Abstract
Systemic inflammation is a hallmark of commonly encountered diseases ranging from bacterial sepsis to sterile syndromes such as major trauma. Derangements in the host vasculature contribute to the cardinal manifestations of sepsis in profound ways. Recent studies of control pathways regulating the vascular endothelium have illuminated how this single cell layer toggles between quiescence and activation to affect the development of shock and multiorgan dysfunction. This article focuses on one such control pathway, the Tie2 receptor and its ligands the angiopoietins, to describe a growing body of genetic, biochemical, mechanistic, and human studies that implicate Tie2 as a critical switch. In health, activated Tie2 maintains the endothelium in a quiescent state characterized by dynamic barrier function and antiadhesion against circulating leukocytes. In sepsis and related diseases, expression of the angiopoietins becomes markedly imbalanced and Tie2 signaling is greatly attenuated. These rapid molecular changes potentiate pathophysiologic responses throughout the body, resulting in injurious vascular leakage and organ inflammation. The Tie2 axis, therefore, may be a promising avenue for future translational studies.
Keywords: acute renal failure, cardiovascular, chronic kidney disease, vascular, endothelial cells, kidney stones
The Vasculature and Systemic Inflammation
Systemic derangements in inflammation populate the spectrum of medicine, ranging from rare autoinflammatory diseases to bacterial sepsis and other common sterile syndromes such as major trauma. Death from severe infection is not only a widespread and growing cause of adult mortality in the United States, but fundamentally a global public health threat.1–3 An argument can be made that, of all acute illnesses, the worldwide public health burden of sepsis is the most pressing—patients spend longer in intensive care units and hospitals and more often suffer long-term health impairments compared with any other admission diagnosis.1 In 2015, the Lancet Infectious Diseases Commission published an in-depth report describing how “sepsis has stubbornly resisted all efforts to successfully develop … new and improved treatments.”4 In their 10-year research prescription, the topmost preclinical priority identified was understanding “endothelial cell function.”
The vasculature contributes to the cardinal manifestations of sepsis in profound ways. Leaky microvessels promote tissue edema: in the lung, this directly impairs gas exchange5; in the kidneys and other organs, edema increases the diffusion distance for nutrient delivery and cellular waste removal. Systemically, loss of intravascular fluid leads to shock, which is why isotonic fluid resuscitation remains the cornerstone of adjuvant therapy.6 The microvascular endothelium becomes proadhesive, posting an array of molecules that facilitate the retardation of bloodborne leukocytes and their diadepedesis into tissue parenchyma, driving cellular inflammation and “bystander” organ injury. Finally, the endothelium regulates coagulation through cell-surface molecules that bind coagulation factors, anticoagulation factors, and fibrinolytic enzymes. Inflammatory stimuli applied to the endothelium may therefore contribute to disseminated intravascular coagulation and other states of altered hemostasis associated with infections.7 Abnormal clotting exacerbates tissue ischemia and may also further potentiate innate inflammatory responses.8
When these vascular responses occur in a spatially and temporally restricted fashion, clinicians observe the classic tetrad of dolor, tumor, rubor, and calor (pain, swelling, redness, and warmth). The localized vascular response to tissue injury or infection is likely adaptive, providing a means to contain an infection from spreading while delivering humoral and cellular effectors of immunity to neutralize invasive pathogens. However, when this same response occurs diffusely and is unchecked, the host can suffer dire consequences.
Introduction to the Tie2 Signaling Axis
Several endothelial control pathways have been implicated in sepsis; examples include vascular endothelial growth factor (VEGF), sphingosine-1-phosphate (S1P), and Slit-Robo.9–12 This update will focus on Tie2, reflecting the preponderance and concordance of experimental and human data from multiple laboratories around the world suggesting its involvement in the host vascular response to diverse states of systemic inflammation.
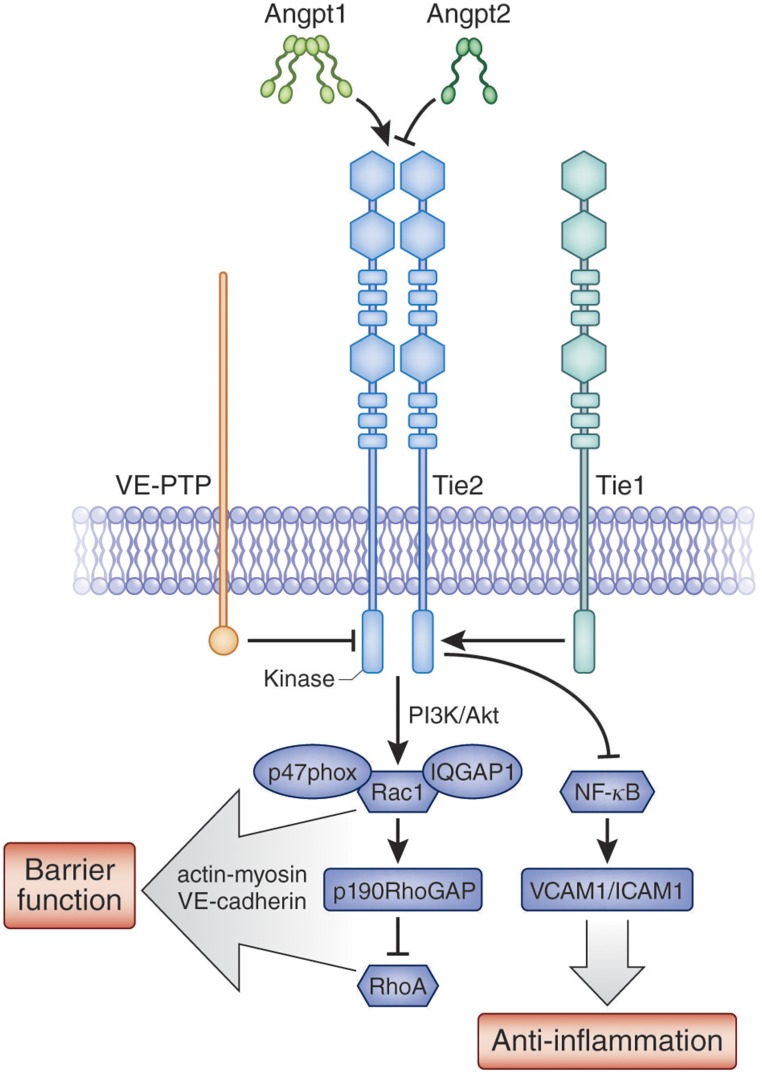
A cell-surface receptor tyrosine kinase (RTK) that is highly enriched in the endothelium, Tie2 is unusual for two major reasons. First, it is tonically phosphorylated in the quiescent adult vasculature, whereas many other vascular RTKs are activated at sites of angiogenesis. This suggests one or more maintenance functions for activated Tie2. Second, Tie2’s phosphorylation is fine-tuned by a unique combination of ligands and other cell-surface proteins: Angiopoietin-1 (Angpt-1), Angiopoietin-2 (Angpt-2), Tie1, and vascular endothelial protein tyrosine phosphatase (VE-PTP) (Figure 1).
