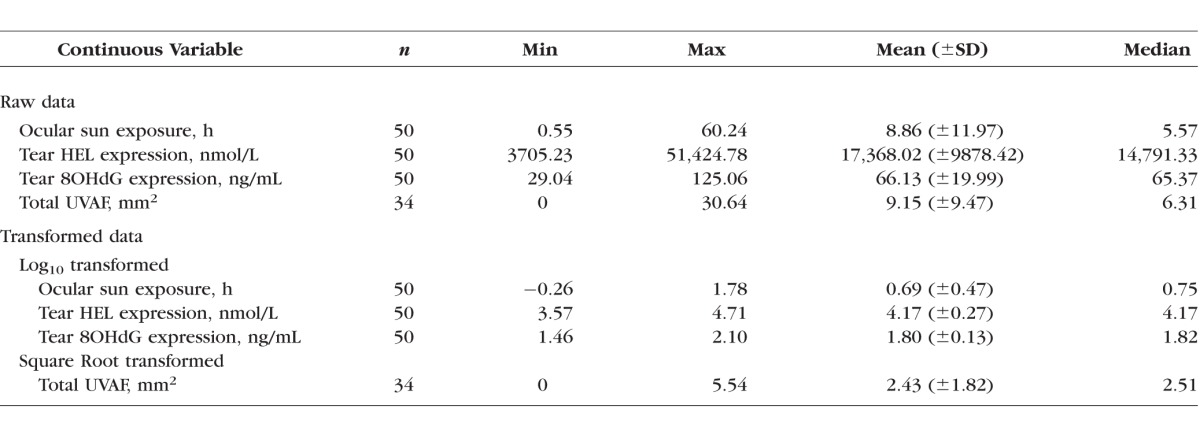
Descriptive statistics were generated for measures of ocular sun exposure, tear HEL, tear 8OHdG, and conjunctival UVAF (Table 1). Violations of assumptions for normal data distribution were evident (Shapiro-Wilk); thus, to better approximate normal distribution log10 or square root transformation of the data was performed.
Table 1.
Descriptive Statistics for Continuous Outcome Variables for Raw and Transformed Data
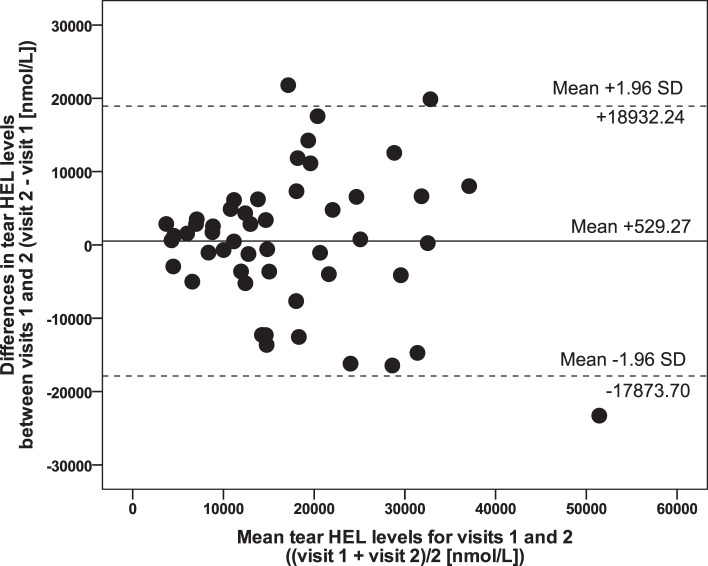
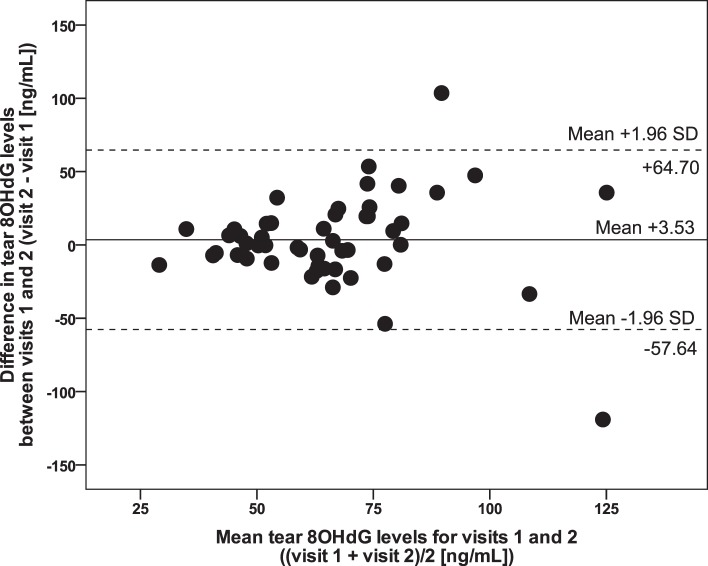
Bland-Altman scatter plots were generated for tear HEL expression (Fig. 1), and tear 8OHdG expression (Fig. 2) to obtain estimates of repeatability based on test–retest variability, with mean differences (bias) and 95% limits of agreement were calculated (Table 1). A range of agreement was defined as mean bias ± 1.96 SDs.
