Abstract
Cytomegalovirus (CMV) infection is a significant source of morbidity and mortality in allogeneic stem cell transplantation (SCT). We identified a cohort of 91 pediatric SCT patients at risk (defined as either donor and/or recipient seropositivity) for CMV infection at our institution. We retrospectively categorized at-risk SCT recipients as those who (1) were at risk of CMV infection in the post-SCT period, (2) had documented CMV infection before SCT, (3) experienced recurrence of post-SCT CMV viremia, or (4) experienced late post-SCT CMV viremia; categories were not mutually exclusive. We analyzed the impact of SCT-related factors on incidence of CMV infection and outcome, and we described the outcome of each of these cohorts. In univariate analysis, recipient CMV seropositivity, use of umbilical cord blood graft, and acute graft-versus-host disease (GVHD) predicted post-SCT CMV viremia, and the effects of acute GVHD (odds ratio, 4.018; 95% confidence interval, 1.032 to 15.643) and CMV seropositivity (odds ratio, 16.525; 95% confidence interval, 2.041 to 133.803) were confirmed in multivariate analysis. Patients with recurrence of post-SCT CMV viremia had a 50% all-cause mortality rate, compared with 12% in all 91 patients. Patients with pre-SCT CMV infection had a high incidence of post-SCT CMV infection but could successfully undergo SCT with antiviral prophylaxis and pre-emptive CMV treatment. All patients with late CMV infection had prior GVHD. Theses findings identify risk factors for post-SCT CMV infection and provide novel descriptions of childhood SCT recipients with pre-SCT, recurrent, and late CMV infection, which may contribute to risk stratification strategies for CMV at-risk patients in pediatric allogeneic SCT.
Keywords: Cytomegalovirus, Umbilical cord transplant, Graft-versus host disease
INTRODUCTION
Human cytomegalovirus (CMV) is a beta-herpesvirus that infects leukocytes, epithelial cells, and neural cells, with high frequency of CMV seropositivity in the general population, illustrating its commonality. It can remain latent for decades before reactivating, typically in situations of immunosuppression, and it poses an ongoing risk for patients with inherently abnormal immune function. Even in the current era of improved screening and antiviral therapy, CMV remains a significant source of morbidity and mortality in allogeneic stem cell transplantation (SCT). Before SCT, donors and recipients are routinely screened for CMV serostatus. Those recipients with serologic evidence of CMV exposure and/or those receiving grafts from seropositive donors are considered to be at risk for viral reactivation and consequent target-organ disease in the profoundly immunosuppressed post-SCT state. CMV infection, defined as the presence of CMV viremia (antigenemia or DNAemia), viruria, or mucosal shedding in the absence of discernable organ disease, which may occur because of latent CMV reactivation or primary infection, occurs in approximately 45% to 65% [1,2] of at-risk SCT patients. CMV disease is involvement of target tissues such as lung, intestine, retina, or liver. Pre-emptive antiviral treatment of those with CMV infection after SCT usually prevents progression to disease, although some patients either do not respond to antiviral therapy or experience recurrence of CMV viremia [1,3–5]. CMV infection has been associated with increased risk of mortality during SCT [4,6] although the reasons for this are not well understood and the deaths are not usually attributable to CMV. Use of prophylactic antivirals for all at-risk patients during SCT decreases rates of CMV viremia [4,7]. However, the ability to detect individualized risk for CMV infection would be of great benefit, as prophylaxis has associated toxicity and may delay acquisition of CMV-specific graft-derived immunity [4,7,8].
It is known that factors affecting post-SCT T cell reconstitution and function impact the risk of CMV infection and disease [9]. During SCT, lymphocyte depletion by use of alemtuzumab or antithymocyte globulin (ATG) [10] and use of umbilical cord blood (UCB) grafts [9,11], which contain lower donor T cell doses and lack cells with CMV-specific immunologic memory, increase CMV infection risk. Immune dysregulation due to both the presence of graft-versus-host disease (GVHD) and its therapies is associated with increased rates of CMV infection [1–3,10–12]. Although the majority of patients resolve CMV infection with preemptive antiviral therapy, infection is persistent or recurrent in some, and there are scant data to assign risk or guide management in this setting. Additionally, some patients who undergo SCT have a prior history of CMV infection because of longstanding congenital immunodeficiency or acquired immunosuppression from chemotherapy. The course and outcome of these groups of patients has not been previously studied.
Although CMV infection in SCT has been investigated extensively in adults, considerably less data exist within the pediatric population because of both the smaller number of SCTs performed and lower rates of CMV seropositivity in this population [13,14]. We retrospectively analyzed pediatric patients at risk for CMV at a single large transplantation center over a 4-year period. We identified risk factors for post-SCT CMV infection that can be used to guide future CMV prophylaxis and treatment algorithms. Moreover, we provide a description of specific subgroups of pediatric SCT recipients: patients with persistent/recurrent CMV infection, with late CMV infection (defined as occurring after day 100 after SCT [15,16]), and those with documented CMV infection before SCT.
MATERIALS AND METHODS
Patients
Records of all 239 patients undergoing allogeneic SCT at Dana-Farber/Children’s Hospital Cancer Center from January 1, 2011 to December 31, 2014 were reviewed. The inclusion criteria for the study was at risk for CMV viral reactivation/disease on the basis of seropositivity of the donor and/or recipient, and 91 patients met this criteria. Of note, UCB patients were classified as at risk for CMV based solely on recipient seropositivity because of unknown UCB serostatus. Records of at-risk patients were reviewed for demographic information (age, sex) and baseline characteristics pertinent to SCT (indication for SCT, history of prior documented CMV infection, prior chemotherapy exposure). The source of stem cells and histocompatibility match were also recorded. The post-SCT course was reviewed from day 0 (the day of donor cell infusion) to the last documented clinical evaluation. Outcome measures including vital status, cause of mortality, chronic GVHD, CMV infection, and duration of follow-up were captured. The Dana-Farber/Harvard Cancer Center Institutional Review Board approved this study. The study was carried out in accordance with the Declaration of Helsinki.