Figure 1.
Vascular quiescence and the Angiopoietin-Tie2 axis. Expression of the receptor tyrosine kinase Tie2 is heavily enriched in the endothelium. Tie2 is activated by Angpt-1 which is secreted by platelets and peri-endothelial cells. In the context of inflammation, Angpt-2 antagonizes Angpt-1. Tie1 is an orphan receptor homolog of Tie2 whose intact expression enhances Tie2 activation. A transmembrane tyrosine phosphatase VE-PTP deactivates Tie2. Downstream of activated Tie2, a PI3K signaling cascade culminates in defense of barrier function by signaling members of the Rho family of GTPases as depicted. Inhibition of the inflammatory transcription factor NF-κB suppresses the expression of key adhesion molecules. Other potential receptors of angiopoietins, such as integrins, are not depicted for simplicity.
The cloning of Tie2 from the innermost tunica intima of blood vessels was described in 1992.13 Shortly thereafter, Tie2 was determined to be essential for cardiovascular development as knockout mice died in utero with multifocal hemorrhages, diffuse edema, and an underdeveloped vascular system.14 The agonist ligand, Angpt-1, was identified through an adaptation of expression cloning.15 Angpt-1 is predominantly made and secreted by platelets and peri-endothelial cells (e.g., vascular smooth muscle cells). Angpt-1 avidly binds the extracellular matrix, and an N-terminal superclustering domain is considered important for its ability to cluster Tie2 monomers at the endothelial cell surface.16 Angpt-2 is highly homologous to Angpt-1, but is preferentially made by endothelial cells and stored with von Willebrand factor (vWF) in Weibel–Palade bodies before release into the circulation.17,18 Angpt-2 competes with Angpt-1 for the same binding site on Tie2,17 but in the context of inflammation, antagonizes Tie2. Several reasons for this differing function have been proposed as discussed in the section below, Mechanisms from Preclinical Models. Tie1 is an orphan receptor that is highly homologous to Tie2.19 Tie1 modulates Tie2 signaling, with the most recent results suggesting that intact Tie1 promotes Tie2 signaling during inflammation.20,21 Finally, VE-PTP forms hetero-oligomers with Tie2 to hydrolyze critical phosphotyrosines, thus attenuating Tie2 signaling.22
Although beyond the scope of this review, it is also important to note that a large literature links the Tie2 axis to many other aspects of vascular biology. For example, this pathway is involved in developmental, adaptive, pathologic, and cancer-related angiogenesis23; in lymphangiogenesis and the function of mature lymphatic vessels24,25; and in vessel-derived signaling that regulates the local parenchyma.26
Evidence Implicating the Tie2 Axis from Human Studies
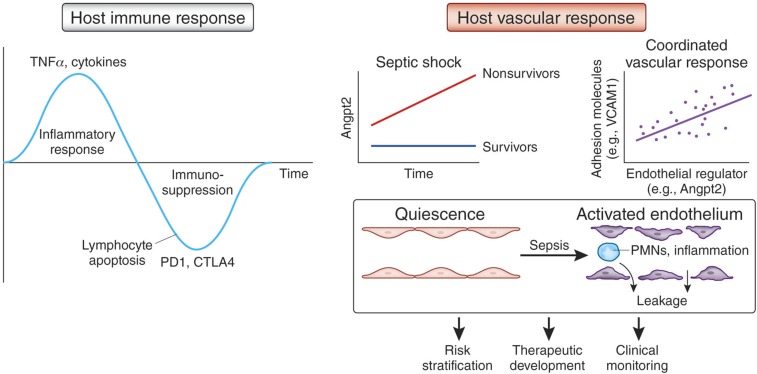
In contrast to the complex temporal kinetics of the immune response during systemic inflammation,27 the host vascular response exhibited by critically ill patients appears to be coordinately regulated,28 more monotonic,29 and more proximate to the clinical complications that result in shock and death (Figure 2). These biologic features may enable measures of the host vascular response to become superior tools for risk stratification and clinical decision-making. Indeed, markers describing the state of endothelial activation may help define distinct molecular phenotypes—so-called “endotypes”—within the broad spectrum of critical illness.30
Figure 2.
Opportunities to investigate the host vascular response. (Left) The host immune response to sepsis is characterized by an initial inflammatory burst driven by TNFα and other cytokines, followed later by profound immune suppression resulting from massive lymphocyte apoptosis via induction of negative costimulatory pathways such as PD-1 and CTLA-4. (Right) In contrast, the host vascular response has a more monotonic and coordinated temporal profile. Strong associations have been described between circulating levels of control molecules such as Angpt-2 and effector molecules such as adhesion proteins. This coordinated humoral profile appears to reflect a shift of endothelium from quiescence to an activated state. Physiologically, this state is manifest by excess permeability and adhesiveness to leukocytes, which in turn lead to organ edema and inflammation. Measurement of the host vascular response may facilitate future risk stratification, therapeutic development, and clinical monitoring of critically ill patients. Adapted in part from reference 27.