Figure 1.
Bland-Altman plot illustrating variability of raw data for tear HEL levels (n = 50). Solid line shows bias (mean differences). Dotted lines show 95% limits of agreement.
Figure 2.
Bland-Altman plot illustrating variability of raw data for tear 8OHdG levels (n = 50). Solid line shows bias (mean differences). Dotted lines show 95% limits of agreement.
Linear Regression Analyses
Multivariate linear regression forward stepwise models were built. Independent variables considered for each model included age, sex, occupation (indoor or outdoor), season (winter or spring), contact lens wear, tear HEL levels, tear 8OHdG levels, ocular sun exposure, and total UVAF. Sensitivity testing subsequently was done to evaluate the effect if the four subjects determined to have mild dry eye based on their responses to the OSDI at visit one were eliminated from the data set; no significantly different results were found for any of the models. Furthermore, although not significant in linear regression models, with univariate linear regression contact lens wear was found to predict higher tear HEL expression (P = 0.042), yet was not predictive of higher tear 8OHdG expression (P = 0.545). Multiple linear regression models were built and tested using the best fit raw data (Tables 2, 3) and using transformed data. For ease of interpretation, regression coefficients are discussed here only for the raw data linear regression models.
Table 2.
Multiple Linear Regression Model for Tear HEL Expression
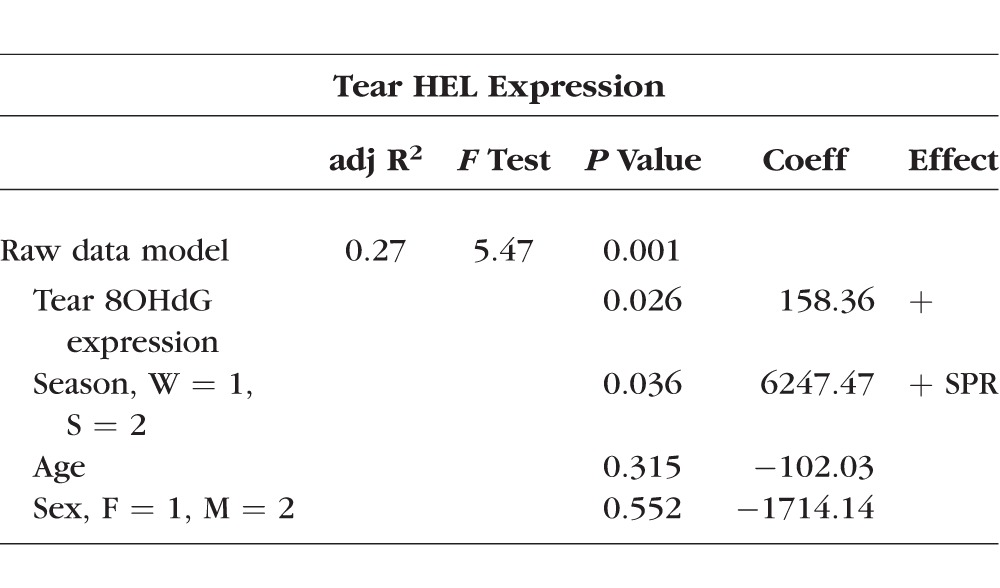
Table 3.
