Abstract
Aminopeptidase A (APA) is expressed in glomerular podocytes and tubular epithelia and metabolizes angiotensin II (AngII), a peptide known to promote glomerulosclerosis. In this study, we tested whether APA expression changes in response to progressive nephron loss or whether APA exerts a protective role against glomerular damage and during AngII-mediated hypertensive kidney injury. At advanced stages of FSGS, fawn-hooded hypertensive rat kidneys exhibited distinctly increased APA staining in areas of intact glomerular capillary loops. Moreover, BALB/c APA-knockout (KO) mice injected with a nephrotoxic serum showed persistent glomerular hyalinosis and albuminuria 96 hours after injection, whereas wild-type controls achieved virtually full recovery. We then tested the effect of 4-week infusion of AngII (400 ng/kg per minute) in APA-KO and wild-type mice. Although we observed no significant difference in achieved systolic BP, AngII-treated APA-KO mice developed a significant rise in albuminuria not observed in AngII-treated wild-type mice along with increased segmental and global sclerosis and/or collapse of juxtamedullary glomeruli, microcystic tubular dilation, and tubulointerstitial fibrosis. In parallel, AngII treatment significantly increased the kidney AngII content and attenuated the expression of podocyte nephrin in APA-KO mice but not in wild-type controls. These data show that deficiency of APA increases susceptibility to glomerular injury in BALB/c mice. The augmented AngII-mediated kidney injury observed in association with increased intrarenal AngII accumulation in the absence of APA suggests a protective metabolizing role of APA in AngII-mediated glomerular diseases.
Keywords: angiotensin II, intrarenal, podocyte, glomerulosclerosis, murine, albuminuria
Aminopeptidase A (APA; glutamyl aminopeptidase; EC 3.4.11.7) is a homodimeric membrane–bound zinc metallopeptidase capable of hydrolyzing angiotensin (Ang) peptides. Through cleavage at the amino terminus, APA initiates the metabolism of the octapeptide AngII by converting it into the heptapeptide AngIII, which is further metabolized rapidly by aminopeptidase N (APN) into AngIV.1,2 Other peptidases are capable of metabolizing AngII at the carboxy terminus to convert it into Ang1–7 (e.g., angiotensin-converting enzyme 2 [ACE2], prolyl endopeptidase, and prolyl carboxypeptidase), whereas neprilysin (NEP) converts AngII into two tetrapeptides, Ang1–4 and Ang5–8.3–8 Although these peptidases are ubiquitously expressed throughout the body, differences in their cellular localization and tissue distribution may have implications in tissue concentration of AngII in health and disease.
Studies indicate that APA, APN, NEP, ACE2, and prolyl carboxypeptidase are involved in AngII enzymatic processing in the kidney.1,9–12 However, intrarenal APA has the highest relative tissue abundance and enzymatic activity compared with the other peptidases.13–15 Because APA is localized in glomerular podocytes,16,17 tubular epithelia,17 and medullary endothelial cells,16 it is critical for AngII metabolism in all kidney compartments. However, the notable localization of APA in podocytes is highlighted by studies showing that intravenous injection of mAb against APA in mice induces overt podocyte foot process effacement, glomerular granular IgG deposition, and albuminuria.18,19 Importantly, the kidneys possess the most robust enzymatic capacity to degrade AngII in the body, with up to 93% of AngII being degraded as it passes through the kidney compared with 60% through systemic circulation and only 5% through pulmonary circulation, and up to 60% of that intrarenal metabolism is mediated by APA.1 Despite those observations regarding APA localization and function and the widely established notion that AngII promotes progressive glomerulosclerosis,20–22 the role of APA-mediated AngII degradation in attenuating AngII-mediated kidney injury has not been elucidated. Furthermore, studies examining the glomerular expression of APA in experimental and human glomerular disease have revealed conflicting data: some reporting an increase in APA expression and others reporting a decrease.23–26
Using a robust model of spontaneous FSGS, the fawn-hooded hypertensive (FHH) rat, we sought to explore whether an adaptive change in pattern of glomerular APA expression is present during progressive nephron loss. Subsequently, we tested whether APA deficiency could influence the reversibility of damage acquired in an established mouse model of glomerular injury, the sheep anti-rat glomerular lysate antiserum (nephrotoxic serum [NTS]) injection model.27–29 Next, because of its inherent property as an AngII-degrading enzyme, we examined the role of APA in a well established mouse model of AngII-dependent hypertensive kidney injury, the chronic AngII infusion model.30–32 We hypothesized that glomerular APA expression changes as part of an adaptive response to injury. Then, we hypothesized that deficiency of APA could result in exacerbated glomerular injury in response to a noxious stimuli, such as the NTS. In addition, we hypothesized that, during systemic exposure to exogenous AngII, full deficiency of APA may lead to reduced AngII degradation, increased intrarenal accumulation of AngII, and subsequent augmented renal parenchymal injury.
Results
Pattern of Adaptive Kidney APA Expression during Progressive FSGS
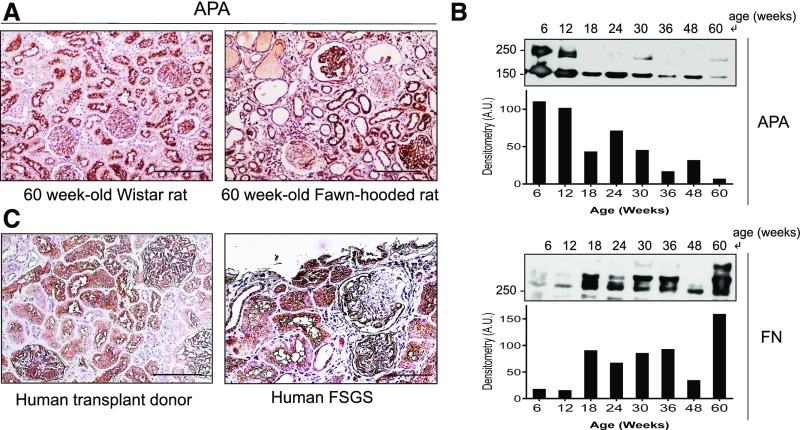
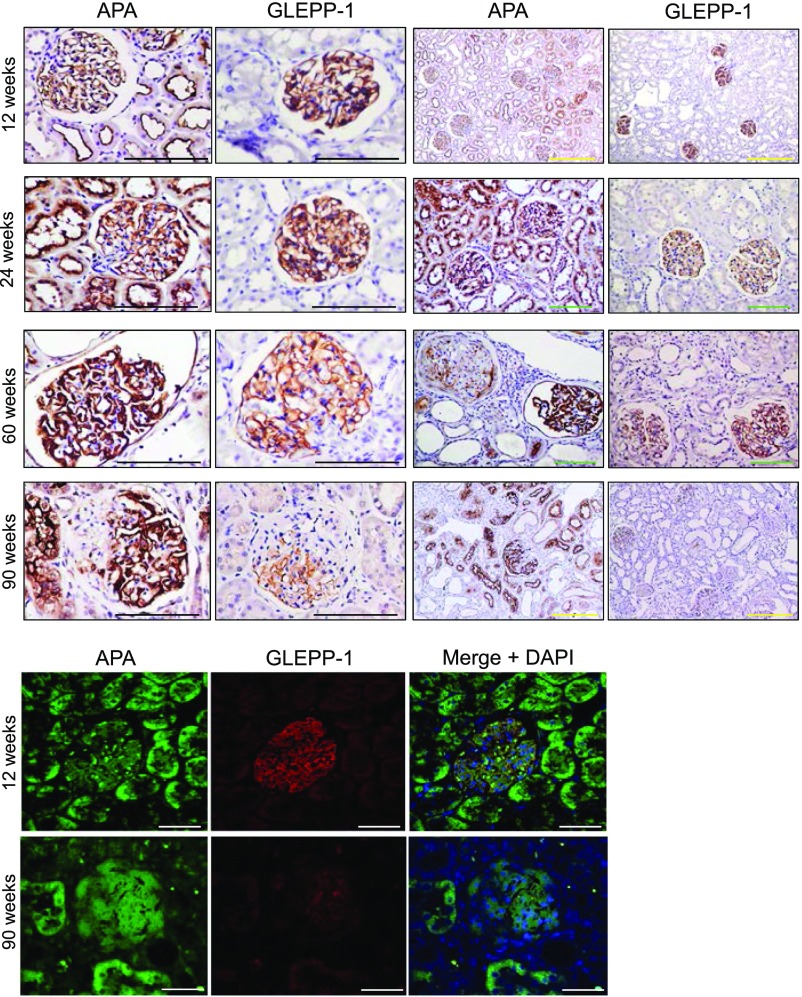
Using the FHH rat model, we examined the pattern of glomerular expression of APA during the course of the disease. In comparison with normal age-matched Wistar rats, kidney APA expression changed at advanced stages (Figure 1A). Obsolescent glomeruli lost virtually all expression of APA. However, coexisting surviving glomeruli exhibited prominent APA staining. Additionally, intact areas within segmentally sclerotic glomeruli also showed prominent APA staining. Notwithstanding the observed heterogeneity in glomerular APA expression, the total glomerular APA expression decreased over time as shown by Western blotting of glomerular homogenates. The upper band for APA represents a mature transmembrane domain protein post-Golgi processing, whereas the lower band corresponds to an immature endoplasmic reticulum fraction.33 In contrast, glomerular fibronectin abundance increased over time (Figure 1B). Furthermore, a kidney specimen from a subject with idiopathic FSGS showed a similar pattern of glomerular APA expression (Figure 1C). To ascertain whether the observed pattern of expression was not a consequence of a nonspecific podocyte hypertrophic adaptation, we compared the pattern of glomerular expression of APA with that of glomerular epithelial protein 1 (GLEPP-1), a podocyte marker. Unlike APA, GLEPP-1 expression was not found to be increased in either intact glomeruli or the nonsclerotic segments of segmentally sclerosed glomeruli during advanced glomerulosclerosis (Figure 2).
