Abstract
Kidney transplant recipients often receive antibody induction. Previous studies of induction therapy were often limited by short follow-up and/or absence of information about complications. After linking Organ Procurement and Transplantation Network data with Medicare claims, we compared outcomes between three induction therapies for kidney recipients. Using novel matching techniques developed on the basis of 15 clinical and demographic characteristics, we generated 1:1 pairs of alemtuzumab–rabbit antithymocyte globulin (rATG) (5330 pairs) and basiliximab-rATG (9378 pairs) recipients. We used paired Cox regression to analyze the primary outcomes of death and death or allograft failure. Secondary outcomes included death or sepsis, death or lymphoma, death or melanoma, and healthcare resource utilization within 1 year. Compared with rATG recipients, alemtuzumab recipients had higher risk of death (hazard ratio [HR], 1.14; 95% confidence interval [95% CI], 1.03 to 1.26; P<0.01) and death or allograft failure (HR, 1.18; 95% CI, 1.09 to 1.28; P<0.001). Results for death as well as death or allograft failure were generally consistent among elderly and nonelderly subgroups and among pairs receiving oral prednisone. Compared with rATG recipients, basiliximab recipients had higher risk of death (HR, 1.08; 95% CI, 1.01 to 1.16; P=0.03) and death or lymphoma (HR, 1.12; 95% CI, 1.01 to 1.23; P=0.03), although these differences were not confirmed in subgroup analyses. One-year resource utilization was slightly lower among alemtuzumab recipients than among rATG recipients, but did not differ between basiliximab and rATG recipients. This observational evidence indicates that, compared with alemtuzumab and basiliximab, rATG associates with lower risk of adverse outcomes, including mortality.
Keywords: cancer, acute rejection, transplant outcomes, immunosuppression, survival
Antibody induction is a common and costly therapy during kidney transplantation.1 As of 2014, approximately 90% of adult kidney transplant recipients (KTRs) received some form of antibody induction, more than double the percentage in 1999.2–5 Induction therapy offers several potential benefits in kidney transplantation, including reduced risk of acute rejection and, potentially, longer graft survival.6–9 Antibody induction agents may have particular value for patients with high immunologic reactivity and elevated probability of acute T cell rejection.9,10
Less is known about long-term consequences of the choice of induction therapy on death, allograft failure, sepsis, and cancers, particularly in real-world practice. Infection is the second-highest cause of death among kidney transplant patients,11,12 and a particular concern for elderly recipients.13 KTRs also have an elevated risk of cancer relative to the general population.14 For example, immunosuppression intensity is an important determinant of the risk of post-transplant lymphoproliferative disease after kidney transplantation.15–19 Studies assessing the relative risks of these agents have often been hampered by short follow-up (in the case of randomized trials), limited power (with single-center studies), or the use of registry data, which have incomplete ascertainment of malignancy outcomes and sepsis. Furthermore, healthcare resource utilization of patients following various induction therapies is poorly understood.
In 2014, the most commonly used induction therapies in kidney transplantation were rabbit antithymocyte globulin (rATG) (approximately 50%), basiliximab (approximately 20%), and alemtuzumab (approximately 15%).4,20,21 rATG is a poly-clonal T cell–depleting antibody manufactured in rabbits.20 Alemtuzumab is a humanized mAb that depletes B and T cells by targeting the CD-52 glycoprotein cell surfaces.22 Some proponents of alemtuzumab endorsed the idea that this therapy could facilitate steroid-free transplants.20,23,24 Basiliximab is a nondepleting mAb that prevents T cell activation by blocking the IL-2 receptor on cell surfaces. A randomized trial suggests basiliximab is associated with fewer infections overall than rATG, but is less effective at preventing acute allograft rejection.11,20 Thus, patients with high risk of acute rejection typically receive a more potent agent such as alemtuzumab or rATG.25–27
To compare a broad range of outcomes by induction strategy, we linked data from the Organ Procurement and Transplantation Network (OPTN) and the Centers for Medicare and Medicaid Services (CMS). To generate transparent results and minimize confounding, we leveraged recent advances in multivariable matching28–32 and created precisely matched pairs of KTRs exposed to rATG versus alemtuzumab, and rATG versus basiliximab.
Results
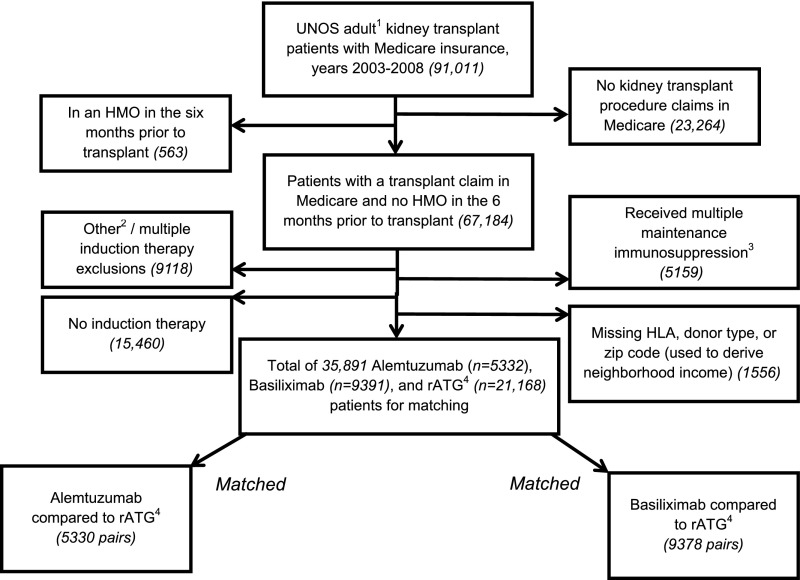
Figure 1 shows cohort generation. Among the 21,168 rATG recipients, we generated matches to 5330 alemtuzumab recipients and 9378 basiliximab recipients (>99% of alemtuzumab and basiliximab recipients meeting study criteria were matched).
Figure 1.
Generation of two cohorts of kidney transplant recipients matched on induction therapy. 1Adults defined as 18-90 years of age. 2Evidence of any of the following drugs: alg, anti-cam-1, anti-IL-6, anti-LFA-1, anti-tnf, atg, IL-1-receptor antagonist, okt3, okt4, rituximab, t10b9, xomazymecd5, zenapax, soluble il-1 receptor, nratgnrats. 3Evidence of multiple cell cycle targets, cytosolic protein inhibitors, or azathioprine. 4Rabbit antithymocyte globulin. UNOS, United Network for Organ Sharing; HMO, health maintenance organization; ALG, antilymphocyte globulin; anti-cam-1, anti-intercellular adhesion molecule 1; anti-LFA-1, anti-lymphocyte function-associated antigen 1; anti-tnf, anti-tumor necrosis factor; atg, equine antithymocyte globulin; nratgnrats, Nashville rabbit antithymocyte globulin/Nashville rabbit antithymocyte serum; okt3, Muromonab-CD3; okt4, Anti-CD4 Antibody-Cytokine Fusion Protein; t10b9, T10B9 monoclonal antibody; xomazymedcd5, Xoma Zyme - CD5+; zenapax, Zenapax - Daclizumab.
Table 1 shows the characteristics of the initial rATG cohort and the matched pairs. Before matching, a higher percentage of rATG relative to basiliximab recipients were black (30% versus 19%), had a previous kidney transplant (15% versus 7%), had peak panel reactive antibody (PRA) between 80% and 100% (12% versus 4%), and received deceased donor organs (58% versus 54%). These findings suggest that before matching, the basiliximab group was at lower immunologic risk than the rATG group. Before matching, a higher percentage of patients who received rATG relative to alemtuzumab recipients were black (30% versus 26%), had a previous kidney transplant (15% versus 11%), had peak PRA between 80% and 100% (12% versus 9%), and received deceased donor organs (58% versus 51%).