Definitions
For bone marrow sources of stem cells, SCT was defined as matched if donor and recipient were identical by high-resolution typing at 10 of 10 HLA loci (A, B, C, DR, and DQ). For UCB SCT sources, matched SCTs included donors and recipients matched at least at 4 out of 6 of the HLA-A, HLA-B, and HLA-DR loci. UCB HLA typing was typically intermediate resolution. CMV infection before SCT was defined as documented CMV viremia of at least 500 copies per milliliter detected by quantitative PCR. CMV infection after SCT was defined as the presence of CMV viremia as detected by quantitative polymerase chain reaction (qPCR) as performed at Boston Children’s Hospital. Positive CMV qPCR required at least 500 copies of the viral genome/mL. CMV infection could arise from latent viral reactivation (in seropositive recipients) or presumed primary infection (in seronegative recipients). CMV disease was defined as biopsy-proven target-organ involvement. The time to clearance of CMV was defined as the time to the first of 2 negative blood CMV qPCR assays after the first positive assay. Treatment-related morality (TRM) was defined as death within 180 days of transplantation, secondary to early or late effects of treatment; patients whose underlying malignant disease recurred were not considered at risk of TRM. Late CMV infection was defined as infection detected after day 100 after SCT [15,16].
Conditioning Regimens
Conditioning regimens varied based on transplantation indication. Patients with acute myeloid leukemia typically received myeloablative busulfan and cyclophosphamide. Patients with acute lymphoblastic leukemia typically received total body irradiation and cyclophosphamide. Reduced-intensity regimens frequently incorporated fludarabine or alemtuzumab. All recipients of UCB transplants received equine ATG as part of the conditioning regimen.
Supportive Care
All patients received prophylaxis against Pneumocystis jiroveci. Patients also received fluconazole as prophylaxis against fungal infection. Routine fungal surveillance cultures were obtained and prophylaxis was adjusted based on sensitivities of isolates. Patients received supportive transfusions of irradiated packed red blood cells and platelets when needed.
GVHD Prophylaxis
In most cases, GVHD prophylaxis included a calcineurin inhibitor, typically cyclosporine. For related donors, cyclosporine was continued through day 100 after SCT and then weaned over the course of 10 to 12 weeks. Most unrelated donors also received SCTs, it was continued through day 180 after SCT and then weaned over the same time period. Unrelated donors also received corticosteroid prophylaxis (at 1 mg/kg prednisolone) starting shortly after SCT, which was typically weaned at day 21 after transplantation over 10 to 12 weeks if no GVHD occurred. Methotrexate (15 mg/m2 on day 1 after transplantation, followed by 10 mg/m2 on days 3, 6, and 11 after transplantation) was given to most patients [17]. For UCB SCT recipients, calcineurin inhibitor plus mycophenolate mofetil or corticosteroids were used without methotrexate.
GVHD Treatment
Diagnosis of GVHD was made based on established criteria [18]. Initial treatment for GVHD included methylprednisolone given intravenously or prednisone given orally (1 mg/kg to 2 mg/kg per day). In corticosteroid-refractory patients, additional immunomodulatory modalities, such as infliximab or extracorporeal photopheresis, were utilized. In corticosteroid-responsive patients, corticosteroids were slowly tapered when stable response was achieved.
CMV Surveillance
Patients considered to be at risk for CMV infection were screened with weekly CMV qPCR assays within 30 days before hospital admission and from time of SCT through day 100 after SCT. Some patients who experienced early CMV infection were screened to day 180 after SCT. Patients who were CMV seropositive received prophylaxis with acyclovir, 500 mg/m2 given intravenously every 8 hours from 1 day before transplantation through day 30 after transplantation. Before June 2012, CMV-seronegative recipients who received grafts from CMV-seropositive donors also received acyclovir prophylaxis.
CMV Treatment
If 1 positive qPCR result was obtained of at least 500 copies of the viral genome per mL of plasma, patients were initiated on treatment with CMV hyperimmune globulin (100 mg/kg 3 times per week given intravenously for 14 days) as well as either ganciclovir (5 mg/kg every 12 hours given intravenously) or foscarnet (60 mg/kg every 8 hours given intravenously) for a minimum treatment course of 14 days. This was extended if CMV viremia persisted longer than 14 days or if CMV disease was documented. After this treatment course and documentation of clearance of CMV viremia, patients received secondary prophylaxis with ganciclovir (5 mg/kg/day) or valganciclovir (500 mg/m2 daily) as well as CMV hyperimmune globulin weekly, typically through day 120 after SCT.
Statistical Analysis
Fisher’s exact test was applied to test the association of transplantation-related factors and the occurrence of post-SCT CMV infection, TRM, and chronic GVHD. A multivariate logistic regression model with backward selection was used to identify factors independently predictive of post-SCT CMV infection. Time-to-event (CMV reactivation or TRM by day 180) was calculated from date of SCT until event or until day 180 after SCT if the patient did not have an event by day 180. CMV infection-free survival curves and TRM-free survival curves were generated using the methods of Kaplan and Meier, with standard errors according to Peto [19], and comparison of survival curves was performed using a 2-sided log rank test. P values less than .05 were considered statistically significant. No adjustment was made for multiple testing because of the exploratory nature of this study.
RESULTS
Patients
Ninety-one CMV at-risk SCT recipients met study eligibility (Table 1) out of a total of 239 patients undergoing allogeneic transplantation during the study period (38% of all patients at risk for post-transplantation CMV). Forty-six of 91 (50%) underwent SCT for malignant conditions. All UCB SCTs were from unrelated sources. Fifty (55%) recipients received systemic corticosteroids after SCT, with 45 (49%) recipients exposed to corticosteroids before day +50. Twenty-four (26%) recipients received ATG and 9 (10%) were given alemtuzumab during conditioning. Recipient and donor serology combinations were nearly equally distributed (as follows [recipient/donor]: positive/positive, 28; positive/negative, 36; negative/positive, 26). Thirteen (14%) recipients developed acute GVHD: 3 grade were I and 10 were grade II or higher.