Figure 1.
Pattern of glomerular APA expression changes during progressive glomerulosclerosis. Examination of glomerular APA expression in 60-week-old FHH rat kidneys by (A) immunohistochemistry. In comparison with normal age-matched Wistar rat kidneys that revealed uniform glomerular APA staining, FHH rat glomeruli showed a heterogeneous pattern of expression, with a mixture of globally sclerotic glomeruli showing virtually no APA expression, segmentally sclerotic glomeruli with enhanced APA staining in the nonsclerotic segments, and fully preserved glomeruli with overall increased APA staining. Examination of APA expression in glomerular extracts (pooled from three rats per lane) by (B) Western blotting showed progressive loss of total APA over time along with a parallel progressive increase in fibronectin (FN). The upper band for APA represents a mature transmembrane domain, whereas the lower band corresponds to an immature endoplasmic reticulum fraction. Examination of APA expression in (C) human kidneys of a transplant healthy donor and an individual with FSGS also revealed a similar distinct patter of APA expression. Scale bars, 200 µm.
Figure 2.
Podocyte APA and GLEPP-1 expression evolve during early and advanced stages of FSGS. Glomerular APA expression examined by immunohistochemistry in kidney sections from FHH rats in specimens obtained at 12, 24, 60, and 90 weeks of age. Sections revealed contrasting degrees of glomerular APA expression at advanced stages. Although APA staining increased in intact glomeruli as well as intact areas of segmentally sclerosed glomeruli, it was absent in globally sclerotic glomeruli. Staining for GLEPP-1 did not show a similar expression pattern. Representative immunofluorescence images show APA staining along with faint GLEPP-1 staining in a segmentally sclerotic glomerulus at advanced stages of disease. Scale bars, 200 μm in black and green; 500 μm in yellow; 50 μm in white.
Renal Characterization of APA-Knockout Mice
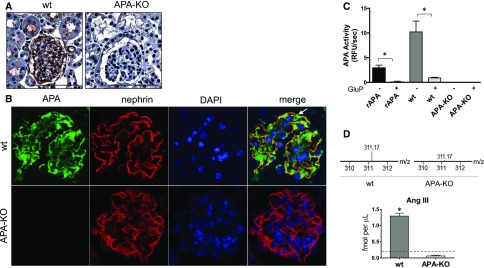
Lack of expression of APA in kidneys of BALB/c APA-knockout (KO) mice was verified by immunohistochemistry. Staining in BALB/c wild-type mice showed expression in podocytes and proximal tubules (Figure 3A). Immunofluorescence studies confirmed colocalization of APA and nephrin in wild-type mouse podocytes and complete absence of APA in APA-KO mice (Figure 3B). Images show APA localization at the body of the podocyte as well as colocalization of APA and nephrin at the slit diaphragms, consistent with a previous report.18 In addition, functional deficiency of intrarenal APA was verified in whole-kidney extracts of APA-KO mice by a fluorogenic substrate–based enzyme activity assay (Figure 3C) as well as a mass spectrometry–based assay (Figure 3D). The heptapeptide AngIII, a product of the cleavage of AngII by APA, was not detected in glomerular suspensions of APA-KO mice (Figure 3D). Altogether, these studies confirmed intrarenal deficiency of APA in APA-KO mice.
Figure 3.
Lack of expression and activity of APA were verified in APA-KO mice. Examination of APA expression by (A) immunohistochemistry in kidney sections of wild-type (wt) and APA-KO mice showed glomerular and apical tubular distribution of APA in wt mice and negative staining in APA-KO mice. Scale bars, 200 µm. Examination by (B) immunofluorescence revealed colocalization of APA with nephrin, a podocyte marker, in wt mice and lack of glomerular APA expression in APA-KO mice. The white arrow indicates the body of the podocyte only showing APA staining (green), whereas colocalization of APA and nephrin corresponds to the podocyte slit diaphragms (yellow). DAPI, 4′,6-diamidino-2-phenylindole. Fluorometry-based APA enzymatic activity (C) was measured in whole-kidney homogenates harvested from wt or APA-KO mice. Bars represent mean values, and error bars show SEMs. APA activity was measured with or without the presence of the APA inhibitor glutamate phosphonate (GluP; 10 μm). Recombinant aminopeptidase A (rAPA) was used as a positive control. (D) Conversion of AngII into AngIII was verified by LC-MS/MS, showing detection of AngIII (represented by the +3 parent ion 311.17 m/z) in glomerular suspensions of wt mice and almost complete absence of AngIII in those of APA-KO mice. The horizontal dashed line in the bar graph denotes the lower limit of detection. *P<0.001.
Renal Phenotype of Aged APA-KO Mice
To determine whether APA-KO mice develop spontaneous kidney disease, kidneys from 12-month-old APA-KO and wild-type control mice were examined histologically. A few glomeruli per section were found to display mild mesangial hypercellularity in APA-KO mice, but this finding did not reach statistical significance. In addition, no significant difference between groups was found in urine albumin-to-creatinine ratio (UACR), although a trend was noted (Supplemental Figure 1).
Effect of NTS in APA-KO Mice
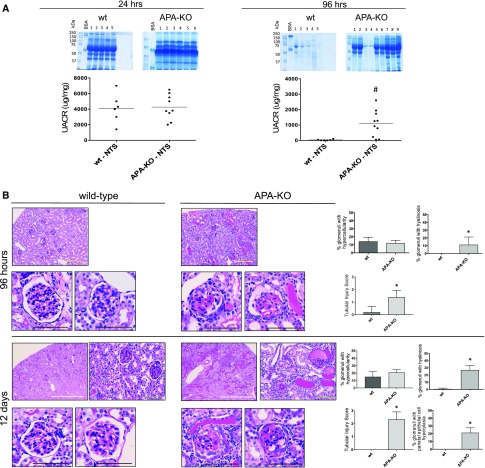
Wild-type and APA-KO mice were subjected to single retro-orbital injections of NTS (50 μl). Within 24 hours, massive albuminuria was induced in both wild-type and APA-KO mice. However, although wild-type mice exhibited an almost complete recovery at 96 hours postinjection, persistent albuminuria was observed in APA-KO mice (Figure 4A). This finding was accompanied by evidence of glomerular hyalinosis and tubular atrophy in APA-KO mice that was not observed in wild-type controls (Figure 4B). Heterogeneity in response to the insult was observed. At 12 days, in addition to glomerular hyalinosis and tubular atrophy, evidence of glomerular parietal cell hyperplasia and glomerular collapse was also detected in APA-KO mice (Figure 4B).