Table 1.
Subject characteristics
| Characteristics | Match 1: Alemtuzumab and rATG | All rATG (n=21,168) n (%) | Match 2: Basiliximab and rATG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Matched Alemtuzumab (n=5330), n (%) | Matched rATG (n=5330), n (%) | Stand. Diff. after Match | P Value after Match | Matched Basiliximab (n=9378), n (%) | Matched rATG (n=9378), n (%) | Stand. Diff. after Match | P Value after Match | ||
| Mean age | 50.5 | 50.6 | 0.00 | 0.85 | 50.2 | 51.3 | 51.3 | 0.00 | 0.88 |
| Age 65–90 yra | 857 (16) | 857 (16) | 0.00 | 1.00 | 3324 (16) | 1755 (19) | 1755 (19) | 0.00 | 1.00 |
| Men | 3185 (60) | 3201 (60) | −0.01 | 0.77 | 12,489 (59) | 5993 (64) | 5928 (63) | 0.01 | 0.33 |
| Black racea | 1382 (26) | 1382 (26) | 0.00 | 1.00 | 6431 (30) | 1781 (19) | 1781 (19) | 0.00 | 1.00 |
| Median neighborhood income, $ | 42,766 | 42,103 | 0.04 | 0.46 | 42,519 | 42,798 | 43,198 | −0.03 | 0.10 |
| Prior kidney transplanta | 603 (11) | 603 (11) | 0.00 | 1.00 | 3228 (15) | 640 (7) | 640 (7) | 0.00 | 1.00 |
| Peak PRAa | |||||||||
| 0%–20% | 3489 (65) | 3489 (65) | 0.00 | 1.00 | 13,417 (63) | 6931 (74) | 6931 (74) | 0.00 | 1.00 |
| 20%–80% | 608 (11) | 608 (11) | 0.00 | 1.00 | 2915 (14) | 879 (9) | 879 (9) | 0.00 | 1.00 |
| 80%–100% | 458 (9) | 458 (9) | 0.00 | 1.00 | 2452 (12) | 400 (4) | 400 (4) | 0.00 | 1.00 |
| Missing | 775 (15) | 775 (15) | 0.00 | 1.00 | 2384 (11) | 1168 (12) | 1168 (12) | 0.00 | 1.00 |
| HLA mismatcha | 4772 (90) | 4772 (90) | 0.00 | 1.00 | 19,134 (90) | 8330 (89) | 8330 (89) | 0.00 | 1.00 |
| Transplant year | 2006.5 | 2006.5 | 0.01 | 0.78 | 2006.2 | 2005.9 | 2006.0 | −0.02 | 0.23 |
| Recipient BMI | 27.7 | 27.6 | 0.02 | 0.90 | 27.6 | 27.4 | 27.4 | 0.00 | 0.54 |
| Weight, kg | 81.0 | 80.8 | 0.01 | 0.87 | 80.4 | 80.5 | 80.5 | 0.00 | 1.00 |
| Missing BMIa | 236 (4) | 236 (4) | 0.00 | 1.00 | 1944 (9) | 573 (6) | 573 (6) | 0.00 | 1.00 |
| Missing weight | 115 (2) | 112 (2) | 0.00 | 0.89 | 969 (5) | 210 (2) | 212 (2) | 0.00 | 0.96 |
| Missing BMI and weighta | 112 (2) | 112 (2) | 0.00 | 1.00 | 965 (5) | 210 (2) | 210 (2) | 0.00 | 1.00 |
| Time on dialysis | |||||||||
| 0–1 yr | 1083 (20) | 1118 (21) | −0.02 | 0.42 | 3573 (17) | 2010 (21) | 2043 (22) | −0.01 | 0.57 |
| 1–3 yr | 1426 (27) | 1388 (26) | 0.02 | 0.42 | 5181 (24) | 2618 (28) | 2650 (28) | −0.01 | 0.62 |
| 3–6 yr | 1300 (24) | 1306 (25) | 0.00 | 0.91 | 5531 (26) | 2143 (23) | 2180 (23) | −0.01 | 0.53 |
| 6–10 yr | 451 (8) | 464 (9) | −0.01 | 0.68 | 1825 (9) | 572 (6) | 577 (6) | 0.00 | 0.90 |
| >10 yr | 98 (2) | 81 (2) | 0.02 | 0.23 | 534 (3) | 126 (1) | 126 (1) | 0.00 | 1.00 |
| Missing | 972 (18) | 973 (18) | 0.00 | 1.00 | 4524 (21) | 1909 (20) | 1802 (19) | 0.03 | 0.05 |
| Cause of ESRD | |||||||||
| GN | 1040 (20) | 1035 (19) | 0.00 | 0.92 | 4034 (19) | 1841 (20) | 1844 (20) | 0.00 | 0.97 |
| HTN | 1078 (20) | 1041 (20) | 0.02 | 0.38 | 4365 (21) | 1764 (19) | 1785 (19) | −0.01 | 0.71 |
| PKD | 410 (8) | 382 (7) | 0.02 | 0.32 | 1463 (7) | 776 (8) | 769 (8) | 0.00 | 0.87 |
| Congenital | 77 (1) | 65 (1) | 0.02 | 0.35 | 336 (2) | 166 (2) | 156 (2) | 0.01 | 0.61 |
| Other | 1440 (27) | 1494 (28) | −0.02 | 0.25 | 6506 (31) | 2673 (29) | 2644 (28) | 0.01 | 0.65 |
| Diabetes | 1768 (33) | 1771 (33) | −0.00 | 0.97 | 6549 (31) | 3032 (32) | 3042 (32) | 0.00 | 0.89 |
| Hepatitis C+ | 172 (3) | 162 (3) | 0.01 | 0.62 | 1230 (6) | 494 (5) | 486 (5) | 0.00 | 0.82 |
| Donor type | |||||||||
| Living | 1856 (35) | 1880 (35) | −0.01 | 0.64 | 6192 (29) | 3364 (36) | 3336 (36) | 0.01 | 0.68 |
| Deceased | 2703 (51) | 2734 (51) | −0.01 | 0.56 | 12,186 (58) | 5042 (54) | 5018 (54) | 0.01 | 0.74 |
| Extended criteria | 771 (15) | 716 (13) | 0.03 | 0.13 | 2790 (13) | 972 (10) | 1024 (11) | −0.02 | 0.23 |
| Propensity score | 0.221 | 0.218 | 0.04 | 0.37 | 0.196 | 0.348 | 0.346 | 0.02 | 0.16 |
| Immunosuppression (not used for match) | |||||||||
| MMF or mycophenolate sodium | 4530 (85) | 5070 (95) | −0.34 | <0.001 | 20,135 (95) | 8836 (94) | 8932 (95) | −0.05 | 0.002 |
| Prednisone use | 1543 (29) | 3529 (66) | −0.82 | <0.001 | 14,685 (69) | 8143 (87) | 6177 (66) | 0.52 | <0.001 |
| Calcineurin or mTOR use | |||||||||
| Tacrolimus | 4514 (85) | 4590 (86) | −0.04 | 0.04 | 17,929 (85) | 6482 (69) | 7792 (83) | −0.34 | <0.001 |
| Cyclosporine | 345 (6) | 411 (8) | −0.05 | 0.01 | 1876 (9) | 1972 (21) | 970 (10) | 0.30 | <0.001 |
| Sirolimus | 74 (1) | 122 (2) | −0.07 | <0.001 | 499 (2) | 414 (4) | 235 (3) | 0.11 | <0.001 |
| None | 397 (7) | 207 (4) | 0.15 | <0.001 | 864 (4) | 510 (5) | 381 (4) | 0.06 | <0.001 |
Stand. Diff., standardized difference; HTN, hypertension; PKD, polycystic kidney disease; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin.
