Abstract
Activated fibroblasts are deemed the main executors of organ fibrosis. However, regulation of the pathologic functions of these cells in vivo is poorly understood. PDGF receptor β (PDGFRβ) is highly expressed in activated pericytes, a main source of fibroblasts. Studies using a PDGFRβ promoter–driven Cre system to delete αv integrins in activated fibroblasts identified these integrins as core regulators of fibroblast activity across solid organs, including the kidneys. Here, we used the same PDGFRβ-Cre line to isolate and study renal fibroblasts ex vivo. We found that renal fibroblasts express three αv integrins, namely αvβ1, αvβ3, and αvβ5. Blockade of αvβ1 prevented direct binding of fibroblasts to the latency-associated peptide of TGF-β1 and prevented activation of the latent TGF-β complex. Continuous administration of a recently described potent small molecule inhibitor of αvβ1, compound 8, starting the day of unilateral ureteral obstruction operation, inhibited collagen deposition in the kidneys of mice 14 days later. Compound 8 also effectively attenuated renal failure, as measured by BUN levels in mice fed an adenine diet known to cause renal injury followed by fibrosis. Inhibition of αvβ1 integrin could thus hold promise as a therapeutic intervention in CKD characterized by renal fibrosis.
Keywords: Integrin, TGFβ, renal fibrosis, CKD, UUO, Adenine
CKD is an increasingly significant public health concern affecting 26 million Americans.1 Currently, no effective agent exists that can directly halt disease progression.
Regardless of the cause, renal fibrosis is a final common pathway of CKD. Fibroblasts are key effectors of fibrosis and contribute to excessive production of extracellular matrix. We recently reported that fibroblast-derived αv integrins contribute importantly to fibrosis.2 Selective αv deletion using PDGF receptor β (PDGFRβ)–Cre protected mice from renal fibrosis after UUO, bleomycin-induced lung fibrosis, and carbon tetrachloride–induced liver fibrosis.2 Integrins are transmembrane receptors with an α and a β subunit. There are five αv integrins, αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8. Global deletion of αvβ3 or αvβ5 or fibroblast-specific deletion of αvβ8 (mice with global β8 deletion die in utero) did not protect mice against liver fibrosis.2 Therefore, either multiple fibroblast αv integrins contribute to tissue fibrosis or the protection is mostly due to loss of αvβ1. Until now, it was impossible to study the in vivo role of αvβ1 because mice lacking β1 on fibroblasts do not survive. To address this, we developed a highly potent small molecule inhibitor of αvβ1 called Compound 8 (C8). Our colleagues Reed et al. recently published that C8 specifically reduced cell adhesion through αvβ1 and protected mice from pulmonary and hepatic fibrosis.3 Here, using the same C8 as described by Reed et al.3 and αv blocking antibodies, we studied the contribution of fibroblast αv integrins to the development of renal fibrosis. We found that renal fibroblasts express three of five αv integrins (αvβ1, αvβ3, and αvβ5). Among them, αvβ1 is the main αv integrin used by renal fibroblasts to directly bind to latency-associated peptide (LAP) of TGFβ1. Inhibition of either αvβ1 or αvβ3 reduced activation of latent TGF-β in vitro, but the effects of αvβ3 inhibition appeared to be an in vitro artifact mediated by loss of adhesion to serum-coated tissue culture plates. Therapeutic delivery of αvβ1 inhibitor C8 significantly attenuated UUO-induced renal fibrosis in vivo, whereas global αvβ3 genetic deletion did not protect mice from renal fibrosis. More importantly, C8 partially rescued renal failure in mice fed an adenine diet known to cause elevated BUN, renal tubular toxicity, and interstitial fibrosis. These results identified αvβ1 integrin as a promising target for antifibrosis therapy.