Human studies of the Tie2 axis have largely focused on circulating levels of the secreted ligands, Angpt-1 and Angpt-2, and to a lesser extent on soluble levels of Tie2 and Tie1. The first study in human sepsis showed that high circulating Angpt-2 was associated with impaired lung gas exchange and proposed that Angpt-2 may be involved in lung vascular leak.31 Indeed, in severe sepsis, levels of circulating Angpt-2 can rise 10–200-fold above baseline within hours of presentation, higher than in any other described human disease. The general relationship of high circulating Angpt-2 to states of vascular leakage and injury has been confirmed by many groups.32–35
Circulating Angpt-2 levels are proportional to the severity of the sepsis syndrome, antecedent to vascular-related complications such as acute respiratory distress syndrome (ARDS), and linked to lung extravascular water content.36,37 Circulating Angpt-2 rises in human volunteers infused with low-dose bacterial endotoxin.38 As discussed next, the inflammatory induction of Angpt-2 likely reflects a two-fold response—initial release of preformed protein from endothelial Weibel–Palade bodies in response to ligation of a number of innate immunity receptors, followed by de novo biosynthesis from the ANGPT2 gene locus.18,39
Human studies have also revealed that critically ill patients develop low circulating Angpt-1.40 The mechanisms underlying Angpt-1 suppression are not as well described. A ratio of circulating Angpt-2/Angpt-1 has been proposed as an indicator of Tie2 signaling impairment that may possess better sensitivity and specificity for predicting adverse outcomes than either protein in isolation.35,40 The settings in which Angpt imbalance may affect outcomes extend beyond sepsis to include viral infections such as influenza, hantavirus, and dengue21,41–45; protozoal infection such as falciparum malaria46–48; lethal anthrax (in a primate model);49 and sterile inflammation syndromes arising from severe trauma or major surgery.50–52 Soluble ectodomains of Tie2 and Tie1 also become elevated in the blood during sepsis and related conditions.21,53
If these molecular changes observed in the blood reflect the milieu at the endothelium, then the drop in Angpt-1, rise in Angpt-2, and loss of receptor ectodomains may collaborate to attenuate the homeostatic signal transduced by Tie2 during vascular quiescence. Several such “hits” to normal Tie2 signaling may, in turn, shift the vascular phenotype toward inflammation and leakage. That so many infectious and noninfectious syndromes managed in the intensive care unit converge on deranged Tie2 signaling proposes this molecular axis as an important effector of the human host vascular response.
Finally, common variants in this family of genes have been implicated in the risk for ARDS. The first study linked polymorphisms in ANGPT2 to the development of ARDS arising specifically from extrapulmonary sources of lung injury—e.g., abdominal sepsis or pancreatitis rather than pneumonia.54 So-called “indirect” ARDS is differentiated from “direct” causes that primarily target the airspace, such as pneumonia or inhalational injury. It is highly plausible that primary abdominal processes secondarily involve the lung through intravascular mechanisms such as humoral elevation of Angpt-2. A larger study of critically ill patients discovered a common variant affecting splicing of the Angpt-2 mRNA that was associated with trauma-associated acute lung injury (ALI) and altered circulating Angpt-2 protein isoforms.52 A more recent study has linked common variants in TIE2 to the development of ARDS by describing single nucleotide polymorphisms that may both reduce TIE2 gene expression and predict ARDS development.44 These early results require further investigation, but raise the possibility that otherwise modest inherited differences in vascular-encoding genes may exert a clinically meaningful effect under the stress of severe inflammation.
Mechanisms from Preclinical Models
Angpt-1
Applying genetic, and then transient, overexpression, excess Angpt-1 was shown in 1999 and 2000 to prevent vascular leakage as triggered by VEGF and chemical irritants.55,56 Witzenbichler and colleagues then reported that excess Angpt-1 prevented leakage and cellular inflammation in endotoxemic mice.57 Angpt-1 is now known to counteract vascular hyperpermeability induced by TNFα and other cytokines, by thrombin, and by anthrax lethal toxin among numerous other triggers (reviewed by Parikh58). To achieve this remarkable defense against unrelated ligands acting on the endothelium, we and others have proposed that Angpt-1 achieves vascular barrier defense by signaling to conserved downstream regulators of endothelial cell shape and intercellular connectivity.59–64
Angpt-1 application reorganizes the actin-myosin cytoskeleton of the endothelial cell such that its boundaries spread centrifugally and tensile strength develops at the cell’s edges. The Rho family GTPases Rac1 and RhoA coordinate this cytoskeletal reorganization. Rac1 is activated by Angpt-1, and, through p190RhoGAP, Rac1 inhibits RhoA.61 While activated Tie2 itself migrates to the cell junction—where transinteraction through Angpt-1 may further strengthen intercellular connections65—the adherens junction protein VE-cadherin appears to be even more critical for barrier defense against inflammatory stressors. Through Rac1, Angpt-1 stabilizes VE-cadherin at the adherens junction. Angpt-1 may also exert additional actions to increase junctional accumulation of VE-cadherin, although these remain to be fully defined. Application of Angpt-1 also reduces vascular inflammation, thereby attenuating the parenchymal infiltration of immune cells. The antileakage and antiadhesive properties of Angpt-1 could be highly desirable in a critical care setting. Notably, Angpt-1 has been proposed as major secreted molecule by which infused mesenchymal stem cells may be protective in critically ill patients.66
Angpt-2
Whereas Angpt-1 is a pure agonist of Tie2, Angpt-2 is better described as a partial agonist or antagonist of Tie2 depending on context.67,68 In the original cloning paper, Angpt-2 competitively inhibited Angpt-1–induced Tie2 activation; this antagonistic effect required the context of the endothelium.17 In models of sepsis, Angpt-2 has been depleted by antibody, deleted genetically, or knocked down by RNA interference.21,29,69–72 Each of these loss-of-function approaches shows the same result—less vascular leakage, better organ function, and improved survival in models of sepsis. Because Angpt-2 inhibition has mirrored the results of Angpt-1 excess—down to the level of measured Tie2 activation69—these results support the notion of Angpt-2 as an antagonist of Tie2 during systemic inflammation.