Variable matched exactly.
After matching, the demographic and clinical characteristics were extremely similar in both matches, with differences in means that were <5% of the SD and P values >0.05 for all matched characteristics. Among the alemtuzumab-rATG pairs, 78 centers used alemtuzumab and 188 centers used rATG. Among the basiliximab-rATG pairs, 196 centers used basiliximab and 201 centers used rATG.
As noted in the Concise Methods, we purposefully did not match on oral immunosuppression in the primary analyses. Over 75% of matched patients received tacrolimus. Prednisone was much more commonly prescribed to basiliximab (87%) and rATG (66%) versus alemtuzumab (29%) recipients (Supplemental Table 1, Table 1). Secondary analyses were conducted to compare outcomes among matched pairs who received tacrolimus and mycophenolate mofetil or mycophenolate sodium; these analyses separated pairs in which either both received maintenance prednisone or neither received prednisone.
Primary Outcomes
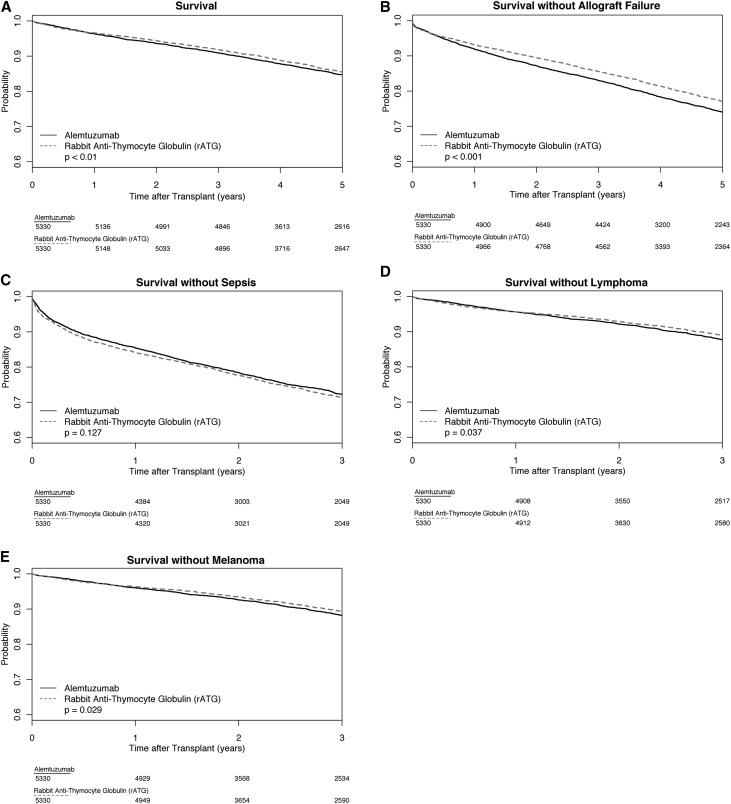
Relative to matched rATG recipients, alemtuzumab and basiliximab recipients had higher mortality risk (alemtuzumab hazard ratio [HR], 1.14; 95% confidence interval [95% CI], 1.03 to 1.26; P<0.01; basiliximab HR, 1.08; 95% CI, 1.01 to 1.16; P=0.03). Alemtuzumab recipients also had higher risk of death or allograft failure (HR, 1.18; 95% CI, 1.09 to 1.28; P<0.001) versus rATG recipients (Table 2). Figures 2 and 3 show Kaplan–Meier survival estimates for the outcomes, with scaled versions in the Supplemental Appendix (Supplemental Figure 1, A and B). Additional analyses of outcomes are presented in Supplemental Tables 2–7.
Table 2.
Outcomes after kidney transplantation among matched pairs
| Clinical Outcomes | HR | 95% CI Lower | 95% CI Upper | Paired Cox Model P Value |
|---|---|---|---|---|
| Alemtuzumab-rATG Matches | ||||
| Death | 1.14 | 1.03 | 1.26 | <0.01 |
| Death or allograft failure | 1.18 | 1.09 | 1.28 | <0.001 |
| Death or sepsis | 0.94 | 0.86 | 1.02 | 0.13 |
| Death or lymphoma | 1.15 | 1.01 | 1.32 | 0.04a |
| Death or melanoma | 1.17 | 1.02 | 1.34 | 0.03 |
| Basiliximab-rATG Matches | ||||
| Death | 1.08 | 1.01 | 1.16 | 0.03 |
| Death or allograft failure | 1.04 | 0.98 | 1.10 | 0.25a |
| Death or sepsis | 1.05 | 0.99 | 1.12 | 0.13a |
| Death or lymphoma | 1.12 | 1.01 | 1.23 | 0.03 |
| Death or melanoma | 1.10 | 1.00 | 1.21 | 0.05 |
rATG is the reference group. HRs, 95% CIs, and P values computed using a paired Cox proportional hazards model. Outcomes that include those derived from Medicare claims (those using sepsis, lymphoma, melanoma) are censored at 3 years post-transplant.
Subsequent analyses suggested the possibility that hazards were nonproportional over time (see Kaplan–Meier figures). For alemtuzumab-rATG pairs, the 3-year differences across groups for death or lymphoma were 1.3% (95% CI, −0.1% to 2.7%). For basiliximab-rATG pairs, the 5-year difference in death or allograft failure was 1.1% (95% CI, −0.1% to 2.4%), and 3-year differences in death or sepsis were 1.9% (95% CI, 0.4% to 3.2%). 95% CIs for differences in survival probabilities were generated using bootstrapping.
Figure 2.
Kaplan–Meier survival estimates for alemtuzumab-rATG pairs with five outcomes. (A) Survival. (B) Survival without allograft failure. (C) Survival without sepsis. (D) Survival without lymphoma. (E) Survival without melanoma.
Figure 3.
Kaplan–Meier survival estimates for basiliximab-rATG pairs with five outcomes. (A) Survival. (B) Survival without allograft failure. (C) Survival without sepsis. (D) Survival without lymphoma. (E) Survival without melanoma.
Secondary Outcomes
Patients who received alemtuzumab experienced modestly elevated risk of death or melanoma (HR, 1.17; 95% CI, 1.02 to 1.34; P=0.03) relative to patients who received rATG. We found that alemtuzumab recipients had a higher risk of death or lymphoma (HR, 1.15; 95% CI, 1.01 to 1.32; P=0.04), although subsequent analyses and inspection of the Kaplan–Meier figure revealed the possibility that the risk associated with alemtuzumab was nonproportional over time. At 3 years, alemtuzumab recipients had a 1.3% higher rate of death or lymphoma (95% CI, −0.1% to 2.7%) than rATG recipients (Supplemental Table 7).
Patients who received basiliximab had higher risk of death or lymphoma (basiliximab HR, 1.12; 95% CI, 1.01 to 1.23; P=0.03) versus patients who received rATG (Table 2).
Secondary Analyses of Pairs with Identical Maintenance Immunosuppression Medications
Among the subset of pairs in which both patients received prednisone (513 pairs), alemtuzumab recipients had higher risk of death or allograft failure relative to rATG recipients (HR, 1.35; 95% CI, 1.04 to 1.77; P=0.03). Matched alemtuzumab-rATG pairs without prednisone did not differ significantly in post-transplant outcomes. Basiliximab-rATG pairs with equivalent oral immunosuppression medications also did not differ in post-transplant outcomes (Table 3).
Table 3.
