Abstract
IgA nephropathy (IgAN), an important cause of kidney failure, is characterized by glomerular IgA deposition and is associated with changes in O-glycosylation of the IgA1 molecule. Here, we sought to identify genetic factors contributing to levels of galactose-deficient IgA1 (Gd-IgA1) in white and Chinese populations. Gd-IgA1 levels were elevated in IgAN patients compared with ethnically matched healthy subjects and correlated with evidence of disease progression. White patients with IgAN exhibited significantly higher Gd-IgA1 levels than did Chinese patients. Among individuals without IgAN, Gd-IgA1 levels did not correlate with kidney function. Gd-IgA1 level heritability (h2), estimated by comparing midparental and offspring Gd-IgA1 levels, was 0.39. Genome-wide association analysis by linear regression identified alleles at a single locus spanning the C1GALT1 gene that strongly associated with Gd-IgA1 level (β=0.26; P=2.35×10−9). This association was replicated in a genome-wide association study of separate cohorts comprising 308 patients with membranous GN from the UK (P<1.00×10−6) and 622 controls with normal kidney function from the UK (P<1.00×10−10), and in a candidate gene study of 704 Chinese patients with IgAN (P<1.00×10−5). The same extended haplotype associated with elevated Gd-IgA1 levels in all cohorts studied. C1GALT1 encodes a galactosyltransferase enzyme that is important in O-galactosylation of glycoproteins. These findings demonstrate that common variation at C1GALT1 influences Gd-IgA1 level in the population, which independently associates with risk of progressive IgAN, and that the pathogenic importance of changes in IgA1 O-glycosylation may vary between white and Chinese patients with IgAN.
Keywords: IgA nephropathy, glomerulonephritis, human genetics, IgA, SNP, Genome Wide Association Study
IgA nephropathy (IgAN) is the commonest GN worldwide and is a major cause of kidney failure.1 The prevalence of IgAN shows marked differences across different ethnic groups, being more prevalent in people with East Asian ancestry and less prevalent in people with African ancestry compared with whites.2 In addition, differences in the clinical features of IgAN in white compared with Chinese patients have been recognized for a long time,3 most notably the clear male preponderance of IgAN studies of IgAN in whites that is absent (or even reversed) in East Asian populations, suggesting that important and incompletely understood differences in disease pathophysiology exist across different populations.4–8 Recent work has identified a number of genetic factors, mostly associated with mechanisms of defense against infection, that are associated with altered risk of disease,9–11 and although the prevalence of the known genetic factors vary across different ethnic groups, the observed differences fall some way short of explaining the differences in prevalence of the disease in different regions.2,12
The human IgA1 molecule differs from the conserved IgA2 subclass by the presence of an additional 13-residue motif in the hinge region that undergoes O-linked glycosylation. The function of this post-translational modification is incompletely understood, but it is known that IgA1 O-linked glycans lacking a galactose moiety are more abundant in the circulation of patients with IgAN,13–15 and that such galactose-deficient IgA1 (Gd-IgA1) is disproportionately found in IgAN glomerular immune deposits.16 In Tn syndrome, a similarly undergalactosylated protein is present on the surfaces of blood cells that leads to autoantibody generation and disease.17 A plausible hypothesis is that Gd-IgA1 plays an important role in the pathophysiology of IgAN by functioning as an autoantigen leading to autoantibody production and formation of circulating immune complexes in susceptible individuals.18,19 In vitro evidence supports a role for these IgA-containing immune complexes in driving glomerular injury through mesangial cell proliferation and secretion of cytokines, chemokines, growth factors, and extracellular matrix components promoting glomerular inflammation and glomerulosclerosis.20–22 Consistent with previous data, we show that Gd-IgA1 level is a heritable trait23–26 and use a genome-wide approach to identify the common genetic factor that influences Gd-IgA1 levels in white and East Asian populations.
Results
Gd-IgA1 Is Elevated in IgAN, Associated with Disease Severity, and Heritable
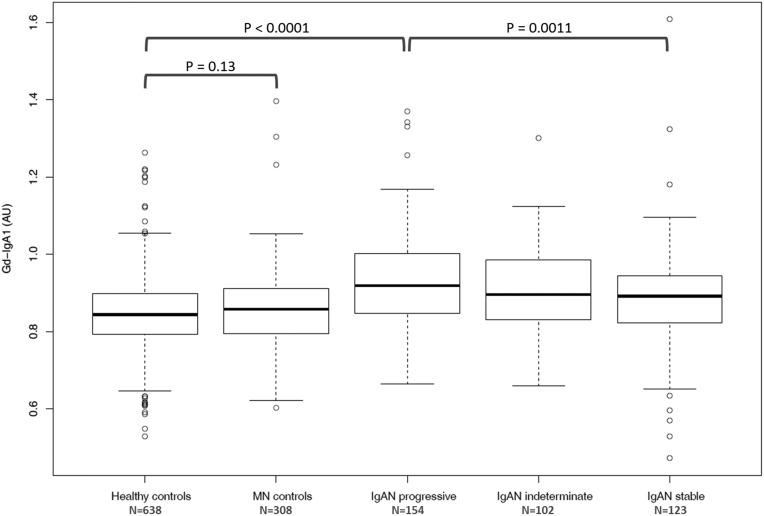
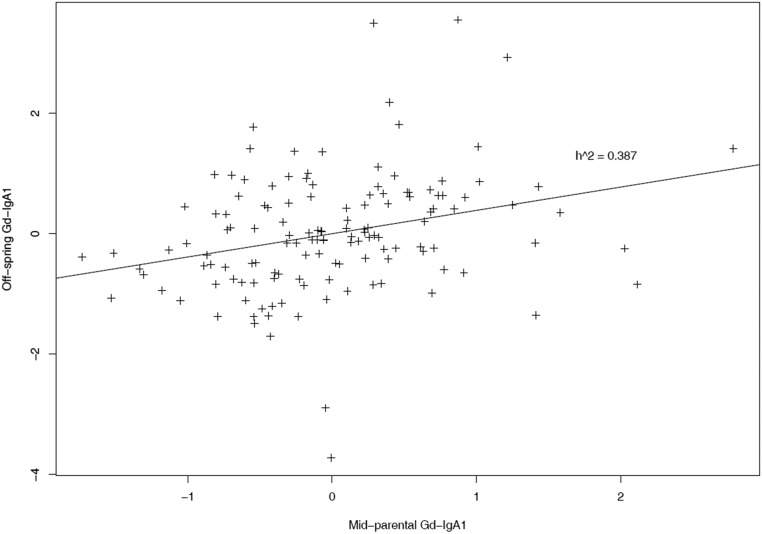
In the discovery UK cohort, Gd-IgA1 levels were normally distributed (Supplemental Figure 1) and elevated in patients. Follow-up data were available for the majority of patients allowing classification into 154 “progressors,” defined as doubling of serum creatinine or needing renal replacement therapy, and 123 “nonprogressors,” defined as serum creatinine <1.35 mg/dl and <20% increase over at least 5 years of follow-up, with the remainder “indeterminate.” Gd-IgA1 levels were significantly higher in progressors compared with nonprogressors (P=0.001; Figure 1). Correlation (P=0.031) was seen between Gd-IgA1 level and serum creatinine−1 (Supplemental Figure 2). In a separate longitudinal cohort of patients with IgAN and healthy subjects (Leicester Research Archive) we observed that the Gd-IgA1 level in an individual did not change significantly over >5 years and changes in renal function in individuals with IgAN were not correlated with changes in Gd-IgA1 level (Supplemental Figure 3). Heritability (h2) was 0.387, estimated by parent-offspring regression of standardized Gd-IgA1 levels (Figure 2), broadly consistent with previously published data.23–26
Figure 1.