Figure 4.
Urine albumin excretion and glomerular injury persist in APA-KO mice injected with NTS. (A) Persistence of albuminuria was observed in APA-KO mice 96 hours after injection of NTS but not in wild-type (wt) mice as shown by albumin gel electrophoresis and UACR by ELISA obtained 24 and 96 hours after NTS injections. (B) Histologic examination shows exacerbated renal parenchymal injury in APA-KO mice injected with NTS. Representative light microscopy images of kidney sections stained with periodic acid–Schiff. Tissue was harvested from wt or APA-KO mice 96 hours or 12 days after treatment with NTS injection. At 96 hours, mesangial hypercellularity was observed in both groups. However, only APA-KO mouse glomeruli showed hyalinosis, tuft adhesion to the Bowman’s capsule, and microaneurysms. Tubular atrophy with protein lakes was also seen in APA-KO mice. At 12 days, in addition to the aforementioned changes, parietal cell hyperplasia was noted in APA-KO mice. Scale bars, 200 μm in black and green; 500 μm in yellow; 2 mm in white. *P<0.001; #P<0.001.
Effect of Chronic AngII Infusion on BP in APA-KO Mice
Osmotic minipumps were implanted in wild-type and APA-KO mice for a 4-week subcutaneous administration of vehicle (isotonic saline) or AngII (400 ng/kg per minute). Tail cuff sphygmomanometry-based systolic BP (SBP) was measured weekly throughout the experiment. The magnitude of the increase in SBP in AngII-treated APA-KO mice was not significantly different than that of wild-type mice at 1, 2, 3, or 4 weeks of infusion (Supplemental Figure 2). At 4 weeks, SBP reached 232±7 and 235±7 mmHg for AngII-treated wild-type and APA-KO mice groups, respectively. The observed degree of hypertension precluded testing higher doses of AngII.
Effect of Chronic AngII Infusion on Albuminuria in APA-KO Mice
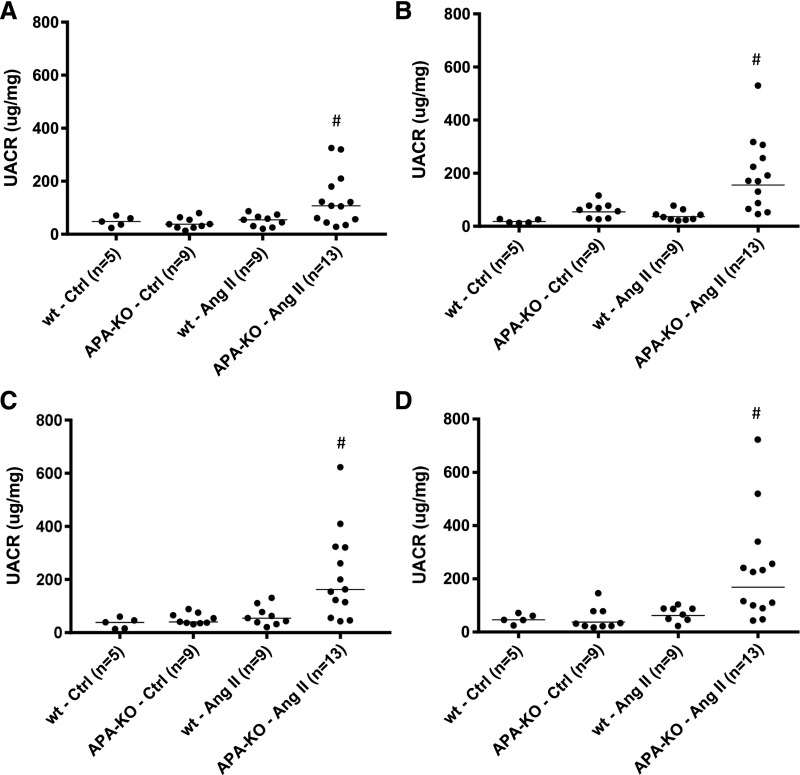
Low levels of UACR were observed in vehicle-treated wild-type and APA-KO mice. Similarly, AngII-treated wild-type mice did not exhibit a significant increase in UACR during the 4 weeks of AngII exposure. However, AngII-treated APA-KO mice developed a significantly greater degree of albuminuria at 1, 2, 3, and 4 weeks (Figure 5). Heterogeneity in the albuminuric response to AngII was observed.
Figure 5.
Global deficiency of APA leads to increased albuminuria during chronic AngII infusion. Values of urinary albumin excretion collected during a 4-week infusion of AngII (400 ng/kg per minute) in APA-KO mice. Urinary albumin excretion rates expressed in UACR in wild-type (wt) or APA-KO mice treated with chronic subcutaneous infusion of either vehicle control (Ctrl) or AngII (400 ng/kg per minute). Data presented in scatter dot plots of values obtained at (A) 1, (B) 2, (C) 3, and (D) 4 weeks showed greater magnitude of UACR in AngII-treated APA-KO mice. #P<0.01 versus all three groups.
Intrarenal Ang Peptide Abundance after Chronic AngII Infusion in APA-KO Mice
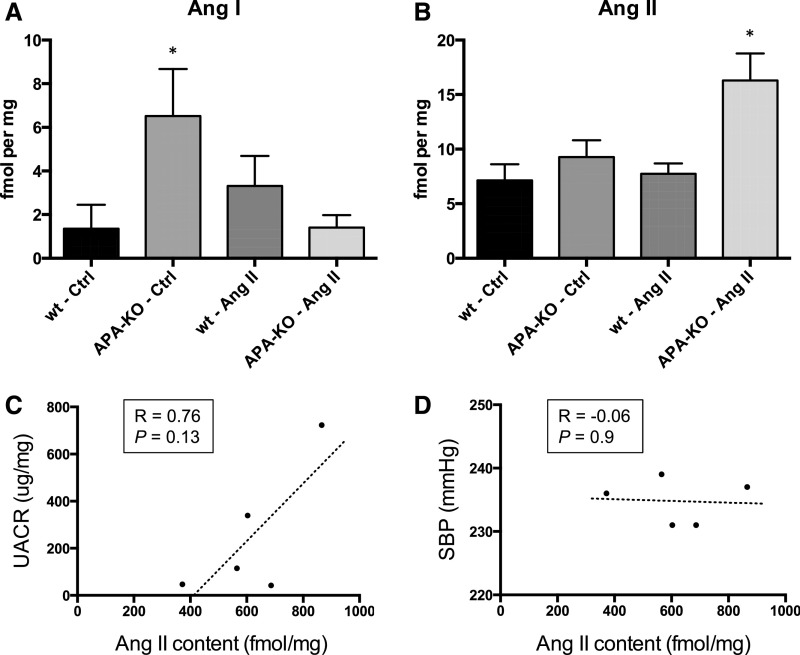
Whole-kidney homogenates were processed and analyzed by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS). AngI abundance was significantly greater in vehicle-treated APA-KO mice compared with wild-type mice but not in APA-KO mice infused with AngII (Figure 6A). However, kidney AngII abundance was not different among both vehicle-treated groups and AngII-treated wild-type mice. However, kidney AngII abundance significantly increased in AngII-treated APA-KO mice (Figure 6B). Moreover, kidney AngII content seemed to correlate better to UACR than to SBP (Figure 6, C and D).