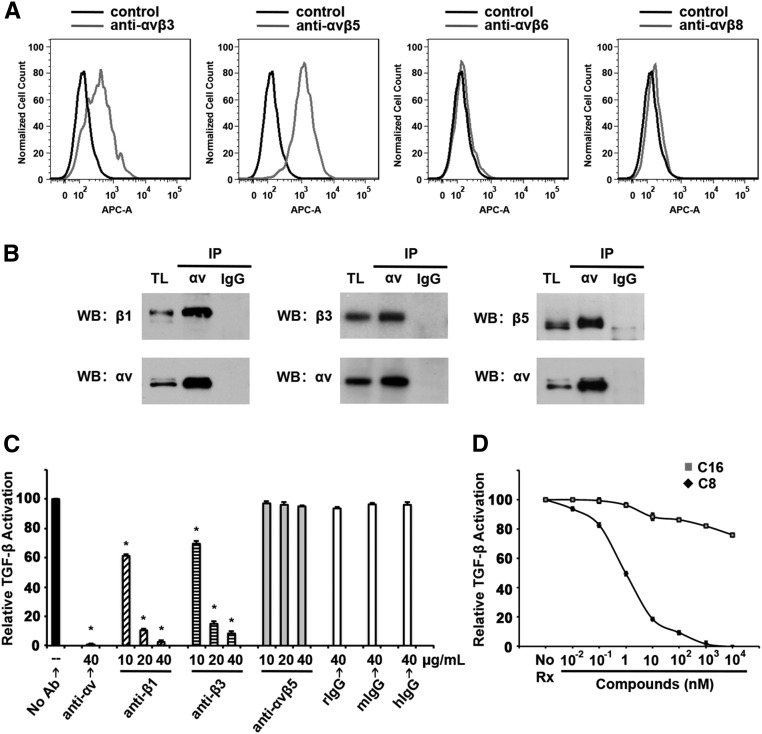
We first examined the expression profile of αv integrins in activated renal fibroblasts. Fibroblasts were isolated from PDGFRβ-Cre, Ai14tdtomatoFlox/Flox mouse kidneys using Fluorescence Activated Cell Sorting (FACS) and then differentiated in culture for 1 week before experiments. Flow cytometry with specific antibodies showed that these cells express αvβ3 and αvβ5 (Figure 1A). They do not express αvβ6, an epithelium-restricted integrin, or αvβ8 (Figure 1A). Flow cytometry using an antibody against mouse αvβ1 was not done because no such antibody exists. However, we were able to detect αvβ1 expression by coimmunoprecipitation (co-IP) followed by western blot (WB) (Figure 1B). Co-IP experiments also confirmed the expression of αvβ3 and αvβ5 on these cells (Figure 1B).
Figure 1.
Renal fibroblasts express multiple αv integrins and activate TGF-β. (A) Tdtomato-positive kidney cells isolated from PDGFRβ-Cre, Ai14tdtomatoFlox/Flox mice and differentiated in culture were subjected to flow cytometry analysis. Cells were incubated with either secondary antibody alone (control, in black) or first with mouse mAbs (in gray) for αvβ3 (Axum-4), αvβ5 (Alula), αvβ6 (3G9), or αvβ8 (Adwa11), all generated by immunizing knockout mice with integrin subunits. (B) Co-IP and WB of cell lysates of isolated Tdtomato-positive mouse kidney cells show that these cells express αvβ1, αvβ3, and αvβ5. Cell lysates were immunoprecipitated with anti-αv antibodies (RMV-7) or isotype control rat IgG1κ. Each immunoprecipitate was divided in half and each half was subjected to WB with antibodies against αv and a respective β integrin subunit. TL, total lysates. (C) Isolated Tdtomato-positive cells were used in coculture TGF-β activation assay in the presence or absence of blocking antibodies against αv (rat monoclonal IgG1κ), β1 (hamster monoclonal IgG1κ), β3 (hamster monoclonal IgG1κ), or αvβ5 (mouse monoclonal IgG). *P<0.05, specific antibody versus corresponding isotype control-treated samples). hIgG, hamster IgG1κ; mIgG, mouse IgG; rIgG, rat IgG1κ. (D) TGF-β activation assays with isolated Tdtomato-positive cell in the presence of C8 (line with black markers) or inactive control compound C16 (line with gray markers) at indicated concentrations. No Rx, no treatment. For both (C and D), relative TGF-β activation is expressed as percentage luminescence of each treatment sample compared with untreated samples after subtraction of TGF-β–independent luminescence. Data shown are the mean±SEM from three experiments.
We and others have shown that αv integrins can bind LAP through the RGD motif,2,4–7 thereby activating latent TGF-β. To investigate whether renal fibroblasts can directly activate TGF-β and, if so, which αv integrins are responsible for that action, we performed TGF-β activation assays in the presence of specific integrin–blocking antibodies (Figure 1C). Isolated and differentiated Tdtomato-positive renal fibroblasts were cocultured with mink lung epithelial cells (TMLC) expressing firefly luciferase under the control of a TGF-β responsive portion of plasminogen activator inhibitor 1 (PAI-1) promoter. Renal fibroblast–induced luciferase activity was well inhibited by pan-αv antibodies. β1 or β3 blocking antibodies also significantly reduced luciferase activity, whereas antibodies against αvβ5 had no effect. We next used the αvβ1 inhibitor C8 described by Reed et al.3 C8 attenuated TGF-β activation by fibroblasts starting at 0.01 nM concentration (Figure 1D). At 1 nM, C8 reduced TGF-β activation by >50%, consistent with the described IC50 for this compound.3 These data demonstrated that renal fibroblasts can activate TGF-β in vitro and that blockade of either αvβ1 or αvβ3 reduces that capacity.