Several reasons have been proposed for a dual agonist/antagonist role of Angpt-2. As noted above, Angpt-2 is highly homologous to Angpt-1 and both bind the same region of Tie2. One model on the basis of cocrystal structures implicates specific amino acid differences in the receptor-binding portions of Angpt-1 versus Angpt-2.73–75 Another model proposes that a superclustering domain unique to Angpt-1’s N-terminus specifically enables this ligand to cluster Tie2 monomers whereas Angpt-2 ligation maintains Tie2 in a more scattered cell surface arrangement that is less efficient for phosphorylation among the C-terminal intracellular tails of the receptor.16,76 A recently described experimental antibody that clusters Angpt-2 and converts its apparent action from Tie2 antagonist to agonist in septic mice supports the latter model and emphasizes the functional importance of the quarternary structure of Tie2 ligands.71
When endothelial cells are exposed to inflammatory cytokines such as TNFα, they respond by weakening their intercellular connections, posting inflammatory adhesion molecules on the cell surface that enable leukocytes to traffic out of blood vessels and into the tissue, and releasing preformed Angpt-2 protein from Weibel–Palade bodies. Angpt-2 potentiates the cytokine-triggered effects on the vasculature as demonstrated by genetic deletion in models of sterile inflammation and bacterial sepsis.29,77,78 Moreover, released Angpt-2 protein may stimulate its own transcription via Foxo1 as described next. Therefore, Angpt-2 may not only potentiate the leak and adhesion responses of blood vessels to cytokines, but may also propagate its own production, setting off a pernicious positive feedback loop.
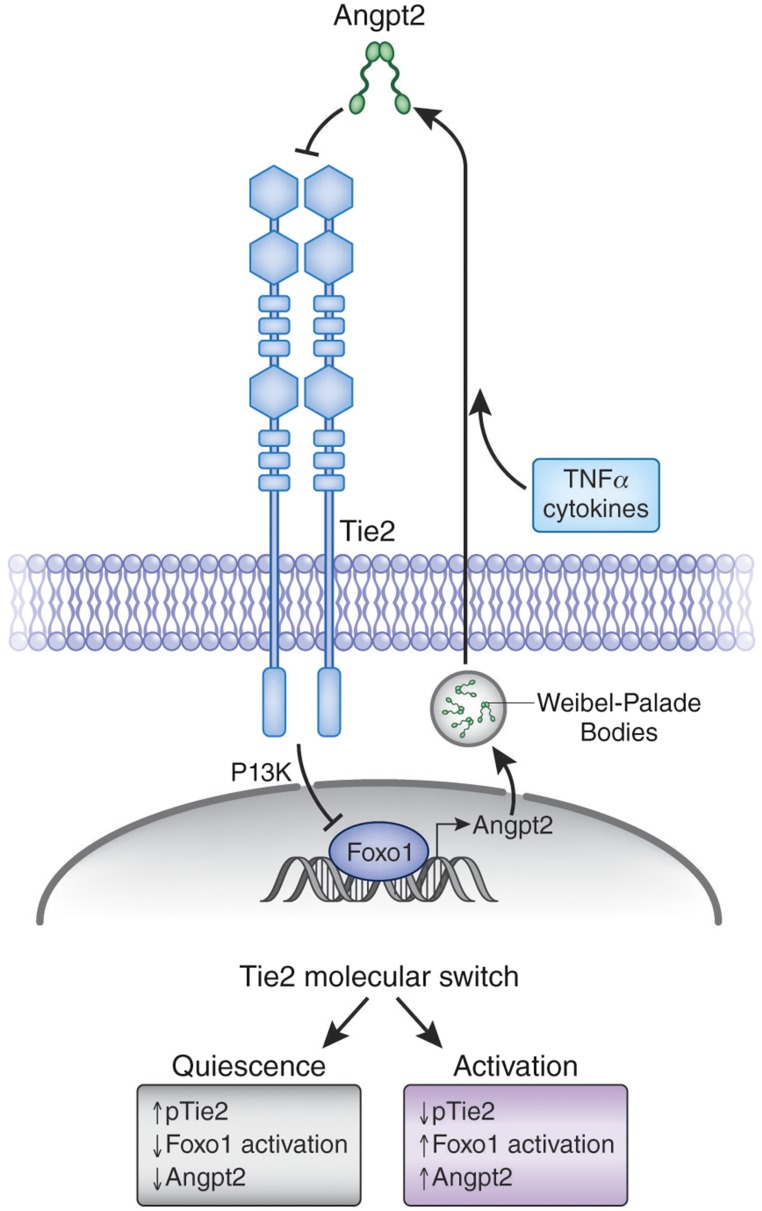
Foxo1
In quiescent endothelium, activated Tie2 transduces a signal that inhibits Foxo1 from transcribing the ANGPT2 gene.79 So, only a low level of Angpt-2 is made and stored in Weibel–Palade bodies. However, exposure to inflammatory stimuli triggers the rapid release of preformed Angpt-2 protein, which is thought to antagonize Tie2 in an autocrine or perhaps even intracrine fashion.39,78,80 With the brake on Foxo1 released, inflamed endothelial cells now synthesize Angpt-2 protein de novo, which in turn further promotes Tie2 inhibition and more ANGPT2 gene transcription (Figure 3).
Figure 3.
Pernicious positive feedback loop for Angpt-2. In quiescent endothelium, Tie2 is highly activated (increased pTie2), which in turn suppresses the transcription factor Foxo1 from transcribing the ANGPT2 gene. Inflammatory cytokines trigger the release of preformed Angpt-2 protein, an event that may initiate a positive feedback loop that results in the induction of de novo Angpt-2 biosynthesis. Loss of signaling through Tie2 may constitute a “switch” that shifts the endothelium from quiescence to activation.
The most direct evidence for a Tie2–Foxo1–Angpt-2 positive feedback loop arising in sepsis comes from in vivo RNA interference experiments that do not affect the preformed Angpt-2 protein, but only inhibit de novo production. Angpt-2 RNA interference has been shown to enhance Tie2 phosphorylation in mice and rescue septic animals from vascular leakage and death.69 Two subsequent studies have also implicated Foxo1 activation during endothelial inflammation.60,72 This amplifier role of Angpt-2 downstream of acute phase reactants aligns well with the clinical results that implicate Angpt-2—and, indeed, more global endothelial activation—in the pathogenesis of adverse outcomes.