Gd-IgA1 levels were higher in 379 patients with IgAN compared with 638 healthy control subjects (P<0.0001). Levels were higher in 154 patients with progressive IgAN (defined as doubling of serum creatinine or ESRD) compared with 123 patients with IgAN in whom good renal function remained stable over >5 years (P=0.0011). Gd-IgA1 levels were not significantly elevated in 308 patients with Membranous Nephropathy (MN controls). AU, arbitrary units of optical absorbance.
Figure 2.
Heritability (h2) of Gd-IgA1 in UK whites is 0.387. This was estimated using 134 complete trios by plotting Gd-IgA1 of patients against their mean parental Gd-IgA1. Values plotted are Gd-IgA1 levels in arbitrary units of optical absorbance standardized to mean of 0 and SD of 1 to allow narrow sense heritability estimation by calculation of the slope.
C1GALT1 Is Principal Genetic Determinant of Gd-IgA1 Level in Multiple Cohorts
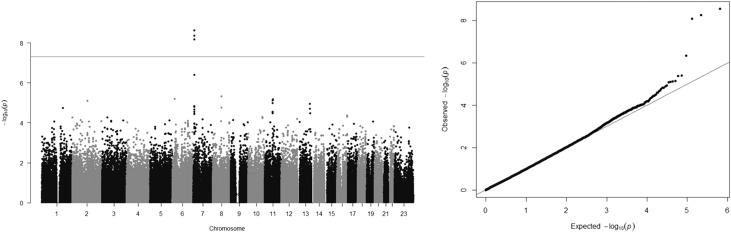
Linear regression genome-wide association study (GWAS) using standardized Gd-IgA1 levels in 513 founder members of the discovery cohort identified alleles at a single locus, spanning the C1GALT1 gene, that were strongly associated with Gd-IgA level (β=0.26; P=2.35×10−9), with no other significantly associated alleles elsewhere in the genome (Figure 3, Table 1). Repeating the analysis conditioned on the most strongly associated SNP (rs1008897) showed no association with any independent alleles, either at C1GALT1 or elsewhere in the genome (Supplemental Figure 4). The association with C1GALT1 was robust to correction for age, sex, renal function, and progression, and genomic inflation factor (λ) was 1.00196, suggesting the analysis was unlikely to be confounded by unidentified population substructure. Haplotype analyses confirmed the findings of the conditional analysis, which was that the association was attributable to the presence of a single haplotype, termed H1, present at a frequency of >0.3 in the UK population and strongly associated with increased Gd-IgA1 levels (β=0.34; P=4.4 x10−11; Supplemental Figure 5, Table 2). Additional haplotype association analysis conditioned on the presence of H1 indicated that no other haplotypes were significantly associated with Gd-IgA1 at either the genome-wide or nominal level corrected for multiple tests (Supplemental Table 1).
Figure 3.
GWAS of Gd-IgA1 levels in 513 unrelated UK individuals showing association with alleles at a single locus on Chromosome 7. Manhattan plot (left panel) showing significance of the association of each SNP allele with Gd-IgA1 level by plotting the negative logarithm to the base 10 of the P value against the genomic position. Horizontal line indicates conventional genome-wide significance (P=5×10−8). Quantile-quantile plot (right panel) is a plot of the observed −log10(P) against the −log10(P) values that would be expected under the null hypothesis of no association. Deviation above the y=x line indicates lower P values than would be expected to occur by chance and implies statistically significant association. The genomic inflation factor was 1.00196.
Table 1.
All SNPs associated with Gd-IgA1 with P<5×10−5 in discovery cohort
| Chromosome | SNP | Raw P Value | GC | Bonferroni | GRAPHIC P Value |
|---|---|---|---|---|---|
| 7 | ars1008897 | 2.35E−09 | 2.55E−09 | 0.000745 | 5.49E−11 |
| 7 | ars758263 | 4.34E−09 | 4.70E−09 | 0.001377 | 1.90E−07 |
| 7 | ars13226913 | 6.55E−09 | 7.07E−09 | 0.002077 | 1.19E−08 |
| 7 | ars4720724 | 3.91E−07 | 4.14E−07 | 0.1238 | 7.51E−09 |
| 17 | rs3803780 | 4.06E−06 | 4.26E−06 | 1 | 0.982 |
| 8 | rs1344616 | 4.62E−06 | 4.85E−06 | 1 | 0.510 |
| 6 | rs9383456 | 6.48E−06 | 6.80E−06 | 1 | 0.755 |
| 11 | rs519380 | 6.79E−06 | 7.12E−06 | 1 | 0.598 |
| 11 | rs2279865 | 7.52E−06 | 7.88E−06 | 1 | 0.0545 |
| 2 | rs294657 | 7.89E−06 | 8.27E−06 | 1 | 0.807 |
| 11 | rs11550299 | 1.03E−05 | 1.08E−05 | 1 | 0.427 |
| 13 | rs1540510 | 1.13E−05 | 1.19E−05 | 1 | 0.621 |
| 7 | ars2108780 | 1.46E−05 | 1.53E−05 | 1 | 3.10E−06 |
| 7 | rs2060163 | 1.59E−05 | 1.66E−05 | 1 | 0.00115 |
| 8 | rs609760 | 1.73E−05 | 1.80E−05 | 1 | 0.605 |
| 1 | rs12140760 | 1.82E−05 | 1.90E−05 | 1 | 0.0227 |
GC, corrected for genomic control; Bonferroni, corrected stringently for 302,210 SNPs analyzed.