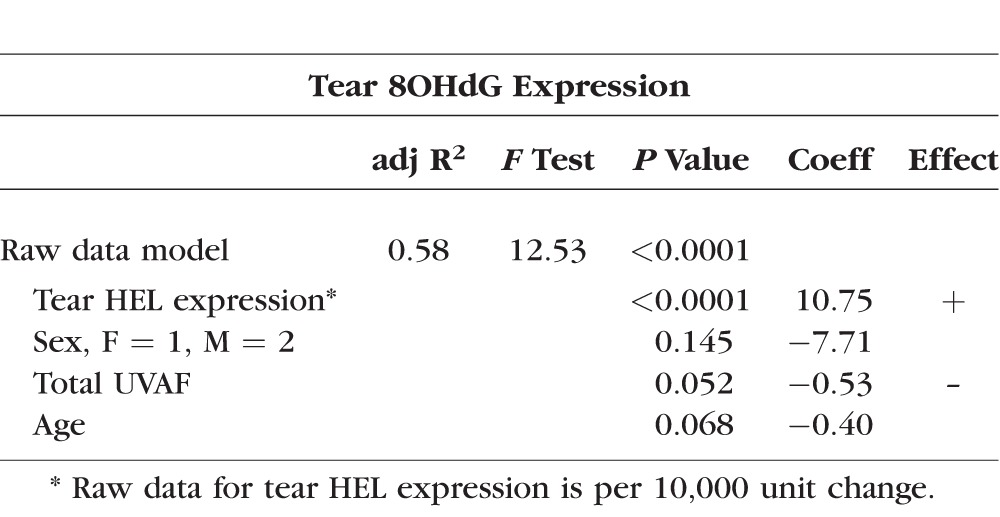
Multivariate Linear Regression Model for Tear 8OHdG Expression
Tear HEL Expression.
Univariate predictors significantly associated with tear HEL expression were tear 8OHdG expression, sex, occupation, contact lens wear, and season of collection. Using transformed data, tear 8OHdG expression (P = 0.017) was identified as a predictor of higher levels of tear HEL expression. Additionally, female sex (P = 0.024), indoor occupation (P = 0.003), spring season of collection (P = 0.003), and contact lens wear (P = 0.042) predicted higher tear HEL expression. For the raw data, significant univariate predictors of tear HEL expression were higher tear 8OHdG expression (P = 0.001), female sex (P = 0.011), and indoor occupation (P = 0.009). Spring season of collection (P = 0.003) continued to predict higher tear HEL expression; contact lens wear was not significant (P = 0.071) using the raw data.
The best fit multiple linear regression model for describing tear HEL expression using raw data as a possible predictor included tear 8OHdG expression and season (Table 2). This model provided a weakly moderate description of tear HEL expression. After adjusting for age and sex, tear 8OHdG expression and season of collection remained significant. Based on this model, for one unit of increase in tear 8OHdG expression, we predicted on average an increase of 158 units of tear HEL expression. For spring season of collection, we predicted on average a 6247-unit increase in tear HEL expression. Age and sex were not significant predictors of tear HEL expression in this model.
Tear 8OHdG Expression.
Significant univariate regression relationships using transformed variables for tear 8OHdG expression were found for total UVAF, tear HEL expression, and sex. Increased total UVAF predicted lower tear 8OHdG expression (P = 0.015). On the other hand, increased tear HEL expression (P = 0.017) and female sex (P = 0.002) predicted higher tear 8OHdG expression. Using the raw data the same predictors of tear 8OHdG were identified with the same directions of influence as found using the transformed data.
Analysis of the raw data generated a model for tear 8OHdG expression including tear HEL expression, sex, and total UVAF (Table 3). This model was robust. After adjusting for age and sex, tear HEL expression remained a significant predictor and total UVAF nearly achieved significance as a predictor. Based on coefficients in this model, we predicted that for a 10,000 unit increase in tear HEL expression we expected on average an increase of 10.75 units of tear 8OHdG expression. In addition, there was a strong suggestion that for a one-unit increase in total UVAF on average we expected tear 8OHdG to decrease by 0.53 units.