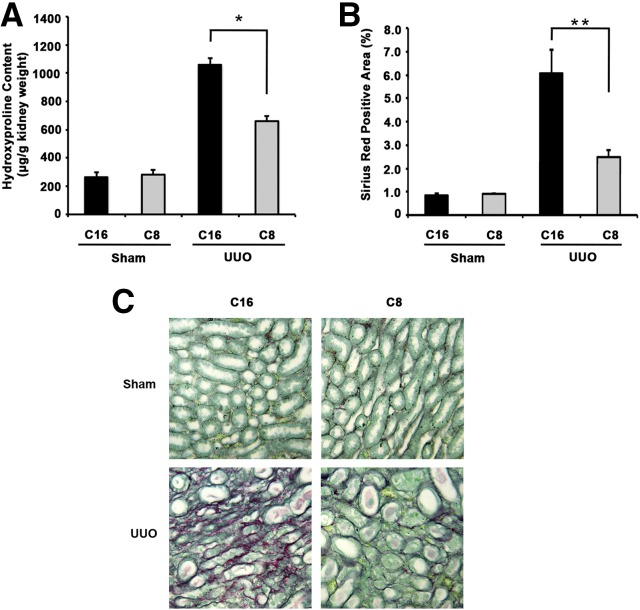
We next examined the effects of in vivo αvβ1 or αvβ3 inhibition on renal fibrosis. To inhibit αvβ1 in vivo, C8 (or the inactive control, C16) was delivered by subcutaneous Alzet pump, placed on the day of UUO surgery, at 70 mg/kg per day. Collagen deposition in the kidneys was evaluated 14 days after surgery by two methods. First, we measured tissue content of hydroxyproline, a major component of collagen (Figure 2A). We next compared picrosirius red staining of fixed kidney tissue sections (Figure 2, B and C). C8 did not alter baseline collagen content. UUO surgery increased collagen content compared with sham operation in C16-treated groups. C8 treatment reduced collagen deposition after UUO by 38% as measured by hydroxyproline assay (Figure 2A) and by 59% as measured by quantification of picrosirius red staining (Figure 2B), respectively. Neither C16 nor C8 had any effect on sham-operated mouse kidneys (Supplemental Figure 1). Thus, C8 treatment does not directly cause structural changes in the kidneys at baseline, a finding that is encouraging because C8 may be a prototype for development of clinically potent antifibrotics.
Figure 2.
Specific αvβ1 blockade with small molecule inhibitor C8 ameliorates UUO-induced renal fibrosis in mice. (A) Hydroxyproline assay of kidney tissues harvested from wild-type mice 14 days after compound treatment. In both compound treatment groups, mice were further divided to undergo either UUO or sham operations (n=4–7 in each group). There is a significant increase in hydroxyproline content after UUO compared with sham operation. This increase is largely attenuated with C8 treatment (*P=0.001, C8 versus C16 treatment in UUO groups). (B) Percentage of sirius red–positive area was quantified. UUO kidneys treated with C8 showed 59% less sirius red positivity compared with C16 treatment group (**P=0.03, C8 versus C16 treatment in UUO groups). (C) Representative images of picrosirius red staining of kidney sections from C16 and C8 treatment groups are shown here. All images are original magnification, ×200. All kidneys were harvested 14 days postsham or -UUO operation.
Because αvβ3 inhibition in vitro also decreased TGF-β activation, we examined the consequences of αvβ3 deletion in vivo using Itgb3−/− mice. In contrast to αvβ1 blockade, genetic deletion of Itgb3 had no effect on collagen deposition in the kidneys after UUO as measured by hydroxyproline assays (Figure 3A) and picrosirius red staining of collagen (Figure 3, B and C).
Figure 3.
αvβ3 gene deletion does not protect mice from UUO-induced renal fibrosis. (A) Hydroxyproline assay of kidney tissues from wild-type and Itgb3−/− mice 14 days after sham or UUO operations (n=4–10 in each group). (B) Percentage of sirius red–positive area was quantified. There is no significant difference in hydroxyproline content (P=0.33) or percentage sirius red positivity (P=0.44) between UUO kidneys of wild-type and Itgb3−/− mice. NS, not significant. (C) Representative images of picrosirius red staining of kidney sections from wild-type and Itgb3−/− mice after sham or UUO operations are shown here. All images are original magnification, ×200. All kidneys were harvested 14 days postsham or -UUO operation.
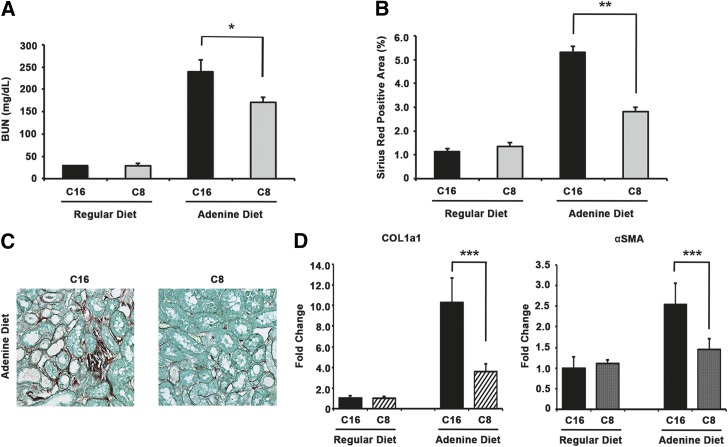
To examine whether αvβ1 blockade in vivo can rescue renal failure in addition to reducing tissue fibrosis, we treated mice with C8 after induction of renal failure by adenine diet. Adenine diet has been shown to cause renal injury characterized by increase in BUN levels and tubulointerstitial fibrosis.8 Mice were fed adenine diet for 28 days total. By day 12, mice developed significant renal failure and renal fibrosis as evidenced by elevated BUN levels and tissue collagen deposition in adenine diet groups (data not shown). On day 14, Alzet pumps containing C8 (or C16) were placed to deliver compounds continuously from days 14–28. By day 28, BUN levels were highly elevated in control compound C16-treated mice. In contrast, C8 treatment reduced the increase in BUN by 28% (BUN 240±27 mg/dl in C16 group, 171±11 mg/dl in C8 group; P=0.04) (Figure 4A). Although it does not completely reverse renal failure, αvβ1 blockade by C8 can reduce the severity of renal failure. Similar to the UUO model, we observed that C8 treatment attenuated the degree of renal fibrosis on the basis of tissue collagen deposition (Figure 4, B and C). Quantitative RT-PCR showed that expression of COL1a1 and αSMA was also lower in C8-treated mouse kidneys compared with C16-treated ones after adenine diet (Figure 4D). Thus, αvβ1 inhibition both reduced the degree of renal fibrosis and effectively preserved residual renal function in a therapeutic model where intervention was begun after the onset of renal failure.