Outcomes after kidney transplantation among maintenance immunosuppression matched pairs; rATG is the reference group
| Clinical Outcomes | Prednisone | No Prednisone | ||
|---|---|---|---|---|
| HR (95% CI) | Paired Cox Model P Value | HR (95% CI) | Paired Cox Model P Value | |
| Alemtuzumab-rATG matches | n=513 Matched Pairs | n=973 Matched Pairs | ||
| Death | 1.37 (0.97 to 1.93) | 0.07 | 1.08 (0.82 to 1.42) | 0.58 |
| Death or allograft failure | 1.35 (1.04 to 1.77) | 0.03 | 1.16 (0.93 to 1.44) | 0.18 |
| Death or sepsis | 1.10 (0.85 to 1.44) | 0.46 | 0.98 (0.79 to 1.22) | 0.87 |
| Death or lymphoma | 1.05 (0.68 to 1.64) | 0.82 | 1.29 (0.90 to 1.86) | 0.17 |
| Death or melanoma | 1.09 (0.68 to 1.74) | 0.72 | 1.26 (0.86 to 1.84) | 0.25 |
| Basiliximab-rATG matches | n=2882 Matched Pairs | n=299 Matched Pairs | ||
| Death | 0.96 (0.85 to 1.09) | 0.56 | 1.15 (0.72 to 1.84) | 0.55 |
| Death or allograft failure | 0.97 (0.87 to 1.08) | 0.62 | 1.21 (0.81 to 1.81) | 0.36 |
| Death or sepsis | 1.03 (0.92 to 1.15) | 0.58 | 0.98 (0.65 to 1.48) | 0.92 |
| Death or lymphoma | 0.97 (0.82 to 1.16) | 0.76 | 1.06 (0.55 to 2.01) | 0.87 |
| Death or melanoma | 0.98 (0.81 to 1.17) | 0.78a | 0.82 (0.44 to 1.53) | 0.53 |
rATG is the reference group. HRs, 95% CIs, and P values computed using a paired Cox proportional hazards model. Outcomes that include those derived from Medicare claims (those using sepsis, lymphoma, melanoma) are censored at 3 years post-transplant.
Subsequent analyses suggested the possibility that hazards were nonproportional over time (see Kaplan–Meier figure). The 3-year difference with a bootstrapped 95% CI was −0.1% (−1.9% to 1.8%).
Health Care Utilization in the Year after Transplantation
After 1 year, the maximum likelihood type estimate (m-estimate) of total cost for alemtuzumab recipients was $3083 lower than for rATG recipients (P<0.001), although this difference was only 4% of the total cost for alemtuzumab recipients ($84,291). Costs were not significantly different between basiliximab and rATG recipients (P=0.13) (Table 4).
Table 4.
Comparison of costs and payments after 1 year, relative to rATG
| Cost and Payment Outcomes | Alemtuzumab Match (n=5330 Matched Pairs) | Basiliximab Match (n=9378 Matched Pairs) | ||||||
|---|---|---|---|---|---|---|---|---|
| Alemtuzumab | Matched rATG | Paired Difference (95% CI) | P Value | Basiliximab | Matched rATG | Paired Difference (95% CI) | P Value | |
| 1-yr total cost, $ | ||||||||
| Mean | 86,290 | 88,948 | −2658 (−4648 to −668) | <0.01 | 87,926 | 88,679 | −753 (−2324 to 819) | 0.35 |
| M-estimate | 84,291 | 87,597 | −3083 (−4858 to −1303) | <0.001 | 85,990 | 87,134 | −1058 (−2427 to 313) | 0.13 |
| 1-yr total payment, $ | ||||||||
| Mean | 75,382 | 79,112 | −3729 (−6614 to −844) | 0.01 | 79,589 | 76,814 | 2775 (356 to 5195) | 0.03 |
| M-estimate | 73,500 | 76,346 | −2847 (−5345 to −355) | 0.03 | 76,102 | 74,183 | 1782 (−118 to 3685) | 0.07 |
P values were calculated using the test of the weighted m-statistic for continuous m-estimates and the permutational t test for continuous means. M-estimate, maximum likelihood type estimate.
Effect Modification by Age
We examined whether the effect of induction therapy was modified by elderly status (age 65–90 years). For the outcome of death or sepsis, alemtuzumab (versus reference rATG) provided a benefit to nonelderly recipients (HR, 0.89; 95% CI, 0.81 to 0.98; P=0.02) that was absent for elderly recipients (HR, 1.14; 95% CI, 0.95 to 1.37; P=0.17; elderly pairs versus nonelderly interaction HR, 1.28; 95% CI, 1.04 to 1.57; P=0.02). However, an inspection of all of the outcomes did not suggest consistent advantages for alemtuzumab versus rATG in the nonelderly subgroup (Supplemental Table 2).
Analyses of effect modification were nonsignificant among the basiliximab-rATG pairs (Supplemental Table 2).
Subcohort Analysis of Nonblack Patients
Nonblack alemtuzumab and basiliximab patients did not differ from patients who received rATG in risk of death or melanoma (Supplemental Table 4).
Exploratory Analyses of Rejection
Compared with rATG matched pairs, alemtuzumab and basiliximab patients showed statistically different risk of the composite outcome of death, allograft failure, or acute rejection (Supplemental Table 4; Stuart–Maxwell P<0.01 for alemtuzumab; P=0.04 for basiliximab). Relative to rATG recipients, alemtuzumab and basiliximab patients had higher odds of acute rejection by 1 year (alemtuzumab odds ratio, 1.31; 95% CI, 1.14 to 1.51; P<0.001; basiliximab odds ratio, 1.16; 95% CI, 1.04 to 1.30; P<0.01). Overall, 84.3% of patients who received alemtuzumab were alive at 1 year without allograft failure or rejection versus 86.6% of patients who received rATG (P<0.001).
Discussion
This matched-cohort study suggests that matched KTRs who receive rATG experience better or similar post-transplant outcomes relative to patients who receive alemtuzumab or basiliximab. Compared with rATG matched pairs, patients who received alemtuzumab had a 14% higher hazard of death and an 18% higher hazard of either death or allograft failure. Patients who received basiliximab had slightly greater risk of death versus matched patients who received rATG. These findings should motivate clinicians to consider the advantages of rATG when attempting to reduce complications after kidney transplantation.
Our study advances the knowledge about induction therapy in several ways. First, by linking CMS administrative claims to OPTN registry data, we report a wider range of adverse outcomes than many prior studies. Second, the analysis leverages major advances in multivariable matching methods, which enable transparent reporting of outcomes across highly similar pairs of KTRs. We generated 1:1 matches for >99% of basiliximab and alemtuzumab recipients meeting inclusion criteria. Matching comprised multiple demographic and clinical characteristics, including an estimate of neighborhood poverty. Third, the study is unique in its presentation of 1-year healthcare resource utilization at >200 centers.
In our primary approach, we did not adjust for oral immunosuppression because induction therapies are often intentionally prescribed with particular combinations of oral immunosuppression. Rather, we compared induction therapies in conjunction with the other immunosuppression with which they are commonly combined. Most notably, alemtuzumab has been promoted as an agent that facilitates corticosteroid withdrawal. In practice, prednisone was much more commonly prescribed to basiliximab (87%) and rATG (66%) versus alemtuzumab (29%) recipients. As a result, the underlying reasons and threshold for prescribing prednisone to patients may differ by induction strategy. However, we recognize that many clinicians may attribute differences in outcomes to oral immunosuppression. We addressed this concern with subgroup analyses of pairs who received tacrolimus, mycophenolate mofetil, or mycophenolate sodium, and who both either received prednisone or no prednisone. They demonstrated advantages for rATG versus alemtuzumab for death and death or allograft failure among prednisone pairs, but differences were not significant among pairs without prednisone. Differences between the basiliximab-rATG pairs were not statistically significant. These subgroup analyses had less power than the main analyses.