Table 1.
Baseline Characteristics of Pediatric Allogeneic Stem Cell Transplantation Patients at Risk for CMV
| Characteristics | Value |
|---|---|
| Overall | 91 |
| Age, median (range), yr | 7 (.3–23) |
| Sex | |
| Male | 44 (48) |
| Female | 47 (52) |
| Indication | |
| Leukemia | 42 (46) |
| MDS | 3 (3) |
| HLH | 6 (7) |
| Immunodeficiency | 17 (19) |
| Bone marrow failure | 11 (12) |
| Other | 12 (13) |
| Donor source | |
| Matched related | 36 (40) |
| Mismatched related | 1 (1) |
| Matched unrelated | 38 (42) |
| Mismatched unrelated | 7 (8) |
| UCB | 9 (10) |
| Reduced intensity conditioning | 9 (10) |
| GVHD prophylaxis calcineurin inhibitor | |
| Cyclosporine | 80 (88) |
| Tacrolimus | 11 (12) |
| Systemic corticosteroid use | |
| None | 41 (45) |
| Before day +50 | 45 (49) |
| After day +50 | 5 (5) |
| CMV serologic status (recipient/donor) | |
| Pos/pos | 28 (31) |
| Pos/neg | 36 (40) |
| Neg/pos | 26 (29) |
| Pos/ind | 1 (1) |
| CMV infection before SCT | |
| Yes | 6 (7) |
| No | 85 (93) |
MDS indicates myelodysplastic syndrome; HLH, hemophagocytic lymphohistiocytosis; pos, positive; neg, negative; ind, indeterminate.
Data presented are n (%), unless otherwise indicated.
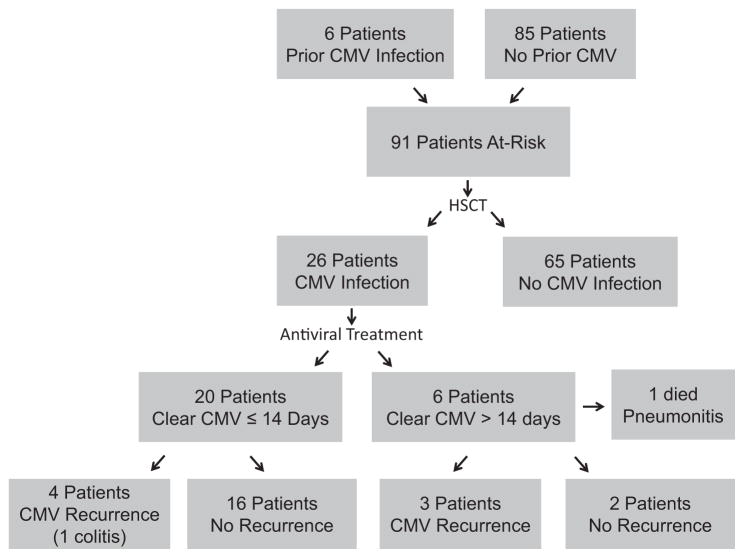
CMV Infection before SCT
Six patients in the cohort (7%) had documented CMV infection within 6 months before SCT, all with viremia but without organ involvement (Figure 1, Supplemental Table S1). Three of these patients had immunodeficiency disorders and 3 had hematologic malignancies. CMV viremia was most often identified during routine surveillance of immunodeficient patients or as part of the evaluation of fever. Infection was first detected 15 to 160 (median, 39) days before SCT and all cleared after CMV-directed therapy with negative PCR before the start of SCT conditioning. Four of these 6 patients received treatment-dosing antivirals during SCT, whereas the other 2 received standard acyclovir prophylaxis.
Figure 1.
Flow diagram of CMV infection and treatment in a cohort of 91 SCT recipients at risk for CMV infection.
CMV Infection and Disease
Out of a total of 91 patients at risk, 26 (29%) recipients experienced CMV infection (Figure 1) during the post-SCT surveillance period. CMV infection occurred at a median of 46 days after graft infusion (range, 9 to 127 days) (Figure 2A). The median CMV viral load at the time of initial infection was 2772 copies/mL (range, 552 to 24,611 copies/mL). Two of the 26 (8%) with CMV infection had biopsy-proven target-organ involvement: 1 patient with pneumonitis and another with colitis. Four of 6 (67%) patients with pre-SCT CMV viremia experienced post-SCT CMV infection, 1 developed pneumonitis, and 22 of 85 (26%) with no prior documented CMV viremia developed infection after SCT. The median time to post-SCT CMV infection for patients with pre-SCT CMV viremia (n = 6) was 15.5 days compared with 48 days for patients without prior documented CMV infection (n = 22; P = .07 by Wilcoxon rank sum test).
Figure 2.
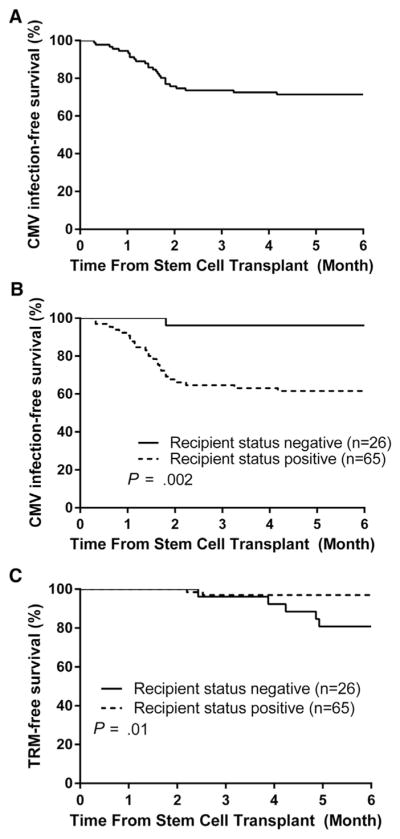
(A) CMV infection-free survival over time in a cohort of 91 SCT recipients at risk for CMV infection, starting from the day of stem cell infusion. (B) CMV infection-free survival over time in a cohort of 91 SCT recipients at risk for CMV infection, starting from day of stem cell infusion, by SCT recipient CMV serostatus (P = .002). (C) Treatment-related morality–free survival, starting from day of stem cell infusion, by SCT recipient CMV serostatus (P = .01).