Figure 4.
αvβ1 blockade with small molecule inhibitor C8 reduces adenine diet–induced renal failure and tissue fibrosis in mice. (A) Mice fed an adenine diet had increased BUN levels. This increase was attenuated in mice treated with C8 compared with C16 treatment (average BUN: 240±27 mg/dl in C16 group with adenine diet, n=7; 170±11 mg/dl in C8 group with adenine diet, n=6; *P=0.04, C8 versus C16 treatment in adenine diet groups). (B) Adenine diet induced significant interstitial fibrosis in mouse kidneys as evidenced by increased picrosirius red–positive area. This was significantly reduced with C8 treatment (average picrosirius red–positive area: 5.30%±0.25% in C16 group and 2.82%±0.2% in C8 group after adenine diet, **P<0.001). (C) Shown here are representative images of picrosirius red staining of kidney sections from C16- and C8-treated mice fed an adenine diet. All images are original magnification, ×200. (D) Kidney tissue expression of Col1a1 and αSMA was measured using qRT-PCR. Both markers increased after adenine diet but the increase was significantly smaller in the C8 treatment group (***P=0.02 for Col1a1 and P=0.04 for αSMA, C8 versus C16 treatment in adenine diet groups). Kidneys and blood samples were obtained 28 days after initiation of adenine diet. Mice were treated with C8 (or C16) on days 14–28.
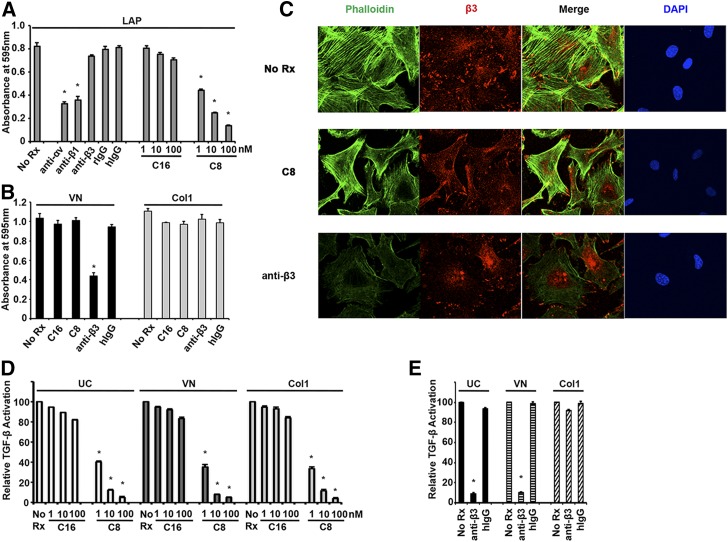
One well described mechanism to activate TGF-β is integrin binding to the RGD motif of LAP, thereby causing a conformational change of the latent TGF-β complex and freeing active TGF-β1.9 We thus determined whether isolated renal fibroblasts could directly bind LAP using either αvβ1 or αvβ3. Isolated renal fibroblasts were plated on LAP-coated dishes with or without specific antibodies or inhibitors. Cell adhesion to LAP was significantly reduced by pan-αv antibody or β1 antibody (Figure 5A), but not by β3 blocking antibody. C8 also caused dose-dependent inhibition of adhesion to LAP (Figure 5A). Therefore, αvβ1 (not αvβ3) is the key αv integrin used by renal fibroblasts to directly bind LAP. We next set out to determine why αvβ3 blockade also appeared to inhibit TGF-β activation (Figure 1C). Because TGF-β activation assays were carried out in the presence of calf serum containing vitronectin, a known substrate for αvβ3, we hypothesized that β3 antibody interfered with cell adhesion to vitronectin rather than directly affecting TGF-β activation. β3 antibody indeed reduced cell adhesion to vitronectin by 55% compared with no treatment or isotype control IgG treatment (Figure 5B). As expected, C8 did not change cell adhesion to vitronectin because αvβ1 is not a principle receptor for vitronectin. Neither β3 antibody nor C8 suppressed cell adhesion to collagen 1 (Figure 5B). Renal fibroblasts formed well organized focal contacts and stress fibers when plated on vitronectin (Figure 5C). These were preserved in the presence of αvβ1 inhibitor C8 but largely diminished by anti-β3 antibody. We then performed TGF-β activation assays with renal fibroblasts plated on different matrix proteins (Figure 5, D and E). Like cells plated on uncoated dishes in the presence of serum, cells adherent to either vitronectin or collagen 1 can activate TGF-β (Figure 5D). C8 decreased TGF-β activation by fibroblasts regardless of the matrix protein they attached to (Figure 5D), whereas inhibition by β3 antibodies was specific for cells plated on uncoated dishes or vitronectin (Figure 5E). Thus, in vitro effects of β3 blockade were likely explained by inhibition of firm cell adhesion rather than any specific effect on TGF-β activation.
