Abstract
The mucoprotein uromodulin is the most abundant protein in mammalian urine and has important roles in ion transport, maintenance of water and electrolyte balance, and clearance of bacteria from the urinary tract. Low urinary uromodulin concentrations have been associated with increased mortality risk. However, measuring uromodulin in urine has several preanalytic drawbacks, and sensitive assays for the detection of uromodulin in blood have become available. In this study, we investigated the association of serum uromodulin concentration with cardiovascular biomarkers and mortality risk in a large cohort of patients referred for coronary angiography. Uromodulin concentrations were available in 3057 of 3316 participants of the Ludwigshafen Risk and Cardiovascular Health Study. Higher serum uromodulin concentration associated with a favorable metabolic profile, lower prevalence rates of comorbidities (arterial hypertension, diabetes mellitus, and heart failure), and a lower risk for 10-year mortality, with hazard ratios (95% confidence intervals) of 0.65 (0.54 to 0.78), 0.71 (0.58 to 0.88), and 0.57 (0.45 to 0.73) in the second, third, and fourth quartiles, respectively, compared with the first quartile. The association with reduced mortality was independent of other cardiovascular risk factors, including eGFR, and stronger after adjustment for the genotype of the rs12917707 polymorphism at the UMOD locus. Adding serum uromodulin concentration to established cardiovascular risk prediction scores improved risk prediction. Uromodulin may, therefore, be a useful marker for cardiovascular and renal health.
Keywords: cardiovascular events, cardiovascular disease, clinical epidemiology, glomerular disease, mortality risk, renal tubular epithelial cells
Uromodulin was isolated in 1950 as a mucoprotein from human urine that inhibits the agglutination of viruses1 and later, named according to its discoverers Tamm and Horsfall. Uromodulin is synthesized on the rough ER of epithelial cells of the thick ascending limb of the loop of Henle. After transport to the apical plasma membrane, it is cleaved and released into the tubular fluid, where it constitutes the most abundant protein present in the urine of humans. Extracellularly, it polymerizes into a high molecular weight polymer resembling a three-dimensional matrix with pores.2 The filamentous gel–like structures made up by uromodulin serve as a physical barrier to water permeation.3 It also has important roles in ion transport, maintenance of water and electrolyte balance,4 and clearing bacteria from the urinary tract (e.g., by direct binding to type-1 fimbriated Escherichia coli).5
Animal studies with uromodulin knockout mice showed an increased susceptibility to urinary tract infections.6 In addition, uromodulin seems to play a key role in innate immunity of the kidney. Uromodulin is able to activate myeloid dendritic cells via Toll-like receptor 4, and TLR4 knockout mice have been reported to be severely impaired in the uromodulin-specific humoral immune response.7 An interaction of the uromodulin protein with lymphotoxin-α has been inferred from interaction databases. Lymphotoxin-α mediates a large variety of inflammatory, immunostimulatory, and antiviral responses. Furthermore, lower urinary uromodulin has been linked to increased all-cause mortality in an observational study of risk factors for cardiovascular disease8 and increased cardiovascular mortality in patients with type 1 diabetes.9
Recently, genetic and genome-wide association studies linking polymorphisms in the UMOD gene located on 16p12.3 with eGFR10 and increased risk of CKD,10–12 hypertension,13 and diabetic nephropathy14 renewed the interest in uromodulin. Although most studies of uromodulin have been conducted using urinary uromodulin, new and more sensitive assays measuring uromodulin in blood have become available. Apart from the intracellular route of uromodulin to the apical cell pole, a second pathway to the basolateral cell site was recently identified, in which the protein is released from the endoplasmic reticulum via the Golgi apparatus and transferred by cytoplasmic vesicles to the contraluminal plasma membrane. From there, it is then shed into the renal interstitium and blood compartment.15 An association with low concentrations of serum or plasma uromodulin with increased risk of CKD has been reported previously.16,17
The aim of our study was to analyze the association of serum uromodulin with all-cause and cardiovascular mortality in a large and well characterized cohort of patients who had been referred for coronary angiography and investigate its potential predictive value in addition to established cardiovascular risk scores.
Results
Serum Uromodulin and Anthropometric Characteristics
Uromodulin was available in 3057 of 3316 participants of the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. We split our cohort into quartiles of uromodulin and investigated the association with anthropometric data and biomarkers by ANOVA and chi-squared test (Table 1). Study participants with higher serum uromodulin were younger, had a lower body mass index (BMI), and had lower blood pressure (BP) as well as had lower triglycerides, fasting glucose, hemoglobin A1c, high-sensitivity C-reactive protein (hsCRP), parathyroid hormone, galectin-3, and N-terminal pro–B-type natriuretic peptide (NT-proBNP). LDL cholesterol, HDL cholesterol, 25OH vitamin D, and 1,25(OH)2 vitamin D were higher in patients with higher uromodulin. Uromodulin was inversely associated with serum creatinine, cystatin C, and β-trace protein and directly correlated with eGFR. Mean serum uromodulin concentration was slightly higher in women compared with men (Supplemental Figure 1).
Table 1.