Predictors of Post-SCT CMV Infection
We examined the relationship of SCT characteristics to the development of post-SCT CMV infection in at-risk patients (Table 2). There was a significantly increased risk of CMV infection in those undergoing UCB SCTs compared with those undergoing bone marrow SCTs (P = .01), with 6 of 9 UCB recipients developing CMV infection. Seven of 13 (54%) patients diagnosed with acute GVHD experienced CMV infection, which was a significant positive association (P = .04). There was no evidence to support increased risk of CMV infection in patients receiving SCT for malignancy (P = .10), recipients of unrelated grafts (P =.20), recipients exposed to corticosteroids within day 50 after SCT (P = .20) and those receiving total body irradiation (P = .80) or recipients exposed to ATG (P = .80), fludarabine (P = .50), or alemtuzumab (P = 1.00) in the conditioning regimen. Use of reduced-intensity conditioning regimens does not appear to affect CMV infection risk (P = .30). There was no apparent effect of age (P = .40). There was a trend for recipients who had documented CMV viremia before SCT (n = 6) to be at increased risk of CMV after SCT (P =.053), though this cohort was small.
Table 2.
Identification of SCT-related Risk Factors for Post-SCT CMV Infection
| Risk Factor | CMV Reactivation
|
Total n = 91 | P Value | |
|---|---|---|---|---|
| No n = 65 | Yes n = 26 | |||
| Age | ||||
| <7 yr | 33 | 10 | 43 | .40 |
| ≥7 yr | 32 | 16 | 48 | |
| Disease category | ||||
| Immunodeficiency | 16 | 3 | 19 | .30 |
| Other | 49 | 23 | 72 | |
| Disease category | ||||
| Malignant | 29 | 17 | 46 | .10 |
| Other | 36 | 9 | 45 | |
| Donor source | ||||
| Matched related donor | 29 | 7 | 36 | .20 |
| Other | 36 | 19 | 55 | |
| Donor source | ||||
| UCB | 3 | 6 | 9 | .01 |
| Other | 62 | 20 | 82 | |
| Donor source | ||||
| Unrelated donor (cord or marrow) | 35 | 19 | 54 | .20 |
| Matched related donor (marrow) | 29 | 7 | 36 | |
| Donor source | ||||
| Mismatched unrelated marrow donor | 5 | 2 | 7 | 1.00 |
| Matched marrow donor (related or unrelated) | 56 | 18 | 74 | |
| CMV status (recipient/donor) | ||||
| Pos/pos | 17 | 11 | 28 | .002* |
| Pos/neg | 23 | 13 | 36 | |
| Neg/pos | 25 | 1 | 26 | |
| Pos/ind | 0 | 1 | 1 | |
| CMV status (recipient/donor) | ||||
| Pos/pos | 17 | 11 | 28 | .80 |
| Pos/neg | 23 | 13 | 36 | |
| CMV status (recipient/donor) | ||||
| Pos/pos | 17 | 11 | 28 | .002 |
| Neg/pos | 25 | 1 | 26 | |
| Recipient CMV status | ||||
| Pos | 40 | 25 | 65 | .0006 |
| Neg | 25 | 1 | 26 | |
| Donor CMV status | ||||
| Pos | 42 | 12 | 36 | .20 |
| Neg | 23 | 13 | 54 | |
| Receipt of TBI | ||||
| Yes | 25 | 9 | 34 | .80 |
| No | 40 | 17 | 57 | |
| History of CMV before transplantation | ||||
| Yes | 2 | 4 | 6 | .053 |
| No | 63 | 22 | 85 | |
| Reduced-intensity regimen | ||||
| Yes | 9 | 1 | 10 | .30 |
| No | 56 | 25 | 81 | |
| ATG use in conditioning | ||||
| Yes | 18 | 6 | 24 | .80 |
| No | 47 | 20 | 67 | |
| Alemtuzumab use in conditioning | ||||
| Yes | 7 | 2 | 9 | 1.00 |
| No | 58 | 24 | 82 | |
| Fludarabine use in conditioning | ||||
| Yes | 26 | 8 | 34 | .50 |
| No | 39 | 18 | 57 | |
| ATG, alemtuzumab, or fludarabine use in conditioning | ||||
| Yes | 34 | 12 | 46 | .60 |
| No | 31 | 14 | 45 | |
| Steroid exposure within day +50 after transplantation | ||||
| Yes | 29 | 16 | 45 | .20 |
| No | 36 | 10 | 46 | |
| Acute GVHD | ||||
| Yes | 6 | 7 | 13 | .04 |
| No | 59 | 19 | 78 | |
| Neutrophil engraftment within day 21 | ||||
| Yes | 28 | 14 | 42 | .40 |
| No | 37 | 12 | 49 | |
TBI indicates total body irradiation.
P value of Fisher’s exact test.
One patient with “positive/indeterminate” (positive recipient but indeterminate donor serostatus) was not included in the Fisher’s exact test.
Among at-risk patients, recipient seropositivity was a strong predictor of both the occurrence of post-SCT CMV infection (25 of 64 [38%] with seropositivity versus 1 of 26 [4%] in those without; P =.0006) (Table 3) and earlier time to occurrence of post-SCT CMT infection (P = .002) (Figure 2B). Among CMV-seropositive recipients, receiving a CMV-seronegative graft was not associated with increased CMV infection risk compared with recipients of a seropositive graft (P = .20). The combination of a CMV-seronegative recipient and CMV-seropositive donor was associated with a significantly decreased risk of CMV infection compared with other serostatus combinations (P = .002) (Table 2).
Table 3.