Our results showing superior outcomes for rATG are supported by many19,21,26,33,34 but not all prior observational studies and clinical trials. In a retrospective analysis of United States registry data (n=14,336), Schold et al. observed less allograft failure associated with rATG versus alemtuzumab among retransplant patients specifically.21 In a literature review of both observational studies and trials, Gaber et al. concluded that rATG provides better allograft survival versus basiliximab among patients with higher immunologic risk.26 Additionally, a recent trial suggested inferior death-censored graft survival with alemtuzumab induction compared with rATG.34
In particular, our results should be contrasted with the Induction with Tacrolimus (INTAC) trial. INTAC participants at high risk of rejection were randomized to alemtuzumab versus rATG whereas low-risk participants were randomized to alemtuzumab versus basiliximab; all 501 participants received tacrolimus, mycophenolate mofetil, and a 5-day glucocorticoid taper. INTAC reported no difference in 3-year mortality or graft failure across the agents.23 In analyses of patients with equal maintenance immunosuppression, we still find lower mortality or graft failure for rATG patients versus alemtuzumab patients when the pairs received prednisone. However, when patients did not receive prednisone, no difference in outcomes was observed, consistent with INTAC. The INTAC trial had lower rates of death or allograft failure at 3 years post-transplant than our study, perhaps explained by the trial’s exclusion of expanded criteria donors (ECD), donation after cardiac death (DCD), and hepatitis C–positive organs. INTAC participants may also have been healthier and better monitored under the trial infrastructure than individuals in our cohort. Therefore, our study adds important new information from real-world practice, suggesting meaningful risks associated with alemtuzumab compared with rATG. We estimate that the observed rates of death or allograft failure are 3 percentage points higher for alemtuzumab versus rATG recipients by 5 years after transplantation, a clinically important difference.
In our study, differences in outcomes between the basiliximab-rATG pairs were smaller in magnitude and less robust in subgroup analyses. The hazard of death was only 8% higher among basiliximab versus rATG recipients, and death or allograft failure did not differ between groups. A clinical trial by Brennan et al. (n=288 recipients) reported that mortality between rATG and basiliximab was not significantly different, although the trial was not powered to detect differences in mortality alone.11 Despite concerns that induction might confer greater risks among elderly patients, our analyses detected no evidence to suggest that basiliximab has substantial advantages among elderly kidney transplant patients. Some observational studies and randomized controlled trials saw no difference in mortality,21,35,36 allograft failure,35,36 and/or acute rejection21,23,34–36 for alemtuzumab and basiliximab recipients relative to rATG recipients.
In an exploratory analysis, the composite outcome of death or allograft failure or acute rejection was lower among rATG recipients versus matched pairs who received alemtuzumab. In particular, 12-month rejection rates were higher for alemtuzumab (9.9% versus 7.7%; P<0.001) and basiliximab (8.1% versus 7.1%; P<0.01) recipients relative to patients who received rATG. This finding of lower rejection with rATG adds important new information to the conclusions from the randomized trial of rATG and basiliximab by Brennan et al.11 because our study was not limited to patients at high risk of early adverse outcomes. In a study of elderly KTRs using OPTN data, Gill et al. similarly observed lower risk of acute rejection for rATG versus alemtuzumab and basiliximab patients.33 The reduced rejection associated with rATG is plausible, because this agent causes persistent depletion of T cell populations and likely targets several pathways of alloimmune activation.23
We acknowledge some emerging evidence that alemtuzumab is associated with lower risk of acute rejection for low-risk patients versus basiliximab or rATG, but similar acute rejection risk to rATG among high-risk patients.7,21,23,34–38 Specifically, one randomized controlled trial found that patients who received alemtuzumab had significantly lower 6-month acute rejection risk than patients who received basiliximab.38 We considered our analysis of acute rejection to be exploratory because of an earlier validation study by our group that acute rejection is reported with variable sensitivity across centers.39 However, we have no reason to believe that reporting rates would correlate with induction therapy choice.
Existing literature comparing sepsis, lymphoma, and skin cancer across induction therapies is limited. The elevated risk of death or lymphoma associated with basiliximab versus rATG in our study is novel. By contrast, in a study of KTRs from 2000 to 2004, Kirk et al. used the OPTN database to suggest higher risk of post-transplant lymphoproliferative disease among rATG relative to basiliximab patients.1 This difference could be explained in part by differences in outcome ascertainment. First, OPTN data have limited sensitivity (52%) related to malignancy outcomes, whereas we used CMS claims.40 Second, we combined the outcomes of death or lymphoma; an examination of lymphoma alone would be problematic given higher mortality rates associated with basiliximab. From a mechanistic perspective, we hypothesize that the higher risk of rejection with basiliximab could have exposed these groups to higher long-term doses of maintenance immunosuppression, which could promote lymphoma. However, unmeasured confounding is also possible. For example, frail KTRs are at risk for several adverse outcomes including delayed graft function and mortality. It is plausible that clinicians may have preferentially selected basiliximab for recipients with more frailty or worse functional status.11,20 Although we matched on over a dozen clinical and demographic factors, we did not have access to frailty data.41,42
We also observed a higher risk of death or melanoma among alemtuzumab recipients relative to rATG recipients. Given our limited data on these outcomes, further studies should explore comparative post-transplant outcomes of sepsis, lymphoma, and melanoma by induction therapy.
We compared 1-year health care utilization by induction therapy and measured significantly greater 1-year costs among rATG versus alemtuzumab recipients, although the difference ($3083) was a small percentage of total costs. One-year costs were not different between basiliximab and rATG recipients. In CMS claims, we cannot disentangle payments to hospitals for induction therapy from the lump sum payments that CMS pays for the transplant hospitalization. Additionally, differences in mortality rates may influence the accumulation of costs. Yet, despite these limitations, the minor differences in costs suggest that it is reasonable to focus on clinical outcomes when selecting induction therapy.
These results should be considered in the context of their limitations. Although we matched on multiple important characteristics, unmeasured confounding remains an important possibility. While our matching techniques were robust, they can only adjust for differences among pairs that were measurable in the data. For example, as noted above, we did not have information on important characteristics that could affect outcomes such as health literacy or prior cancers.11,20,33,41,42 We also did not have information on induction therapy dose, which may vary considerably for rATG. Although recipients in each induction therapy group received their transplants at dozens of centers, outcomes might be confounded by variation and overall quality of care by transplant center.
In conclusion, antibody induction therapy has potent effects on the immune system with important implications for outcomes after kidney transplantation. In a large cohort of KTRs matched on diverse characteristics, rATG recipients enjoyed longer survival and generally similar or better outcomes compared with alemtuzumab and basiliximab recipients. Clinically, alemtuzumab recipients had 18% higher risk of death or allograft failure post-transplant compared with rATG recipients (estimated rates of 25.9% versus 22.9% at 5 years for alemtuzumab-rATG pairs). Basiliximab recipients had 8% higher risk of death compared with rATG recipients (estimated rates of 16.3% versus 15.2% at 5 years for basiliximab-rATG pairs). These results should help guide clinicians’ selection of induction therapy to prolong survival and avoid important complications including allograft failure after kidney transplantation.
Concise Methods
Overview
This retrospective cohort study using registry data analyzes outcomes after the administration of alemtuzumab, rATG, and basiliximab (labeled as “Campath - Alemtuzumab,” “Thymoglobulin,” and “Simulect - Basiliximab” in OPTN data) to KTRs in the United States. The study was approved by the University of Pennsylvania Institutional Review Board (protocol no. 821200).