SNPs at the C1GALT1 locus—only these SNPs also showed significant association with Gd-IgA1 in the GRAPHIC cohort.
Table 2.
Association of haplotypes across C1GALT1 with Gd-IgA1 showing a similar effect of the H1 haplotype in both populations
| Cohort | CHR | SNP1 | SNP2 | Haplotype | Frequency | β | P Value | Bonferroni |
|---|---|---|---|---|---|---|---|---|
| White | 7 | rs4720724 | rs2190935 | GTCCGCa | 0.319 | 0.343 | 4.40E−11 | 3.96E−10 |
| 7 | rs4720724 | rs2190935 | ATCTAT | 0.207 | 0.0498 | 0.397 | 1 | |
| 7 | rs4720724 | rs2190935 | AGCTAC | 0.17 | −0.315 | 1.36E−06 | 1.22E−05 | |
| 7 | rs4720724 | rs2190935 | AGTCAT | 0.134 | −0.237 | 0.00153 | 0.01 | |
| 7 | rs4720724 | rs2190935 | GGTCAT | 0.0479 | −0.19 | 0.118 | 1 | |
| 7 | rs4720724 | rs2190935 | AGTTAC | 0.0429 | −0.258 | 0.0421 | 0.38 | |
| 7 | rs4720724 | rs2190935 | ATCCGC | 0.0154 | 0.058 | 0.78 | 1 | |
| 7 | rs4720724 | rs2190935 | GTCTAT | 0.012 | 0.304 | 0.214 | 1 | |
| 7 | rs4720724 | rs2190935 | GTCCAC | 0.0113 | 0.248 | 0.265 | 1 | |
| Chinese | 7 | rs4720724 | rs2190935 | AGCTAC | 0.523 | −0.184 | 0.000592 | 0.005 |
| 7 | rs4720724 | rs2190935 | AGTCAT | 0.162 | 0.0761 | 0.307 | 1 | |
| 7 | rs4720724 | rs2190935 | AGTTAC | 0.162 | 0.18 | 0.0165 | 0.15 | |
| 7 | rs4720724 | rs2190935 | GTCCGCa | 0.0359 | 0.536 | 6.21E−05 | 5.59E−04 | |
| 7 | rs4720724 | rs2190935 | GGTCAT | 0.0211 | 0.0119 | 0.949 | 1 | |
| 7 | rs4720724 | rs2190935 | ATTCAT | 0.0195 | −0.0189 | 0.928 | 1 | |
| 7 | rs4720724 | rs2190935 | GTTCGC | 0.0142 | −0.392 | 0.102 | 0.92 | |
| 7 | rs4720724 | rs2190935 | ATCCGC | 0.0107 | −0.148 | 0.542 | 1 | |
| 7 | rs4720724 | rs2190935 | AGCTAC | 0.01 | 0.224 | 0.431 | 1 |
P values represent the effect of testing each haploptype against all of the others. The commonest haplotype in the UK population is almost ten-fold less common in the Chinese population. SNPs defining this haplotype are rs4720724, rs758263, rs4263662, rs10259085, rs1008897, and rs2190935. Bonferroni, with correction for nine haplotypes tested.
H1 haplotype.
In a cohort of 318 UK white patients with biopsy-proven membranous nephropathy (MN), sera for Gd-IgA1 measurements were available for 308 and values were lower than patients with IgAN and similar to values in healthy subjects (Figure 1). Among the MN cohort, >30% of subjects had eGFR<60 ml/min but, unlike in patients with IgAN, we observed no correlation between Gd-IgA1 levels and serum creatinine−1 (Supplemental Figure 2). Genome-wide linear regression analysis of Gd-IgA1 levels within the MN cohort revealed association between Gd-IgA1 and the same alleles as observed in the discovery cohort (Supplemental Figure 6). In the combined analysis of 821 individuals, no associations at or approaching genome-wide significance (defined as P<5×10−8) at other loci were detected. Intriguingly, weak evidence of association (P approximately 10−5) was seen at the X-chromosomal C1GALT1C1 locus encoding Cosmc (Supplemental Figure 7), which is necessary for the C1GALT1 gene product to function.
Among 622 participants in the UK Genetic Regulation of Arterial Pressure of Humans in the Community (GRAPHIC) cohort (who all lacked evidence of renal impairment), strong association was again observed with alleles at the C1GALT1 locus (β=0.38, P<3.6×10−11; Supplemental Figure 8), with no other loci implicated. Univariate and multivariate analyses demonstrated no significant association with age, sex, or other baseline characteristics including creatinine clearance and eGFR (β<0.001 and P>0.5 for both). This therefore replicates the association of the H1 haplotype with elevated Gd-IgA1 in whites, and implies that differences in renal function per se do not influence Gd-IgA1 level.