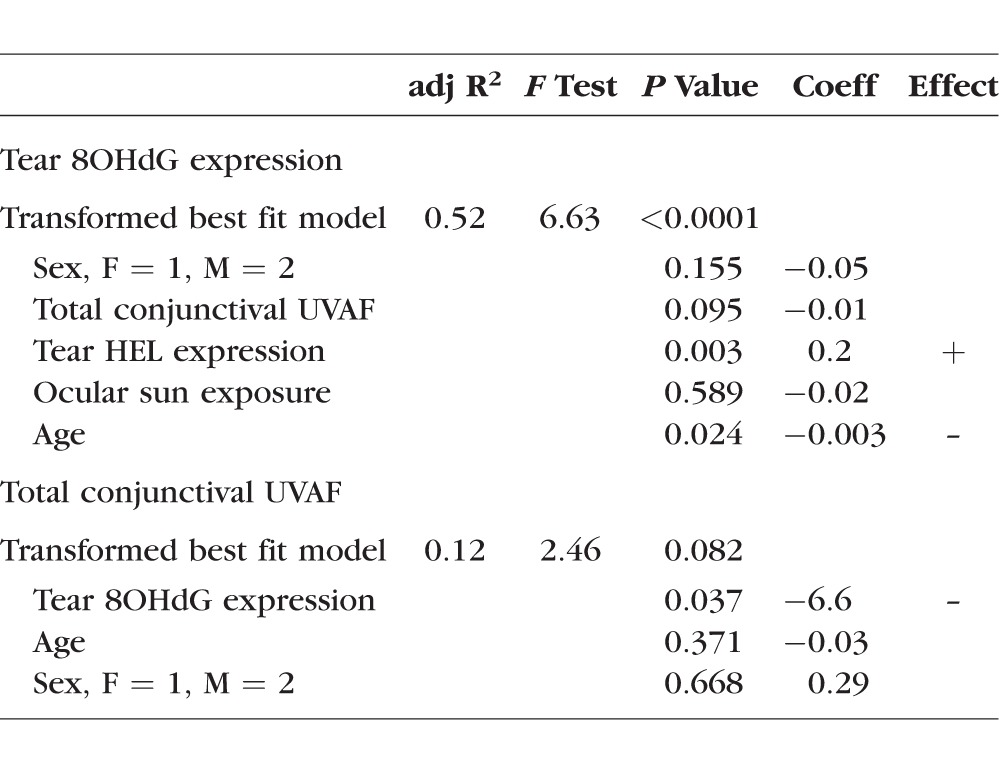
When considering transformed data, outcomes followed a similar pattern (Table 4). The linear regression model for tear 8OHdG expression using transformed data was highly significant and showed tear HEL expression (P = 0.003) and age (P = 0.024) as predictors. Sex, UVAF, and ocular sun exposure did not remain significant in this model. However, the model generated with transformed conjunctival UVAF data as the outcome variable was not significant (P = 0.082), yet 8OHdG remained a significant predictor (P = 0.037; Haworth K and Chandler HL. IOVS 2016;57:ARVO E-Abstract 3509).
Table 4.
Linear Regression Models Using Transformed Data for Tear 8OHdG Expression and Total Conjunctival UVAF as Outcome Variables
Discussion
Assessment of Oxidative Stress
It is challenging to directly detect ROS due to their high reactivity and short half-lives.17 Therefore, it is more common to measure the deleterious products formed by ROS actions.18 Biologically relevant oxidative stress markers include those for damage to lipids, proteins, and DNA, as well as assessment for antioxidant activity.
Lipid Damage.