Figure 5.
αvβ1, but not αvβ3, mediates direct interaction of renal fibroblasts to TGF-β1 LAP. Inhibitory effects of β3 blocking antibodies are due to inhibition of cell tethering. (A) Adhesion assays of isolated Tdtomato-positive mouse kidney cells to LAP. Anti-αv and anti-β1 antibodies, but not anti-β3, significantly inhibited cell adhesion to LAP (*P<0.001, specific antibody versus its isotype control). αvβ1 inhibitor C8 also decreased cell binding to LAP starting at 1 nM concentration (*P<0.001, C8 versus C16 treatment at the same concentrations). All antibodies were used at 40 μg/ml. C16 and C8 were used at the concentrations indicated (1–100 nM). hIgG, hamster IgG1κ; no Rx, no treatment; rIgG, rat IgG1κ. (B) Adhesion assays of the same cells to vitronectin (VN) or collagen 1 (Col1). C8 did not alter cell adhesion to either VN or Col1. Anti-β3 decreased cell adhesion to VN but not Col1 (*P<0.001, anti-β3 versus hIgG). All antibodies were used at 40 μg/ml concentration. C8 and C16 were used at 100 nM concentrations. (C) Confocal microscopy images of cells plated on VN. Anti-β3 blocking antibodies reduced β3 recruitment to focal contacts and stress fiber formation (β3 staining in red, phalloidin staining in green, DAPI in blue). C8 had no effect on the formation of either focal contacts or stress fibers of cells adherent to VN. All images shown are original magnification, ×400. (D) TGF-β activation assays of isolated Tdtomato-positive cells plated on dishes that were uncoated (UC), or coated with VN or Col1. Cells were either untreated (No Rx) or treated with increasing concentrations of C16 or C8. C8 reduced cell activation of TGF-β regardless of the matrix they attached to (*P<0.001, C8 versus C16 treatment at the same concentrations). (E) TGF-β activation assays of cells either untreated (No Rx) or treated with anti-β3 antibody or its isotype control (hIgG). Anti-β3 antibody inhibited TGF-β activation by cells plated on uncoated dishes or dishes coated with VN (*P<0.001, anti-β3 versus hIgG). Anti-β3 antibodies did not affect TGF-β activation by cells adhering to Col1. Antibodies were used at 40 μg/ml. Data shown in (A, B, D, and E) are the mean±SEM from three experiments.
In summary, our study identified fibroblast αvβ1 as a critical regulator of renal fibrosis. It is the only αv integrin on renal fibroblasts that can bind and activate TGF-β. Small molecule inhibitor C8 was highly effective in blocking αvβ1 in vitro and in attenuating renal fibrosis and preserving renal function in vivo. This lead compound provides an excellent basis for designing pharmacologic agents to treat renal fibrosis and CKD.
The signaling pathways engaged by αvβ1 to promote fibrosis remain to be fully explored. One such pathway may involve interplay between αvβ1 and other integrins. Hartner et al. described de novo α8β1 expression on renal fibroblasts after UUO.10 They found that mice lacking α8β1 suffered worse UUO-induced renal fibrosis compared with wild-type controls, suggesting a possible protective effect of α8β1. α8β1 has been reported to bind LAP but does not activate TGFβ1.11 As mentioned in our earlier study, C8 is highly selective for αvβ1 versus α8β1 (IC50 approximately 1 nM for αvβ1, IC50 >100,000 nM for α8β1).3 It is conceivable that, by blocking αvβ1, C8 indirectly clears the way for α8β1 to bind to LAP-TGFβ1 thereby trapping TGFβ1 in its latent form. To test this hypothesis, kidney fibrosis models can be established in itga8−/− mice in future studies to examine the effects of C8.