Multivariate Logistic Regression Models Testing the Association of Transplantation-related Factors with CMV infection (n = 91)
| Parameter | Model 1
|
Model 2
|
|||||
|---|---|---|---|---|---|---|---|
| Wald Chi-Square Value | P Value | Wald Chi-Square Value | P Value | OR Estimate | 95% Confidence Limits on the OR |
||
| Intercept | 2.0355 | .1537 | 6.1932 | .0128 | |||
| Recipient CMV | |||||||
| Pos versus neg | 5.9772 | .0145 | 6.9089 | .0086 | 16.5 | 2.0 | 133.8 |
| Acute GVHD | |||||||
| Yes versus no | 3.0671 | .0799 | 4.0215 | .0449 | 4.0 | 1.0 | 15.6 |
| Source of transplant | |||||||
| UCB | 2.2492 | .1337 | |||||
OR indicates odds ratio.
Model 2 (the final parsimonious model) identifies an association of recipient CMV and acute GVHD with the occurrence of post-SCT CMV infection.
On multivariate analysis, recipient CMV seropositivity (odds ratio, 16.525; 95% confidence interval, 2.041 to 133.803) and acute GVHD (odds ratio, 4.018; 95% confidence interval, 1.032 to 15.643) were significantly and independently associated with post-SCT CMV infection (Table 3).
Antiviral Treatment and Efficacy
All patients with CMV viremia detected in the post-SCT surveillance period received a 14-day course of antiviral and thrice weekly immune globulin therapy. Twenty patients (77%) cleared CMV viremia within this period (Figure 1). Median time to CMV viremia clearance was 9.5 days (range, 1 to 34 days). Fifteen of 20 (75%) treated initially with ganciclovir cleared viremia within 14 days. Four of 5 (80%) patients treated initially with foscarnet cleared within 14 days. One patient treated with valganciclovir cleared CMV viremia in 3 days. Six patients did not clear CMV infection within 14 days (5 treated with ganciclovir, 1 with foscarnet), with 1 of the ganciclovir-treated patients developing CMV pneumonitis, with death secondary to this.
Recurrent CMV Infection
A total of 7 of 26 (27%) SCT recipients with initial post-SCT CMV infection experienced recurrence at a median of 33 days (range, 9 to 74 days) after clearance of initial infection (Figure 1). All episodes occurred in unrelated-donor SCTs, with 4 patients having received UCB transplants (Supplemental Table S2). None of these patients had CMV infection before transplantation. Three of these recurrent episodes occurred in recipients who did not clear CMV viremia within 14 days of upfront treatment. Four of these patients had at least grade II acute GVHD. Five of these patients developed recurrent infection while on secondary CMV prophylaxis. All CMV infection recurrences were in CMV-seropositive recipients who were exposed to systemic corticosteroids within 50 days after SCT for various indications. Five of 7 recipients with CMV recurrence had received alemtuzumab, ATG, or fludarabine during conditioning, and 6 of 7 had a peak viral load of at least 2000 copies per μL (range, 1569 to 85,276 copies/mL) during the initial CMV infection. Because of persistent CMV viremia on ganciclovir, 1 patient’s isolate was tested for antiviral resistance and was determined to be resistant to ganciclovir and sensitive to foscarnet and cidofovir. Treatment was changed to foscarnet with clearance CMV within 3 days. One patient with recurrent CMV developed CMV colitis.
Late CMV Infection
Patients with early CMV infection were screened past day 100 after SCT. A total of 3 recipients in our cohort developed late CMV infection at 105 to 127 days after SCT (Supplemental Table S3). All underwent SCT for malignant indications and had unrelated donors. All of these 3 recipients had experienced early CMV infection before day 100 after SCT and had cleared the initial infection within 14 days with antiviral treatment. One developed biopsy-proven CMV colitis. All 3 of these individuals had acute GVHD of at least grade II and had received corticosteroids for GVHD prophylaxis starting at day 7 after SCT, as well as for acute GVHD.
SCT Outcome
We assessed factors influencing SCT outcomes, including day 180 TRM and development of chronic GVHD (Table 4). A higher proportion of CMV-seronegative recipients who received seropositive grafts had day 180 TRM (P = .02) (Table 4) and decreased TRM-free survival (P = .01) (Figure 2C) compared with all seropositive recipients. Examination of these cases individually did not disclose a recurrent specific cause of TRM, and none of these patients experienced relapse of their initial disease. CMV-seropositive recipients who received a seronegative graft had no difference in the occurrence of day 180 TRM compared with seropositive recipients of a seropositive graft (P = .50, Fisher’s exact test) (Table 4). TRM occurred in 2 of 6 who did not clear CMV infection within 14 days of antiviral treatment. CMV infection itself did not confer an increased risk of the occurrence of TRM (P = .70) (Table 4).
Table 4.