Tie2
Tie2 itself is dynamically regulated during sepsis and other severe infectious syndromes. Ghosh and colleagues recently reported that Tie2 expression is reduced not only in sepsis, but also in experimental influenza, malaria, and anthrax, conditions that have all been linked to severe vascular complications.44 Although the mechanisms leading to infection-related attenuation of Tie2 itself require more study, these experiments did demonstrate that acutely reduced Tie2 expression suffices to weaken the endothelial barrier in vivo. Moreover, they described evidence that preexisting, genetically determined reduction in Tie2 expression renders endothelial cells and animals hyper-sensitive to triggers of leakage and hypo-responsive to the protective effect of Angpt-1. These results corroborate the human findings linking common low-expressor TIE2 alleles to the development of ARDS.
Tie1
Understanding of Tie1’s role in inflammation has evolved in recent years. Earlier studies proposed Tie1 as an antagonist of Tie2 signaling on the basis of functional and structural results.81,82 More recent studies have suggested that full-length Tie1 may be necessary to extend the duration of Tie2 phosphorylation.20 The rapid cleavage of Tie1 upon exposure to inflammatory stimuli reduces the ability of Angpt-1 or Angpt-2 to activate Tie2.21 The biophysical mechanism by which Tie1 promotes Tie2 phosphorylation remains to be elucidated, but these new results once again emphasize the exquisite fine-tuning of Tie2 signaling.
VE-PTP
In the last several years, VE-PTP has also emerged as an important regulator of Tie2 signaling. VE-PTP inhibition is being developed clinically as a method for therapeutic Tie2 activation.83 The effect of VE-PTP inhibition on vascular barrier function is complex to consider because this tyrosine phosphatase is known to interact with at least three important regulators of the barrier—Tie2, VEGFR2, and VE-cadherin.22,84,85 Inhibition of VE-PTP can stabilize phosphate groups on all three. As described above, activated Tie2 protects barrier function. Activated VEGFR2, in contrast, promotes permeability. And phosphorylation of VE-cadherin at key residues promotes its endocytic removal from the adherens junction, thus also weakening barrier function.86 Consistent with the physiology being determined by the actual binding partners of VE-PTP, a recent study from a leading group investigating VE-PTP showed that the effects of VE-PTP inhibition on barrier function depend to a large extent on the level of Tie2 expression.62
Renal Health and Diseases
Only a handful of experimental studies thus far have examined the effect of the Tie2 axis on renal biology. During development, deletion of Angpt-2 drives dysmorphogenesis of cortical peritubular capillaries.87 In endotoxemic or septic AKI, either excess Angpt-1 or less Angpt-2 is protective.29,88 Whether these encouraging results are due to a direct effect on the renal microvasculature or an indirect effect of modulating vascular health elsewhere—e.g., less lung injury leading to less severe AKI—remains to be determined. A peptide-based Tie2 activator compound was reported to ameliorate postischemic AKI.89 In contrast, Angpt-1 therapy was found to be detrimental after folate-induced kidney injury.90
In solid organ transplantation, Angpt-1 has been shown to protect against allograft arteriosclerosis, calcineurin inhibitor toxicity, and late rejection.88,91–93 Analogously, Angpt-2 inhibition appears to prevent ischemia-reperfusion injury and subsequent chronic rejection.94 In models of diabetic kidney disease, Angpt-1 excess may be protective whereas Angpt-1 deletion is injurious.95–97 Podocyte overexpression of Angpt-2 is sufficient to provoke mild proteinuria.98 Finally, excess circulating Angpt-2 has been linked to adverse outcomes in CKD99 and, experimentally, to increased arterial stiffness.100
Hurdles to Translation
Results from human genetics, biochemistry, and mechanistic studies in preclinical models all implicate the Tie2 axis in the pathogenesis of adverse host vascular responses to severe infections and sterile acute systemic inflammatory syndromes. Developing one or more successful adjuvant therapies targeting the Tie2 axis in affected humans remains the long-term challenge. As described above, there are several reasons for optimism. Nonetheless, acute systemic inflammation is characterized by profound derangements in dozens of pathways across thousands of molecules, so a cautious approach is warranted as translational options are considered.
A “targeted therapy” should not only specifically target a host molecule, but also target a defined population. In trials of adjuvant therapies for sepsis, enrolled subjects have seldom been defined by molecular features. Yet, cytogenetics and gene expression patterns are routinely used in other fields such as oncology to match therapy and patient. Part of this is undoubtedly operational. Sepsis progresses at such a rapid pace in affected individuals that an investigational trial should strive to mirror the rapid triaging often necessary to identify and treat the most severe patients. On the other hand, a neutralizing antibody against Angpt-2 makes little sense to administer in septic individuals who exhibit minimal elevation in circulating Angpt-2. As a result, point-of-care diagnostics should be developed. Such tools would not only enhance initial stratification, but also help track over time the Angpt-Tie2 status of a given individual. Rapid molecular measurements could help with the initial enrollment of a desirable target population and also facilitate readouts from early phase clinical trials that could refine the path to clinical development of a companion therapy.
What kind of therapy would be ideal? If signaling through Tie2 is suppressed at a minimum of two different levels, expression and phosphorylation, then the salutary downstream signaling is at least doubly attenuated. This suggests that a therapy achieving high activation through the remaining Tie2 receptors could be more desirable than an approach that alleviates one of the blocks against Tie2 signaling. A recent study made this consideration more concrete. Han et al. directly compared a Tie2-activating strategy against an Angpt-2 neutralizing approach in experimental sepsis.71 They found that either approach was beneficial, but the former even more so. The enthusiasm for superactivation of Tie2 is certainly tempered by safety considerations; for example, Tie2 is known to activate endothelial nitric oxide synthase, whose downstream vasodilatory action could be counterproductive for treating shock. Inhibiting a deleterious molecule such as Angpt-2 may have fewer adverse effects than over-activating Tie2. The only human experience with hyper-active Tie2 comes from people with gain-of-function mutations that enhance kinase activity. These individuals exhibit venous malformations during early development.101,102 A short-term exposure to high levels of phosphorylated Tie2 is unlikely to trigger vascular remodeling, but this, too, remains an open question.