Concise Methods
Mice
Ai14 (Rosa-CAG-LSL-tdTomato-WPRE) mice12 were obtained from Jackson Laboratory and crossed with PDGFRβ-Cre mice13 (obtained from Ralf Adams, University of Münster, Germany). They were maintained on C57BL/6 background. Itgb3−/− mice on 129/svJae background14 were obtained from Richard Hynes (Massachusetts Institute of Technology, Cambridge). Wild-type 129/svJae littermates were used as control in UUO experiments. Itgb8−/− mice were described previously15,16 and were maintained on CD1 background. Genotyping of all mice was performed by PCR as described previously.12–16 Wild-type C57/BL6 mice were purchased from Jackson Laboratory. Mice used for all experiments were 8–12 weeks old and were housed under specific pathogen–free conditions in the Animal Barrier Facility of the University of California San Francisco (UCSF) and University of California Irvine (UCI). All experiments were approved by the Institutional Animal Care and Use Committee of UCSF and UCI.
Unilateral Ureteral Obstruction
For renal fibrosis, UUO was induced by ligation of the left ureter in 8–12-week-old male mice as described previously.17 Sham-operated mice underwent an identical surgical procedure except that ligation of the ureter was not performed. Kidneys were harvested 14 days after surgery. To treat mice with small molecule inhibitors, Alzet pumps containing C8 or C16 were placed subcutaneously on the day of surgery and continued to release compounds for 14 days at a rate of 70 mg/kg per day. The chemical composition and production of the compounds was described in detail in our recent publication.3
Adenine Diet–Induced Renal Injury Model
Mice were fed 0.2% adenine diet to induce renal injury as described previously.8 Wild-type C57/BL6 mice 10–12 weeks old were fed with either 0.2% adenine diet or regular diet as control for a total of 28 days. On day 14, Alzet pumps containing C8 or C16 were placed subcutaneously and continued to release compounds for 14 days at a rate of 70 mg/kg per day. Mice continued to receive adenine diet from days 14–28 while receiving compound treatment. BUN levels were measured on day 12 (before Alzet pump placement) to confirm establishment of renal injury and on day 28. Kidneys were harvested on day 28 of the experiment. Again, the chemical composition and production of the compounds was described in detail in our recent publication.3
Hydroxyproline Assay
The left kidney was extracted from the mouse and cut in half. One half kidney was used for hydroxyproline assay and the other half was fixed in 10% formalin for histology studies. Hydroxyproline assay was described previously.2 Briefly, half kidneys were weighed, homogenized, and then precipitated with trichloroacetic acid. The precipitants were baked overnight at 110°C in HCl. Samples were reconstituted in water, and hydroxyproline content was measured using a colorimetric chloramine T assay.
Primary Cell Isolation and FACS
Mice were perfused with PBS through the left ventricle to remove blood cells. The kidneys were excised and the capsules removed. They were then minced with scissors into pieces 2 mm in size and digested in Dulbecco Modified Eagle Medium (DMEM; Invitrogen) containing 0.13 IU/ml Liberase (Roche) and 0.5 mg/ml collagenase 4 (Sigma). The cell suspension was shaken (200–250 rpm) at 37°C for 30 minutes and then gently vortexed for 30 seconds. The cell suspension was passed through a 40 μM cell strainer and centrifuged at 1000 rpm for 5 minutes to form a pellet. To remove residual red blood cells, the cell pellet was resuspended in red blood cell lysis buffer and left to sit at room temperature for 10 minutes. The cells were washed with DMEM twice to remove cell debris and resuspended in FACS buffer (PBS supplemented with 3% FCS). After live/dead staining with sytox blue (Invitrogen), live Tdtomato-positive cells from PDGFRβ-Cre, Ai14tdtomatoFlox/Flox mice were sorted using FACSAria (BD Biosciences). Tdtomato-positive cells were cultured in DMEM supplemented with glucose, L-glutamine, penicillin/streptomycin, and 15% FCS.
Flow Cytometry
Cell-sorted Tdtomato-positive cells from kidneys of PDGFRβ-Cre, Ai14tdtomatoFlox/Flox mice were plated on tissue culture plastic for 7 days before experiments. Cells were harvested with trypsin, washed twice with PBS, and resuspended in FACS buffer containing 10% normal goat serum (Jackson Laboratory). Cells were then washed twice with PBS and sequentially incubated with primary antibodies and secondary antibodies (or secondary antibodies only for control) at 4°C for 30 minutes each. At the end of incubation, the cells were washed with PBS and analyzed on a Becton Dickinson FACS Canto II. The primary antibodies used were mouse mAbs generated in our laboratory: Axum-4 (αvβ3),18 Alula (αvβ5),19 3G9 (αvβ6),20 and Adwa11 (αvβ8). Mouse monoclonal αvβ8 antibody Adwa11 was generated by injecting Itgb8−/− mice with recombinant human αvβ8 protein (Cat#4135-AV-050; R&D Systems). The specificity of Adwa11 to αvβ8 was tested on 293T cells stably expressing human αvβ8 (293T-β8 cells21) and mouse astrocytes (obtained from ATCC) known to express αvβ8 (Supplemental Figure 2). APC-conjugated goat anti-mouse (Invitrogen) was used as secondary antibody.