Figure 6.
Kidney content of Ang peptides after chronic AngII infusion is altered in APA-KO mice. Quantification of tissue content of (A) AngI and (B) AngII performed by LC-MS/MS in whole-kidney homogenates from wild-type (wt) or APA-KO mice treated with chronic infusion of either vehicle control (Ctrl) or AngII (400 ng/kg per minute) for 4 weeks (n=5 per group). *P<0.05. Correlation between kidney AngII content and (C) UACR and (D) SBP) is shown.
Effect of Chronic AngII Infusion on Kidney Morphology in APA-KO Mice
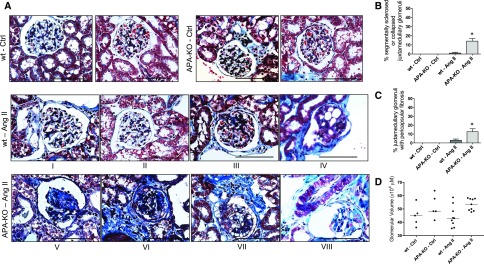
Glomerular and vascular lesions are common morphologic features described in this model.30,34,35 Sections from wild-type and APA-KO mice treated with vehicle showed no evidence of glomerular damage after 4 weeks. Only a few wild-type mice treated with AngII showed discrete evidence of segmental sclerosis and microaneurysms. In contrast, AngII-treated APA-KO mice exhibited significantly more pronounced segmental and global sclerosis and glomerular collapse (Figure 7). Affected glomeruli were almost exclusively found at the juxtamedullary region. Because of the known effects of AngII in cell hypertrophy36,37 and on juxtamedullary glomeruli volume,38 we assessed for glomerular size but did not find a significant difference in glomerular volume among treatment groups.
Figure 7.
Histologic examination shows exacerbated glomerular injury in APA-KO mice after chronic AngII infusion. (A) Representative light microscopy images of kidney sections stained with Masson trichrome. Tissue was harvested from wild-type (wt) or APA-KO mice treated with chronic subcutaneous infusion of either vehicle control (Ctrl) or AngII (400 ng/kg per minute) for 4 weeks. (B and C) Bar graphs show comparison of the percentages of juxtamedullary glomeruli with segmental (III, IV, and VII) or global (V and VI) sclerosis, collapse (V and VIII), or microaneurysms (IV and VIII). (D) Evidence of glomerular enlargement was compared between groups. *P<0.01. Scale bars, 200 μm.
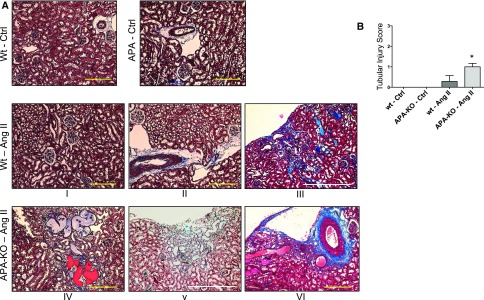
Sections from wild-type and APA-KO mice treated with vehicle control showed no evidence of tubulointerstitial damage. Mild patchy tubular atrophy, microcystic tubular dilation, and interstitial fibrosis were found in AngII-treated wild-type mice, whereas APA-KO mice infused with AngII acquired significantly worse tubulointerstitial injury, including scattered large protein lakes (Figure 8). These lesions were found either surrounding an affected glomerulus within the juxtamedullary region or at the subcapsular region depicting a radial pattern, perhaps after a vascular bed distribution.
Figure 8.
Histologic examination shows exacerbated tubulointerstitial injury in APA-KO mice after chronic AngII infusion. (A) Representative light microscopy images of kidney sections stained with Masson trichrome. Tissue was harvested from wild-type (wt) or APA-KO mice treated with chronic subcutaneous infusion of either vehicle (Ctrl) or AngII (400 ng/kg per minute) for 4 weeks. (B) Bar graph shows comparison of tubulointerstitial injury scores that accounted for patchy subcapsular fibrosis (III and V) and microcystic tubular dilation (III–VI). *P<0.01. Scale bars, 500 μm in yellow; 2 mm in white.
Effect of Chronic AngII Infusion on Podocyte Protein Expression in APA-KO Mice
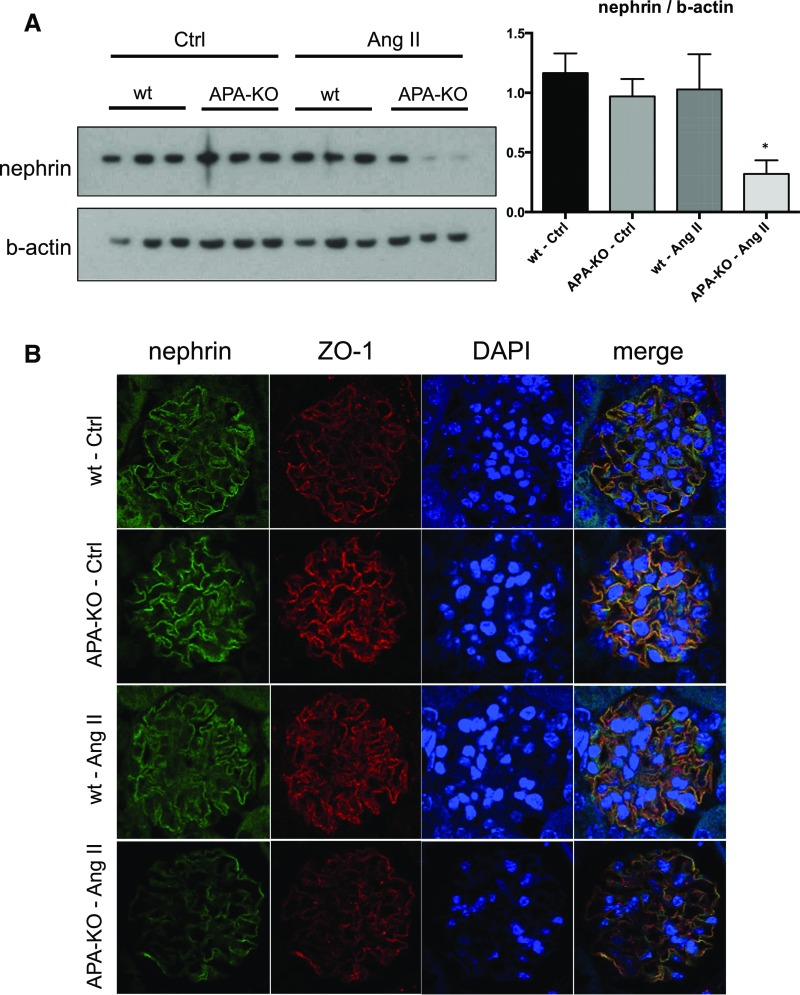
Because AngII has been reported to induce podocyte injury in association with a decrease in nephrin expression,39,40 we examined the effect of chronic AngII infusion on nephrin. We found a reduction in nephrin expression in AngII-treated APA-KO mouse kidneys by Western blotting in whole-kidney homogenates as well as immunofluorescence in tissue sections (Figure 9). Zona occludens 1 (ZO-1) was used as a colocalizing podocyte marker, but its expression was also attenuated in AngII-treated APA-KO mouse kidneys (Figure 9). Others have reported redistribution of ZO-1 and cytoskeletal rearrangement in response to AngII.41
Figure 9.
Podocyte protein expression changes observed in APA-KO mice after chronic AngII infusion. Representative images depicting the effect of chronic infusion of AngII on (A) nephrin and β-actin as examined by Western blotting in whole-kidney homogenates and (B) nephrin and ZO-1 expression as examined by immunofluorescence in 5 µm kidney sections. Graph shown next to the Western blot reflects quantification of band densities from five individual mouse kidney homogenates from the wild-type (wt) control (Ctrl) group and six of each of the other three treatment groups. *P<0.01.