Anthropometric and biochemical characteristics of study participants shown as mean±SD or median (25th–75th percentile) according to quartiles of serum uromodulin
| Characteristic | Uromodulin, ng/ml | P Valuea | |||
|---|---|---|---|---|---|
| <103 | 103–146 | 146–199 | ≥199 | ||
| N | 765 | 765 | 763 | 764 | |
| Age, yr | 65.4±10.6 | 63.6±10.3 | 62.0±10.2 | 59.6±10.4 | <0.001 |
| Sex, % men | 71.2 | 71.7 | 71.6 | 64.4 | 0.003 |
| BMI, kg/m2 | 27.7±4.28 | 27.7±3.95 | 27.7±4.25 | 26.9±3.84 | <0.001 |
| Systolic BP, mmHg | 144±24.5 | 141±23.4 | 141±23.9 | 138±22.0 | <0.001 |
| Diastolic BP, mmHg | 80.7±11.7 | 80.8±11.0 | 81.5±11.9 | 81.0±10.8 | 0.51 |
| LDL-C, mg/dl | 113±33.5 | 116±33.5 | 117±33.3 | 120.0±36.3 | <0.001 |
| HDL-C, mg/dl | 36.5±10.5 | 38.2±10.7 | 38.7±11.0 | 41.2±10.6 | <0.001 |
| TG, mg/dl | 156 (120–219) | 147 (113–201) | 147 (107–200) | 135 (101–181) | <0.001 |
| Fasting glucose, mg/dl | 106 (95.9–129) | 103 (93.7–122) | 102 (93.7–116) | 99.2 (91.6–110) | <0.001 |
| HbA1c, % | 6.55±1.44 | 6.42±1.34 | 6.23±1.12 | 6.07±0.99 | <0.001 |
| HOMA-IR | 2.43 (1.48–4.09) | 2.22 (1.39–3.99) | 2.20 (1.37–3.54) | 1.82 (1.56–2.91) | <0.001 |
| hsCRP, mg/dl | 5.06 (1.87–10.7) | 3.68 (1.44–9.04) | 2.92 (1.22–7.35) | 2.34 (1.05–6.54) | <0.001 |
| Fibrinogen, mg/dl | 402 (342–476) | 374 (318–458) | 372 (319–440) | 359 (310–428) | <0.001 |
| Galectin-3, mg/L | 17.4 (13.4–22.2) | 14.8 (11.5–18.2) | 13.8 (10.7–16.8) | 12.9 (10.3–15.8) | <0.001 |
| NT-proBNP, pg/ml | 618 (201–1772) | 289 (108–845) | 261 (100–678) | 184 (81–530) | <0.001 |
| Renin, pg/ml | 23.0 (12.0–54.0) | 19.0 (10.0–42.0) | 17.0 (9.00–37.0) | 17.0 (9.00–36.0) | <0.001 |
| Aldosterone, ng/L | 77.0 (47.0–127) | 73.0 (46.0–120) | 76.0 (47.0–115) | 79.0 (47.0–122) | 0.18 |
| Uric acid, mg/dl | 5.6 (4.4–7.0) | 5.0 (4.1–6.0) | 4.8 (3.9–5.8) | 4.3 (3.5–5.1) | <0.001 |
| PTH, pg/ml | 33 (24–46.8) | 29 (22–40) | 29 (21–37) | 26 (20–35) | <0.001 |
| 25OH vitamin D , μg/L | 16.3±9.63 | 17.5±10.9 | 18.0±8.97 | 18.3±9.01 | <0.001 |
| 1,25(OH)2 vitamin D, ng/L | 30.7±14.3 | 34.8±12.9 | 36.2±13.5 | 38.5±14.1 | <0.001 |
| β-Trace protein, mg/L | 0.62 (0.49–0.84) | 0.55 (0.44–0.69) | 0.52 (0.41–0.63) | 0.52 (0.43–0.61) | <0.001 |
| Creatinine, mg/dl | 1.0 (0.9–1.2) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | <0.001 |
| Cystatin C, mg/L | 1.07 (0.89–1.38) | 0.93 (0.82–1.06) | 0.89 (0.79–1.01) | 0.84 (0.77–0.94) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 69.1±23.6 | 81.5±18.0 | 85.8±16.3 | 90.7±15.2 | <0.001 |
| CAD, % | 83.9 | 80.8 | 76.7 | 70 | <0.001 |
| Heart failure, % | 45.5 | 33.1 | 29.4 | 24.3 | <0.001 |
| Diabetes mellitus, % | 49.3 | 44.6 | 37.5 | 28.5 | <0.001 |
| Hypertension, % | 77.6 | 75.8 | 70.8 | 66.1 | <0.001 |
| Smoking (active/ex/never), % | 22.7/44.4/32.8 | 23.3/43.1/33.6 | 21.5/40.5/38.0 | 25.3/36.3/38.5 | 0.01 |
| ACE inhibitors, % | 62.4 | 56.2 | 49.9 | 44.2 | <0.001 |
| Diuretics, % | 44.7 | 31.5 | 23.3 | 14.8 | <0.001 |
| Calcium antagonists, % | 19.5 | 15.8 | 14.8 | 12.8 | 0.004 |
| AT2 receptor blocker, % | 6.41 | 4.71 | 4.06 | 3.14 | 0.02 |
| β-Blocker, % | 60.8 | 64.7 | 64.9 | 63 | 0.31 |
| Lipid-lowering therapy, % | 47.8 | 49.7 | 51.6 | 46.2 | 0.17 |
LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TG, triglycerides; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment–insulin resistance; PTH, parathyroid hormone; 25OH vitamin D, 25-hydroxycholecalciferol; 1,25(OH)2 vitamin D, 1,25-dihydroxycholecalciferol; ACE, angiotensin converting enzyme; AT2, angiotensin II.
ANOVA (non-normally distributed variables were log transformed before entering analyses) or chi-squared test; a P value of 0.001 would be regarded as significant after Bonferroni correction for 35 tests.
Regarding prevalent disease, participants with higher uromodulin were less likely to suffer from coronary artery disease (CAD), diabetes mellitus, and hypertension. Accordingly, participants in higher quartiles of uromodulin were less likely to use antihypertensive medication.
We also analyzed the association of uromodulin with different immune cell subtypes. Although there was no association with natural killer cells and monocytes, we found higher percentages of lymphocytes and lower percentages of neutrophils and eosinophils with increasing serum uromodulin concentration (Supplemental Table 1).