Data Sources
We used data from the OPTN, including immunosuppression files reported from the transplant hospitalization. The OPTN data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the OPTN. The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight to the activities of the OPTN contractor. These OPTN data were linked to data from the CMS. Using zip codes, we categorized participants’ neighborhoods into four groups on the basis of the percentage of residents living in poverty.43
Population
The cohort comprised adults (aged 18–90 years) who received a kidney transplant between January 1, 2003, through December 31, 2008, and had CMS insurance coverage at the time of transplantation and a Medicare claim related to the transplant procedure on or near the OPTN transplant date. Exclusion criteria included multiorgan transplantation; induction therapy other than alemtuzumab, rATG, and basiliximab, or multiple induction agents; discharge on azathioprine (because of the enhanced risk of skin cancer44,45 as well as the very rare use of azathioprine at discharge during this period [<1% of recipients]); discharge on a combination of tacrolimus, cyclosporine, and/or sirolimus (because such regimens are uncommon and might drive higher risk of sepsis and allograft failure46–48); missing zip code (because of matching on neighborhood poverty); and body mass index (BMI) <12 or >50 kg/m2.
Outcomes
The primary outcomes were death and death or allograft failure. Secondary outcomes were death or sepsis, death or lymphoma, and death or melanoma. We did not examine post-transplant complications such as sepsis alone because, from a patient’s perspective, an improvement in sepsis rates would not be important unless the combined outcome of death and sepsis were also improved. We also analyzed a composite outcome of death or allograft failure or acute rejection by 1 year post-transplant, using OPTN data. We did not use Cox regression to analyze this composite outcome because the OPTN data do not provide specific dates for acute rejection events; these events are only reported on standard forms as having occurred at the time of transplant and defined intervals afterward (e.g., 6 months and 1 year). This composite outcome was exploratory because of evidence that acute rejection is under-reported at many centers.39 Finally, we prespecified subgroup analyses among elderly (≥65 years) and nonelderly patients, because of the elevated risk of infection13,49 and reduced rejection risk50,51 in elderly KTRs.
Death and allograft failure were determined through the OPTN dataset. Death dates in the OPTN dataset were reported by centers and via linkage to the Social Security Death Master File. The last date of follow-up for analyses of death and death or allograft failure was January of 2012. Sepsis, lymphoma, and melanoma were ascertained through CMS. The last date of follow-up for outcomes involving CMS claims was December 31, 2009, such that every patient had the opportunity for at least 1 year of follow-up for these outcomes. The Supplemental Appendix lists administrative codes used for outcomes ascertained with CMS claims.
One-year post-transplant costs were determined on the basis of resource utilization found in Medicare inpatient, outpatient, and Part B claims.52–54 For the transplant hospitalization, the cost accounted for days in the hospital and level of care (intensive care unit versus floor),53 all procedures for which a bill was charged to CMS (including operating room cost55 and anesthesia units), and total revenue value units determined from all bills. Follow-up costs up to 1 year after the admission date of the transplant hospitalization included all costs from any subsequent hospitalization, as well as any outpatient, physician, or emergency-department bills56 (see Supplemental Appendix). The goal was to compare healthcare resource utilization in matched pairs.57–59 Therefore, the same cost was assigned for specific resources, such as a hospitalization day or procedure, across all areas of the United States regardless of variation in price. Using the Consumer Price Index, all 1-year costs were indexed to 2008, our last year of transplant cases. Notably, the cost of the transplant hospitalization itself is delivered by CMS as a bundled payment to the transplant program, which includes induction therapy given with transplant. Transplant hospitals are also reimbursed with a standard acquisition cost for the organ (included in our analyses). Finally, we calculated CMS payments to hospitals and physicians.
Matching on Recipient and Donor Characteristics
Two independent matches without replacement were conducted to create highly similar alemtuzumab-rATG and basiliximab-rATG pairs. We chose rATG as the reference group, because it is the most commonly used agent in kidney transplantation. We implemented a 1:1 matched-pair design because of advantages over multivariable regression approaches that were used in a number of retrospective analyses of induction therapy.28,60,61 Multivariable regression relies upon construction of a model with a valid functional form, including sufficient attention to complex interactions between recipient and donor characteristics. In contrast, matching enables a transparent comparison of very similar groups of patients receiving different therapies and does not have the same distributional assumptions required in most regression. We completed matches before examining any outcome analyses.29 We used both traditional propensity score matching and other more recently developed matching techniques to create very similar pairs. Matches were created using R MIPMatch with exact matching, fine balance,30,32 and a distance matrix defined through the Mahalanobis distance.31
Recipients were matched exactly for elderly status (<65 years versus ≥65–90 years), race (black versus nonblack), previous kidney transplant (binary), HLA mismatch (zero versus nonzero), PRA category (missing, 0%–20%, 20%–80%, 80%–100%), and availability of BMI (missing versus nonmissing) and weight (missing versus nonmissing) data. We matched age in years, transplant year, neighborhood median income, BMI, and weight (if BMI was missing) as continuous variables by minimizing the total penalized Mahalanobis distance between cases and matched controls. We used near-fine balance30,32 to ensure that the marginal distributions of the following categorical covariates were extremely similar between cases and matched controls: sex (men versus women), hepatitis C serostatus (positive versus not positive), categories of dialysis vintage (0–1, 1–3, 3–6, 6–10, and >10 years, and missing), diabetes (present or absent), cause of ESRD (specified as individual categories of GN, hypertension, polycystic kidney disease, and congenital), and donor type (live, deceased standard criteria, or deceased extended criteria [defined conventionally during the period of study as donor age ≥60 years, or age 50–59 years plus the presence of at least two of the following characteristics: donor hypertension, donor cause of death cerebrovascular accident, and donor terminal creatinine ≥1.5 mg/dl]). All of these were coded as binary covariates and included in the Mahalanobis distance. Finally, in each match we added to the distance a propensity score for receiving the focal agent (alemtuzumab or basiliximab) with scaled penalties added to minimize differences in the propensity score inside pairs.61,62 Notably, diabetes was considered present if coded either as a comorbidity or as cause of ESRD in the OPTN data.
After careful deliberation, we decided not to match on oral immunosuppression at discharge in the primary analyses. The rationale was that induction antibody therapy has commonly been prescribed as a “bundle” of therapies, and oral immunosuppression was sometimes customized on the basis of induction therapy. For example, alemtuzumab is often used in “steroid-free” maintenance regimens.63–65 Adjusting for prednisone use would therefore adjust away the manner in which these agents were combined in real-world practice. However, we performed supplementary analyses restricted to pairs who received tacrolimus and either mycophenolate mofetil or mycophenolate sodium, the most common oral immunosuppression regimen for KTRs. We then divided these pairs into subsets where both patients received prednisone or neither patient received prednisone and compared outcomes between induction therapy groups.
Balance for all matches between treatment and comparison groups was assessed using the Wilcoxon rank sum test for each continuous covariate and the Fisher exact test for binary covariates and using the standardized difference in means after matching.
Statistical Analyses
To compare differences between the treatment and comparison group pairs, HRs with 95% CIs were generated using the paired version of the Cox proportional hazards model.66 Survival curves were estimated using the method of Kaplan and Meier. Pairs were considered censored after 3 years for the Medicare outcomes of sepsis, lymphoma, and melanoma because nonelderly patients commonly lose Medicare coverage after 3 years. Analyses of death and allograft failure were not censored at 3 years because these outcomes were derived using OPTN data. Person-time follow-up is included in Supplemental Table 5 of the Supplemental Appendix.
We tested for violations of the proportional hazards assumption in the Cox models by fitting additional models that included an induction therapy by time interaction. In this large study, there were small violations of proportional hazards that were statistically significant, although perhaps of negligible clinical interest. When this interaction was statistically significant, we presented both Cox regression results and also tested (see Supplemental Tables 6 and 7) the difference between the curves using the Prentice–Wilcoxon statistic67 and reported the difference between the curves at 3 years (when using Medicare data) and 5 years (when using OPTN data only). We obtained standard errors for paired differences in Kaplan–Meier survival curves at 3 or 5 years using the bootstrap method.68
We examined for effect modification of therapy by elderly age category using paired Cox regression.66 We also performed subcohort analyses among nonblack patients for the skin cancer outcomes, because of higher risks of skin malignancy in this group.