The first point of contact for UV exposure on the ocular surface is the tear film. At the forefront of the tear film is the lipid bilayer. Furthermore, just posterior to the tear film is the ocular surface with its large surface area composed, in part, by several layers of epithelial cells, with each cell enclosed by a lipid bilayer membrane. These lipid bilayer structures are likely subject to ROS-mediated UV damage, and lipid peroxidation may be the first evidence of ocular surface oxidative damage. HEL is a lipid hydroperoxide-derived oxidation product from omega-6 fatty acids.19 A limited number of publications investigating biomarkers in human tears for other steps in the lipid peroxidation pathway are available; increased expression was reported for two later stage products, 4-hydroxy-2-nonenal and malondialdehyde, in those with dry eye and increased age.20,21 As HEL represents only one step in the lipid peroxidation pathway, future additional studies evaluating tears and ocular surface samples for different types of lipid peroxidation products would help clarify the potential significance of other lipid peroxidation effects.
The present study has identified tear HEL values that are considerably higher than those previously reported for human tears.14,15,20,22,23 Reasons for this could be experimental differences in tear sample preparation and analysis, environmental-induced variation (i.e., previous reports are all from Japan), dietary differences, or genetic variation in HEL production. In support of diet-induced variation, it is well established that consumption of omega-6 fatty acids is much higher for those who choose a Western diet compared to those who consume a traditional Japanese diet.24 Furthermore, increased consumption of omega-6 fatty acids may lead to altered lipogenic mRNA transcription,25 possibly facilitating increased production of HEL. Further research is needed to clarify the source and determining factors of HEL expression on the ocular surface.
DNA Damage.
Following its discovery in 1984, 8OHdG has become the most widely accepted assay for oxidative DNA damage.26 While many oxidative mechanisms may contribute to its production, 8-OHdG is formed primarily from oxidative DNA damage by the interaction of hydroxyl radicals with the DNA base guanosine.27 Relevant to the ocular surface, 8OHdG has been detected in surgically obtained human conjunctival specimens,23,28 cultured human corneal epithelial cells,29 and murine corneal tissue samples.7 For the first time to our knowledge, we report detection of tear 8OHdG levels. We expect that the origin of tear 8OHdG is from damage to DNA in the ocular surface epithelium with subsequent accumulation in the tears.
Bland-Altman Plot Assessment of Repeatability
Tear HEL Levels.
While the mean difference for tear HEL levels may appear high (+529.27), it should be stressed that this only represents approximately 3% of the limits of agreement values, and, thus, is comparatively near zero. Although the data point spread is somewhat wide, there is no apparent systematic variation of the means. To date, clinically acceptable variation in tear HEL levels has not been well defined, yet the test–retest variability found here is acceptable for use of this measure in future studies.
Tear 8OHdG Levels.
Repeatability for tear 8OHdG levels also was good, with the mean difference close to zero, as +3.53 is approximately 5% of the limits of agreement. The majority of scatter plot points cluster very near the mean difference; however, a possible increase in variability with increased levels of tear 8OHdG is apparent. This variability may be explained by factors affecting ocular sun exposure, such as time spent outdoors due to occupation, ambient temperature, and use of ocular protective measures. Variability of the 8OHdG ELISA also may have a role. The ELISA manufacturer does not provide data on the assay's intra- or interassay precision. As this is the first time for tear 8OHdG levels to be reported to our knowledge, clinically acceptable variation is yet to be determined; however, we considered the test–retest variability found here to be acceptable.
Linear Regression Models
Tear HEL Expression.
Increased tear 8OHdG expression was identified consistently as a predictor of higher levels of tear HEL expression using univariate and multivariate models, and when using either transformed or raw data (Table 2). This positive predictive relationship strongly suggested that lipid modification is accompanied by DNA modification on the ocular surface.
Univariate predictors for tear HEL expression demonstrate female sex, indoor occupation, spring season of collection, and contact lens wear may predict higher expression. Collectively these predictors support increased tear lipid peroxidation may be present in these subject groups when compared to male sex, outdoor occupation, winter season of collection, and no contact lens wear. However, when considering them together with the fact that univariate logistic regression demonstrated male sex predicted winter season of collection (P = 0.005), it is strongly suggested that the present study data were confounded by the fact that most samples collected from males and those with outdoor occupations were collected during the winter season. Furthermore, spring season of collection may have an influence on tear HEL expression directly through increased tear HEL expression and indirectly by possible increased release of cellular HEL into the tears. Subsequently, there is limited evidence in the present study to support the significance of sex, occupation, season of collection, and contact lens wear to tear HEL expression.