Correlation of Serum Uromodulin with Markers of Kidney Function
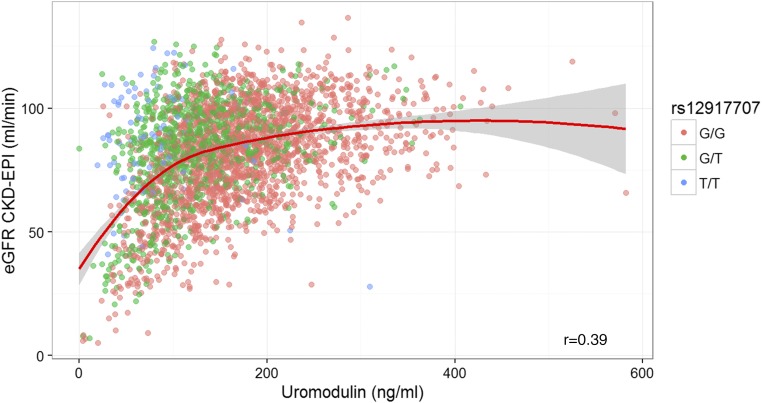
Uromodulin showed a strong direct correlation with eGFR at lower concentrations that flattens in the higher ranges (i.e., in people with normal kidney function) (Figure 1). Serum concentrations of uromodulin were directly correlated with LDL cholesterol and HDL cholesterol and inversely correlated with age, BMI, systolic BP, triglycerides, glucose, hemoglobin A1c, hsCRP, fibrinogen, galectin-3, uric acid, and NT-proBNP (Table 2). After adjustment for age, sex, and eGFR, the correlation coefficients were attenuated but still nominally significant. For urinary uromodulin, meta-analyses have identified a genetic variant in the UMOD promotor rs12917707, the minor allele of which is associated with lower uromodulin but higher eGFR.12 Using genotypic data of 2984 participants in the LURIC Study that had been imputed to the HapMap2 reference panel, we performed a genome-wide association study with serum uromodulin as phenotype, identified the same variant as significantly associated with the serum concentration (Supplemental Figures 2 and 3), and replicated the association of the T allele of rs12917707 with higher eGFR (Figure 1, Table 3). Genetic data were only available for 2826 study participants, limiting our sample size for all analyses involving rs12917707.
Figure 1.
Higher serum uromodulin correlates with higher estimated GFR. Correlation of serum uromodulin with eGFR. Data points are color coded according to rs12917707 genotype. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Table 2.
Correlation of serum uromodulin with biomarkers
| Marker | Crude | Adjusted for Age and Sex | Additionally Adjusted for eGFR | |||
|---|---|---|---|---|---|---|
| R | P Value | R | P Value | R | P Value | |
| Age, yr | −0.21 | <0.001 | — | — | — | — |
| eGFR, ml/min | 0.38 | <0.001 | 0.35 | <0.001 | — | — |
| BMI, kg/m2 | −0.06 | 0.002 | −0.08 | <0.001 | −0.06 | 0.002 |
| Systolic BP, mmHg | −0.10 | <0.001 | −0.02 | 0.26 | −0.04 | 0.04 |
| Diastolic BP, mmHg | 0.02 | 0.24 | 0.03 | 0.10 | 0.01 | 0.54 |
| LDL-C, mg/dl | 0.08 | <0.001 | 0.08 | <0.001 | 0.05 | <0.01 |
| HDL-C, mg/dl | 0.17 | <0.001 | 0.16 | <0.001 | 0.11 | <0.001 |
| TG, mg/dl | −0.13 | <0.001 | −0.14 | <0.001 | −0.11 | <0.001 |
| Fasting glucose, mg/dl | −0.14 | <0.001 | −0.11 | <0.001 | −0.10 | <0.001 |
| HbA1c, % | −0.14 | <0.001 | −0.11 | <0.001 | −0.08 | <0.001 |
| hsCRP, mg/dl | −0.17 | <0.001 | −0.07 | <0.001 | −0.04 | 0.05 |
| Fibrinogen, mg/dl | −0.15 | <0.001 | −0.10 | <0.001 | −0.04 | 0.02 |
| Galectin-3, mg/L | −0.31 | <0.001 | −0.27 | <0.001 | −0.09 | <0.001 |
| Uric acid, mg/dl | −0.30 | <0.001 | −0.28 | <0.001 | −0.15 | <0.001 |
| NT-proBNP, ng/ml | −0.24 | <0.001 | −0.17 | <0.001 | −0.06 | 0.001 |
LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TG, triglycerides; HbA1c, hemoglobin A1c.
Table 3.