The composite outcome of 1-year death, allograft failure, or acute rejection was compared using the Stuart–Maxwell test.69,70 The McNemar test was used to compare differences in overall acute rejection rates and specific components of the composite 1-year death, allograft failure, or rejection outcome. When examining the continuous outcomes of CMS payments and 1-year costs, we used robust tests, specifically m-statistics71–73 (Maximum Likelihood Type Estimates) and the permutational t test.71–73 All analyses were conducted using R 2.13.1 (R Foundation) and SAS 9.3 (SAS Institute Inc).
Disclosures
J. Schold is on the Speakers Bureau for Sanofi and Novartis.
Supplementary Material
Acknowledgments
We acknowledge the efforts of Vishnu Potluri for data related to trends in induction therapy utilization. We thank Donald Tsai for providing expertise and advice in reviewing Centers for Medicare and Medicaid Services codes related to lymphoma after transplantation.
Our study’s abstract was presented on a poster at the 2016 American Transplant Congress, Boston, MA, June 13, 2016.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016070768/-/DCSupplemental.
References
- 1.Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, Potdar S, Shapiro R, Dharnidharka VR, Kauffman HM: Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant 7: 2619–2625, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danovitch G: Handbook of Kidney Transplantation, Philadelphia, PA, Lippincott Williams & Wilkins, 2010 [Google Scholar]
- 3.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 4. United Network for Organ Sharing Standard Transplant and Analysis Research (STAR) 2015. Available at https://optn.transplant.hrsa.gov/data/request-data/#. Accessed June 26, 2015.
- 5.James A, Mannon RB: The cost of transplant immunosuppressant therapy: Is this sustainable? Curr Transplant Rep 2: 113–121, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai J, Terasaki PI: Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: An analysis of united network for organ sharing registry data. Transplantation 90: 1511–1515, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Hardinger KL, Brennan DC, Klein CL: Selection of induction therapy in kidney transplantation. Transpl Int 26: 662–672, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Vardhan H, Prasad N, Jaiswal A, Yadav B, Kumar S, Bhadauria D, Kaul A, Gupta A, Srivartava A, Sharma RK: Outcomes of living donor renal transplant recipients with and without basiliximab induction: A long-term follow-up study. Indian J Transplant 8: 44–50, 2014 [Google Scholar]
- 9.Szczech LA, Berlin JA, Feldman HI; Anti-Lymphocyte Antibody Induction Therapy Study Group: The effect of antilymphocyte induction therapy on renal allograft survival. A meta-analysis of individual patient-level data. Ann Intern Med 128: 817–826, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Opelz G, Unterrainer C, Süsal C, Döhler B: Efficacy and safety of antibody induction therapy in the current era of kidney transplantation. Nephrol Dial Transplant 31: 1730–1738, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D; Thymoglobulin Induction Study Group: Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 355: 1967–1977, 2006 [DOI] [PubMed] [Google Scholar]
- 12.de Carvalho MA, Freitas FGR, Silva Junior HT, Bafi AT, Machado FR, Pestana JOM: Mortality predictors in renal transplant recipients with severe sepsis and septic shock. PLoS One 9: e111610, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B: Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int 59: 1539–1543, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M: Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306: 1891–1901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, Leichtman AB, Held PJ, Port FK: Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant 4: 87–93, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Cherikh WS, Kauffman HM, McBride MA, Maghirang J, Swinnen LJ, Hanto DW: Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation 76: 1289–1293, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Yoon J-H, Lee S, Kim H-J, Lee JW, Min WS, Chung BH, Yang CW, Kim YS, Kim JI, Moon IS, Oh EJ, Park GS, Cho SG: Comparative analysis of post-transplant lymphoproliferative disorder after kidney transplantation versus hematopoietic stem cell transplantation. Transpl Int 27: 721–732, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Lentine KL, Schnitzler MA, Xiao H, Brennan DC: Long-term safety and efficacy of antithymocyte globulin induction: Use of integrated national registry data to achieve ten-year follow-up of 10-10 study participants. Trials 16: 365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst FP, Altieri M, Nee R, Agodoa LY, Abbott KC, Jindal RM: Poor outcomes in elderly kidney transplant recipients receiving alemtuzumab induction. Am J Nephrol 34: 534–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Afaneh C, Aull M, Schubl S, Leeser D, Kapur S: Induction therapy: a modern review of kidney transplantation agents. J Transplant Technol Res 03: S4–001, 2013.
- 21.Schold J, Poggio E, Goldfarb D, Kayler L, Flechner S: Clinical outcomes associated with induction regimens among retransplant kidney recipients in the United States. Transplantation 99: 1165–1171, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, Albitar M, Brettman L, Santabarbara P, Wacker B, Rai KR: Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood 99: 3554–3561, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, Croy R, Holman J; INTAC Study Group: Alemtuzumab induction in renal transplantation. N Engl J Med 364: 1909–1919, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Muthusamy ASR, Vaidya AC, Sinha S, Roy D, Elker DE, Friend PJ: Alemtuzumab induction and steroid-free maintenance immunosuppression in pancreas transplantation. Am J Transplant 8: 2126–2131, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Gabardi S, Martin ST, Roberts KL, Grafals M: Induction immunosuppressive therapies in renal transplantation. Am J Health Syst Pharm 68: 211–218, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Gaber AO, Knight RJ, Patel S, Gaber LW: A review of the evidence for use of thymoglobulin induction in renal transplantation. Transplant Proc 42: 1395–1400, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Knight RJ, Kerman RH, Schoenberg L, Podder H, Van Buren CT, Katz S, Kahan BD: The selective use of basiliximab versus thymoglobulin in combination with sirolimus for cadaveric renal transplant recipients at low risk versus high risk for delayed graft function. Transplantation 78: 904–910, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Li YP, Propert KJ, Rosenbaum PR: Balanced risk set matching. J Am Stat Assoc 96: 870–882, 2001 [Google Scholar]
- 29.Rubin DB: The design versus the analysis of observational studies for causal effects: Parallels with the design of randomized trials. Stat Med 26: 20–36, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum PR, Ross RN, Silber JH: Minimum distance matched sampling with fine balance in an observational study of treatment for ovarian cancer. J Am Stat Assoc 102: 75–83, 2007 [Google Scholar]
- 31.Zubizarreta J: Using mixed integer programming for matching in an observational study of kidney failure after surgery. J Am Stat Assoc 107: 1360–1371, 2012 [Google Scholar]
- 32.Yang D, Small DS, Silber JH, Rosenbaum PR: Optimal matching with minimal deviation from fine balance in a study of obesity and surgical outcomes. Biometrics 68: 628–636, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill J, Sampaio M, Gill JS, Dong J, Kuo HT, Danovitch GM, Bunnapradist S: Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol 6: 1168–1178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, Roth D, Kupin W, Tueros L, Flores S, Hanson L, Vianna R, Burke GW 3rd: Randomized trial of three induction antibodies in kidney transplantation: Long-term results. Transplantation 97: 1128–1138, 2014 [DOI] [PubMed] [Google Scholar]
- 35. Serrano OK, Friedmann P, Ahsanuddin S, Millan C, Ben-Yaacov A, Kayler LK. Outcomes associated with steroid avoidance and alemtuzumab among kidney transplant recipients. Clin J Am Soc Nephrol 10: 2030–2038, 2015. [DOI] [PMC free article] [PubMed]