The Same C1GALT1 Haplotype Is Rare but Also Associated with Gd-IgA1 in the Chinese Population
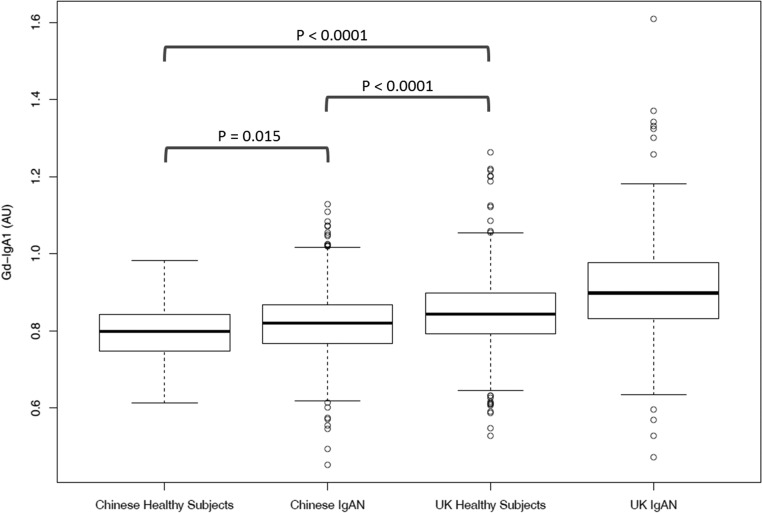
Data from the HapMap project indicate that, although common in Europeans, the H1 haplotype is less common in African and East Asian populations.27 To investigate how genetic variation across this locus influences Gd-IgA1 levels in a Chinese population we measured serum Gd-IgA1 levels in a cohort of 704 Chinese patients (49% male) with IgAN, and 111 ethnicity- and age-matched controls. This demonstrated that Gd-IgA1 levels were significantly higher in Chinese patients than Chinese healthy subjects, but in both these groups Gd-IgA1 levels were lower than in UK subjects (Figure 4).
Figure 4.
Gd-IgA1 levels were elevated in 704 Han Chinese patients with IgAN compared with 111 healthy Chinese subjects (P=0.015). Levels in both Chinese groups were significantly lower than those in UK healthy subjects and UK patients with IgAN (P<0.0001 for both). Ten percent of Chinese and 24% of UK subjects with IgAN exhibited Gd-IgA1 levels above the 95th percentile of their respective control populations. AU, arbitrary units of optical absorbance.
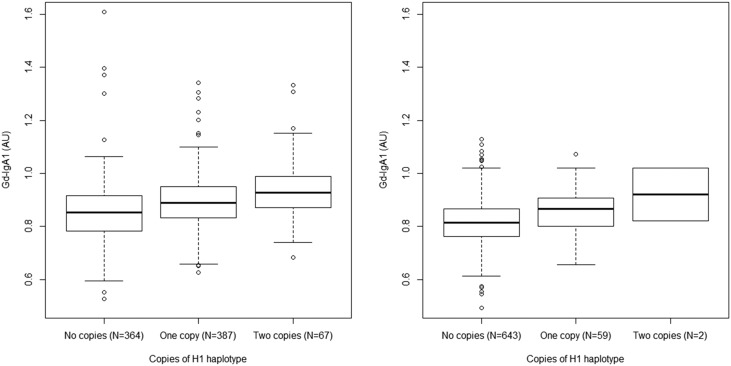
We next performed a candidate locus association study by genotyping 38 SNPs across the C1GALT1 locus and observed association of alleles at this locus with Gd-IgA1 level (P=5×10−4; Supplemental Figure 9). Consistent with HapMap data, we observed that the H1 haplotype was present but rare in this population, with a frequency of 0.035. The presence of this haplotype was again associated with elevated Gd-IgA1 levels (Table 2, P=6×10−5). Multiple regression analyses showed that disease status, ethnicity, and the presence of the H1 haplotype were all independently correlated with Gd-IgA1 level, but age and sex were not: Gd-IgA1 levels remained significantly higher in white compared with Chinese subjects when stratified by number of copies of the H1 haplotype (Figure 5; P<10−8).
Figure 5.
Gd-IgA1 levels increase with copies of the H1 haplotype in Chinese and white populations. In the combined IgA and MN (white) cohort (left panel) haplotype frequency was 0.32 (n=821, R2=0.033, P<0.0001). In Chinese patients with IgAN (right panel) haplotype frequency was 0.04 (n=704, R2=0.019, P<0.001). In multiple regression analysis, Chinese ethnicity was associated with lower Gd-IgA1 levels than in whites, even correcting for haplotype frequency and disease status (P<0.0001). AU, arbitrary units of optical absorbance.
Discussion
Elevation of serum Gd-IgA1 is a consistent finding in IgAN that, in susceptible individuals, is associated with autoantibody production and the formation of circulating immune complexes that trigger glomerular injury through mesangial cell activation, endocapillary proliferation, podocyte injury, and tubulointerstitial inflammation and fibrosis.16,18,20–22 Delineating the genetic control of Gd-IgA1 production is therefore important in understanding this common GN.
In white and Chinese populations we identified association between Gd-IgA1 levels and a haplotype spanning the C1GALT1 gene. C1GALT1 encodes the enzyme core 1 synthase, glycoprotein-N-acetylgalactosamine 3-β-galactosyltransferase (C1GALT1), which catalyzes the transfer of galactose (Gal) from UDP-Gal to N-acetylgalactosamine (GalNAc) O-linked esters of threonine and serine residues of target proteins, including IgA1, to form the T antigen. This requires the chaperone Cosmc, encoded by the X-linked gene C1GALT1C1, which prevents rapid degradation of C1GALT1.28 We also observed possible association between Gd-IgA1 and variation at C1GALT1C1, although this was not statistically significant at the genome-wide level. Mice lacking C1GALT1 develop thrombocytopenia, proteinuria, and renal failure associated with anomalous glycosylation of proteins.29 Somatic mutations inactivating Cosmc (leading to increased degradation of C1GALT1) cause Tn syndrome in humans, which is characterized by autoantibodies forming against the undergalactosylated O-linked glycoproteins on red cell membranes.17 It therefore appears likely that variation at C1GALT1 might similarly modulate galactosylation of O-glycosylated proteins, including IgA1, resulting in generation of IgA1 neo-epitopes capable of triggering autoantibody production and disease in susceptible individuals.