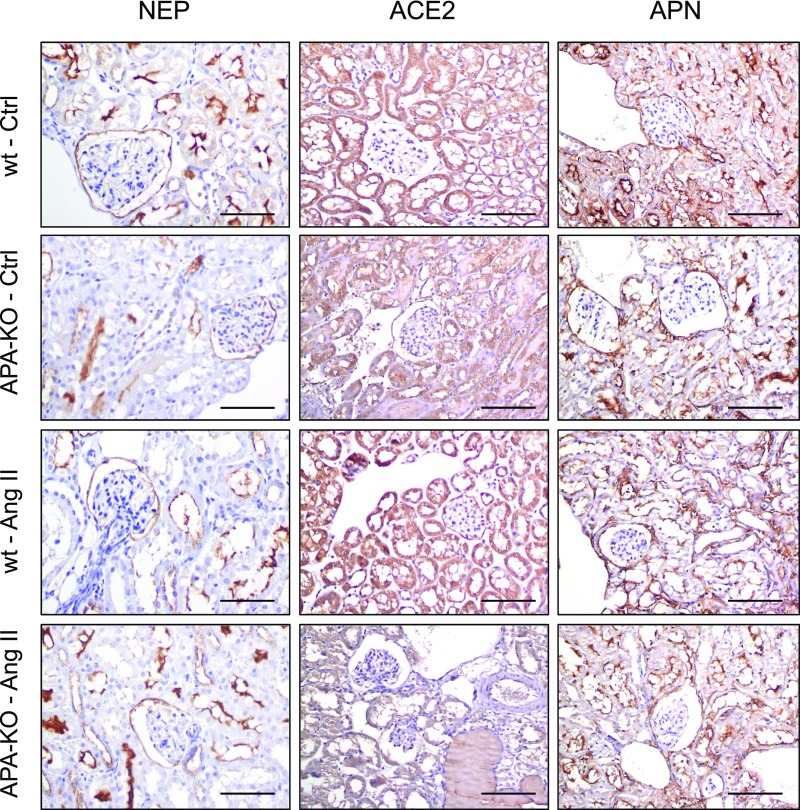
Effect of Chronic AngII Infusion on Expression of Angiotensinases in APA-KO Mice
We examined the intrarenal expression of key renin-angiotensin system (RAS) peptidases known to metabolize AngII by immunohistochemistry, searching for a compensatory upregulation in response to APA deficiency. However, no difference in expression of ACE2, NEP, or APN was found among all four treatment groups (Figure 10).
Figure 10.
RAS peptidase expression does not change in APA-KO mice after chronic AngII infusion. Representative images depicting the effect of chronic infusion of AngII on expression of NEP, ACE2, and APN as examined by immunofluorescence. Glomerular parietal epithelial and tubular epithelial cell distribution was noted for all three enzymes, with faint podocyte localization. No difference in degree of staining was observed across treatment groups. Ctrl, control; wt, wild type. Scale bars, 200 μm.
Discussion
We report a distinct pattern of glomerular expression of APA during progressive glomerulosclerosis. Although the overall expression of glomerular APA decreases over time in parallel with the development of glomerulosclerosis, a heterogeneous population of glomeruli is recognizable at advanced stages of disease. Strong APA staining was observed in the surviving intact glomeruli as well as the segmental areas where the glomerular capillary loops remain intact, whereas virtually no APA staining was found in globally sclerotic glomeruli. Using enzyme histochemistry, a similar adaptive pattern of APA activity was reported in advanced human CKD.42 The authors labeled the glomeruli displaying high APA activity as “highly resistant” because of their normal morphology, despite the severe damage of the surrounding tissue, and implicated APA in mechanisms of cellular adaptation to progressive nephron loss.42,43 Our findings are in line with those observations and provide a protein abundance correlate to their functional studies. In addition, the pattern of APA expression did not seem to correspond to nonspecific podocyte hypertrophy, because the expression of GLEPP-1, a podocyte marker, was not enhanced in the surviving glomeruli at advanced stages. In agreement with our findings, others reported gradual fall in GLEPP-1 expression in a rat model of podocyte injury.44,45 Thus, augmentation of podocyte APA abundance seems to be an adaptive response to progressive glomerulosclerosis.
To further investigate the role of APA in mechanisms of adaptation to glomerular injury, we subjected APA-KO mice to a glomerular injury model. Compared with wild-type controls, APA-KO mice exhibited persistent albuminuria and glomerular hyalinosis in response to NTS, suggesting that APA participates in glomerular repair mechanisms. Because APA is an angiotensinase and AngII can promote podocyte loss and injury,44,46 it is plausible that the lack of recovery to the NTS injection in APA-KO mice corresponds to an exacerbated AngII-mediated mechanism.
Our studies using a chronic AngII infusion model show that mice fully deficient in APA exhibit increased susceptibility to AngII-mediated kidney injury as manifested by changes in albuminuria, kidney morphology, and nephrin expression. These findings were observed in association with increased intrarenal abundance of AngII in APA-KO mice infused with AngII. Thus, our data suggest that the increased susceptibility to AngII-mediated kidney injury may correspond to reduced metabolism of AngII. Intrarenal AngII content in vehicle-treated APA-KO mice was not increased, suggesting that, under basal conditions and in the absence of an amplified RAS state, other mechanisms of AngII degradation might be sufficient to counterbalance APA deficiency. However, this equilibrium may be lost during a perturbation that leads to amplification in AngII generation or enhanced AngII delivery to the kidney.
Despite pronounced hypertension, only minimal renal parenchymal damage was observed in AngII-treated wild-type mice, likely due to resistance inherent to their background strain.47 In contrast, although acquiring a similar degree of hypertension, APA-KO mice developed marked glomerulosclerosis, glomerular collapse, and tubular damage in response to AngII treatment. This pattern of injury resembled that described in other susceptible mouse strains subjected to the AngII infusion model.34,35,48 Thus, APA deficiency increased the susceptibility of BALB/c mice to the pathologic actions of AngII in the kidney. Notably, affected glomeruli were almost exclusively localized at the juxtamedullary region, zonal localization that fits with previous descriptions of vulnerability of juxtamedullary glomeruli in animal models of glomerular capillary hypertension49 or FSGS50 and human FSGS.51,52
Unlike our study, APA-KO mice on a hybrid C57BL/6J-129Sv background strain exhibited an augmented pressor response to chronic AngII. However, the authors did not report whether that pressor susceptibility was accompanied with greater kidney injury.53 Although systemic hypertension is a critical element driving the pathogenesis of AngII-dependent glomerulosclerosis, local nonpressor effects of AngII in the kidney are thought to play an independent role.54–56 Thus, the greater renal parenchymal injury in AngII-treated APA-KO mice likely resulted from local intrarenal effects of AngII amplified by APA deficiency.
A vast array of evidence indicates that AngII can directly exert deleterious effects in podocyte biology, such as derangements of the slit diaphragm composition40,57,58 or the cytoskeleton,38,59,60 activation of profibrotic pathways,61 apoptosis,62–64 and podocyte detachment.44 In our studies, AngII infusion induced attenuation of nephrin expression in APA-KO mice but not in wild-type mice. These findings suggest that the noxious effects of AngII on podocyte slit diaphragm integrity of BALB/c mice were enhanced in a state of global APA deficiency. Because APA deficiency led to greater kidney AngII content, direct effects of AngII on podocytes were likely amplified. In support of this contention, AngII has been shown to reduce nephrin expression40 and mRNA57 in mouse podocytes in vitro as well as nephrin mRNA abundance after acute subcutaneous AngII injection in rats.57 The observed decrease in nephrin expression should not be viewed as a sole mediator of the albuminuric glomerular phenotype. Rather, it is interpreted as a surrogate indicator for distressed glomeruli caused by AngII. The lack of nephrin attenuation in wild-type AngII-treated mice likely corresponded to the relative renal resistance of BALB/c mice to the AngII infusion model.47
Interestingly, vehicle-treated APA-KO mice were found to have increased kidney AngI abundance. Because APA is the primary glomerular peptidase responsible for the conversion of AngI to Ang2–10,65 the greater content of AngI in APA-KO mice likely corresponds to diminished AngI metabolism. In AngII-treated APA-KO mice, the intrarenal AngI content was not increased. This may pertain to the known negative feedback mechanism by which AngII inhibits renin release via stimulation of Angtype 1 receptors. Thus, AngII infusion may suppress renin release, thereby diminishing AngI generation from angiotensinogen. Altogether, our findings of intrarenal AngI content are in accordance with the known RAS feedback loops. Interestingly, we did not detect any compensatory increase in kidney expression of ACE2, NEP, or APN in our studies. ACE2 has been implicated as a renoprotective molecule in models of diabetic kidney disease.66–68 Nonetheless, peptidomic and network modeling approaches have revealed dominance of APA over ACE2 as Ang peptidase.12,69
In conclusion, deficiency of APA leads to increased susceptibility to glomerular injury as shown by the NTS injection model and the AngII-mediated renal parenchymal injury model. In lieu of the relative abundance and enzymatic activity of APA in the kidney and the adaptive changes in glomerular APA expression during glomerulosclerosis, our findings suggest a key role of APA in maintenance of intrarenal RAS equilibrium.