Identification of Risk Factors for the Occurrence of TRM by Day 180 after SCT in Patients at Risk for CMV
| Risk Factors | TRM at day 180
|
Total n = 91 | P Value | |
|---|---|---|---|---|
| No n = 84 | Yes n = 7 | |||
| Age | ||||
| <7 yr | 40 | 3 | 43 | 1.00 |
| ≥7 yr | 44 | 4 | 48 | |
| Disease category | ||||
| Immunodeficiency | 17 | 2 | 19 | .60 |
| Other | 67 | 5 | 72 | |
| Disease category | ||||
| Malignant | 44 | 2 | 46 | .30 |
| Other | 40 | 5 | 45 | |
| Donor source | ||||
| Matched related donor | 34 | 2 | 36 | .70 |
| Other | 50 | 5 | 55 | |
| Donor source | ||||
| UCB | 9 | 0 | 9 | 1.00 |
| Other | 75 | 7 | 82 | |
| Donor source | ||||
| Unrelated donor (cord or marrow) | 49 | 5 | 54 | .70 |
| Matched related donor (marrow) | 34 | 2 | 36 | |
| Donor source | ||||
| Mismatched unrelated marrow donor | 7 | 0 | 7 | 1.00 |
| Matched marrow donor CMV status (recipient/donor) | 67 | 7 | 74 | |
| Pos/pos | 28 | 0 | 28 | .02* |
| Pos/neg | 34 | 2 | 36 | |
| Neg/pos | 21 | 5 | 26 | |
| Pos/ind | 1 | 1 | ||
| CMV status (recipient/donor) | ||||
| Pos/pos | 28 | 0 | 28 | .50 |
| Pos/neg | 34 | 2 | 36 | |
| CMV status (recipient/donor) | ||||
| Pos/pos | 28 | 0 | 28 | .02 |
| Neg/pos | 21 | 5 | 26 | |
| Recipient CMV status | ||||
| Pos | 63 | 2 | 65 | .02 |
| Neg | 21 | 5 | 26 | |
| Donor CMV status | ||||
| Pos | 49 | 5 | 54 | .70 |
| Neg | 34 | 2 | 36 | |
| Receipt of TBI | ||||
| Yes | 33 | 1 | 34 | .30 |
| No | 51 | 6 | 57 | |
| History of CMV before transplantation | ||||
| Yes | 5 | 1 | 6 | .40 |
| No | 79 | 6 | 85 | |
| Reduced-intensity regimen | ||||
| Yes | 8 | 2 | 10 | .20 |
| No | 76 | 5 | 81 | |
| ATG use in conditioning | ||||
| Yes | 22 | 2 | 24 | 1.00 |
| No | 62 | 5 | 67 | |
| Alemtuzumab use in conditioning | ||||
| Yes | 7 | 2 | 9 | .10 |
| No | 77 | 5 | 82 | |
| Fludarabine use in conditioning | ||||
| Yes | 29 | 5 | 34 | .10 |
| No | 55 | 2 | 57 | |
| ATG, alemtuzumab, or fludarabine use in conditioning | ||||
| Yes | 41 | 5 | 46 | .40 |
| No | 43 | 2 | 45 | |
| Steroid exposure within day +50 after transplantation | ||||
| Yes | 41 | 4 | 45 | .70 |
| No | 43 | 3 | 46 | |
| Acute GVHD | ||||
| Yes | 11 | 2 | 13 | .30 |
| No | 73 | 5 | 78 | |
| Neutrophil engraftment within day 21 | ||||
| Yes | 37 | 5 | 42 | .20 |
| No | 47 | 2 | 49 | |
| Reactivation of CMV | ||||
| Yes | 25 | 1 | 26 | .70 |
| No | 59 | 6 | 65 | |
| Re-reactivation of CMV | ||||
| Yes | 6 | 0 | 6 | 1.00 |
| No | 78 | 7 | 85 | |
P value of Fisher’s exact test.
One patient with “pos/ind” (positive recipient but indeterminate) was not included in the Fisher’s exact test.
Disease, donor source, conditioning regimens, and CMV serostatus were not significantly associated with the development of chronic GVHD. Nine of 65 (14%) CMV-seropositive recipients developed chronic GVHD compared with 0% of seronegative recipients, though this result did not reach statistical significance (P = .056). CMV infection did not confer an increased risk of chronic GVHD in our cohort (P = .30).
A similar proportion of patients with pre-SCT CMV infection had day 180 TRM compared with others (P = .40) (Table 4). One of these 6 developed CMV pneumonitis and experienced TRM at day 77 after SCT, whereas the other 5 are alive at long-term follow-up (Supplemental Table S1). None of these patients developed chronic GVHD.
Among patients experiencing CMV recurrence, 2 out of 7 (29%) experienced TRM (Supplemental Table S2), compared with 5% among patients with a single episode of CMV infection and 12% among all at-risk patients. Three out of 7 (43%) patients with CMV recurrence died (from TRM [n = 2] and disease relapse [n = 1]) (Supplemental Table S2). Six of 26 (15%; TRM, n = 3; disease relapse, n = 3) patients with a single episode of CMV infection died and 19 (21%) of all 91 patients at risk died. Two patients with multiple episodes of CMV infection developed chronic GVHD. Among the 3 patients who experienced late CMV infection, 1 experienced TRM (Supplemental Table S3). Two patients with late CMV infection developed chronic GVHD.
DISCUSSION
We have described the course of 91 pediatric SCT patients at risk for CMV infection at a single center over a 4-year period. We retrospectively evaluated risk factors affecting CMV infection in this group, as well as the impact of CMV infection on SCT outcomes. We additionally described the incidence of recurrent CMV infection, the impact of multiple episodes of CMV infection on outcome, our experience with late CMV, and the SCT course of a cohort of patients with pre-SCT CMV infection.
The course and outcome of pediatric patients with documented CMV infection before SCT are previously not described. We found that these patients had a high incidence of post-SCT CMV infection and had a tendency toward earlier reactivation compared with other patients at risk for CMV. One developed CMV pneumonitis that resulted in TRM. It is important to emphasize that the other 5 patients survived without CMV organ disease, indicating that patients with prior CMV infection can safely navigate SCT; however, these patients have a high risk of viremia and should be carefully monitored.
Without prophylactic antiviral use, rates of CMV infection during allogeneic SCT among at-risk adult patients range from 45% to 65% [1,2,12], though this is likely dependent on the prevalence of latent CMV infection in donor populations, which may vary with geography and socioeconomic status [20]. Among pediatric centers, use of antiviral prophylaxis for CMV at-risk recipients is variable [21], and although this strategy can decrease infection risk considerably [4,7,8], long-term impact on CMV immune control is not known. Our center routinely uses prophylactic acyclovir for 30 days after SCT in at-risk patients, and our reported frequency of CMV reactivation compares favorably with those reported in adult and other pediatric series [2,9,12,14,22].
Donor and recipient serostatus has been extensively investigated with regard to effect on CMV reactivation as well as SCT outcome. The ideal combination is clearly seronegative recipient and donor; however, use of a seropositive graft for seronegative patients is often unavoidable. Among seronegative patients, those recipients who receive seropositive grafts have been reported to have worse overall survival compared with those who receive seronegative grafts [12,23,24], suggestive of possible graft-derived infection. In series of pediatric patients, incidence of CMV infection among seronegative donors receiving seropositive grafts was reported to range from 0% [14,22] to 25% [23]. We found a very low incidence of CMV infection in this cohort, suggesting that use of CMV-seropositive grafts for seronegative recipients is a reasonable approach that does not create a large risk of CMV-related morbidity. As we historically utilized antiviral prophylaxis in all at-risk patients at our institution, this may explain our low incidence of 4% in this group.