Concluding Remarks
Despite progress in the field, important questions need to be addressed. For example, the community is still striving to develop a comprehensive and agreed-upon model that accounts for Angpt-2’s dual roles at the Tie2 receptor. Second, the reduction of Tie2 expression during infections appears to be an independent contributor beyond the imbalance in the ligands themselves, yet little is known regarding transcriptional and post-translational regulation of receptor levels.103 Third, the endothelium responds in a highly coordinated fashion to inflammatory stress. How does the endothelium integrate signals not only from Tie2, but other control pathways, to switch its phenotype from the quiescent state to one that is leaky, prothrombotic, and proadhesive? Finally, even as acute care improves, survivors of critical illness face a long road to recovery. Complete versus incomplete organ repair in the kidney and beyond may well be related to the endothelium. Angpt-2 has already been described as an “angiocrine” factor secreted from blood vessels to direct the regeneration of the injured liver.26 Addressing how the Tie2 axis modulates organ repair responses may therefore be of fundamental and translational interest. Indeed, recent progress in the Angpt-Tie2 field has been marked by parallel growth of fundamental knowledge in lockstep with budding translational enthusiasm.
Disclosures
S.M.P. is listed as an inventor on patent applications filed by Beth Israel Deaconess Medical Center regarding angiopoietins in critical diseases. S.M.P. is a consultant to Eunoia Biotech.
Acknowledgments
Research in the laboratory is supported by National Institutes of Health grants R01-DK095072, R01-HL093234, R01-HL125275, and R41-GM121153.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Daniels R: What next for sepsis? Lancet Infect Dis 15: 499–501, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Kissoon N, Carcillo JA, Espinosa V, Argent A, Devictor D, Madden M, Singhi S, van der Voort E, Latour J: World federation of pediatric intensive care and critical care Societies: Global sepsis initiative. Pediatr Crit Care Med 12: 494–503, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Seymour CW, Rea TD, Kahn JM, Walkey AJ, Yealy DM, Angus DC: Severe sepsis in pre-hospital emergency care: Analysis of incidence, care, and outcome. Am J Respir Crit Care Med 186: 1264–1271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, Tracey K, van der Poll T, Pelfrene E: Sepsis: A roadmap for future research. Lancet Infect Dis 15: 581–614, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC; ProCESS Investigators : A randomized trial of protocol-based care for early septic shock. N Engl J Med 370: 1683–1693, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aird WC: Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Rittirsch D, Flierl MA, Ward PA: Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8: 776–787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W: Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452: 654–658, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WC: Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med 203: 1447–1458, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY: Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med 2: 23ra19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Stankovic M, Bonder CS, Hahn CN, Parsons M, Pitson SM, Xia P, Proia RL, Vadas MA, Gamble JR: Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood 111: 3489–3497, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML: tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 7: 1471–1480, 1992 [PubMed] [Google Scholar]
- 14.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML: Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8: 1897–1909, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, Xu A, Leidich R, Radziejewska E, Rafique A, Goldberg J, Jain V, Bailey K, Karow M, Fandl J, Samuelsson SJ, Ioffe E, Rudge JS, Daly TJ, Radziejewski C, Yancopoulos GD: Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol 10: 38–44, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG: The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Korhonen J, Partanen J, Armstrong E, Vaahtokari A, Elenius K, Jalkanen M, Alitalo K: Enhanced expression of the tie receptor tyrosine kinase in endothelial cells during neovascularization. Blood 80: 2548–2555, 1992 [PubMed] [Google Scholar]
- 20.Savant S, La Porta S, Budnik A, Busch K, Hu J, Tisch N, Korn C, Valls AF, Benest AV, Terhardt D, Qu X, Adams RH, Baldwin HS, Ruiz de Almodóvar C, Rodewald HR, Augustin HG: The orphan receptor Tie1 controls angiogenesis and vascular remodeling by differentially regulating Tie2 in Tip and stalk cells. Cell Reports 12: 1761–1773, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korhonen EA, Lampinen A, Giri H, Anisimov A, Kim M, Allen B, Fang S, D’Amico G, Sipilä TJ, Lohela M, Strandin T, Vaheri A, Ylä-Herttuala S, Koh GY, McDonald DM, Alitalo K, Saharinen P: Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest 126: 3495–3510, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fachinger G, Deutsch U, Risau W: Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene 18: 5948–5953, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Augustin HG, Koh GY, Thurston G, Alitalo K: Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165–177, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD: Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 3: 411–423, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Zheng W, Nurmi H, Appak S, Sabine A, Bovay E, Korhonen EA, Orsenigo F, Lohela M, D’Amico G, Holopainen T, Leow CC, Dejana E, Petrova TV, Augustin HG, Alitalo K: Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev 28: 1592–1603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, Terhardt D, Vogel MJ, Cao L, Korn C, Bartels S, Thomas M, Augustin HG: Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science 343: 416–419, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Opal S: Immunotherapy for sepsis--A new approach against an ancient foe. N Engl J Med 363: 87–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro NI, Schuetz P, Yano K, Sorasaki M, Parikh SM, Jones AE, Trzeciak S, Ngo L, Aird WC: The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care 14: R182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David S, Mukherjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, Karumanchi SA, Shapiro NI, Parikh SM: Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis*. Crit Care Med 40: 3034–3041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB; NIH NHLBI ARDS Network : Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 147: 1539–1548, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP: Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3: e46, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C: Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med 35: 199–206, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Bhandari V, Elias JA: The role of angiopoietin 2 in hyperoxia-induced acute lung injury. Cell Cycle 6: 1049–1052, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Kümpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT: Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 12: R147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong T, McClintock DE, Kallet RH, Ware LB, Matthay MA, Liu KD: Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med 38: 1845–1851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB: Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 63: 903–909, 2008 [DOI] [PubMed] [Google Scholar]