Concise Methods
Animal Care
Our protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and in accordance with the procedures and practices of the National Insititutes of Health Guide for the Care and Use of Laboratory Animals. All rodents were housed at a certified facility at 22°C and in a 12:12-hour light/dark cycle, allowed free access to tap water, fed a standard diet, and provided environmental enrichment. All surgeries were performed under isoflurane anesthesia (5% induction and 2% maintenance) at 37°C and perioperative buprenorphine.
Rodents
BALB/c APA-KO (ENPEP−/−) mice were provided by Renata Pasqualini (University of New Mexico, Albaquerque, NM). Generation of APA-KO mice was previously reported in detail.70 Wild-type BALB/c control mice were purchased from Envigo (Indianapolis, IN). Male FHH rats were an in-house colony established from animals obtained from Charles River Laboratories (Wilmington, MA). Male Wistar rats were purchased from Envigo (Indianapolis, IN).
Human Tissue
Our study protocol was approved by the Medical University of South Carolina Institutional Review Board. Archived formalin-fixed, paraffin-embedded kidney specimens were processed for examination by immunohistochemistry. Five-μm sections were obtained from biopsy cores from three healthy donors and five patients with diagnosis of idiopathic FSGS.
Glomerular Injury Model
Mice were retro-orbitally injected with 50 μl NTS (PTX-001S; Probetex Inc., San Antonio, TX) at 12 weeks of age as previously reported.29,71 Pilot studies were carried out to select the dosage. Urine samples were collected at preinjection and 24 and 96 hours postinjection. Kidney tissues were collected at euthanasia.
AngII Infusion Model
An established model for AngII-mediated hypertensive kidney injury was used with some modifications.30 Eight- to 12-week-old male mice underwent osmotic minipump implantation (Alzet, Palo Alto, CA) for delivery of AngII (400 mg/kg per minute) for 4 weeks. SBP was measured with a tail cuff sphygmomanometer at baseline and weekly throughout the experiment.
Albuminuria
Mice were placed in metabolic cages for 24-hour urine collection weekly. UACR was used as a marker of glomerular injury. Urine albumin and creatinine were measured by ELISA (Exocell, Philadelphia, PA) as per the manufacturer’s protocol. Samples were diluted (1:10–1:100) before assay. For the NTS model, 2.5-μl urine aliquots were run on a 10% SDS-PAGE, and the gels were stained with Coomassie Brilliant Blue G-250 (0.25%) prepared in 50% methanol and 10% acetic acid for 30 minutes as previously reported.72,73
Immunohistochemistry for Kidney Morphology and RAS Peptidase Expression
Parenchymal renal injury was assessed by immunohistochemistry. Kidneys harvested at euthanasia were fixed in 4% paraformaldehyde and paraffin embedded. Morphology was examined in sections stained with hematoxylin-eosin, Jones silver, periodic acid–Schiff, and Masson trichrome. Expression RAS peptidases and GLEPP-1 were examined using an antigen retrieval technique. Briefly, 4- to 5-μm sections were deparaffinized in xylene, washed in ethanol gradient, and microwaved for 20 minutes with Cymatin Citrate (pH 6.0) for antigen retrieval (DAKO, Carpinteria, CA) followed by washes with 3% hydrogen peroxide, PBS, and 2.5% horse serum (ImmPRESS Reagent Peroxidase Anti-Goat Ig Kit; Vector Laboratories, Burlingame, CA). Primary antibodies were goat anti-APA (anti–BP-1; Abcam Laboratories, Cambridge, MA), 1:167 (mouse specimens) and 1:150 (rat and human specimens); mouse anti-NEP (anti-CD10; Vector Laboratories), 1:50; goat anti-ACE2 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:50; rabbit anti-APN (anti-CD13; Origene, Rockville, MD), 1:250; and mouse anti-GLEPP-1 (gift from Roger Wiggins, University of Michigan, Ann Arbor, MI), 1:50. The anti–GLEPP-1 antibody was not reactive in mouse tissue. Tissues were mounted with Cytoseal 60 (ThermoFisher Scientific, Carlsbad, CA).
Scoring of specimens were adjudicated blindly by a trained pathologist. Glomerular damage was rated by percentage of affected glomeruli and determined by the presence of segmental or global sclerosis or hyalinosis, glomerular collapse, microaneurysms, pericapsular fibrosis, mesangial hypercellularity, or parietal epithelial cell hyperplasia. Glomerulomegaly was assessed by measurement of glomerular volume using the software AxioVision 4.6 (Dublin, CA) and standard morphometric equations.74 An average of 15 glomeruli per tissue section per kidney was obtained. Tubulointerstitial damage was determined by the presence of interstitial fibrosis, tubular atrophy, and microcystic tubular dilation and rated by a standard scale: 0, 1 (<25%), 2 (25%–50%), and 3 (>50%).
Immunofluorescence for Glomerular Protein Expression
Procedures were performed as previously described.75 Primary antibodies for APA (1:10; Abcam Laboratories), nephrin76 (1:150), and ZO-1 (1:300; Invitrogen, Carlsbad, CA) were diluted in 5% BSA in PBS and incubated overnight at 4°C. Sections were washed, incubated with Alexa Fluor–labeled secondary antibodies at 1:1000 for 1 hour at 37°C, and mounted with antifade reagent containing 4′,6-diamidino-2-phenylindole.
Western Blotting
Kidney homogenates were loaded in Novex 4%–12% Bis-Tris NuPage Gels (Invitrogen) as previously described with minor modifications.2 Primary antibodies used were goat anti-APA (Abcam), 1:500; rabbit antifibronectin (Abcam), 1:1000; rabbit antinephrin (gift from Puneet Garg, University of Michigan, Ann Arbor, MI), 1:2000; and mouse anti–β-actin (Sigma-Aldrich, St. Louis, MO), 1:5000.
APA Activity by Fluorometry
Mouse kidney homogenates (n=3 per group) were prepared with triethanolamine sucrose buffer (10 mM triethanolamine and 250 mM sucrose, pH 7.6) and diluted in assay buffer (25 mM Tris, 50 mM CaCl2, and 0.2 M NaCl, pH 8.0) to a 2 μg/μl concentration. Lysates were pretreated with or without 4-amino-4-phosphonobutyric acid (glutamate phosphonate, 10 μM final; APA inhibitor77,78; gift from Robert Speth, Nova Southeastern University, Fort Lauderdale FL). The fluorogenic substrate H-Glu-AMC (Bachem, Torrance, CA) was added to 100 μg protein per well at a final concentration of 100 μM. Excitation and emission were measured for 30 minutes at 380 and 460 nm, respectively. Recombinant APA (0.02 μg; R&D Systems, Minneapolis, MN) was used as a positive control.