Recipient CMV seropositivity is generally thought to be an adverse factor for outcome in SCT although the exact mechanism for this is unclear in the age of effective CMV screening and pre-emptive therapy [24]. In the unrelated donor setting, CMV-seropositive recipients receiving seronegative grafts have been suggested to have decreased survival compared with those receiving seropositive grafts in both adult and pediatric series [14,25], although this has not been specifically linked to CMV-related mortality. A large retrospective analysis of 49,542 patients in the European Bone Marrow Transplantation registry supported this notion, but only among those donors who received myeloablative conditioning [13]. This effect has also been reported in a small pediatric cohort [14]. Therefore, it has been proposed that CMV-seropositive recipients should receive grafts from seropositive donors to confer graft-derived acquired adaptive immunity and, thereby, curb viral reactivation. We did not observe increased occurrence of TRM in CMV-seropositive patients who received seronegative grafts compared with those who received seropositive grafts. However, we did observe an effect of CMV serostatus on outcome, finding that CMV-seropositive patients had a lower occurrence of TRM within 180 days after SCT compared with seronegative patients who received CMV-seropositive grafts. This outcome appeared to be unrelated to CMV infection, as we observed a markedly lower rate of CMV infection in seronegative recipients compared with in seropositive patients. Our data show that recipient serostatus appears to be an important determinant of CMV infection risk, and this does not appear to be affected by donor serostatus.
Acute GVHD has been reproducibly associated with CMV infection in both pediatric and adult patients [2,14,26], in accordance with our findings. It has been speculated previously that this association was due to an immune dysregulatory state [15], and it was recently shown that CMV infection in SCT patients disrupts the T cell repertoire of the graft [27]. Acute GVHD is almost always treated with initially with systemic corticosteroids and so the relationship of corticosteroid exposure and CMV infection has previously been analyzed [22]. Therefore, it could be hypothesized that corticosteroid exposure might be a surrogate marker for acute GVHD. Our center routinely uses corticosteroids for GVHD prophylaxis for unrelated donor sources (a practice that may not be routine at other centers). As such, recipients of unrelated donor SCTs are included in our cohort of corticosteroid-exposed recipients. As we found no evidence of a significant association of corticosteroid exposure and CMV infection, corticosteroid GVHD prophylaxis is likely not a risk factor for CMV infection in our cohort.
The effect of alternative donors compared to matched related stem cell donors on CMV infection has been previously described in adult and pediatric patients [12,22,28,29]. Among our cohort, we did not observe an increased rate of initial CMV infection in the group of SCT recipients who received non–matched related marrow grafts compared with those who received fully matched sibling marrow grafts. Among pediatric patients, alternative donors confer increased risk of CMV infection in some but not all series [14,22]. We did, however, find a significantly increased risk of CMV infection in recipients of UCB SCTs compared with in recipients of bone marrow SCTs. UCB SCT has been reported to be associated with a 50% to 55% incidence rate of CMV antigenemia after SCT [11,30]. Based on these results and our own, we can speculate that this may be due to the absence of anti-CMV acquired immune function in UCB-derived lymphoid cells [31] and slower hematopoietic recovery compared with those after bone marrow–derived grafts [32]. We did find that all cases of late and recurrent CMV infection occurred in recipients of unrelated grafts, suggestive of a relationship between unrelated donor sources a longer period of susceptibility to post-SCT CMV infection.
Pre-emptive treatment for CMV infection has been shown to be effective in decreasing the incidence of post-transplantation CMV end-organ disease [4,33]. Two recipients with CMV infection(8%) developedCMV disease inour cohort—1 with persistence of viremia despite pre-emptive treatment and the other with recurrent CMV infection. Our results compare favorably with prior published series of pediatric patients, reporting 12% and 25% SCT recipients with CMV infection developing end-organ disease [14,22].
We described SCT recipients who experienced multiple episodes of CMV, a cohort where there is limited evidence to guide management [16,22,34]. Among those with initial infection, incidence of recurrence has been reported to be 10% to 38% [16,22,34], consistent with our rate of 27%. Reported risk factors for recurrence include unrelated donor SCT and antiviral treatment duration beyond 4 weeks for first infection [34]. With a total of 7 recipients who experienced at least 2 episodes of CMV, our study was not sufficiently powered to statistically evaluate factors impacting infection recurrence. Five of 7 recipients who experienced recurrent CMV were exposed the potent lympholytics during conditioning, suggesting that prolonged lymphopenia may predispose to CMV recurrence. All 7 of these patients had been exposed to systemic corticosteroids within 50 days after SCT, potentially delaying functional lymphoid reconstitution. Finally, our data suggest that patients with multiple episodes of CMV may be at higher risk of mortality.
In our cohort, a total of 3 SCT recipients experienced CMV infection after day 100, defined as late CMV infection. Proposed risk factors for late CMV infection in adults include GVHD, early CMV infection, a CMV-seronegative graft, and persistent lymphopenia [16]. All 3 in our cohort had acute GVHD, 2 had early CMV infection, and 1 received a seronegative graft. Larger cohorts are required to investigate prognostic factors for late CMV infection in pediatric patients.
We have reported on our experience in pediatric allogeneic SCT in patients at risk for CMV infection. Although current prophylaxis, surveillance, and treatment strategies have markedly decreased the incidence of CMV infection and target-organ disease in SCT [7,33], our findings show that the patients who experience persistent viremia, late infection, and recurrent infection bear the burden of CMV morbidity in modern pediatric SCT. Our data suggest that patients with acute GVHD, and possibly those receiving UCB grafts, deserve close attention and surveillance. Therefore, risk-adapted CMV surveillance and treatment schema should ideally be designed for these patients. Though we are a large pediatric SCT center, our cohort is too small to definitively identify risk factors for poor CMV outcome that occurs only in a small minority of patients using our surveillance and treatment strategies. However, we found that patients with adverse CMV outcome tended to be seropositive, have unrelated donors, experience acute GVHD, and be exposed to lympholytic agents during SCT, consistent with the proposed link between immune reconstitution and dysregulation and handling of CMV infection [24]. As such, SCT recipients at risk for CMV with these characteristics deserve meticulous management with more aggressive prophylaxis and screening.