Immunoprecipitation and WB
Isolated and cultured Tdtomato-positive cells were solubilized in HBSM buffer (20 mM Hepes, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 5 mM MgCl2) containing 1% Triton-100 detergent and protease inhibitors. Cleared lysates from 2 × 106 cells were divided into two tubes. Cell lysates in each tube were incubated with rotation with 10 μg of either αv integrin antibody (clone RMV-7; the hybridoma was a generous gift from Dr. Hideo Yagita, Juntendo University, Japan22) or rat IgG1κ isotype control (BioLegend) for 2 hours at 4°C. Immune complexes were collected with protein G agarose (GE Health Care) and eluted with reducing Laemmli sample buffer by boiling for 5 minutes. The samples were then subjected to SDS-PAGE and WB. Antibodies used for WB were mouse anti-αv integrin mAb (clone 21/CD51, Cat#611012; BD Biosciences), rabbit anti-β1 integrin polyclonal antibody (Cat#ab1952; Millipore), rabbit anti-β3 integrin mAb (clone EPR2417Y, Cat#ab75872; Abcam), and rabbit anti-β5 integrin polyclonal antibody (Cat#ab15459; Abcam).
TGF-β Activation Assay
Isolated Tdtomato-positive cells were cultured for 7 days before experiments. For TGF-β activation assay, cells were plated at 50,000 cells/well density in 96-well plates with mink lung epithelial cells (TMLC) expressing firefly luciferase downstream of a TGF-β–sensitive portion of the PAI-1 promoter (15,000 cells/well). Cells were cocultured with or without specific antibodies or compounds for 16 hours before lysis. TGF-β activity was calculated by measurement of luminescence of cell lysates from each well. There was baseline TGF-β–independent PAI-1 activity in samples treated with TGF-β blocking antibody (1D11, generated in our laboratory from a hybridoma obtained from ATCC). We subtracted the luminescence of 1D11 treatment samples (TGF-β–independent activity) from the luminescence of other treated or untreated conditions and consider the remaining portion as specific TGF-β activation. The specific functional blocking antibodies used were rat anti-αv mAb (clone RMV-7, Cat#CBL1346Z-I; Millipore), hamster anti-β1 mAb (clone HMβ1–1, Cat#102209; BioLegend), hamster anti-β3 monoclonal (clone 2C9.G2, Cat#104309; BioLegend), and mouse anti-αvβ5 mAb (Alula, produced in our laboratory18). Isotype control antibodies used were rat IgG1κ, mouse IgG1, and hamster IgG1κ (all from BioLegend).
Immunohistochemistry and Immunofluorescence
Paraffin-embedded sections were processed for immunohistochemistry as described previously.23 Five-micrometer sections were stained with picrosirius red or hematoxylin and eosin. To quantify picrosirius red positivity, a Leica CTR5000 microscope was used to capture ten nonoverlapping fields of each kidney section at a final magnification of 200× and analysis was performed using ImageJ as described previously.23,24 The same microscope was used to capture images from hematoxylin and eosin–stained kidney sections at a final magnification of 200×. For immunofluorescence staining, cells were plated on either uncoated cover slips or cover slips coated with 1 μg/ml vitronectin (R&D Systems) or 10 μg/ml collagen 1 (BD Biosciences). Cells were incubated with or without anti-β3 antibodies (BioLegend) or compounds (C8) at 37°C for 3 hours. Cells were then fixed in ice-cold acetone, and incubated with rabbit anti-β3 antibody mAb (clone EPR2417Y, Cat#ab75872; Abcam) and Alexa 647 Fluor-conjugated secondary antibodies (Invitrogen). FITC-conjugated phalloidin was used to stain stress fibers (Sigma) and DAPI (Invitrogen) was used for nuclear staining. Images were acquired using a Leica LSM510 NLO Meta confocal microscopy system. Representative single scan images were recompiled in Adobe Photoshop Elements 12 software in accordance with image manipulation guidelines of this journal.
Quantitative RT-PCR
Total RNA was isolated from mouse kidneys using an RNeasy kit (Qiagen). cDNA was analyzed by SYBR Green real-time PCR with a ViiA 7 System thermocycler and normalized to 18S expression. Primers used were as follows: Col1A1 forward: GCTCCTCTTAGGGGCCACT, Col1A1 reverse: CCACGTCTCACCATTGGGG; α-SMA forward: GTCCCAGACATCAGGGAGTAA, α-SMA reverse: TCGGATACTTCAGCGTCAGGA; 18S forward: TAGAGGGACAAGTGGCGTTC, 18S reverse: CGCTGAGC-CAGTCAGTGT.
Adhesion Assay
Cell adhesion assays were performed on 96-well tissue culture plates precoated with vitronectin (R&D Systems), collagen I (BD Biosciences), LAP (recombinant human LAP TGF-β1), or BSA as controls, as described previously.2 Briefly, cells were allowed to adhere for 60 minutes, washed with PBS, and fixed and stained with solution containing 0.5% crystal violet, 1% formaldehyde, and 20% methanol. Cells were then extracted with 2% Triton X-100 before luminescence was read at 595 nm.