Association of rs12917707 with serum uromodulin and eGFR
| rs12917707 | N | Uromodulin, ng/ml | eGFR, ml/min |
|---|---|---|---|
| G/G | 1870 | 175±75.3 | 81.0±20.6 |
| G/T | 865 | 125±56.8 | 82.8±19.4 |
| T/T | 91 | 85.7±51.2 | 86.5±20.0 |
| P<0.001 | P<0.01 |
Serum Uromodulin and Mortality
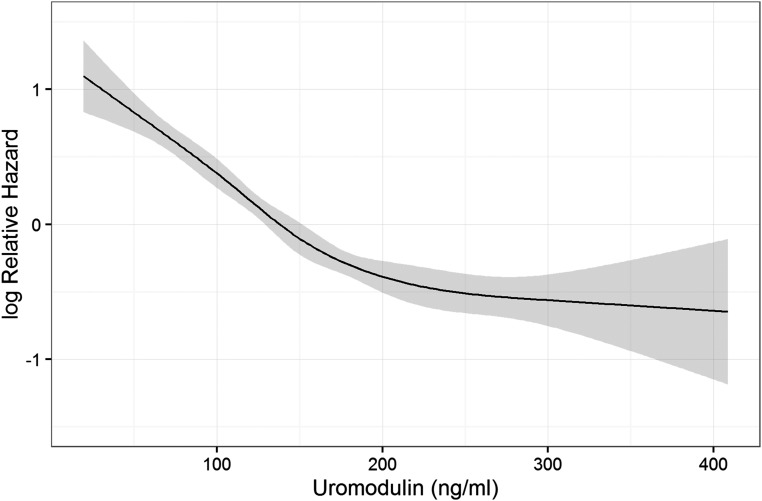
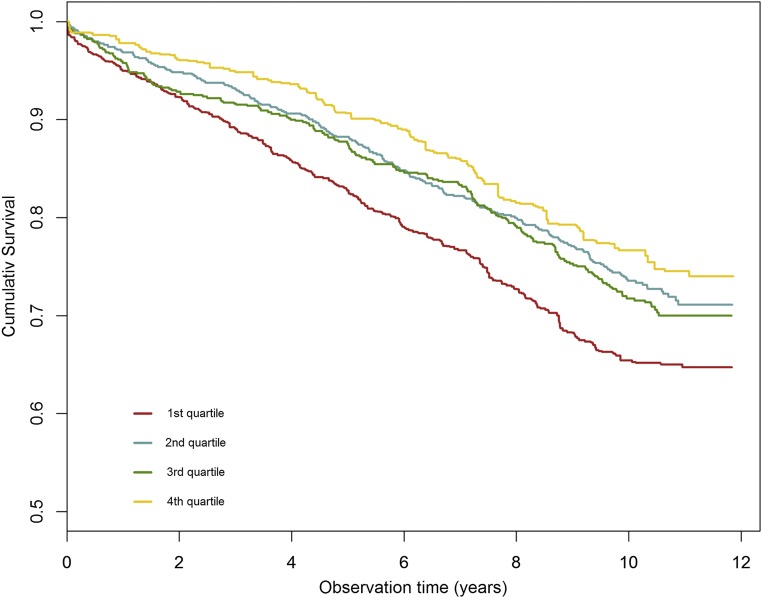
We examined the association of uromodulin with all-cause and cause-specific mortality by means of Cox regression. Modeling uromodulin as restricted cubic spline, we plotted the predicted hazard against the uromodulin concentration (Figure 2). The risk decreased strongly up to a uromodulin concentration of about 200 ng/dl, after which the slope of the curve flattened. Sex-stratified analysis revealed a more linear relationship in women (Supplemental Figure 4). Overall, the rs12917707 variant was not significantly associated with mortality, but we found evidence for a possible protective effect of the minor uromodulin-lowering allele in study participants younger than 67 years old (now the regular retirement age in Germany) with hazard ratios (HRs) of 0.74 (95% confidence interval [95% CI], 0.58 to 0.94) and 0.71 (95% CI, 0.37 to 1.39) for study participants heterozygous or homozygous for the T allele, respectively. Because this stands in contrast to the association of low serum uromodulin with increased risk, we adjusted the Cox regression models additionally for rs12917707, except for that the basic model was adjusted only for age and sex. Model 2 was additionally adjusted for diabetes mellitus, BMI, arterial hypertension, smoking, and eGFR, and model 3 was additionally adjusted for hsCRP, NT-proBNP, and medication (ACE inhibitors, AT2 receptor blockers, calcium antagonists, diuretics, β-blockers, and lipid-lowering drugs). Results without adjustment for rs12917707 are shown in Supplemental Table 2. During a median follow-up of 9.9 years, 838 patients (29.7%) died; 390 of these patients (13.9%) died from cardiovascular causes. Adjusted survival curves for all-cause mortality are shown in Figure 3. Higher quartiles of uromodulin were associated with a significantly reduced risk to die from any cause with HRs of 0.65 (95% CI, 0.54 to 0.78), 0.71 (95% CI, 0.58 to 0.88), and 0.57 (95% CI, 0.45 to 0.73) in the second, third, and fourth quartiles in model 4, respectively (Table 4). HRs for cardiovascular mortality were 0.54 (95% CI, 0.41 to 0.71), 0.53 (95% CI, 0.39 to 0.72), and 0.44 (95% CI, 0.31 to 0.63) in the second, third, and fourth quartiles, respectively. Regarding death due to fatal infection, we found a significant inverse association only without adjustment for cardiovascular risk factors with HRs of 0.62 (95% CI, 0.34 to 1.10), 0.28 (95% CI, 0.13 to 0.61), and 0.27 (95% CI, 0.12 to 0.61) in the second, third, and fourth quartiles in model 2, respectively.
Figure 2.
Nonlinear relationship between serum uromodulin and all-cause mortality. In a Cox regression model, uromodulin was modeled as restricted cubic spline and plotted against the log hazard.
Figure 3.
Lower serum uromodulin is associated with higher mortality. Adjusted survival curves for all-cause mortality. Quartiles of serum uromodulin were balanced for age, sex, BMI, diabetes mellitus, smoking, eGFR, hypertension, and medication by inverse variance weighting. HRs for the second, third, and fourth quartiles compared with the first quartile were 0.74 (95% CI, 0.67 to 0.81), 0.79 (95% CI, 0.72 to 0.86), and 0.64 (95% CI, 0.58 to 0.70), respectively. The P value of the robust score test was <0.001.
Table 4.