- 37.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, van Hinsbergh VW, Bouw MP, van der Hoeven JG, Groeneveld AB: Circulating angiopoietin-2 levels in the course of septic shock: Relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med 35: 1567–1574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kümpers P, van Meurs M, David S, Molema G, Bijzet J, Lukasz A, Biertz F, Haller H, Zijlstra JG: Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Crit Care 13: R64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh CC, Thamm K, Berghelli AV, Schrimpf C, Maski MR, Abid T, Milam KE, Rajakumar A, Santel A, Kielstein JT, Ahmed A, Thickett D, Wang K, Chase M, Donnino MW, Aird WC, Haller H, David S, Parikh SM: Drug repurposing screen identifies foxo1-dependent angiopoietin-2 regulation in sepsis. Crit Care Med 43: e230–e240, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano JS Jr, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS: Admission angiopoietin levels in children with septic shock. Shock 28: 650–654, 2007 [PMC free article] [PubMed] [Google Scholar]
- 41.Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER: Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol 82: 5797–5806, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yacoub S, Lam PK, Vu le HM, Le TL, Ha NT, Toan TT, Van NT, Quyen NT, Le Duyen HT, Van Kinh N, Fox A, Mongkolspaya J, Wolbers M, Simmons CP, Screaton GR, Wertheim H, Wills B: Association of microvascular function and endothelial biomarkers with clinical outcome in dengue: An observational study. J Infect Dis 214: 697–706, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiyama MG, Armstrong SM, Wang C, Hwang D, Leong-Poi H, Advani A, Advani S, Zhang H, Szaszi K, Tabuchi A, Kuebler WM, Van Slyke P, Dumont DJ, Lee WL: The Tie2-agonist Vasculotide rescues mice from influenza virus infection. Sci Rep 5: 11030, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh CC, David S, Zhang R, Berghelli A, Milam K, Higgins SJ, Hunter J, Mukherjee A, Wei Y, Tran M, Suber F, Kobzik L, Kain KC, Lu S, Santel A, Yano K, Guha P, Dumont DJ, Christiani DC, Parikh SM: Gene control of tyrosine kinase TIE2 and vascular manifestations of infections. Proc Natl Acad Sci USA 113: 2472–2477, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michels M, van der Ven AJ, Djamiatun K, Fijnheer R, de Groot PG, Griffioen AW, Sebastian S, Faradz SM, de Mast Q: Imbalance of angiopoietin-1 and angiopoetin-2 in severe dengue and relationship with thrombocytopenia, endothelial activation, and vascular stability. Am J Trop Med Hyg 87: 943–946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conroy AL, Lafferty EI, Lovegrove FE, Krudsood S, Tangpukdee N, Liles WC, Kain KC: Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J 8: 295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC, Kain KC: Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4: e4912, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, Price RN, Duffull SB, Celermajer DS, Anstey NM: Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 105: 17097–17102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghosh CC1, Mukherjee A, David S, Knaus UG, Stearns-Kurosawa DJ, Kurosawa S, Parikh SM: Impaired function of the Tie-2 receptor contributes to vascular leakage and lethality in anthrax. Proc Natl Acad Sci U S A 109: 10024–10029, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR, Matthay MA, May AK, Ware LB: Acute lung injury in patients with traumatic injuries: Utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma 68: 1121–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, Christiaans SC, Bir ND, Pittet JF: Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg 247: 320–326, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, Fuchs BD, Lanken PN, Albelda SM, Rushefski M, Aplenc R, Abramova H, Atochina-Vasserman EN, Beers MF, Calfee CS, Cohen MJ, Pittet JF, Christiani DC, O’Keefe GE, Ware LB, May AK, Wurfel MM, Hakonarson H, Christie JD: ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med 183: 1344–1353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Heijden M, van Nieuw Amerongen GP, van Hinsbergh VW, Groeneveld AB: The interaction of soluble Tie2 with angiopoietins and pulmonary vascular permeability in septic and nonseptic critically ill patients. Shock 33: 263–268, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Su L, Zhai R, Sheu CC, Gallagher DC, Gong MN, Tejera P, Thompson BT, Christiani DC: Genetic variants in the angiopoietin-2 gene are associated with increased risk of ARDS. Intensive Care Med 35: 1024–1030, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD: Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6: 460–463, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM: Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C: Protective role of angiopoietin-1 in endotoxic shock. Circulation 111: 97–105, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Parikh SM: Dysregulation of the angiopoietin-Tie-2 axis in sepsis and ARDS. Virulence 4: 517–524, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David S, Ghosh CC, Mukherjee A, Parikh SM: Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol 31: 2643–2652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh CC, Mukherjee A, David S, Milam KE, Hunter JT, Parikh SM: Angiopoietin-1 requires oxidant signaling through p47phox to promote endothelial barrier defense. PLoS One 10: e0119577, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP: Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 282: 23910–23918, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Frye M, Dierkes M, Küppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, Vestweber D: Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med 212: 2267–2287, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, Vestweber D: VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med 205: 2929–2945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gavard J, Patel V, Gutkind JS: Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14: 25–36, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K: Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol 10: 527–537, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Fang X, Neyrinck AP, Matthay MA, Lee JW: Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 285: 26211–26222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM: Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol 29: 2011–2022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, Ioffe E, Daly TJ, Fandl JP, Papadopoulos N, McDonald DM, Thurston G, Yancopoulos GD, Rudge JS: Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA 103: 15491–15496, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stiehl T, Thamm K, Kaufmann J, Schaeper U, Kirsch T, Haller H, Santel A, Ghosh CC, Parikh SM, David S: Lung-targeted RNA interference against angiopoietin-2 ameliorates multiple organ dysfunction and death in sepsis. Crit Care Med 42: e654–e662, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S, Hu J, Soehnlein O, Franz WM, Sperandio M, Pohl U, Thomas M, Weber C, Augustin HG, Fässler R, Deutsch U, Kupatt C: Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest 123: 3436–3445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han S, Lee SJ, Kim KE, Lee HS, Oh N, Park I, Ko E, Oh SJ, Lee YS, Kim D, Lee S, Lee DH, Lee KH, Chae SY, Lee JH, Kim SJ, Kim HC, Kim S, Kim SH, Kim C, Nakaoka Y, He Y, Augustin HG, Hu J, Song PH, Kim YI, Kim P, Kim I, Koh GY: Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med 8: 335ra55, 2016 [DOI] [PubMed] [Google Scholar]