It was expected that increased levels of UV exposure would result in increased tear HEL expression; however, this hypothesis was not supported by the present data. Specifically, it was expected that those with outdoor occupations would have higher levels of tear HEL when compared to those with indoor occupations. Consequently, increased exposure to UV by those with outdoor occupations was expected to cause increased levels of lipid peroxidation. However, as reported previously, study subjects with outdoor occupations had only marginally higher hours of ocular sun exposure as they completed the study during the cold 2014 Ohio winter season.9 This may explain why tear HEL expression was not increased in those with outdoor occupations. Alternatively, it was not surprising that sample collection in the spring season was associated with increased tear HEL expression. When compared to winter, ambient UV levels increase30,31 along with outdoor activities during warmer seasons, potentially resulting in increased UV exposure.
Tear 8OHdG Expression.
Increased tear HEL expression consistently predicted higher tear 8OHdG expression. This positive predictive relationship was evident for univariate analysis and multivariate models using transformed or raw data. These results provide strong support that DNA damage occurs concurrently with lipid damage on the ocular surface.7,23 Although the significance of tear HEL expression as a predictor of tear 8OHdG expression is strong in the transformed (P = 0.003) and raw (P < 0.0001) data-based models, the coefficient of determination is small at a 10.09-unit increase in the raw data model per 10,000-unit increase of tear HEL expression. Hence, relevance of this finding to ocular surface disease remains to be evaluated.
While increased tear HEL expression predicted higher tear 8OHdG expression, we previously reported that increased UVAF predicted lower tear 8OHdG expression in univariate analyses and nearly reached significance in multivariate models (Haworth K and Chandler HL. IOVS 2016;57:ARVO E-Abstract 3509). Therefore, data herein provided moderate support for conjunctival UVAF being associated with decreased tear DNA damage expression. Given the small sample size, it is possible the present study is underpowered to detect a significant relationship in the multivariate model.
It is possible conjunctival UVAF results in decreased tear 8OHdG expression as a result of altered collagen, as occurs with collagen cross-linking, shown to cause autofluorescence following UV exposure.32 Moreover, surgically excised pterygia tissue was demonstrated to have increased 8OHdG expression when compared to adjacent normal tissue.28 Chronic UV exposure may result in increased conjunctival UVAF. Given conjunctival collagen is located primarily in the posterior region of the substantia propria, changes to this region may be permanent and may affect overall conjunctival tissue function; one altered function may be reduced epithelial shedding. Moreover, anterior surface conjunctival epithelial cells may be affected more transiently by recent UV exposure, and may result in simultaneous expression of tear HEL and tear 8OHdG. Consequently, it is suggested that UV exposure-associated altered collagen and cells may have reduced function and, rather than shedding into the tear film, are more frequently retained on the ocular surface, subsequently reducing tear film 8OHdG expression.
Detection of 8OHdG in tears in the present study suggested that shedding from the conjunctival and corneal epithelial cells may accumulate in the tear film, and suggested that DNA repair may accompany lipid peroxidative damage. Hence, it has been shown to our knowledge for the first time on the ocular surface using human in vivo samples that lipid damage is associated with DNA damage. Future studies will help delineate the clinical relevance of this relationship to ocular surface disease.
Conclusions
Feasibility and repeatability are demonstrated for the previously unreported ELISA measurement of oxidative stress levels in human tears (8OHdG). Furthermore, linear regression analysis suggested that lipid damage is associated with DNA damage on the ocular surface. Additionally, findings supported that an inverse relationship may exist between conjunctival UVAF and tear 8OHdG expression. Findings from this study established a baseline in normal subjects for future studies comparing these outcome measures with ocular surface disease groups.