Survival according to quartiles of serum uromodulin
| Endpoint | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All-cause mortality (838 events) | ||||||||
| First quartile | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Second quartile | 0.57 (0.48 to 0.67) | <0.001 | 0.52 (0.43 to 0.62) | <0.001 | 0.59 (0.49 to 0.71) | <0.001 | 0.65 (0.54 to 0.78) | <0.001 |
| Third quartile | 0.55 (0.46 to 0.65) | <0.001 | 0.49 (0.40 to 0.59) | <0.001 | 0.61 (0.49 to 0.74) | <0.001 | 0.71 (0.58 to 0.88) | 0.001 |
| Fourth quartile | 0.41 (0.34 to 0.50) | <0.001 | 0.36 (0.29 to 0.44) | <0.001 | 0.47 (0.37 to 0.59) | <0.001 | 0.57 (0.45 to 0.73) | <0.001 |
| Cardiovascular mortality (513 events) | ||||||||
| First quartile | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Second quartile | 0.45 (0.35 to 0.56) | <0.001 | 0.45 (0.36 to 0.56) | <0.001 | 0.53 (0.42 to 0.67) | <0.001 | 0.58 (0.46 to 0.74) | <0.001 |
| Third quartile | 0.40 (0.31 to 0.51) | <0.001 | 0.40 (0.31 to 0.51) | <0.001 | 0.52 (0.40 to 0.68) | <0.001 | 0.62 (0.47 to 0.81) | <0.001 |
| Fourth quartile | 0.28 (0.21 to 0.37) | <0.001 | 0.28 (0.21 to 0.37) | <0.001 | 0.38 (0.28 to 0.52) | <0.001 | 0.48 (0.35 to 0.66) | <0.001 |
| Cancer mortality (128 events) | ||||||||
| First quartile | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Second quartile | 0.89 (0.56 to 1.42) | 0.63 | 0.86 (0.53 to 1.41) | 0.55 | 0.86 (0.52 to 1.42) | 0.56 | 0.89 (0.54 to 1.47) | 0.65 |
| Third quartile | 0.96 (0.60 to 1.54) | 0.88 | 1.02 (0.62 to 1.68) | 0.92 | 1.11 (0.66 to 1.88) | 0.69 | 1.24 (0.73 to 2.11) | 0.43 |
| Fourth quartile | 0.76 (0.46 to 1.28) | 0.30 | 0.78 (0.45 to 1.37) | 0.40 | 0.81 (0.44 to 1.48) | 0.49 | 0.91 (0.50 to 1.67) | 0.77 |
| Death due to fatal infection (66 events) | ||||||||
| First quartile | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Second quartile | 0.64 (0.37 to 1.12) | 0.12 | 0.62 (0.34 to 1.10) | 0.101 | 0.80 (0.44 to 1.46) | 0.47 | 0.86 (0.47 to 1.58) | 0.62 |
| Third quartile | 0.35 (0.17 to 0.72) | 0.004 | 0.28 (0.13 to 0.61) | 0.001 | 0.44 (0.19 to 0.99) | 0.05 | 0.48 (0.21 to 1.10) | 0.08 |
| Fourth quartile | 0.32 (0.15 to 0.71) | <0.01 | 0.27 (0.12 to 0.61) | 0.002 | 0.48 (0.20 to 1.17) | 0.11 | 0.56 (0.23 to 1.34) | 0.19 |
Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for rs12917707 genotype. Model 3 was additionally adjusted for BMI, diabetes mellitus, hypertension, smoking, and eGFR group. Model 4 was additionally adjusted for hsCRP, NT-proBNP, and medication (angiotensin converting enzyme inhibitors, angiotensin II receptor blocker, calcium antagonists, diuretics, β-blocker, and lipid-lowering drugs).
We also analyzed the association of uromodulin with mortality in different patient subgroups using uromodulin as a continuous Z-transformed value for all patients (Supplemental Figure 5) as well as stratified for different subgroups (Supplemental Figure 6). The association of uromodulin with reduced risk was slightly stronger in women compared with men, patients free from CAD compared with patients with CAD, patients with reduced kidney function compared with patients with an eGFR>90 ml/min, and patients without diabetes mellitus compared with patients with diabetes mellitus. Furthermore, we calculated a ratio of cystatin C and uromodulin and compared its association with mortality with uromodulin alone (Supplemental Figure 5). Higher values of the ratio were associated with increased risk.
Performance of Serum Uromodulin in Risk Prediction Models
We investigated whether the addition of uromodulin to established risk prediction algorithms for persons with or without a clinical history of cardiovascular disease would improve risk prediction. We selected a subgroup of patients with measured uromodulin who had not experienced myocardial infarction or stroke at study baseline (n=1338) and tested both the European Society of Cardiology (ESC) heart score and the Pooled Cohort Equation (PCE)18 with and without uromodulin (Table 5). The inclusion of uromodulin slightly increased the conventional area under the curve and Harrell C for the end point all-cause mortality, with larger improvements for the end point cardiovascular death. We additionally calculated the net reclassification index (NRI). Inclusion of uromodulin led to a categorical NRI of approximately 9%, whereas the continuous NRIs were 27.4% and 29.3% when adding uromodulin to either the ESC heart score or the PCE, respectively (Table 6).
Table 5.
Risk prediction models with and without inclusion of serum uromodulin
| Model | All-Cause Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| AUC (95% CI) | Harrell C Statistic | AUC (95% CI) | Harrell C Statistic | |
| No history of CVD | ||||
| ESC heart score | 0.721 (0.69 to 0.75) | 0.70 | 0.724 (0.69 to 0.76) | 0.71 |
| ESC heart score and uromodulin | 0.729 (0.70 to 0.76) | 0.70 | 0.744 (0.71 to 0.78)a | 0.73 |
| PCE | 0.732 (0.70 to 0.76) | 0.71 | 0.733 (0.70 to 0.77) | 0.72 |
| PCE and uromodulin | 0.736 (0.70 to 0.79) | 0.71 | 0.750 (0.71 to 0.79)b | 0.74 |
| Previous CVD | ||||
| Marshner score | 0.727 (0.70 to 0.76) | 0.69 | 0.696 (0.66 to 0.73) | 0.69 |
| Marshner and uromodulin | 0.735 (0.70 to 0.77) | 0.70 | 0.704 (0.67 to 0.74) | 0.70 |
| VILCAD risk score | 0.744 (0.71 to 0.77) | 0.71 | 0.740 (0.71 to 0.77) | 0.74 |
| VILCAD risk score and uromodulin | 0.748 (0.72 to 0.78) | 0.72 | 0.741 (0.71 to 0.78) | 0.74 |
Model including uromodulin versus base model. AUC, area under the curve; CVD, cardiovascular disease; VILCAD, Vienna and Ludwigshafen Coronary Artery Disease.
P<0.05.
P<0.10.
Table 6.