Supplementary Material
Acknowledgments
This research was supported by the Natalia Fund at the Dana-Farber Cancer Institute.
Authorship statement: W.B.L. and L.L. contributed equally to this work.
Financial disclosure statement: The authors have no financial conflicts of interest to disclose.
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.bbmt.2016.04.004.
References
- 1.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12:322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller W, Flynn P, McCullough J, et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood. 1986;67:1162–1167. [PubMed] [Google Scholar]
- 3.Broers AE, van Der Holt R, van Esser JW, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95:2240–2245. [PubMed] [Google Scholar]
- 4.Goodrich JM, Bowden RA, Fisher L, et al. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118:173–178. doi: 10.7326/0003-4819-118-3-199302010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Reusser P, Einsele H, Lee J, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002;99:1159–1164. doi: 10.1182/blood.v99.4.1159. [DOI] [PubMed] [Google Scholar]
- 6.Humar A, Wood S, Lipton J, et al. Effect of cytomegalovirus infection on 1-year mortality rates among recipients of allogeneic bone marrow transplants. Clin Infect Dis. 1998;26:606–610. doi: 10.1086/514569. [DOI] [PubMed] [Google Scholar]
- 7.Winston DJ, Ho WG, Bartoni K, et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993;118:179–184. doi: 10.7326/0003-4819-118-3-199302010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Prentice HG, Gluckman E, Powles RL, et al. Impact of long-term acyclovir on cytomegalovirus infection and survival after allogeneic bone marrow transplantation. European Acyclovir for CMV Prophylaxis Study Group. Lancet. 1994;343:749–753. doi: 10.1016/s0140-6736(94)91835-x. [DOI] [PubMed] [Google Scholar]
- 9.Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett. 2014;342:1–8. doi: 10.1016/j.canlet.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 10.George B, Kerridge IH, Gilroy N, et al. A risk score for early cytomegalovirus reactivation after allogeneic stem cell transplantation identifies low-, intermediate-, and high-risk groups: reactivation risk is increased by graft-versus-host disease only in the intermediate-risk group. Transpl Infect Dis. 2012;14:141–148. doi: 10.1111/j.1399-3062.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura T, Narimatsu H, Kami M, et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biol Blood Marrow Transplant. 2007;13:577–583. doi: 10.1016/j.bbmt.2006.12.454. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91:78–83. [PubMed] [Google Scholar]
- 13.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2014;59:473–481. doi: 10.1093/cid/ciu364. [DOI] [PubMed] [Google Scholar]
- 14.Paris C, Kopp K, King A, et al. Cytomegalovirus infection in children undergoing hematopoietic stem cell transplantation in Chile. Pediatr Blood Cancer. 2009;53:453–458. doi: 10.1002/pbc.22060. [DOI] [PubMed] [Google Scholar]
- 15.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozdemir E, Saliba RM, Champlin RE, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40:125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 17.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 18.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier MP. Nonparametric estimation from incomplete observations. J Am Stat Assn. 1958;53:457–481. [Google Scholar]
- 20.N’Diaye DS, Yazdanpanah Y, Krivine A, et al. Predictive factors of cytomegalovirus seropositivity among pregnant women in Paris, France. PLoS One. 2014;9:e89857. doi: 10.1371/journal.pone.0089857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bontant T, Sedlacek P, Balduzzi A, et al. Survey of CMV management in pediatric allogeneic HSCT programs, on behalf of the inborn errors, infectious diseases and pediatric diseases working parties of EBMT. Bone Marrow Transplant. 2014;49:276–279. doi: 10.1038/bmt.2013.164. [DOI] [PubMed] [Google Scholar]
- 22.Yoon HS, Lee JH, Choi ES, et al. Cytomegalovirus infection in children who underwent hematopoietic stem cell transplantation at a single center: a retrospective study of the risk factors. Pediatr Transplant. 2009;13:898–905. doi: 10.1111/j.1399-3046.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 23.Tan PL, Lim LM, Khanlian C, Villegas MS. A single-center experience of cytomegalovirus infections in Asian pediatric patients undergoing allogeneic hematopoietic stem cell transplant for leukemia in Singapore. Transpl Infect Dis. 2014;16:556–560. doi: 10.1111/tid.12238. [DOI] [PubMed] [Google Scholar]
- 24.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103:2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 25.Ljungman P, Brand R, Einsele H, et al. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102:4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 26.Walker CM, van Burik JA, DeFor TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Suessmuth Y, Mukherjee R, Watkins B, et al. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRbeta repertoire. Blood. 2015;125:3835–3850. doi: 10.1182/blood-2015-03-631853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakrabarti S, Mackinnon S, Chopra R, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- 29.Schetelig J, Oswald O, Steuer N, et al. Cytomegalovirus infections in allogeneic stem cell recipients after reduced-intensity or myeloablative conditioning assessed by quantitative PCR and pp65-antigenemia. Bone Marrow Transplant. 2003;32:695–701. doi: 10.1038/sj.bmt.1704164. [DOI] [PubMed] [Google Scholar]
- 30.Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:215–222. doi: 10.1016/j.bbmt.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis ZB, Cooley SA, Cichocki F, et al. Adaptive natural killer cell and killer cell immunoglobulin-like receptor-expressing T cell responses are induced by cytomegalovirus and are associated with protection against cytomegalovirus reactivation after allogeneic donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1653–1662. doi: 10.1016/j.bbmt.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 33.Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325:1601–1607. doi: 10.1056/NEJM199112053252303. [DOI] [PubMed] [Google Scholar]
- 34.Einsele H, Hebart H, Kauffmann-Schneider C, et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000;25:757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.