It is recognized there are limitations to this study. The study was designed as a feasibility study to demonstrate detection ability of outcome measures for in vivo human ocular surface samples, so inclusion criteria were liberal. Inclusion of those with dry eye and contact lens wearers may have confounded the findings. Furthermore, participant smoking status was not queried and is known to increase expression of lipid peroxidation markers.14 Future studies with more restrictive inclusion and exclusion criteria will help to clarify and delineate the significance of the present findings to ocular surface disease. It is hoped the outcome measures reported here will stimulate additional studies to provide further insight into the importance of the tear film lipid layer for the maintenance of ocular surface equilibrium, as well as its potential role in the onset of ocular surface disease.
Acknowledgments
The authors thank Donald O. Mutti (The Ohio State University College of Optometry) for the technical support, camera system design, and construction, and Loraine Sinnott and Lisa Jones-Jordan (The Ohio State University College of Optometry) for statistical analysis. This manuscript comprises a portion of Kristina M. Haworth's doctoral (PhD) dissertation at The Ohio State University College of Optometry.
Supported by National Institutes of Health (Bethesda, MD, USA) Grant NEI U10 EY008893, PI: Karla Zadnik, and by The Ohio State University College of Optometry, provided by Karla Zadnik.
Disclosure: K.M. Haworth, None; H.L. Chandler, None
References
- 1. Lucas RJ,, McMichael T,, Smith W,, Armstrong B. Solar ultraviolet radiation: global burden of disease from solar ultraviolet radiation. : Environmental Burden of Disease Series. Vol. 13. Geneva: World Health Organization; 2006. [Google Scholar]
- 2. Chandler H. Ultraviolet absorption by contact lenses and the significance on the ocular anterior segment. Eye Contact Lens. 2011; 37: 259–266. [DOI] [PubMed] [Google Scholar]
- 3. Williams DL. Oxidative stress and the eye. Vet Clin North Am Small Anim Pract. 2008; 38: 179–192. [DOI] [PubMed] [Google Scholar]
- 4. Chalam KV,, Khetpal V,, Rusovici R,, Balaiya S. A review: role of ultraviolet radiation in age-related macular degeneration. Eye Contact Lens. 2011; 37: 225–232. [DOI] [PubMed] [Google Scholar]
- 5. Mikkelsen RB,, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003; 22: 5734–5754. [DOI] [PubMed] [Google Scholar]
- 6. Chandler HL,, Reuter KS,, Sinnott LT,, Nichols JJ. Prevention of UV-induced damage to the anterior segment using class I UV-absorbing hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010; 51: 172–178. [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim OM,, Kojima T,, Wakamatsu TH,, et al. Corneal and retinal effects of ultraviolet-B exposure in a soft contact lens mouse model. Invest Ophthalmol Vis Sci. 2012; 53: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 8. Youn HY,, Cullen AP,, Chou BR,, Sivak JG. Phototoxicity of ultraviolet (UV) radiation: evaluation of UV-blocking efficiency of intraocular lens (IOL) materials using retinal cell culture and in vitro bioassays. Open Toxicol J. 2010; 4: 13–20. [Google Scholar]
- 9. Haworth KM,, Chandler HL. Seasonal effect on ocular sun exposure and conjunctival uv autofluorescence. Optom Vis Sci. 2017; 94: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiffman RM,, Christianson MD,, Jacobsen G,, Hirsch JD,, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118: 615–621. [DOI] [PubMed] [Google Scholar]
- 11. Sherwin JC,, Hewitt AW,, Kearns LS,, Griffiths LR,, Mackey DA,, Coroneo MT. The association between pterygium and conjunctival ultraviolet autofluorescence: The Norfolk Island Eye Study. Acta Ophthalmol. 2013; 91: 363–370. [DOI] [PubMed] [Google Scholar]