Net reclassification for adding serum uromodulin to the ESC heart score or the PCE in study participants of the LURIC Study without previous cardiovascular events
| Model | Categoriala NRI (95% CI) | Continuous NRI (95% CI) |
|---|---|---|
| ESC heart score and uromodulin | 0.092 (0.01 to 0.19) | 0.274 (0.16 to 0.43) |
| PCE and uromodulin | 0.096 (0.02 to 0.18) | 0.293 (0.15 to 0.42) |
ESC heart score: <5%, 5%–10%, or >10%; PCE: <5%, 5%–10%, 10%–20%, or >20%.
In a subgroup of patients with stable CAD (n=1166), we tested the incremental predictive value of uromodulin in comparison with Marschner score19 and the Vienna and Ludwigshafen Coronary Artery Disease risk score20 (Table 5). The models including uromodulin showed slightly improved performance for both risk scores.
Discussion
Main Findings
This is the first study that measured the concentration of uromodulin in blood samples drawn from a large cohort of patients referred for coronary angiography and correlated it with biomarkers for cardiovascular risk as well as mortality (total and cardiovascular). We had four main findings. First, uromodulin showed associations with total and cardiovascular mortality, even after multivariate adjustment including eGFR. Second, the minor allele of rs129017707 that is associated with lower uromodulin was associated with higher eGFR and reduced mortality in younger study participants. Third, uromodulin showed strong inverse correlations with markers of cardiovascular risk. Fourth, adding serum uromodulin to established risk prediction scores significantly improved the risk prediction in participants free of prior cardiovascular disease.
Serum Uromodulin and Biomarkers
The exact biologic role of uromodulin is still unsolved, and it has been linked with several mechanisms, like the regulation of NaCl transport processes21 as well as innate immunity.7 Recently, the ancestry of the top risk UMOD promoter variant rs4293393 was investigated, strengthening the link to immunity, because the distribution of the UMOD ancestral allele did not follow the ancestral susceptibility model observed for variants associated with salt-sensitive hypertension but instead, correlated with pathogen diversity and the prevalence of antibiotic-resistant urinary tract infections.22
In our study, we observed significant inverse correlations with markers of inflammation and acute-phase proteins, such as fibrinogen. In line with this, we found a positive association of serum uromodulin with lymphocytes and negative associations with neutrophils and eosinophils. However, these data are not sufficient to answer the question of whether the association of uromodulin with mortality reflects a relationship between uromodulin and innate immunity or is caused by other mechanisms. It is in this context that it is interesting to note that uromodulin was also inversely correlated with markers of fibrosis (galectin-3) and heart failure (NT-proBNP). Regarding kidney function, serum uromodulin was consistently and inversely correlated with markers of reduced glomerular filtration (β-trace protein, creatinine, and cystatin C) and directly correlated with 1,25(OH)2 vitamin D, the active form of 25(OH) vitamin D, which is predominantly produced by the kidneys. This strengthens the notion that uromodulin, exclusively produced in thick ascending limb epithelia, represents a specific tissue marker of kidney health independent from markers of glomerular filtration and might potentially help to detect early renal injury.
Morbidity and Mortality
Cross-sectionally, patients with higher serum concentrations of uromodulin not only were less likely to suffer from reduced kidney function, but also, the percentages of patients suffering from CAD, heart failure, diabetes mellitus, or hypertension decreased with increasing uromodulin. Most important, prospectively, low concentrations of uromodulin were strong predictors of all-cause and cardiovascular mortality after multivariate adjustment including eGFR. This is in line with results reported for urinary uromodulin by Garimella et al.8 Higher uromodulin concentrations were also associated with reduced mortality due to fatal infection in a basic model with adjustment only for age, sex, and rs129017707, which further supports a role of uromodulin in the immune defense. However, information on the etiology of lethal infections (bacterial, fungal, or viral) was not available. The finding that the relationship between uromodulin and mortality was stronger after adjustment for rs129017707 may imply that uromodulin becomes most informative if it is interpreted on the background of the rs129017707 genotype.
Adding serum uromodulin to established risk prediction algorithms for primary and secondary prevention resulted in improved C statistics, especially in persons without a prior history of cardiovascular disease. We could also show improved risk classification when we added uromodulin to the Framingham score or the ESC heart score by calculating the NRI.
Strengths and Limitations
All LURIC Study participants were of European origin and recruited at a tertiary referral center. Therefore, our findings may not be representative of a random population sample or applicable to other ethnicities. Furthermore, uromodulin was only measured once in baseline samples, and urine samples were not available to also assess urinary uromodulin. The major strengths of the LURIC Study cohort are, however, the precise clinical and metabolic characterization of the participants, including the availability of coronary angiograms, its cross-sectional and prospective design, and the long-term follow-up (median of 9.9 years). In addition, assessing uromodulin in serum may overcome several preanalytic drawbacks that were reported for the urinary antigen.23
Higher serum uromodulin was significantly associated with a better metabolic profile, lower prevalence rates of comorbidities, and a lower risk for 10-year mortality. The association with reduced mortality was independent of other cardiovascular risk factors, including eGFR. Alleles at the UMOD locus that were associated with lower uromodulin concentration were also associated with lower mortality but only in a subgroup of younger study participants. Adjusting for this polymorphism strengthened the inverse association of uromodulin with mortality. Adding serum uromodulin to established cardiovascular risk prediction scores slightly improved risk prediction, and uromodulin may, therefore, represent a useful novel marker for cardiovascular and renal health.
Concise Methods
Subjects
The LURIC Study consisted of 3316 whites referred for coronary angiography between 1997 and 2000 to the Ludwigshafen Heart Center in southwest Germany.24 Clinical indications for angiography were chest pain or a positive noninvasive stress test suggestive of myocardial ischemia. Individuals suffering from acute illnesses other than acute coronary syndrome, chronic noncardiac diseases, and a history of malignancy within the past 5 years were excluded. The ethics committee of the Landesärztekammer Rheinland-Pfalz approved the study, which was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants.