There is, however, no evidence for a generalized defect in O-galactosylation in IgAN: we have previously shown that in IgAN the patterns of O-galactosylation of C1 inhibitor and IgD, the only other O-glycosylated human immunoglobulin isotype, are unaltered, implying that the reduction in IgA1 O-galactosylation in IgAN is a specific feature of IgA1-secreting cells.30 This suggests that the haplotype we identified leads to elevated Gd-IgA1 through altered maturation-dependent transcriptional regulation of C1GALT1, rather than via differences in C1GALT1 protein structure that would be present in all C1GALT1-expressing cell types. Consistent with this, imputation of all of the alleles common to the H1 haplotype using 1000 Genomes data identified no coding variants in linkage disequilibrium with the associated alleles. Furthermore, published data linking genetic variation with gene expression (eQTLs) in lymphoblastoid cell lines show that SNP alleles imputed to lie on the H1 haplotype are strongly associated with reduced C1GALT1 transcript levels (Table 3).31 Additional in silico analyses using ENCODE data show that some strongly associated SNPs lie within consensus transcription factor binding elements, including rs7780273 (imputed P=1.8 x10−8; eQTL P value of 7.5×10−7), which lies at a predicted SOX2-OCT4 site,32,33 and rs758263, which lies within the core promoter region for C1GALT1 and is predicted to affect binding of RUNX3, a transcription factor present in B cells that is necessary for class switching to IgA production.34,35 These in silico predictions coupled with previous in vitro data demonstrating modulation of IgA1 O-galactosylation by Th2 cytokines and IL-6,36,37 as well as our previous observation that IgA1 O-galactosylation varies depending on the site of antigen encounter and B cell activation,38 are consistent with an effect of the H1 haplotype on transcriptional control of C1GALT1, particularly in IgA1-committed B cells.
Table 3.
Expression quantitative trait loci of SNPs associated with Gd-IgA131
| SNP | Position | Gd-IgA1 P Value (Imputed) | GRAPHIC Replication P Value | Cell Type | eQTL P Value |
|---|---|---|---|---|---|
| rs10246303 | 7286445 | 6.488E−10 | 3.906E−08 | LCL | 3.03884E−09 |
| rs6463656 | 7244181 | 7.576E−09 | 1.458E−08 | LCL | 3.00367E−08 |
| rs6463657 | 7244254 | 7.576E−09 | 1.455E−08 | LCL | 3.40312E−08 |
| rs13226913 | 7246846 | 7.576E−09 | 1.19E−08 | LCL | 3.8322E−08 |
| rs10251505 | 7254489 | 1.561E−08 | 1.721E−08 | LCL | 2.03717E−08 |
| rs4318980 | 7256490 | 1.767E−08 | 1.426E−08 | LCL | 1.44846E−08 |
| rs7780273 | 7250449 | 1.883E−08 | 0.00010165 | LCL | 3.11303E−09 |
| rs2881755 | 7256439 | 2.022E−08 | 1.544E−07 | LCL | 1.07186E−07 |
| rs2881756 | 7256439 | 2.022E−08 | N/A | LCL | 1.46728E−07 |
| rs10952047 | 7289543 | 2.358E−08 | 2.003E−06 | LCL | 1.7149E−11 |
| rs4720726 | 7225451 | 3.287E−08 | 1.68E−04 | LCL | 7.5698E−09 |
| rs11771259 | 7277215 | 3.691E−08 | 5.363E−06 | LCL | 1.14591E−11 |
| rs4724958 | 7226553 | 7.369E−08 | 0.00017043 | LCL | 9.72937E−09 |
| rs4720727 | 7226695 | 1.704E−07 | 2.569E−07 | LCL | 6.11804E−08 |
| rs12702588 | 7222806 | 3.597E−07 | N/A | LCL | 7.84334E−07 |
| rs57552003 | 7223104 | 6.837E−07 | 5.952E−06 | LCL | 5.51448E−08 |
| rs11773545 | 7196878 | 8.911E−-07 | 0.00176318 | LCL | 1.59081E−06 |
| rs1047763 | 7283569 | 1.034E−06 | 2.018E−06 | LCL | 3.64039E−11 |
Gd-IgA1 P values are imputed, except for rs13226913 which was directly genotyped. All of these SNPs are on the H1 haplotype and the allele associated with elevated Gd-IgA1 levels is associated with lower transcript levels in all cases. eQTL, expression quantitative trait loci; LCL, lymphoblastoid cell line; N/A, not available.
Our data indicate that C1GALT1 genotype explains approximately 3% of the variability in Gd-IgA1 levels, suggesting other factors are important in determining Gd-IgA1 levels in an individual. Because this is a genome-wide study it is unlikely that variation at any other single locus is responsible for this, and we also exclude the possibility that kidney function itself significantly affects Gd-IgA1 levels. Cytokines, and presumably other stimuli, acting in B cells can influence IgA1 galactosylation,37 and likely contribute to the variability in Gd-IgA1 levels. Identification of the transcriptional mechanisms influencing C1GALT1 expression may provide insights into how Gd-IgA1 levels are controlled in health and disease.
Association between C1GALT1 and IgAN susceptibility has previously been tested in candidate gene studies: Li et al.39,40 genotyped nine SNPs in Asian cohorts and reconstructed haplotypes across the gene. They identified evidence of association between the disease and the presence of haplotypes containing rs1047763(G) allele (designated “YATDG” and “ATDG”). Although this allele was also associated with the disease in an Italian study,41 these associations with disease risk have not been replicated in larger genome-wide studies.9–11,42,43 We found that rs1047763(G) was associated with Gd-IgA1 level in both our discovery (P=8.7×10−6) and replication (P=2×10−6) cohorts, and is present on the H1 haplotype, consistent with increased disease susceptibility being mediated by increased Gd-IgA1 levels.
A limitation of this and other GWASs in IgAN is the lack of clear evidence of association with IgAN susceptibility at this locus. Our data show that for each standard deviation increase in Gd-IgA1, the odds of IgAN increased by a ratio of 1.52 (95% confidence interval, 1.35 to 1.73), and in the white discovery cohort the R2 value associating the H1 haplotype with Gd-IgA1 level was 0.033 (implying that 3.3% of the variation in Gd-IgA1 level is explained by the number of copies of H1 haplotype). Power calculation, performed as described previously,44 indicates that a candidate gene study (with nominal α 0.05) in the white population would need >5300 participants to have >80% power to detect an effect of the H1 haplotype on risk of IgAN. In a GWAS (where α is typically <5×10−8), a study around five times larger would be needed. In Chinese subjects the H1 haplotype is less common, and we observed an R2 of 0.019, implying that previously published studies in this population have been underpowered to detect an association with disease risk: even the largest published meta-GWAS study, comprising 7600 cases and 13,000 controls,45 has <10% power to detect an association between C1GALT1 and disease.