Statistical Analyses
All data are presented as mean±SEM. Statistical significance was calculated using a two-tailed paired t test. Differences with a P value of <0.05 were considered statistically significant.
Disclosures
D.S., W.F.D., H.J., and N.I.R. are inventors of US Patent application 61/884,583 related to this work. D.S. and W.F.D. are cofounders of Pliant Therapeutics, Inc.
Supplementary Material
Acknowledgments
We appreciate expert input from Dr. Xiaozhu Huang on the assessment of mouse renal pathology. We thank Dr. Xin Ren, Dr. Nanyan Wu, and Ms. Yanli Wang for their technical assistance in performing flow cytometry analysis and histochemistry. We also thank Dr. Nosratola D. Vaziri for providing his expertise and the laboratory space to perform part of this work.
This work was supported by a Ben J. Lipps Research Fellowship from the American Society of Nephrology (ASN) (Y.C.), National Institute of Diabetes and Digestive and Kidney Diseases F32DK100084 (Y.C.), National Heart, Lung, and Blood Institute HL123423 (D.S. and W.F.D.), a grant from the University of California, San Francisco Program in Biomedical Breakthrough Research funded in part by the Sandler Foundation (W.F.D. and D.S.), and research funding from the Division of Nephrology and Hypertension at University of California, Irvine funded in part by a generous donation from Dr. Joseph Lee (Y.C.).
Part of the findings of this manuscript was presented as an oral abstract at the ASN Kidney Week 2013 (abstract #TH-OR035) on November 7, 2013, Atlanta, Georgia.
Compound C8 was provided in part by Pliant Therapeutics, Inc. via a material transfer agreement with University of California, Irvine.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050585/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007. 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 2.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D: Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19: 1617–1624, 2013. 10.1038/nm.3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, Sheppard D: The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med 7: 288ra79, 2015. 10.1126/scitranslmed.aaa5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D: The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL: The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 157: 493–507, 2002. 10.1083/jcb.200109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wipff PJ, Rifkin DB, Meister JJ, Hinz B: Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007. 10.1083/jcb.200704042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Xu SW, Blumbach K, Eastwood M, Denton CP, Eckes B, Krieg T, Abraham DJ, Leask A: Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci 123: 3674–3682, 2010. 10.1242/jcs.070672 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Doi K, Maeda-Mamiya R, Negishi K, Portilla D, Sugaya T, Fujita T, Noiri E: Urinary L-type fatty acid-binding protein can reflect renal tubulointerstitial injury. Am J Pathol 174: 1203–1211, 2009. 10.2353/ajpath.2009.080511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson NC, Sheppard D: Integrin-mediated regulation of TGFβ in fibrosis. Biochim Biophys Acta 1832: 891–896, 2013. 10.1016/j.bbadis.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartner A, Menendez-Castro C, Cordasic N, Marek I, Volkert G, Klanke B, Rascher W, Hilgers KF: Tubulointerstitial de novo expression of the α8 integrin chain in a rodent model of renal fibrosis--a potential target for anti-fibrotic therapy? PLoS One 7: e48362, 2012. 10.1371/journal.pone.0048362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM: Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J Cell Sci 115: 4641–4648, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H: A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH: Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006. 10.1016/j.cell.2005.10.034 [DOI] [PubMed] [Google Scholar]
- 14.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Culleré M, Ross FP, Coller BS, Teitelbaum S, Hynes RO: Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 103: 229–238, 1999. 10.1172/JCI5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF: Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci 25: 9940–9948, 2005. 10.1523/JNEUROSCI.3467-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D: Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365, 2007. 10.1038/nature06110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB: Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol 163: 1261–1273, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A, Burlingame AL, Matthay MA, Sheppard D: IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia. Am J Physiol Lung Cell Mol Physiol 303: L12–L19, 2012. 10.1152/ajplung.00375.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su G, Atakilit A, Li JT, Wu N, Luong J, Chen R, Bhattacharya M, Sheppard D: Effective treatment of mouse sepsis with an inhibitory antibody targeting integrin αvβ5. Crit Care Med 41: 546–553, 2013. 10.1097/CCM.0b013e3182711b1e [DOI] [PubMed] [Google Scholar]
- 20.Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, Sheppard D, Violette SM: Function-blocking integrin alphavbeta6 monoclonal antibodies: Distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem 279: 17875–17887, 2004. 10.1074/jbc.M312103200 [DOI] [PubMed] [Google Scholar]
- 21.Nishimura SL, Sheppard D, Pytela R: Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J Biol Chem 269: 28708–28715, 1994 [PubMed] [Google Scholar]
- 22.Takahashi K, Nakamura T, Koyanagi M, Kato K, Hashimoto Y, Yagita H, Okumura K: A murine very late activation antigen-like extracellular matrix receptor involved in CD2- and lymphocyte function-associated antigen-1-independent killer-target cell interaction. J Immunol 145: 4371–4379, 1990 [PubMed] [Google Scholar]
- 23.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T: Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 103: 5060–5065, 2006. 10.1073/pnas.0511167103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM: Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120: 1056–1068, 2010. 10.1172/JCI41563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.