Laboratory Procedures
Fasting blood samples were obtained by venipuncture at study entry. A summary of analytic methods has been reported previously.24 Serum uromodulin was measured using a sensitive ELISA specifically adapted to serum specimens.17 IgG1 of two affinity-purified mAbs directed against uromodulin isolated from urine was used either for coating microtiter plates or as detection antibody and processed according to the manufacturer’s manual (Euroimmun Diagnostik, Lubeck, Germany). More details about assay kits used to measure cardiovascular biomarkers are given in Supplemental Material.
Definition of Clinical Variables and End Points
The presence of a visible luminal narrowing (>20% stenosis) in at least one of 15 coronary segments was used to define CAD according to the classification of the American Heart Association.24 Diabetes mellitus was defined according to 2010 guidelines of the American Diabetes Association as increased fasting (≥126 mg/dl) and/or postchallenge (2 hours after the 75-g glucose load >200 mg/dl) glucose and/or elevated glycated hemoglobin (>6.5%) and/or history of diabetes. Hypertension was defined as a systolic and/or diastolic BP ≥140 and/or ≥90 mmHg, respectively, or a history of hypertension. The GFR was estimated by using the 2012 Chronic Kidney Disease Epidemiology Collaboration eGFRcreat-cys equation,25 and the patients were stratified into categories of their eGFR according to the recent Kidney Disease Improving Global Outcomes guidelines.26
Information on vital status was obtained from local registries. Death certificates, medical records of local hospitals, and autopsy data were reviewed independently by two experienced clinicians who were blinded to patient characteristics and classified the causes of death. In cases of disagreement or uncertainty concerning the coding of a specific cause of death, the decision was made by a principal investigator (W.M.). Nine hundred eighteen participants (29.9%) died during a median follow-up of 9.9 years (range =0.1–11.9 years). Cardiovascular mortality included the following categories: sudden cardiac death (n=240; 7.9%), fatal myocardial infarction (n=95; 3.1%), death due to congestive heart failure (n=136; 4.4%), death after intervention to treat CAD (n=25; 0.8%), fatal stroke (n=55; 1.8%), and other causes of death due to CAD (n=18; 0.6%). Information for vital status is complete for all participants, but the cause of death of 19 deceased was unknown, and these patients were included in calculations of all-cause mortality but were not included in calculations considering different causes of death. For the Cox regression analyses including the rs12917707 polymorphism, fewer events were available for analysis due to missing genotypic data.
Statistical Analyses
Continuous data are presented as the mean and SD when normally distributed or the median and 25th and 75th percentiles for non-normally distributed variables. Categorical data are presented as percentages. Statistical differences between groups and continuous variables were determined using ANOVA. Non-normally distributed variables were log transformed before entering analysis. The chi-squared test was used for categorical variables. Correlations between uromodulin and biomarkers were assessed by Spearman rho. Cox proportional hazard models were built to assess the effect of serum uromodulin on all-cause mortality and cardiovascular mortality. Uromodulin was examined as quartiles or standardized Z-transformed values. HRs given for Z-transformed values show that the HR was associated with a 1-SD increase of the marker. We also adjusted the data distribution by inverse probability weighting, so that each uromodulin quartile’s weighted distribution matched that of the whole cohort, thereby balancing the subgroups for the confounding variables. We plotted the reweighted distribution of confounding variables for each uromodulin quartile to check whether the balancing worked. A weighted Cox model was calculated, and we report the result of the robust score test as implemented in the coxph function in R that corresponds to a log rank test corrected for weighting. The proportional hazard assumption was checked by examination of scaled Schoenfeld residuals. For the generation of HR plots, serum uromodulin was modeled as restricted cubic spline. Details regarding the GWAS analysis are given in Supplemental Material.
All tests were two sided, and a P value <0.05 was considered statistically significant. All analyses were carried out using R v3.3.1 (http://www.r-project.org). HR plots were drawn using the R package rms (v4.5–0). Harrell C was calculated using the R package hmisc (v3.17–4), ROC curves were calculated and compared using the method of Delong27 as implemented in the R package pROC (v1.8),28 and the NRI for censored survival data was calculated using the R package nricens (v1.2).
Disclosures
G.E.D., H.S., and M.E.K. declare no conflicts of interest. B.K.K. reports travel support, lecture fees, and/or advisory board memberships from Astellas, Bayer, BMS, Chiesi, Hexal, Pfizer, and Sanofi (all outside of the submitted work). W.M. reports grants and personal fees from AMGEN, BASF, Sanofi, Siemens Diagnostics, Aegerion Pharmaceuticals, Astrazeneca, Danone Research, Numares, Pfizer, and Hoffmann LaRoche; personal fees from MSD and Alexion; and grants from Abbott Diagnostics (all outside of the submitted work). W.M. is employed with Synlab Holding Deutschland GmbH. J.E.S received travel support from Hofmann-Roche and Euroimmun.
Supplementary Material
Acknowledgments
We thank the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study team members, who were either temporarily or permanently involved in patient recruitment as well as sample and data handling, in addition to the laboratory staff at the Ludwigshafen General Hospital and the Universities of Freiburg and Ulm. We also thank Viktor Herbst, Matthias Block, and Dr. Wolfgang Schlumberger (Institute of Experimental Immunology, Euroimmun AG Lübeck) for providing us with ELISA test kits.
The LURIC Study was supported by 7th Framework Program RiskyCAD (European Union project, Personalized Diagnostics and Treatment for High Risk Coronary Artery Disease Patients) grant agreement number 305739 of the European Union. The work of M.E.K. and W.M. is supported as part of the Competence Cluster of Nutrition and Cardiovascular Health, which is funded by the German Federal Ministry of Education and Research.
The funding sources had no involvement in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Uromodulin in the Bloodstream: Old Wine in a New Wineskin,” on pages 1955–1957.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016111162/-/DCSupplemental.