Perhaps the most surprising finding in this study was the disparity in levels of Gd-IgA1 between white and Chinese subjects, both healthy and with IgAN. All Gd-IgA1 level measurements were performed in the same laboratory using the same batch of Helix aspersa lectin across all samples, with the same standards run in all plates, allowing direct comparison of Gd-IgA1 levels across the different cohorts. This is the first time such a comparison has been reported and indicates that the increased prevalence of IgAN in China cannot be attributed to differences in Gd-IgA1 levels. This, together with the differences in frequency of the H1 haplotype and the clinical manifestations of IgAN described in different continents, suggests that different pathogenic pathways may be operating in different populations. Previous studies have shown that IgAN susceptibility is associated with variation at genes involved in immune defense, and higher-risk alleles are more prevalent in East Asians.2,12 One possibility is that the high burden of immunologic risk alleles in East Asian populations results in a higher likelihood of a damaging immunologic response to Gd-IgA1 in the circulation, and hence a higher likelihood of kidney disease. In this paradigm, the reduced Gd-IgA1 in Chinese subjects might even have resulted from selection against the H1 haplotype in this population due to the high prevalence of other risk alleles that increase disease susceptibility.
In summary, we demonstrate that circulating Gd-IgA1 levels are heritable and influenced by genetic variation at the C1GALT1 gene across different populations. This provides the first direct evidence that common genetic variation can influence O-glycosylation in humans. Our observations suggest that modulation of this pathway might influence susceptibility to, or outcomes in, IgAN, and C1GALT1 activity would be the logical enzymatic step to target in order to test this hypothesis.
Concise Methods
Discovery cohort: UK Glomerulonephritis DNA Bank (UKGDB) from individuals with biopsy-proven IgAN (66% male) and healthy relatives has been previously described.11 After quality control for ethnicity (using principal component analysis), genotyping rate, and excluding cryptic relatedness, estimated from identity-by-state information (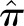 >0.125), serum and genotype data were available for 379 UK white patients and 309 of their parents in 134 parent-affected trios. All individuals were genotyped at 318,127 SNPs using the Illumina Sentrix HumanHap300 BeadChip, of which 302,210 passed quality control (>90% genotyping rate, minor allele frequency >0.05, Hardy–Weinberg equilibrium P>0.001). Follow-up data allowed 277 of these patients to be classed as “progressors” (ESRD or doubling of serum creatinine) or “nonprogressors” (normal renal function and <20% rise in serum creatinine over >5 years’ follow-up), with the remainder classed as “indeterminate.”
>0.125), serum and genotype data were available for 379 UK white patients and 309 of their parents in 134 parent-affected trios. All individuals were genotyped at 318,127 SNPs using the Illumina Sentrix HumanHap300 BeadChip, of which 302,210 passed quality control (>90% genotyping rate, minor allele frequency >0.05, Hardy–Weinberg equilibrium P>0.001). Follow-up data allowed 277 of these patients to be classed as “progressors” (ESRD or doubling of serum creatinine) or “nonprogressors” (normal renal function and <20% rise in serum creatinine over >5 years’ follow-up), with the remainder classed as “indeterminate.”
UK replication cohorts: Replication was performed using serum from 308 white UK patients with biopsy-proven membranous glomerulopathy from the UKGDB,46 genotyped using the Illumina HumanCNV370-Quad SNP chip. Further independent replication was performed using 622 samples from unrelated adults from the GRAPHIC cohort, in which all individuals had normal urinalysis and plasma urea and creatinine at recruitment.47
Chinese replication cohort: DNA and sera were available from 704 Han Chinese patients from the Guangzhou (49% male) with biopsy-proven IgAN and 111 healthy age- and sex-matched subjects. Gd-IgA1 was measured and each patient was genotyped at 38 SNPs across the C1GALT1 gene, selected using Haploview48 to tag >90% of haplotypes present in the Chinese population. All subjects provided informed written consent to genetic analyses and the study was performed according to the principles of the Declaration of Helsinki with local ethical approval at each site.
Genome-wide linear regression association analyses were performed with Plink49,50 using Gd-IgA1 data standardized to a mean of 0 and an SD of 1 to allow interpretation of β, as detailed in the Supplemental Material. Haplotypes were visualized using Haploview.48 Genotypes were imputed using the University of Michigan imputation server.51 ANOVA and linear regression tests were performed using R. Gd-IgA1 levels were measured using a Helix aspersa lectin–based ELISA method as previously described (see Supplemental Material).52
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by a UK National Institute for Health Research Translational Research Collaboration in Rare Disease Grant, The Mayer Family Trust, and Kidney Research UK. D.P.G. is supported by the Medical Research Council. C.P.N. and N.J.S. are supported by the British Heart Foundation and N.J.S. is a UK National Institute for Health Research Senior Investigator.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016091043/-/DCSupplemental.