- 36.Morgan RD, O’Callaghan JM, Knight SR, Morris PJ: Alemtuzumab induction therapy in kidney transplantation: A systematic review and meta-analysis. Transplantation 93: 1179–1188, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Kuypers DRJ: Alemtuzumab induction therapy in kidney transplantation. Lancet 384: 1649–1651, 2014 [DOI] [PubMed] [Google Scholar]
- 38.3C Study Collaborative Group, Haynes R, Harden PN, Parminder J, Friend PJ: Alemtuzumab-based induction treatment versus basiliximab-based induction treatment in kidney transplantation (the 3C Study): A randomised trial. Lancet Lond Engl 384(9955): 1684–1690, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Potluri VS, Parikh CR, Hall IE, Ficek J, Doshi MD, Butrymowicz I, Weng FL, Schröppel B, Thiessen-Philbrook H, Reese PP: Validating early post-transplant outcomes reported for recipients of deceased donor kidney transplants. Clin J Am Soc Nephrol 11: 324–331, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan N, Abbas AM, Lichtenstein GR, Loftus EV Jr, Bazzano LA: Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: A nationwide retrospective cohort study. Gastroenterology 145: 1007–1015.e3, 2013 [DOI] [PubMed] [Google Scholar]
- 41.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, Walston J, Segev DL: Frailty and mortality in kidney transplant recipients. Am J Transplant 15: 149–154, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese PP, Shults J, Bloom RD, Mussell A, Harhay MN, Abt P, Levine M, Johansen KL, Karlawish JT, Feldman HI: Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kidney Dis 66: 837–845, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Census Bureau: Areas with Concentrated Poverty: 1999. July 2005. Available at: https://www.census.gov/prod/2005pubs/censr-16.pdf. Accessed June 1, 2014
- 44.O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P: Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 309: 1871–1874, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiyad Z, Olsen CM, Burke MT, Isbel NM, Green AC: Azathioprine and risk of skin cancer in organ transplant recipients: Systematic review and meta-analysis [published online ahead of print May 10, 2016]. Am J Transplant doi: 10.1111/ajt.13863. [DOI] [PubMed]
- 46.Cortazar F, Molnar MZ, Isakova T, Czira ME, Kovesdy CP, Roth D, Mucsi I, Wolf M: Clinical outcomes in kidney transplant recipients receiving long-term therapy with inhibitors of the mammalian target of rapamycin. Am J Transplant 12: 379–387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leas BF, Uhl S, Sawinski DL, Trofe-Clark J, Tuteja S, Kaczmarek JL, Umscheid CA: Calcineurin Inhibitors for Renal Transplant Rockville, MD, Agency for Healthcare Research and Quality (US), 2016, Available at: http://www.ncbi.nlm.nih.gov/books/NBK356377/. Accessed May 19, 2016 [PubMed] [Google Scholar]
- 48. Sawinski D, Trofe-Clark J, Leas B, Uhl S, Tuteja S, Kaczmarek JL, French B, Umscheid CA: Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in Renal Transplantation: A systematic review and meta-analysis. Am J Transplant 16: 2117–2138, 2016. [DOI] [PubMed]
- 49.Kauffman HM, McBride MA, Cors CS, Roza AM, Wynn JJ: Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation 83: 404–410, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Wu C, Shapiro R, Tan H, Basu A, Smetanka C, Morgan C, Shah N, McCauley J, Unruh M: Kidney transplantation in elderly people: The influence of recipient comorbidity and living kidney donors. J Am Geriatr Soc 56: 231–238, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Meier-Kriesche HU, Ojo A, Hanson J, Cibrik D, Lake K, Agodoa LY, Leichtman A, Kaplan B: Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation 69: 885–889, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Silber JH, Rosenbaum PR, Kelz RR, Reinke CE, Neuman MD, Ross RN, Even-Shoshan O, David G, Saynisch PA, Kyle FA, Bratzler DW, Fleisher LA: Medical and financial risks associated with surgery in the elderly obese. Ann Surg 256: 79–86, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halpern NA, Pastores SM: Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med 38: 65–71, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Wilson LS, Lightwood JM: Pancreatic cancer: Total costs and utilization of health services. J Surg Oncol 71: 171–181, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee A, Chen L, Goldenberg EA, Bae HT, Finlayson SRG: Opportunity cost in the evaluation of surgical innovations: A case study of laparoscopic versus open colectomy. Surg Endosc 24: 1075–1079, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Bamezai A, Melnick G, Nawathe A: The cost of an emergency department visit and its relationship to emergency department volume. Ann Emerg Med 45: 483–490, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Silber JH, Rosenbaum PR, Ross RN, Ludwig JM, Wang W, Niknam BA, Mukherjee N, Saynisch PA, Even-Shoshan O, Kelz RR, Fleisher LA: Template matching for auditing hospital cost and quality. Health Serv Res 49: 1446–1474, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silber JH, Rosenbaum PR, Ross RN, Ludwig JM, Wang W, Niknam BA, Saynisch PA, Even-Shoshan O, Kelz RR, Fleisher LA: A hospital-specific template for benchmarking its cost and quality. Health Serv Res 49: 1475–1497, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silber JH, Rosenbaum PR, McHugh MD, Ludwig JM, Smith HL, Niknam BA, Even-Shoshan O, Fleisher LA, Kelz RR, Aiken LH: Comparison of the value of nursing work environments in hospitals across different levels of patient risk. JAMA Surg 151: 527–536, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baser O: Choosing propensity score matching over regression adjustment for causal inference: when, why and how it makes sense. J Med Econ 10: 379–391, 2007 [Google Scholar]
- 61.Rosenbaum PR, Rubin DB: The central role of the propensity score in observational studies for causal effects. Biometrika 70: 41–55, 1983 [Google Scholar]
- 62.Rosenbaum PR: Design of Observational Studies, New York, NY, Springer, 2010 [Google Scholar]
- 63.Cole E, Landsberg D, Russell D, Zaltzman J, Kiberd B, Caravaggio C, Vasquez AR, Halloran P: A pilot study of steroid-free immunosuppression in the prevention of acute rejection in renal allograft recipients. Transplantation 72: 845–850, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Baez Y, Giron F, Niño-Murcia A, Rodríguez J, Salcedo S: Experience with alemtuzumab (Campath-1H) as induction agent in renal transplantation followed by steroid-free immunosuppression. Transplant Proc 40: 697–699, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Thomas PG, Woodside KJ, Lappin JA, Vaidya S, Rajaraman S, Gugliuzza KK: Alemtuzumab (Campath 1H) induction with tacrolimus monotherapy is safe for high immunological risk renal transplantation. Transplantation 83: 1509–1512, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Holt JD, Prentice RL: Survival analyses in twin studies and matched pair experiments. Biometrika 61: 17–30, 1974 [Google Scholar]
- 67.O’Brien PC, Fleming TR: A paired prentice-wilcoxon test for censored paired data. Biometrics 43: 169–180, 1987 [Google Scholar]
- 68.Efron B, Tibshirani R: Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1: 54–75, 1986 [Google Scholar]
- 69.Stuart A: A test for homogeneity of the marginal distributions in a two-way classification. Biometrika 42: 412–416, 1955 [Google Scholar]
- 70.Maxwell AE: Comparing the classification of subjects by two independent judges. Br J Psychiatry 116: 651–655, 1970 [DOI] [PubMed] [Google Scholar]
- 71.Maritz JS: A note on exact robust confidence intervals for location. Biometrika 66: 163–170, 1979 [Google Scholar]
- 72.Rosenbaum PR: Two R packages for sensitivity analysis in observational studies. Observ Stud 1: 1–17, 2015 [Google Scholar]
- 73.Huber PJ, Ronchetti EM: The basic types of estimates. In: Robust Statistics. New Jersey, John Wiley & Sons, Inc., 1981, pp 43–72. Available at: http://onlinelibrary.wiley.com/doi/10.1002/0471725250.ch3/summary. Accessed May 12, 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.