APA Activity by LC-MS/MS
Conversion of AngII to AngIII was examined in suspensions of glomeruli isolated from wild-type and APA-KO mouse kidneys as previously reported with some modifications.65 Isolation of glomeruli was undertaken following a magnetic beads–based method.79 Two thousand glomeruli per 1 ml were incubated in Krebs buffer with 1 μM AngII for 60 minutes. Buffer aliquots were serially obtained and analyzed by LC-MS/MS to search for generation of AngIII. Each aliquot was centrifuged at 20,000×g for 10 minutes. Supernatant (25 μl) was mixed 1:1 with stable isotope–labeled AngIII in 0.4% formic acid to a final concentration of 22.5 fmol/μl. Samples were loaded into the autosampler, and 4 μl supernatant was injected onto an ACQUITY UPLC M-Class HSS T3 Column (1.8 μm; 75 μm×150 mm; Waters Corp., Milford, MA) at 10 μl/min with 0.2% formic acid in mass spectrometry–grade water. Peptides were separated at 10 μl/min using an M-Class UPLC (Waters Corp.) from zero to 30% mobile phase B (95% acetonitrile/0.1% formic acid) over 4 minutes. Data were acquired with a Triple Quadrupole Mass Spectrometer (TQS; Waters Corp.) using a low-flow probe at the source. The transitions (m/z) 311.2–419.2 and 313.3–425.3 were monitored for quantification of the native and stable isotope–labeled standard. Estimates of concentration were made against an external standard curve (0.5–250 fmol on column) in Targetlynx (Waters Corp.). Lower limit of quantification was determined as 10× SD of five blank injections.
Ang Peptide Content by LC-MS/MS
Tissue abundance of Ang peptides was determined using a Triple-ToF 5600 Mass Spectrometer (SCIEX, Framingham, MA) using previously published methods with some modifications.80 Frozen whole kidney was pulverized in liquid nitrogen, and 40–190 mg were transferred to a glass dounce for homogenization in 60% methanol in water containing 1 mM phenathroline. Stable isotope–labeled standards for AngII, AngIII, AngIV, and Ang2–10 were added to a concentration of 10.8 fmol/mg wet kidney weight.
Statistical Analyses
Figures were generated using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA), and values were displayed as mean and SD. Comparisons between groups were performed using two-way ANOVA with post hoc analysis, repeated measures ANOVA, paired t tests, or Fischer exact tests when appropriate. Correlations were assessed by Spearman test. A P value <0.05 was deemed significant.
Disclosures
J.C.Q.V. has served on advisory boards for Mallinckrodt Pharmaceuticals, Alexion Pharmaceuticals, and Relypsa Inc.
Supplementary Material
Acknowledgments
We thank Alison Bland and Peifeng Deng for technical support.
This work was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health (DK080944, J.C.Q.V.), and American Recovery and Reinvestment Act supplement to DK080944 (W.R.F. and M.P.H.) and Dialysis Clinics Incorporated (J.C.Q.V. and M.G.J.). D.N. is supported by grant R01DK0887956-07 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Part of this work was presented as a poster at American Society of Nephrology Kidney Week in Philadelphia, Pennsylvania on November 11–16, 2014 and in San Diego, California on November 3–8, 2015.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016111166/-/DCSupplemental.
References
- 1.Bauer J, Berthold H, Schaefer F, Ehmke H, Parekh N: Quantification of conversion and degradation of circulating angiotensin in rats. Am J Physiol 277: R412–R418, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG: Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 293: F398–F407, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Velez JC: Prolyl carboxypeptidase: A forgotten kidney angiotensinase. Focus on “Identification of prolyl carboxypeptidase as an alternative enzyme for processing of renal angiotensin II using mass spectrometry.” Am J Physiol Cell Physiol 304: C939–C940, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang HY, Erdös EG, Chiang TS: New enzymatic route for the inactivation of angiotensin. Nature 218: 1224–1226, 1968 [DOI] [PubMed] [Google Scholar]
- 5.Allred AJ, Diz DI, Ferrario CM, Chappell MC: Pathways for angiotensin-(1---7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol 279: F841–F850, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Rosivall L, Narkates AJ, Oparil S, Navar LG: De novo intrarenal formation of angiotensin II during control and enhanced renin secretion. Am J Physiol 252: F1118–F1123, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Li N, Zimpelmann J, Cheng K, Wilkins JA, Burns KD: The role of angiotensin converting enzyme 2 in the generation of angiotensin 1-7 by rat proximal tubules. Am J Physiol Renal Physiol 288: F353–F362, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM: Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383: 45–51, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D: ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Grobe N, Weir NM, Leiva O, Ong FS, Bernstein KE, Schmaier AH, Morris M, Elased KM: Identification of prolyl carboxypeptidase as an alternative enzyme for processing of renal angiotensin II using mass spectrometry. Am J Physiol Cell Physiol 304: C945–C953, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velez JC, Ierardi JL, Bland AM, Morinelli TA, Arthur JM, Raymond JR, Janech MG: Enzymatic processing of angiotensin peptides by human glomerular endothelial cells. Am J Physiol Renal Physiol 302: F1583–F1594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwacke JH, Spainhour JC, Ierardi JL, Chaves JM, Arthur JM, Janech MG, Velez JC: Network modeling reveals steps in angiotensin peptide processing. Hypertension 61: 690–700, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troyanovskaya M, Jayaraman G, Song L, Healy DP: Aminopeptidase-A. I. CDNA cloning and expression and localization in rat tissues. Am J Physiol Regul Integr Comp Physiol 278: R413–R424, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bioinformatics Laboratory at Wilmer Institute, Johns Hopkins University: Tissue-specific Gene Expression and Regulation (TiGER), version 1.0. Available at http://bioinfo.wilmer.jhu.edu/tiger/db_gene/ENPEP-index.html.” Accessed on March 2008
- 15.Scherberich JE, Wiemer J, Herzig C, Fischer P, Schoeppe W: Isolation and partial characterization of angiotensinase A and aminopeptidase M from urine and human kidney by lectin affinity chromatography and high-performance liquid chromatography. J Chromatogr A 521: 279–289, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Mentzel S, Dijkman HB, Van Son JP, Koene RA, Assmann KJ: Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem 44: 445–461, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Kugler P, Wolf G, Scherberich J: Histochemical demonstration of peptidases in the human kidney. Histochemistry 83: 337–341, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Assmann KJ, van Son JP, Dijkman HB, Koene RA: A nephritogenic rat monoclonal antibody to mouse aminopeptidase A. Induction of massive albuminuria after a single intravenous injection. J Exp Med 175: 623–635, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chugh S, Yuan H, Topham PS, Haydar SA, Mittal V, Taylor GA, Kalluri R, Salant DJ: Aminopeptidase A: A nephritogenic target antigen of nephrotoxic serum. Kidney Int 59: 601–613, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G: Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 352: 1252–1256, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Meyer TW, Anderson S, Rennke HG, Brenner BM: Reversing glomerular hypertension stabilizes established glomerular injury. Kidney Int 31: 752–759, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Wolf G, Thaiss F, Mueller E, Disser M, Pooth R, Zahner G, Stahl RA: Glomerular mRNA expression of angiotensinase A after renal ablation. Exp Nephrol 3: 240–248, 1995 [PubMed] [Google Scholar]