References
- 1.D’Amico G: The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727, 1987 [PubMed] [Google Scholar]
- 2.Kiryluk K, Li Y, Sanna-Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, Snyder HJ, Choi M, Hou P, Scolari F, Izzi C, Gigante M, Gesualdo L, Savoldi S, Amoroso A, Cusi D, Zamboli P, Julian BA, Novak J, Wyatt RJ, Mucha K, Perola M, Kristiansson K, Viktorin A, Magnusson PK, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Boland A, Metzger M, Thibaudin L, Wanner C, Jager KJ, Goto S, Maixnerova D, Karnib HH, Nagy J, Panzer U, Xie J, Chen N, Tesar V, Narita I, Berthoux F, Floege J, Stengel B, Zhang H, Lifton RP, Gharavi AG: Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Tao K, Nicholls K, Kincaid-Smith P: [IgA nephropathy in Chinese and Australian patients: A comparison between clinical and pathological features]. Zhonghua Yi Xue Za Zhi 71: 153–155, 112, 1991 [PubMed] [Google Scholar]
- 4.Ştefan G, Ismail G, Stancu S, Zugravu A, Andronesi A, Mandache E, Mircescu G: Validation study of Oxford Classification of IgA Nephropathy: The significance of extracapillary hypercellularity and mesangial IgG immunostaining. Pathol Int 66: 453–459, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Pesce F, Diciolla M, Binetti G, Naso D, Ostuni VC, Di Noia T, Vågane AM, Bjørneklett R, Suzuki H, Tomino Y, Di Sciascio E, Schena FP: Clinical decision support system for end-stage kidney disease risk estimation in IgA nephropathy patients. Nephrol Dial Transplant 31: 80–86, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Floege J, Rauen T, Eitner F: Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 374: 992–993, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Ning Y, Shang W, Li M, Ku M, Li Q, Li Y, Dai W, Shao J, Zeng R, Han M, He X, Yao Y, Lv Y, Liu X, Ge S, Xu G: Analysis of 4931 renal biopsy data in central China from 1994 to 2014. Ren Fail 38: 1021–1030, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Goto S, Kondo D, Takata T, Yamazaki H, Hosojima M, Yamamoto S, Kaneko Y, Aoyagi R, Narita I: Comparison of methods of steroid administration combined with tonsillectomy for IgA nephropathy patients [published online ahead of print May 23, 2016]. Clin Exp Nephrol doi:10.1007/s10157-016-1282-8 [DOI] [PubMed] [Google Scholar]
- 9.Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, Sun LD, Sim KS, Li Y, Foo JN, Wang W, Li ZJ, Yin XY, Tang XQ, Fan L, Chen J, Li RS, Wan JX, Liu ZS, Lou TQ, Zhu L, Huang XJ, Zhang XJ, Liu ZH, Liu JJ: A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 44: 178–182, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, Sanna-Cherchi S, Men CJ, Julian BA, Wyatt RJ, Novak J, He JC, Wang H, Lv J, Zhu L, Wang W, Wang Z, Yasuno K, Gunel M, Mane S, Umlauf S, Tikhonova I, Beerman I, Savoldi S, Magistroni R, Ghiggeri GM, Bodria M, Lugani F, Ravani P, Ponticelli C, Allegri L, Boscutti G, Frasca G, Amore A, Peruzzi L, Coppo R, Izzi C, Viola BF, Prati E, Salvadori M, Mignani R, Gesualdo L, Bertinetto F, Mesiano P, Amoroso A, Scolari F, Chen N, Zhang H, Lifton RP: Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 43: 321–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, Kumar A, Peden JF, Maxwell PH, Morris DL, Padmanabhan S, Vyse TJ, Zawadzka A, Rees AJ, Lathrop M, Ratcliffe PJ: HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 21: 1791–1797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai Z, Li M, Liu W, Foo JN, Mansouri O, Yin P, Zhou Q, Tang X, Dong X, Feng S, Xu R, Zhong Z, Chen J, Wan J, Lou T, Yu J, Zhou Q, Fan J, Mao H, Gale D, Barratt J, Armour JA, Liu J, Yu X: Low α-defensin gene copy number increases the risk for IgA nephropathy and renal dysfunction. Sci Transl Med 8: 345ra88, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Hiki Y, Tanaka A, Kokubo T, Iwase H, Nishikido J, Hotta K, Kobayashi Y: Analyses of IgA1 hinge glycopeptides in IgA nephropathy by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Am Soc Nephrol 9: 577–582, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Allen AC, Bailey EM, Barratt J, Buck KS, Feehally J: Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J Am Soc Nephrol 10: 1763–1771, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J: Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem 280: 19136–19145, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, Feehally J: Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int 60: 969–973, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ju T, Cummings RD: Protein glycosylation: Chaperone mutation in Tn syndrome. Nature 437: 1252, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang ZQ, Raska M, Stewart TJ, Reily C, King RG, Crossman DK, Crowley MR, Hargett A, Zhang Z, Suzuki H, Hall S, Wyatt RJ, Julian BA, Renfrow MB, Gharavi AG, Novak J: Somatic mutations modulate autoantibodies against galactose-deficient IgA1 in IgA nephropathy. J Am Soc Nephrol 27: 3278–3284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amore A, Cirina P, Conti G, Brusa P, Peruzzi L, Coppo R: Glycosylation of circulating IgA in patients with IgA nephropathy modulates proliferation and apoptosis of mesangial cells. J Am Soc Nephrol 12: 1862–1871, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Novak J, Vu HL, Novak L, Julian BA, Mestecky J, Tomana M: Interactions of human mesangial cells with IgA and IgA-containing immune complexes. Kidney Int 62: 465–475, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC: Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 15: 622–634, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ: Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int 80: 79–87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomax-Browne HJ, Visconti A, Pusey CD, Cook HT, Spector TD, Pickering MC, Falchi M: IgA1 glycosylation is heritable in healthy twins. J Am Soc Nephrol 28: 64–68, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hastings MC, Moldoveanu Z, Julian BA, Novak J, Sanders JT, McGlothan KR, Gharavi AG, Wyatt RJ: Galactose-deficient IgA1 in African Americans with IgA nephropathy: Serum levels and heritability. Clin J Am Soc Nephrol 5: 2069–2074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA: Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19: 1008–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Peltonen L, Dermitzakis E, Bonnen PE, Altshuler DM, Gibbs RA, de Bakker PI, Deloukas P, Gabriel SB, Gwilliam R, Hunt S, Inouye M, Jia X, Palotie A, Parkin M, Whittaker P, Yu F, Chang K, Hawes A, Lewis LR, Ren Y, Wheeler D, Gibbs