- 72.Kim M, Allen B, Korhonen EA, Nitschké M, Yang HW, Baluk P, Saharinen P, Alitalo K, Daly C, Thurston G, McDonald DM: Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J Clin Invest 126: 3511–3525, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barton WA, Tzvetkova D, Nikolov DB: Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure 13: 825–832, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Barton WA, Tzvetkova-Robev D, Miranda EP, Kolev MV, Rajashankar KR, Himanen JP, Nikolov DB: Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat Struct Mol Biol 13: 524–532, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Yu X, Seegar TC, Dalton AC, Tzvetkova-Robev D, Goldgur Y, Rajashankar KR, Nikolov DB, Barton WA: Structural basis for angiopoietin-1-mediated signaling initiation. Proc Natl Acad Sci USA 110: 7205–7210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim KT, Choi HH, Steinmetz MO, Maco B, Kammerer RA, Ahn SY, Kim HZ, Lee GM, Koh GY: Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J Biol Chem 280: 20126–20131, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG: Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12: 235–239, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, Fiedler U, Augustin HG: Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One 8: e70459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS: Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev 18: 1060–1071, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG: The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci 118: 771–780, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Yuan HT, Venkatesha S, Chan B, Deutsch U, Mammoto T, Sukhatme VP, Woolf AS, Karumanchi SA: Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J 21: 3171–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Seegar TC, Eller B, Tzvetkova-Robev D, Kolev MV, Henderson SC, Nikolov DB, Barton WA: Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol Cell 37: 643–655, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen J, Frye M, Lee BL, Reinardy JL, McClung JM, Ding K, Kojima M, Xia H, Seidel C, Lima e Silva R, Dong A, Hackett SF, Wang J, Howard BW, Vestweber D, Kontos CD, Peters KG, Campochiaro PA: Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest 124: 4564–4576, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, Vestweber D: Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med 208: 2393–2401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, Hellberg C, Mellberg S, Koch S, Dimberg A, Koh GY, Dejana E, Belting HG, Affolter M, Thurston G, Holmgren L, Vestweber D, Claesson-Welsh L: VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat Commun 4: 1672, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, Weström S, Claesson-Welsh L, Vestweber D, Gerhardt H: The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol 16: 309–321, 2014 [DOI] [PubMed] [Google Scholar]
- 87.Pitera JE, Woolf AS, Gale NW, Yancopoulos GD, Yuan HT: Dysmorphogenesis of kidney cortical peritubular capillaries in angiopoietin-2-deficient mice. Am J Pathol 165: 1895–1906, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee S, Kim W, Kim DH, Moon SO, Jung YJ, Lee AS, Kang KP, Jang KY, Lee SY, Sung MJ, Koh GY, Park SK: Protective effect of COMP-angiopoietin-1 on cyclosporine-induced renal injury in mice. Nephrol Dial Transplant 23: 2784–2794, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Rübig E, Stypmann J, Van Slyke P, Dumont DJ, Spieker T, Buscher K, Reuter S, Goerge T, Pavenstädt H, Kümpers P: The synthetic Tie2 agonist peptide vasculotide protects renal vascular barrier function in experimental acute kidney injury. Sci Rep 6: 22111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, Yancopoulos GD, Rudge JS, Woolf AS: Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int 74: 300–309, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Nykänen AI, Krebs R, Saaristo A, Turunen P, Alitalo K, Ylä-Herttuala S, Koskinen PK, Lemström KB: Angiopoietin-1 protects against the development of cardiac allograft arteriosclerosis. Circulation 107: 1308–1314, 2003 [DOI] [PubMed] [Google Scholar]
- 92.Syrjälä SO, Nykänen AI, Tuuminen R, Raissadati A, Keränen MA, Arnaudova R, Krebs R, Koh GY, Alitalo K, Lemström KB: Donor heart treatment with COMP-Ang1 limits ischemia-reperfusion injury and rejection of cardiac allografts. Am J Transplant 15: 2075–2084, 2015 [DOI] [PubMed] [Google Scholar]
- 93.Thamm K, Njau F, Van Slyke P, Dumont DJ, Park JK, Haller H, David S: Pharmacological Tie2 activation in kidney transplantation. World J Transplant 6: 573–582, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Syrjälä SO, Tuuminen R, Nykänen AI, Raissadati A, Dashkevich A, Keränen MA, Arnaudova R, Krebs R, Leow CC, Saharinen P, Alitalo K, Lemström KB: Angiopoietin-2 inhibition prevents transplant ischemia-reperfusion injury and chronic rejection in rat cardiac allografts. Am J Transplant 14: 1096–1108, 2014 [DOI] [PubMed] [Google Scholar]
- 95.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE: Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dessapt-Baradez C, Woolf AS, White KE, Pan J, Huang JL, Hayward AA, Price KL, Kolatsi-Joannou M, Locatelli M, Diennet M, Webster Z, Smillie SJ, Nair V, Kretzler M, Cohen CD, Long DA, Gnudi L: Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. J Am Soc Nephrol 25: 33–42, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang KY, Lee SY, Park BH, Koh GY, Park SK: Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transplant 22: 396–408, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Davis B, Dei Cas A, Long DA, White KE, Hayward A, Ku CH, Woolf AS, Bilous R, Viberti G, Gnudi L: Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol 18: 2320–2329, 2007 [DOI] [PubMed] [Google Scholar]
- 99.David S, John SG, Jefferies HJ, Sigrist MK, Kümpers P, Kielstein JT, Haller H, McIntyre CW: Angiopoietin-2 levels predict mortality in CKD patients. Nephrol Dial Transplant 27: 1867–1872, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Chang FC, Chiang WC, Tsai MH, Chou YH, Pan SY, Chang YT, Yeh PY, Chen YT, Chiang CK, Chen YM, Chu TS, Wu KD, Lin SL: Angiopoietin-2-induced arterial stiffness in CKD. J Am Soc Nephrol 25: 1198–1209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, Eklund L, Boon LM, Vikkula M: Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet 41: 118–124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR: Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 87: 1181–1190, 1996 [DOI] [PubMed] [Google Scholar]
- 103.Wehrle C, Van Slyke P, Dumont DJ: Angiopoietin-1-induced ubiquitylation of Tie2 by c-Cbl is required for internalization and degradation. Biochem J 423: 375–380, 2009 [DOI] [PubMed] [Google Scholar]