- 12. Kwok RK,, Linet MS,, Chodick G,, et al. Simplified categorization of outdoor activities for male and female U.S. indoor workers-a feasibility study to improve assessment of ultraviolet radiation exposures in epidemiologic study questionnaires. Photochem Photobiol. 2009; 85: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Threlfall TJ,, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999; 128: 280–287. [DOI] [PubMed] [Google Scholar]
- 14. Rummenie VT,, Matsumoto Y,, Dogru M,, et al. Tear cytokine and ocular surface alterations following brief passive cigarette smoke exposure. Cytokine. 2008; 43: 200–208. [DOI] [PubMed] [Google Scholar]
- 15. Wakamatsu TH,, Dogru M,, Ayako I,, et al. Evaluation of lipid oxidative stress status and inflammation in atopic ocular surface disease. Mol Vis. 2010; 16: 2465–2475. [PMC free article] [PubMed] [Google Scholar]
- 16. Su H,, Gornitsky M,, Velly AM,, Yu H,, Benarroch M,, Schipper HM., Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic Biol Med. 2009; 46: 914–921. [DOI] [PubMed] [Google Scholar]
- 17. Freinbichler W,, Colivicchi MA,, Stefanini C,, et al. Highly reactive oxygen species: detection, formation, and possible functions. Cell Mol Life Sci. 2011; 68: 2067–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Labuschagne CF,, Brenkman AB. Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res Rev. 2013; 12: 918–930. [DOI] [PubMed] [Google Scholar]
- 19. Kato Y,, Osawa T. Detection of lipid-lysine amide-type adduct as a marker of PUFA oxidation and its applications. Arch Biochem Biophys. 2010; 501: 182–187. [DOI] [PubMed] [Google Scholar]
- 20. Choi W,, Lian C,, Ying L,, et al. Expression of lipid peroxidation markers in the tear film and ocular surface of patients with non-Sjogren syndrome: potential biomarkers for dry eye disease. Curr Eye Res. 2016; 41: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 21. Benlloch-Navarro S,, Franco I,, Sanchez-Vallejo V,, Silvestre D,, Romero FJ,, Miranda M. Lipid peroxidation is increased in tears from the elderly. Exp Eye Res. 2013; 115: 199–205. [DOI] [PubMed] [Google Scholar]
- 22. Wakamatsu TH,, Dogru M,, Matsumoto Y,, et al. Evaluation of lipid oxidative stress status in Sjogren syndrome patients. Invest Ophthalmol Vis Sci. 2013; 54: 201–210. [DOI] [PubMed] [Google Scholar]
- 23. Ward SK,, Wakamatsu TH,, Dogru M,, et al. The role of oxidative stress and inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2010; 51: 1994–2002. [DOI] [PubMed] [Google Scholar]
- 24. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002; 56: 365–379. [DOI] [PubMed] [Google Scholar]
- 25. Muhlhausler BS,, Cook-Johnson R,, James M,, Miljkovic D,, Duthoit E,, Gibson R. Opposing effects of omega-3 and omega-6 long chain polyunsaturated fatty acids on the expression of lipogenic genes in omental and retroperitoneal adipose depots in the rat. J Nutr Metab. 2010; 2010: 927836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasai H. Chemistry-based studies on oxidative DNA damage: formation, repair, and mutagenesis. Free Radic Biol Med. 2002; 33: 450–456. [PubMed] [Google Scholar]
- 27. Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997; 387: 147–163. [DOI] [PubMed] [Google Scholar]
- 28. Kau HC,, Tsai CC,, Lee CF,, et al. Increased oxidative DNA damage, 8-hydroxydeoxy- guanosine, in human pterygium. Eye (Lond). 2006; 20: 826–831. [DOI] [PubMed] [Google Scholar]
- 29. Pauloin T,, Dutot M,, Joly F,, Warnet JM,, Rat P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol Vis. 2009; 15: 577–583. [PMC free article] [PubMed] [Google Scholar]
- 30. United States Environmental Protection Agency Monthly UV Index. Available at: https://www.epa.gov/sunsafety/sun-safety-monthly-average-uv-index. Accessed September 25, 2014.
- 31. Godar DE,, Wengraitis SP,, Shreffler J,, Sliney DH. UV doses of Americans. Photochem Photobiol. 2001; 73: 621–629. [DOI] [PubMed] [Google Scholar]
- 32. Chai D,, Gaster RN,, Roizenblatt R,, Juhasz T,, Brown DJ,, Jester JV. Quantitative assessment of UVA-riboflavin corneal cross-linking using nonlinear optical microscopy. Invest Ophthalmol Vis Sci. 2011; 52: 4231–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]