RA, Muzny DM, Barnes C, Darvishi K, Hurles M, Korn JM, Kristiansson K, Lee C, McCarrol SA, Nemesh J, Dermitzakis E, Keinan A, Montgomery SB, Pollack S, Price AL, Soranzo N, Bonnen PE, Gibbs RA, Gonzaga-Jauregui C, Keinan A, Price AL, Yu F, Anttila V, Brodeur W, Daly MJ, Leslie S, McVean G, Moutsianas L, Nguyen H, Schaffner SF, Zhang Q, Ghori MJ, McGinnis R, McLaren W, Pollack S, Price AL, Schaffner SF, Takeuchi F, Grossman SR, Shlyakhter I, Hostetter EB, Sabeti PC, Adebamowo CA, Foster MW, Gordon DR, Licinio J, Manca MC, Marshall PA, Matsuda I, Ngare D, Wang VO, Reddy D, Rotimi CN, Royal CD, Sharp RR, Zeng C, Brooks LD, McEwen JE; International HapMap 3 Consortium : Integrating common and rare genetic variation in diverse human populations. Nature 467: 52–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju T, Cummings RD: A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA 99: 16613–16618, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander WS, Viney EM, Zhang JG, Metcalf D, Kauppi M, Hyland CD, Carpinelli MR, Stevenson W, Croker BA, Hilton AA, Ellis S, Selan C, Nandurkar HH, Goodnow CC, Kile BT, Nicola NA, Roberts AW, Hilton DJ: Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc Natl Acad Sci USA 103: 16442–16447, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AC, de Wolff JF, Molyneux K, Feehally J, Barratt J: O-glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol 17: 1192–1199, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Yu CH, Pal LR, Moult J: Consensus genome-wide expression quantitative trait loci and their relationship with human complex trait disease. OMICS 20: 400–414, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerstein MB, Kundaje A, Hariharan M, Landt SG, Yan KK, Cheng C, Mu XJ, Khurana E, Rozowsky J, Alexander R, Min R, Alves P, Abyzov A, Addleman N, Bhardwaj N, Boyle AP, Cayting P, Charos A, Chen DZ, Cheng Y, Clarke D, Eastman C, Euskirchen G, Frietze S, Fu Y, Gertz J, Grubert F, Harmanci A, Jain P, Kasowski M, Lacroute P, Leng J, Lian J, Monahan H, O’Geen H, Ouyang Z, Partridge EC, Patacsil D, Pauli F, Raha D, Ramirez L, Reddy TE, Reed B, Shi M, Slifer T, Wang J, Wu L, Yang X, Yip KY, Zilberman-Schapira G, Batzoglou S, Sidow A, Farnham PJ, Myers RM, Weissman SM, Snyder M: Architecture of the human regulatory network derived from ENCODE data. Nature 489: 91–100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Zhuang J, Iyer S, Lin X, Whitfield TW, Greven MC, Pierce BG, Dong X, Kundaje A, Cheng Y, Rando OJ, Birney E, Myers RM, Noble WS, Snyder M, Weng Z: Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res 22: 1798–1812, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe K, Sugai M, Nambu Y, Osato M, Hayashi T, Kawaguchi M, Komori T, Ito Y, Shimizu A: Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J Immunol 184: 2785–2792, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Zeng J, Mi R, Wang Y, Li Y, Lin L, Yao B, Song L, van Die I, Chapman AB, Cummings RD, Jin P, Ju T: Promoters of human cosmc and T-synthase genes are similar in structure, yet different in epigenetic regulation. J Biol Chem 290: 19018–19033, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada K, Kobayashi N, Ikeda T, Suzuki Y, Tsuge T, Horikoshi S, Emancipator SN, Tomino Y: Down-regulation of core 1 beta1,3-galactosyltransferase and Cosmc by Th2 cytokine alters O-glycosylation of IgA1. Nephrol Dial Transplant 25: 3890–3897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki H, Raska M, Yamada K, Moldoveanu Z, Julian BA, Wyatt RJ, Tomino Y, Gharavi AG, Novak J: Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 289: 5330–5339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AC, Molyneux K, Feehally J, Barratt J: O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol 17: 3520–3528, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Li GS, Zhang H, Lv JC, Shen Y, Wang HY: Variants of C1GALT1 gene are associated with the genetic susceptibility to IgA nephropathy. Kidney Int 71: 448–453, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Zhu L, Tang W, Li G, Lv J, Ding J, Yu L, Zhao M, Li Y, Zhang X, Shen Y, Zhang H, Wang H: Interaction between variants of two glycosyltransferase genes in IgA nephropathy. Kidney Int 76: 190–198, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Pirulli D, Crovella S, Ulivi S, Zadro C, Bertok S, Rendine S, Scolari F, Foramitti M, Ravani P, Roccatello D, Savoldi S, Cerullo G, Lanzilotta SG, Bisceglia L, Zelante L, Floege J, Alexopoulos E, Kirmizis D, Ghiggeri GM, Frascà G, Schena FP, Amoroso A; European IgAN Consortium : Genetic variant of C1GalT1 contributes to the susceptibility to IgA nephropathy. J Nephrol 22: 152–159, 2009 [PubMed] [Google Scholar]
- 42.An J, Lü Q, Zhao H, Cao Y, Yan B, Ma Z: A study on the association between C1GALT1 polymorphisms and the risk of Henoch-Schönlein purpura in a Chinese population. Rheumatol Int 33: 2539–2542, 2013 [DOI] [PubMed] [Google Scholar]
- 43.He X, Zhao P, Kang S, Ding Y, Luan J, Liu Z, Wu Y, Yin W: C1GALT1 polymorphisms are associated with Henoch-Schönlein purpura nephritis. Pediatr Nephrol 27: 1505–1509, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Brion MJ, Shakhbazov K, Visscher PM: Calculating statistical power in Mendelian randomization studies. Int J Epidemiol 42: 1497–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG: Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46: 1187–1196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Tobin MD, Raleigh SM, Newhouse S, Braund P, Bodycote C, Ogleby J, Cross D, Gracey J, Hayes S, Smith T, Ridge C, Caulfield M, Sheehan NA, Munroe PB, Burton PR, Samani NJ: Association of WNK1 gene polymorphisms and haplotypes with ambulatory blood pressure in the general population. Circulation 112: 3423–3429, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Barrett JC, Fry B, Maller J, Daly MJ: Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC: PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purcell S: PLINK v1.9. 2015. Available at http://pngu.mgh.harvard.edu/purcell/plink/
- 51. Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, Vrieze S, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh PR, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C: Next-generation genotype imputation service and methods. Nature Genetics 48: 1284–1287, 2016. [DOI] [PMC free article] [PubMed]
- 52.Allen AC, Harper SJ, Feehally J: Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol 100: 470–474, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.