- 24.Wolf G, Thaiss F, Scherberich JE, Schoeppe W, Stahl RA: Glomerular angiotensinase A in the rat: Increase of enzyme activity following renal ablation. Kidney Int 38: 862–868, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Song L, Healy DP: Kidney aminopeptidase A and hypertension, part II: Effects of angiotensin II. Hypertension 33: 746–752, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA: Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis 43: 636–650, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ: Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 54: 416–425, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Chen SM, Mukoyama T, Sato N, Yamagata S, Arai Y, Satoh N, Ueda S: Induction of nephrotoxic serum nephritis in inbred mice and suppressive effect of colchicine on the development of this nephritis. Pharmacol Res 45: 319–324, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM: Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F: Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Rozenfeld R, Muller L, El Messari S, Llorens-Cortes C: The C-terminal domain of aminopeptidase A is an intramolecular chaperone required for the correct folding, cell surface expression, and activity of this monozinc aminopeptidase. J Biol Chem 279: 43285–43295, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Huang XR, Lan HY: Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am J Physiol Renal Physiol 302: F986–F997, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA: Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension 52: 256–263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson PW, Do YS, Hsueh WA: Angiotensin II causes mesangial cell hypertrophy. Hypertension 21: 29–35, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM: Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Hornych H, Beaufils M, Richet G: The effect of exogenous angiotensin on superficial and deep glomeruli in the rat kidney. Kidney Int 2: 336–343, 1972 [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Chang JH, Paik SY, Tang Y, Eisner W, Spurney RF: Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol 25: 1376–1386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G: Nephrin expression is reduced in human diabetic nephropathy: Evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 52: 1023–1030, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, Remuzzi G: Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol 168: 1073–1085, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherberich JE, Wolf G, Albers C, Nowack A, Stuckhardt C, Schoeppe W: Glomerular and tubular membrane antigens reflecting cellular adaptation in human renal failure. Kidney Int Suppl 27: S38–S51, 1989 [PubMed] [Google Scholar]
- 43.Scherberich JE, Matthess A, Remelius W, Schoeppe W: Aminopeptidase A (= angiotensinase A) in human progressive renal disease. Miner Electrolyte Metab 18: 97–100, 1992 [PubMed] [Google Scholar]
- 44.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC: Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM: Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension 43: 364–369, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, Wu G, Bowling B, Byrd A, Hall G, Sparks M, Zhang ZS, Homstad A, Barisoni L, Birbaumer L, Rosenberg P, Winn MP: TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol 22: 526–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iversen BM, Amann K, Kvam FI, Wang X, Ofstad J: Increased glomerular capillary pressure and size mediate glomerulosclerosis in SHR juxtamedullary cortex. Am J Physiol 274: F365–F373, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, Lee WH: Experimental focal segmental glomerulosclerosis in mice. Nephron 78: 440–452, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Sherman RL, Susin M, Mouradian J: Focal glomerular sclerosis. Perspect Nephrol Hypertens 6: 175–186, 1977 [PubMed] [Google Scholar]
- 52.Danilewicz M, Wagrowska-Danilewicz M: Minimal change disease and idiopathic focal segmental glomerulosclerosis in adults. A quantitative study. Pol J Pathol 47: 209–214, 1996 [PubMed] [Google Scholar]
- 53.Mitsui T, Nomura S, Okada M, Ohno Y, Kobayashi H, Nakashima Y, Murata Y, Takeuchi M, Kuno N, Nagasaka T, O-Wang J, Cooper MD, Mizutani S: Hypertension and angiotensin II hypersensitivity in aminopeptidase A-deficient mice. Mol Med 9: 57–62, 2003 [PMC free article] [PubMed] [Google Scholar]
- 54.Kagami S: Involvement of glomerular renin-angiotensin system (RAS) activation in the development and progression of glomerular injury. Clin Exp Nephrol 16: 214–220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urushihara M, Kinoshita Y, Kondo S, Kagami S: Involvement of the intrarenal renin-angiotensin system in experimental models of glomerulonephritis. J Biomed Biotechnol 2012: 601786, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahmood J, Khan F, Okada S, Kumagai N, Morioka T, Oite T: Local delivery of angiotensin receptor blocker into the kidney ameliorates progression of experimental glomerulonephritis. Kidney Int 70: 1591–1598, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Suzuki K, Han GD, Miyauchi N, Hashimoto T, Nakatsue T, Fujioka Y, Koike H, Shimizu F, Kawachi H: Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol 170: 1841–1853, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wennmann DO, Hsu HH, Pavenstädt H: The renin-angiotensin-aldosterone system in podocytes. Semin Nephrol 32: 377–384, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, Weide T, Schlatter E, Pavenstädt H: Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med (Berl) 86: 1379–1394, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J: Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S, Lee JS, Iglesias-de la Cruz MC, Wang A, Izquierdo-Lahuerta A, Gandhi NK, Danesh FR, Wolf G, Ziyadeh FN: Angiotensin II stimulates alpha3(IV) collagen production in mouse podocytes via TGF-beta and VEGF signalling: Implications for diabetic glomerulopathy. Nephrol Dial Transplant 20: 1320–1328, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, Singhal PC: Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol 28: 500–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC: Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol 283: F173–F180, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Durvasula RV, Shankland SJ: Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol 294: F830–F839, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Velez JC, Ryan KJ, Harbeson CE, Bland AM, Budisavljevic MN, Arthur JM, Fitzgibbon WR, Raymond JR, Janech MG: Angiotensin I is largely converted to angiotensin (1-7) and angiotensin (2-10) by isolated rat glomeruli. Hypertension 53: 790–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D: Glomerular localization and expression of angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD: Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW: Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438–451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wysocki J, Ye M, Batlle D: Plasma and kidney angiotensin peptides: Importance of the aminopeptidase A/angiotensin III axis. Am J Hypertens 28: 1418–1426, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Q, Taniuchi I, Kitamura D, Wang J, Kearney JF, Watanabe T, Cooper MD: T and B cell development in BP-1/6C3/aminopeptidase A-deficient mice. J Immunol 160: 4681–4687, 1998 [PubMed] [Google Scholar]
- 71.Lichtnekert J, Kulkarni OP, Mulay SR, Rupanagudi KV, Ryu M, Allam R, Vielhauer V, Muruve D, Lindenmeyer MT, Cohen CD, Anders HJ: Anti-GBM glomerulonephritis involves IL-1 but is independent of NLRP3/ASC inflammasome-mediated activation of caspase-1. PLoS One 6: e26778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gee HY, Sadowski CE, Aggarwal PK, Porath JD, Yakulov TA, Schueler M, Lovric S, Ashraf S, Braun DA, Halbritter J, Fang H, Airik R, Vega-Warner V, Cho KJ, Chan TA, Morris LG, ffrench-Constant C, Allen N, McNeill H, Büscher R, Kyrieleis H, Wallot M, Gaspert A, Kistler T, Milford DV, Saleem MA, Keng WT, Alexander SI, Valentini RP, Licht C, Teh JC, Bogdanovic R, Koziell A, Bierzynska A, Soliman NA, Otto EA, Lifton RP, Holzman LB, Sibinga NE, Walz G, Tufro A, Hildebrandt F: FAT1 mutations cause a glomerulotubular nephropathy. Nat Commun 7: 10822, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge Y, Si J, Tian L, Zhuang S, Dworkin LD, Gong R: Conditional ablation of glycogen synthase kinase 3β in postnatal mouse kidney. Lab Invest 91: 85–96, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Haas CS, Amann K, Schittny J, Blaser B, Müller U, Hartner A: Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice. J Am Soc Nephrol 14: 2288–2296, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Arif E, Wagner MC, Johnstone DB, Wong HN, George B, Pruthi PA, Lazzara MJ, Nihalani D: Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol Cell Biol 31: 2134–2150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 56: 1481–1491, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Vazeux G, Iturrioz X, Corvol P, Llorens-Cortès C: A tyrosine residue essential for catalytic activity in aminopeptidase A. Biochem J 327: 883–889, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karamyan VT, Gadepalli R, Rimoldi JM, Speth RC: Brain AT1 angiotensin receptor subtype binding: Importance of peptidase inhibition for identification of angiotensin II as its endogenous ligand. J Pharmacol Exp Ther 331: 170–177, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velez JC, Janech MG, Hicks MP, Morinelli TA, Rodgers J, Self SE, Arthur JM, Fitzgibbon WR: Lack of renoprotective effect of chronic intravenous angiotensin-(1-7) or angiotensin-(2-10) in a rat model of focal segmental glomerulosclerosis. PLoS One 9: e110083, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.