References
- 1.Tamm I, Horsfall FL Jr.: Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med 74: 106–108, 1950 [PubMed] [Google Scholar]
- 2.Porter KR, Tamm I: Direct visualization of a mucoprotein component of urine. J Biol Chem 212: 135–140, 1955 [PubMed] [Google Scholar]
- 3.Wiggins RC: Uromucoid (Tamm-Horsfall glycoprotein) forms different polymeric arrangements on a filter surface under different physicochemical conditions. Clin Chim Acta 162: 329–340, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Torffvit O, Melander O, Hultén UL: Urinary excretion rate of Tamm-Horsfall protein is related to salt intake in humans. Nephron Physiol 97: 31–36, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR: Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem 276: 9924–9930, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S: Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: Rapid communication. Kidney Int 65: 791–797, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Säemann MD, Weichhart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, Sobanov Y, Stulnig TM, Akira S, von Gabain A, von Ahsen U, Hörl WH, Zlabinger GJ: Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest 115: 468–475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, Kestenbaum BR, Siscovick DS, Jensen MK, Shlipak MG, Chaves PH, Sarnak MJ: Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int 88: 1126–1134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sejdiu I, Torffvit O: Decreased urinary concentration of Tamm-Horsfall protein is associated with development of renal failure and cardiovascular death within 20 years in type 1 but not in type 2 diabetic patients. Scand J Urol Nephrol 42: 168–174, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köttgen A, Hwang SJ, Larson MG, Van Eyk JE, Fu Q, Benjamin EJ, Dehghan A, Glazer NL, Kao WH, Harris TB, Gudnason V, Shlipak MG, Yang Q, Coresh J, Levy D, Fox CS: Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol 21: 337–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olden M, Corre T, Hayward C, Toniolo D, Ulivi S, Gasparini P, Pistis G, Hwang SJ, Bergmann S, Campbell H, Cocca M, Gandin I, Girotto G, Glaudemans B, Hastie ND, Loffing J, Polasek O, Rampoldi L, Rudan I, Sala C, Traglia M, Vollenweider P, Vuckovic D, Youhanna S, Weber J, Wright AF, Kutalik Z, Bochud M, Fox CS, Devuyst O: Common variants in UMOD associate with urinary uromodulin levels: A meta-analysis. J Am Soc Nephrol 25: 1869–1882, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, Hastie CE, Menni C, Monti MC, Delles C, Laing S, Corso B, Navis G, Kwakernaak AJ, van der Harst P, Bochud M, Maillard M, Burnier M, Hedner T, Kjeldsen S, Wahlstrand B, Sjögren M, Fava C, Montagnana M, Danese E, Torffvit O, Hedblad B, Snieder H, Connell JM, Brown M, Samani NJ, Farrall M, Cesana G, Mancia G, Signorini S, Grassi G, Eyheramendy S, Wichmann HE, Laan M, Strachan DP, Sever P, Shields DC, Stanton A, Vollenweider P, Teumer A, Völzke H, Rettig R, Newton-Cheh C, Arora P, Zhang F, Soranzo N, Spector TD, Lucas G, Kathiresan S, Siscovick DS, Luan J, Loos RJ, Wareham NJ, Penninx BW, Nolte IM, McBride M, Miller WH, Nicklin SA, Baker AH, Graham D, McDonald RA, Pell JP, Sattar N, Welsh P, Munroe P, Caulfield MJ, Zanchetti A, Dominiczak AF; Global BPgen Consortium : Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet 6: e1001177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahluwalia TS, Lindholm E, Groop L, Melander O: Uromodulin gene variant is associated with type 2 diabetic nephropathy. J Hypertens 29: 1731–1734, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, Block M, Kaden J, Schlumberger W: Serum uromodulin - a marker of kidney function and renal parenchymal integrity [published online ahead of print February 16, 2017]. Nephrol Dial Transplant doi: 10.1093/ndt/gfw422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risch L, Lhotta K, Meier D, Medina-Escobar P, Nydegger UE, Risch M: The serum uromodulin level is associated with kidney function. Clin Chem Lab Med 52: 1755–1761, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, Angermann S, Hasenau AL, Stecher L, Heemann U, Renders L, Scherberich J: Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 95: e3011, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr., Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 63[25 Pt B]: 2889–2934, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Marschner IC, Colquhoun D, Simes RJ, Glasziou P, Harris P, Singh BB, Friedlander D, White H, Thompson P, Tonkin A; Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) Study; LIPID Study Investigators : Long-term risk stratification for survivors of acute coronary syndromes. Results from the long-term intervention with pravastatin in ischemic disease (LIPID) study. J Am Coll Cardiol 38: 56–63, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Goliasch G, Kleber ME, Richter B, Plischke M, Hoke M, Haschemi A, Marculescu R, Endler G, Grammer TB, Pilz S, Tomaschitz A, Silbernagel G, Maurer G, Wagner O, Huber K, März W, Mannhalter C, Niessner A: Routinely available biomarkers improve prediction of long-term mortality in stable coronary artery disease: The Vienna and Ludwigshafen coronary artery disease (VILCAD) risk score. Eur Heart J 33: 2282–2289, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, Raffi H, Rampoldi L, Uchida S, Hille C, Dosche C, Kumar S, Castañeda-Bueno M, Gamba G, Bachmann S: Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem 286: 30200–30210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghirotto S, Tassi F, Barbujani G, Pattini L, Hayward C, Vollenweider P, Bochud M, Rampoldi L, Devuyst O: The uromodulin gene locus shows evidence of pathogen adaptation through human evolution. J Am Soc Nephrol 27: 2983–2996, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O: Determination of uromodulin in human urine: Influence of storage and processing. Nephrol Dial Transplant 29: 136–145, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Winkelmann BR, März W, Boehm BO, Zotz R, Hager J, Hellstern P, Senges J; LURIC Study Group (LUdwigshafen RIsk and Cardiovascular Health) : Rationale and design of the LURIC study--a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics 2[Suppl 1]: S1–S73, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrassy KM: Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.’ Kidney Int 84: 622–623, 2013 [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 28.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M: pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: 77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.